Gamma-Irradiation Induced Functionalization of Graphene Oxide with Organosilanes
Abstract
1. Introduction
2. Results and Discussion
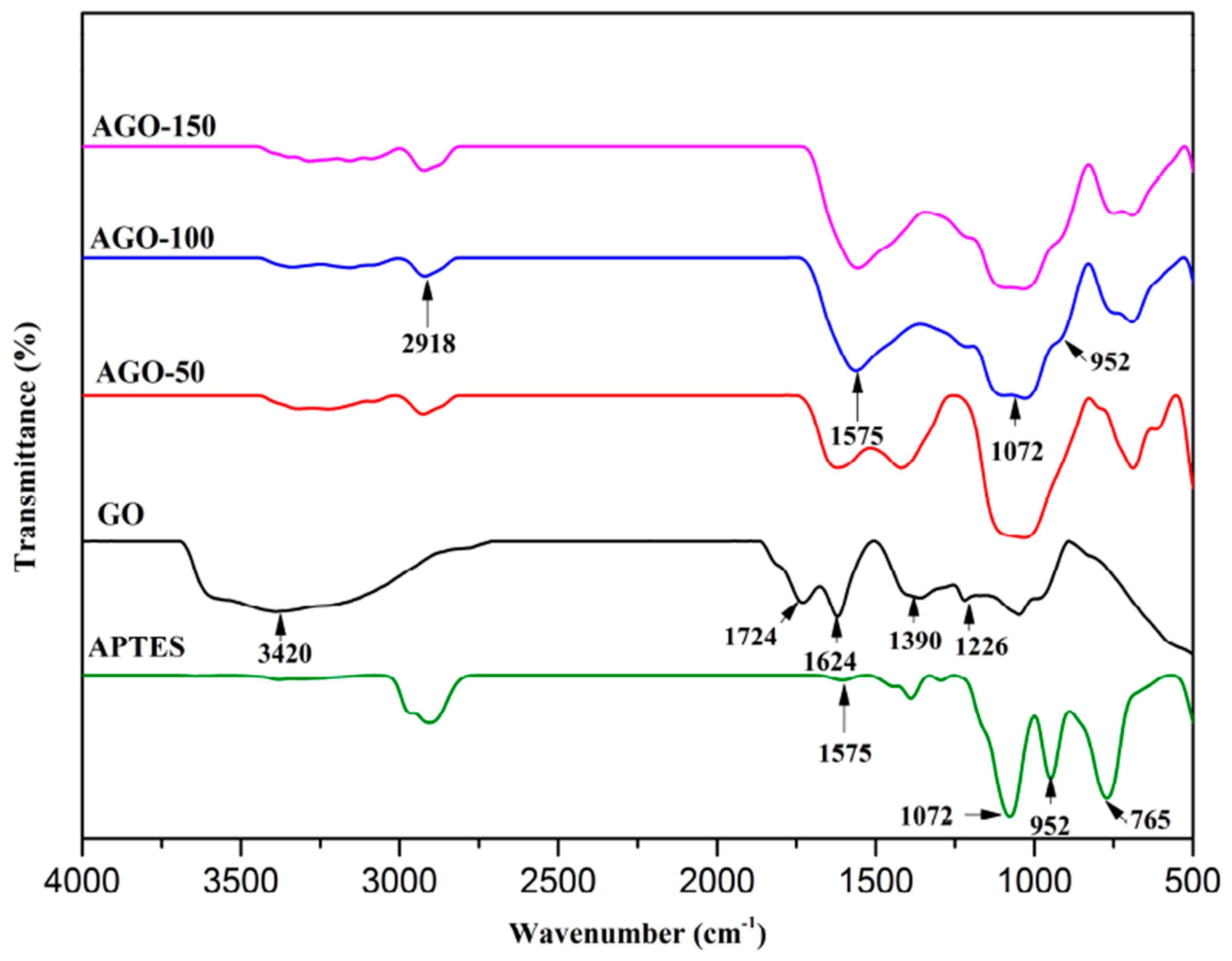
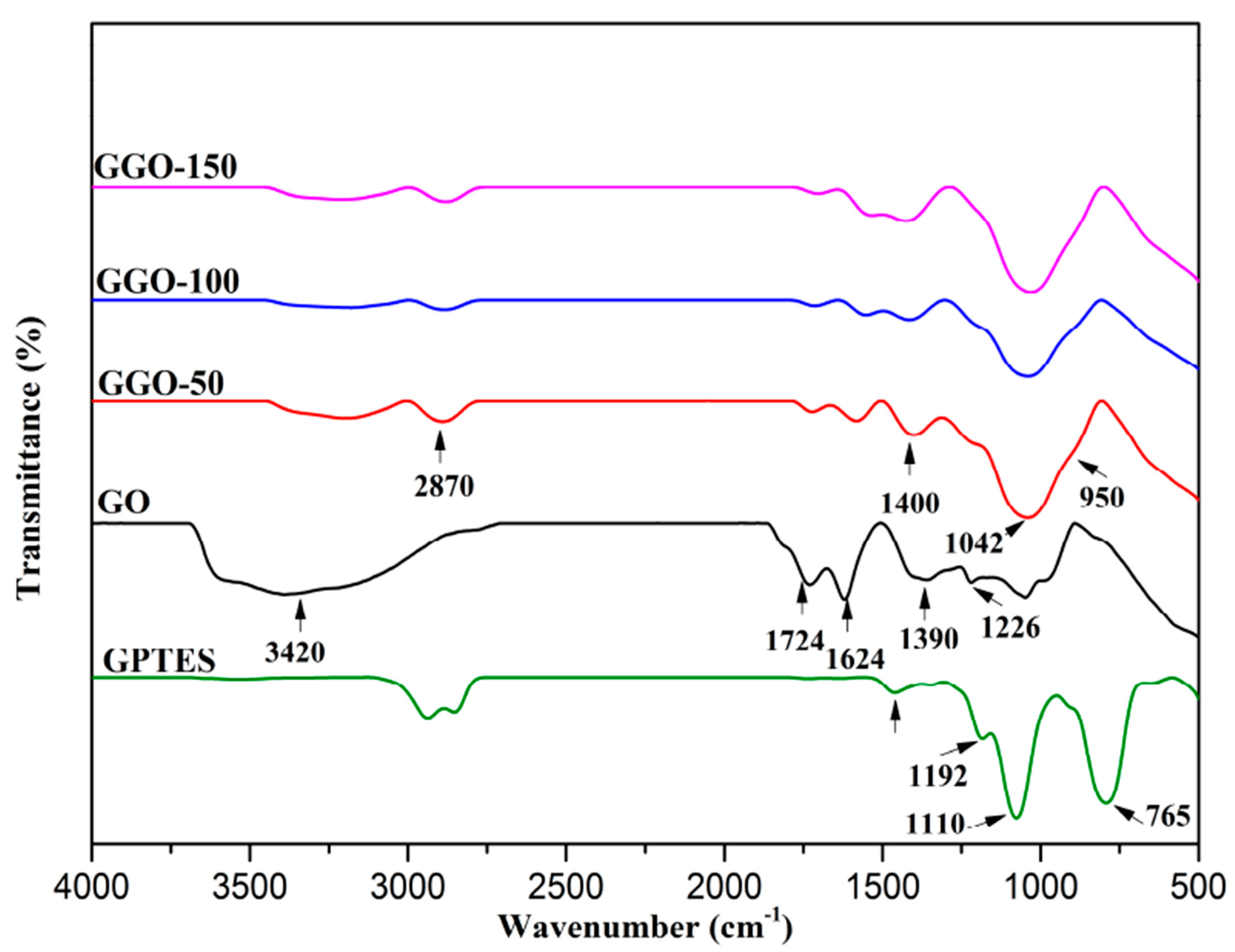
2.1. Fourier Transform Infrared (FTIR)
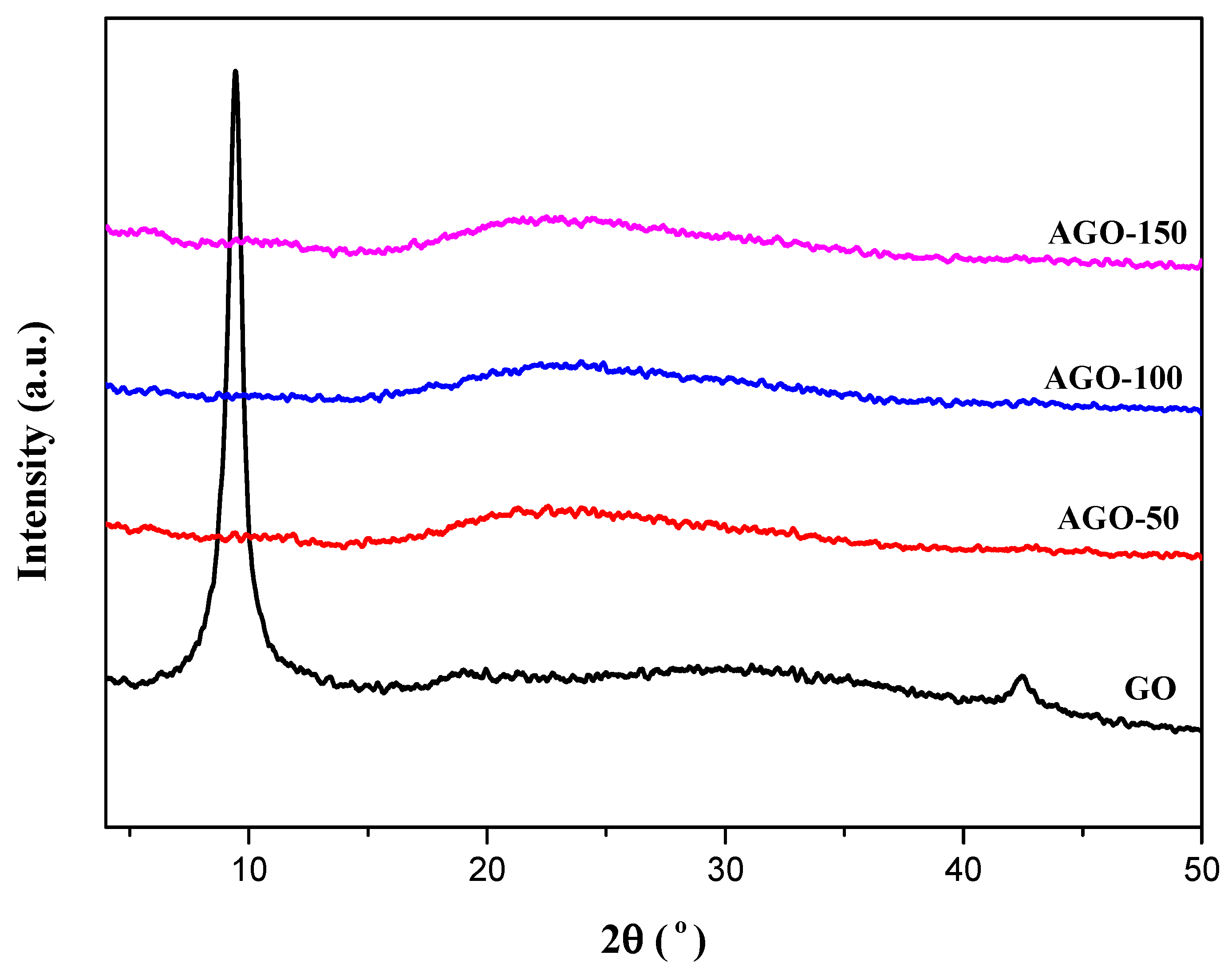
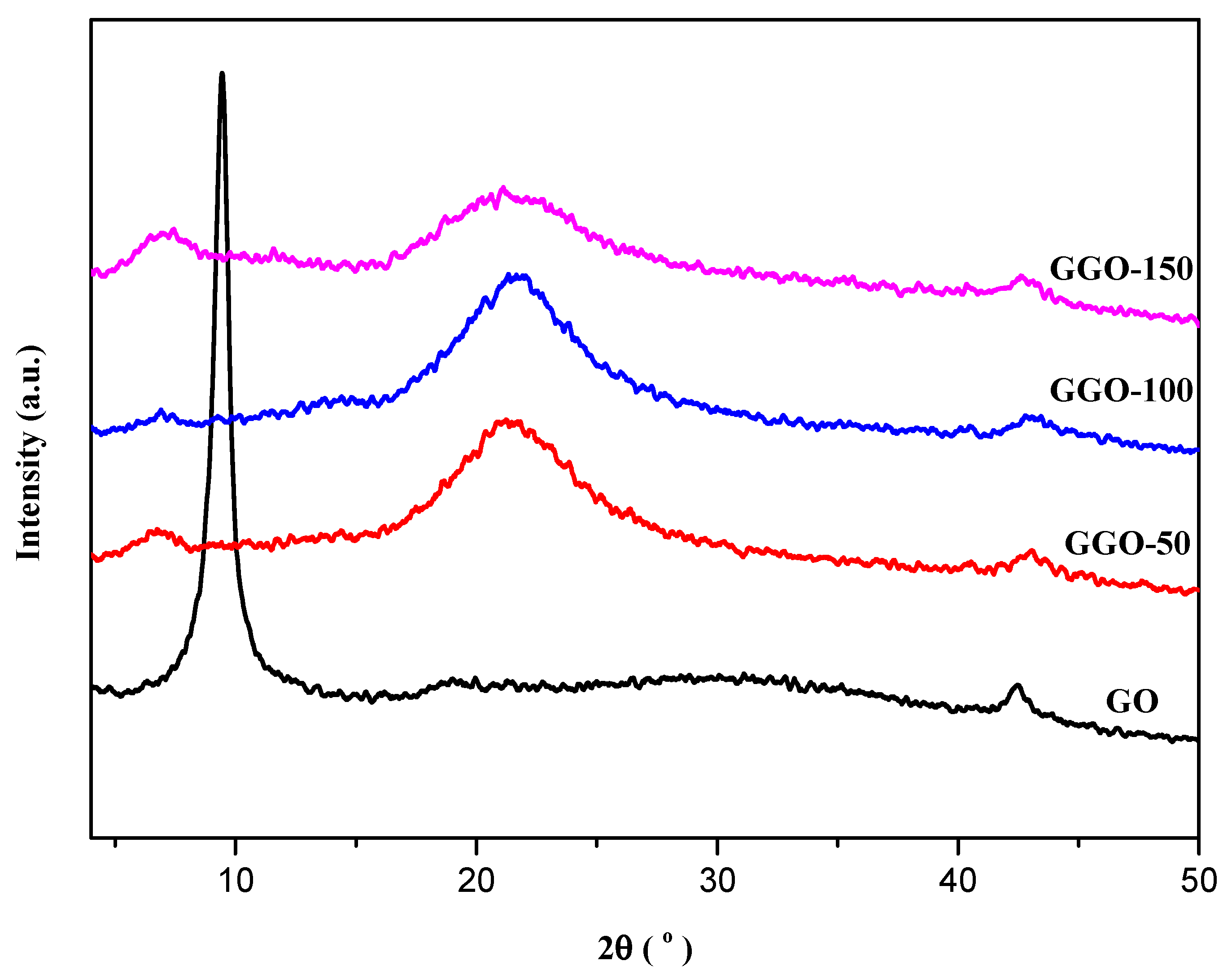
2.2. X-ray Diffraction (XRD) Analysis
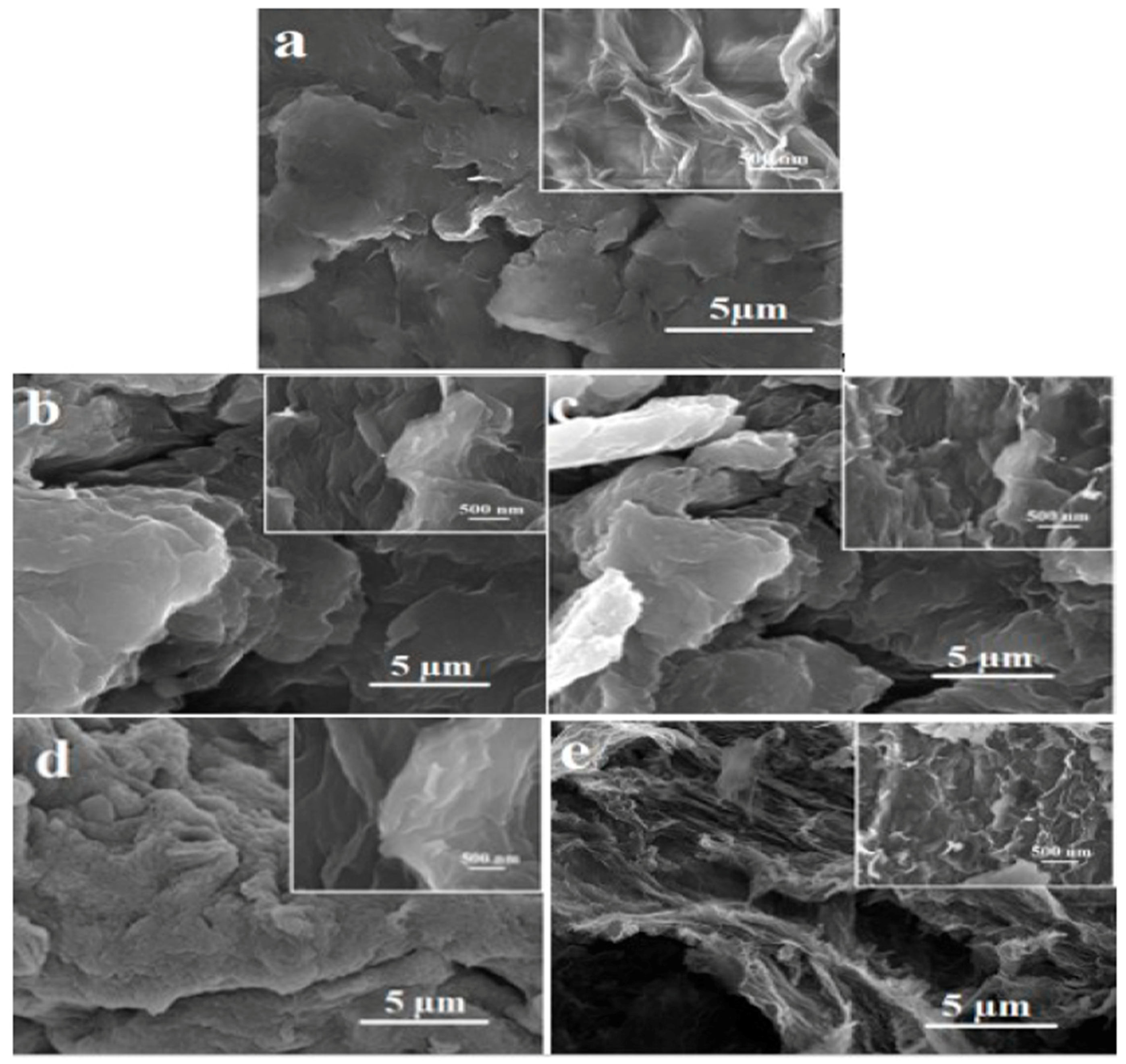
2.3. Field Emission Scanning Electron Microscopy (FE-SEM)
2.4. Raman Spectroscopy
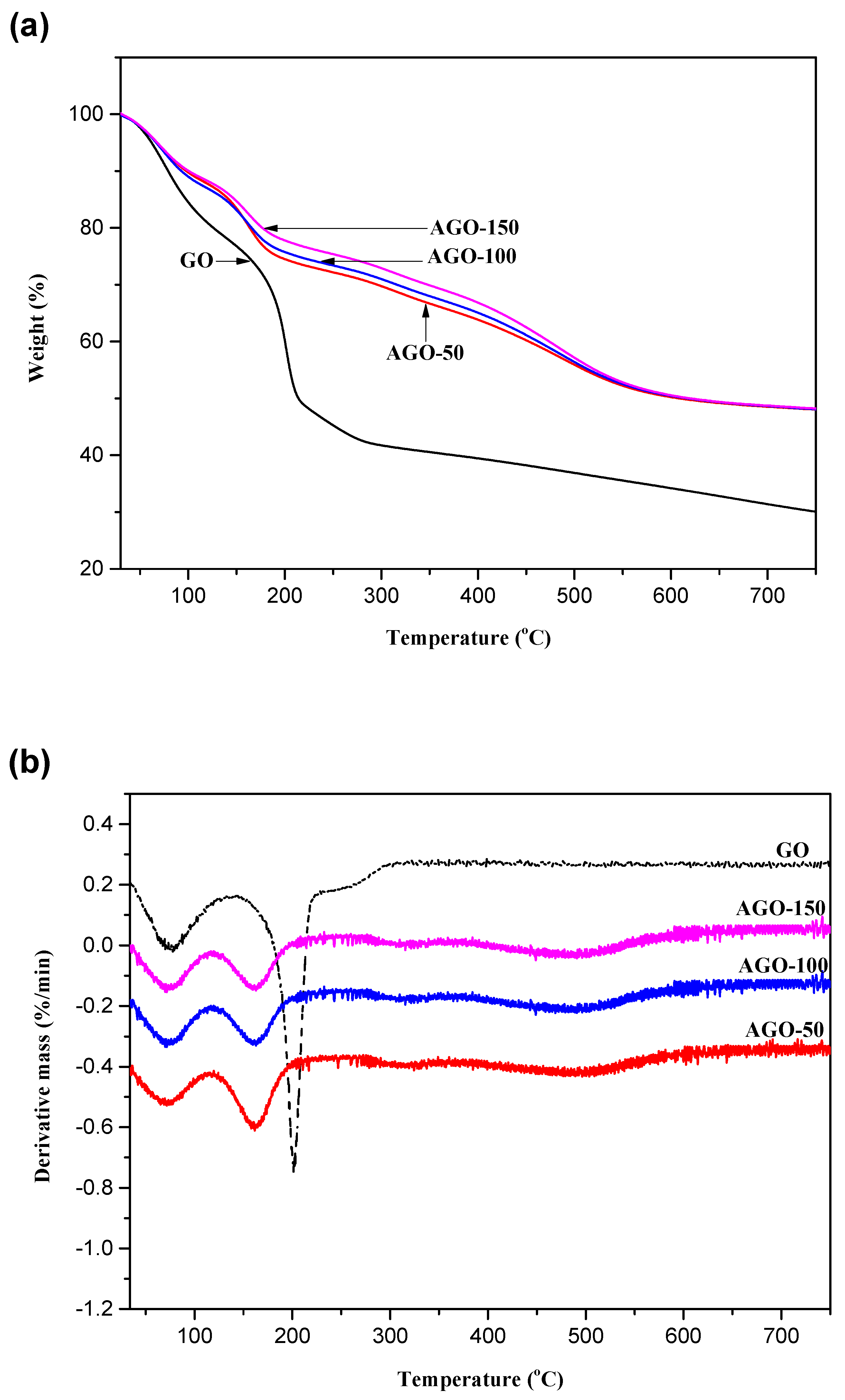
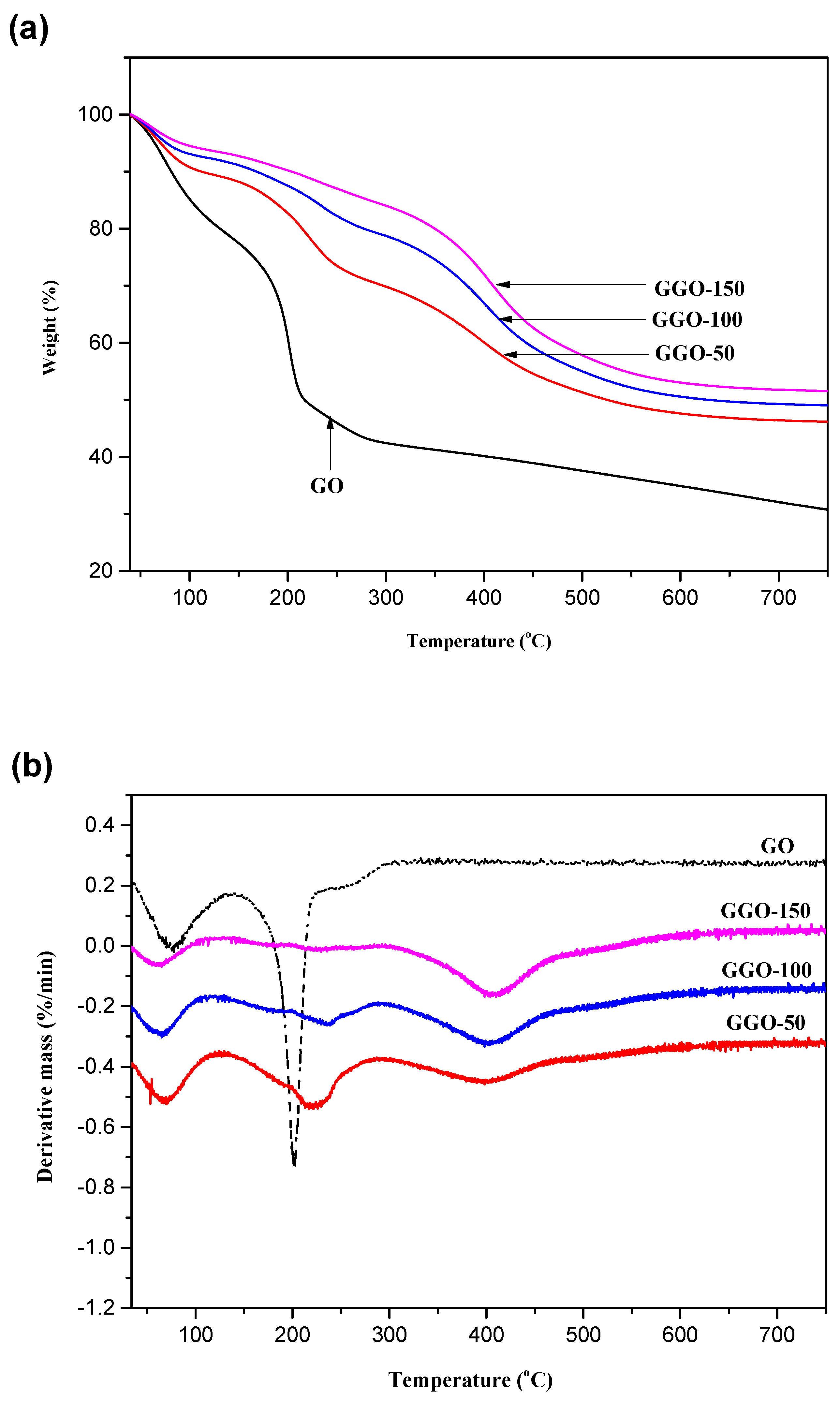
2.5. Thermogravimetric (TG) Analysis
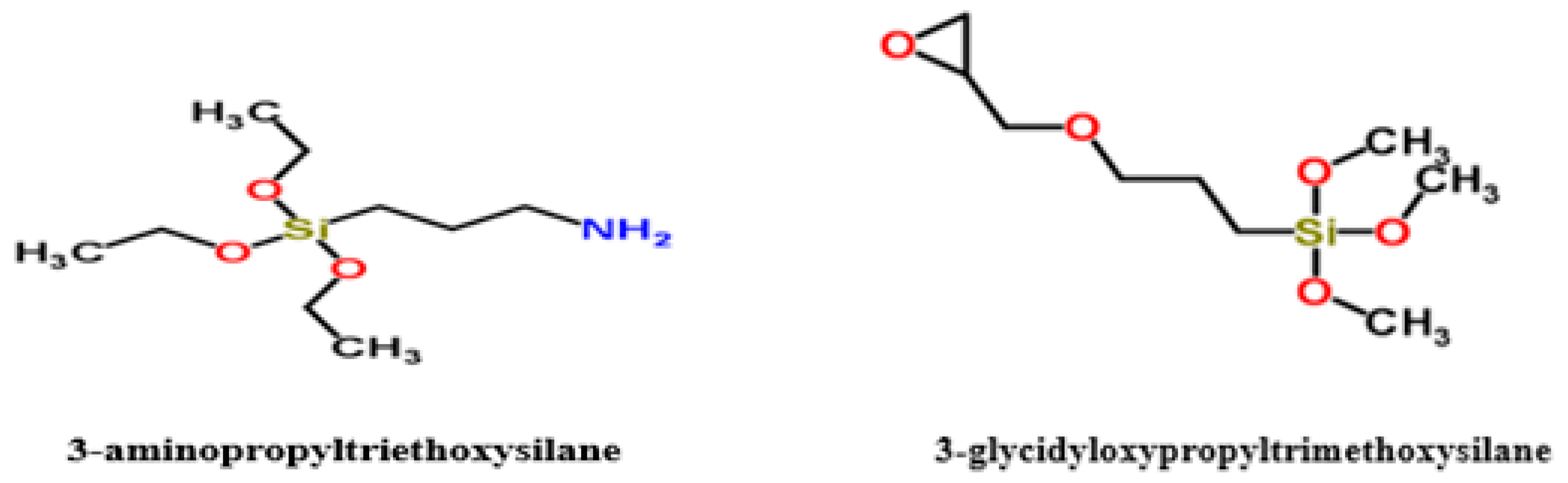
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Graphene Oxide
3.3. Functionalization of Graphene Oxide
3.4. Characterizations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jung, C.-H.; Hong, J.-H.; Jung, J.-M.; Hwang, I.-T.; Jung, C.-H.; Choi, J.-H. Preparation of sulfonated reduced graphene oxide by radiation-induced chemical reduction of sulfonated graphene oxide. Carbon Lett. 2015, 16, 41–44. [Google Scholar] [CrossRef]
- Liu, F.; Lee, C.W.; Im, J.S. Graphene-Based Carbon Materials for Electrochemical Energy Storage. J. Nanomater. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Dumée, L.F.; Feng, C.; He, L.; Allioux, F.-M.; Yi, Z.; Gao, W.; Banos, C.; Davies, J.B.; Kong, L. Tuning the grade of graphene: Gamma ray irradiation of free-standing graphene oxide films in gaseous phase. Appl. Surf. Sci. 2014, 322, 126–135. [Google Scholar] [CrossRef]
- Özçelik, V.O.; Cahangirov, S.; Ciraci, S. Epitaxial growth mechanisms of graphene and effects of substrates. Phys. Rev. B 2012, 85, 235456. [Google Scholar] [CrossRef]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Hussain, S.Z.; Ismail, A.F.; Khan, I.U.; Inamuddin. Graphene and its derivatives: Synthesis, modifications, and applications in wastewater treatment. Environ. Chem. Lett. 2018, 16, 1301–1323. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Liu, J.; Choi, H.J. Graphene and Graphene Oxide Composites and Their Electrorheological Applications. J. Nanomater. 2015, 2015, 8. [Google Scholar] [CrossRef]
- Lubkowski, G.; Kuhnhenn, J.; Suhrke, M.; Weinand, U.; Endler, I.; Meibner, F.; Richter, S. Gamma Radiation Effects in Vertically Aligned Carbon Nanotubes. IEEE Trans. Nuclear Sci. 2012, 59, 792–796. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Li, W.; Zhou, B.; Wang, M.; Li, Z.; Ren, R. Silane functionalization of graphene oxide and its use as a reinforcement in bismaleimide composites. J. Mater. Sci. 2015, 50, 5402–5410. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.-K.; Tang, B.Z. Functionalization of carbon nanotubes using a silane coupling agent. Carbon 2006, 44, 3232–3238. [Google Scholar] [CrossRef]
- Ahmad Daud, N.; Chieng, B.W.; Ibrahim, N.A.; Talib, Z.A.; Muhamad, E.N.; Zainal Abidin, Z. Functionalizing Graphene Oxide with Alkylamine by Gamma-ray Irradiation Method. Nanomaterials 2017, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Chieng, B.W.; Ibrahim, N.A.; Ahmad Daud, N.; Talib, Z.A. Functionalization of Graphene Oxide via Gamma-Ray Irradiation for Hydrophobic Materials. In Synthesis, Technology and Applications of Carbon Nanomaterials; Rashid, S.A., Raja Othman, R.N.I., Hussein, M.Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 177–203. [Google Scholar] [CrossRef]
- Ma, H.-L.; Zhang, L.; Zhang, Y.; Wang, S.; Sun, C.; Yu, H.; Zeng, X.; Zhai, M. Radiation preparation of graphene/carbon nanotubes hybrid fillers for mechanical reinforcement of poly(vinyl alcohol) films. Radiat. Phys. Chem. 2016, 118, 21–26. [Google Scholar] [CrossRef]
- Mathialagan, M.; Ismail, H. Optimization and effect of 3-aminopropyltriethoxysilane content on the properties of bentonite-filled ethylene propylene diene monomer composites. Polym. Compos. 2012, 33, 1993–2000. [Google Scholar] [CrossRef]
- Ling, P.A.; Ismail, H. Tensile Properties, Water Uptake, and Thermal Properties of Polypropylene/Waste Pulverized Tire/Kenaf (PP/WPT/KNF) Composites. Bioresources 2013, 8, 806–817. [Google Scholar] [CrossRef]
- Demjén, Z.; Pukánszky, B.; Nagy, J. Possible coupling reactions of functional silanes and polypropylene. Polymer 1999, 40, 1763–1773. [Google Scholar] [CrossRef]
- Šapić, I.M.; Bistričić, L.; Volovšek, V.; Dananić, V. Vibrational Analysis of 3-Glycidoxypropyltrimethoxysilane Polymer. Macromol. Symp. 2014, 339, 122–129. [Google Scholar] [CrossRef]
- Mauro, M.; Maggio, M.; Cipolletti, V.; Galimberti, M.; Longo, P.; Guerra, G. Graphite oxide intercalation compounds with rotator hexagonal order in the intercalated layers. Carbon 2013, 61, 395–403. [Google Scholar] [CrossRef]
- Pourhashem, S.; Rashidi, A.; Vaezi, M.R.; Bagherzadeh, M.R. Excellent corrosion protection performance of epoxy composite coatings filled with amino-silane functionalized graphene oxide. Surf. Coat. Technol. 2017, 317, 1–9. [Google Scholar] [CrossRef]
- Lee, C.Y.; Bae, J.-H.; Kim, T.-Y.; Chang, S.-H.; Kim, S.Y. Using silane-functionalized graphene oxides for enhancing the interfacial bonding strength of carbon/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2015, 75, 11–17. [Google Scholar] [CrossRef]
- Sheng, X.; Cai, W.; Zhong, L.; Xie, D.; Zhang, X. Synthesis of Functionalized Graphene/Polyaniline Nanocomposites with Effective Synergistic Reinforcement on Anticorrosion. Ind. Eng. Chem. Res. 2016, 55, 8576–8585. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Haeri, Z.; Ramezanzadeh, M. A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance. Chem. Eng. J. 2016, 303, 511–528. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.; Baeck, S.H.; Shim, S.E. Effect of surface treatment of graphene nanoplatelets for improvement of thermal and electrical properties of epoxy composites. Carbon Lett. 2015, 16, 34–40. [Google Scholar] [CrossRef][Green Version]
- Bandyopadhyay, P.; Park, W.B.; Layek, R.K.; Uddin, M.E.; Kim, N.H.; Kim, H.-G.; Lee, J.H. Hexylamine functionalized reduced graphene oxide/polyurethane nanocomposite-coated nylon for enhanced hydrogen gas barrier film. J. Membr. Sci. 2016, 500, 106–114. [Google Scholar] [CrossRef]
- Hack, R.; Correia, C.H.G.; Zanon, R.A.d.S.; Pezzin, S.H. Characterization of graphene nanosheets obtained by a modified Hummer’s method. Matéria (Rio de Janeiro) 2018, 23. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Bae, J.H.; Lee, K.Y.; Noh, W.H.; Lee, S.H.; Ryu, S.H. Physical and chemical characteristics of multiwalled carbon nanotubes functionalized with aminosilane and its influence on the properties of natural rubber composites. Compos. Sci. Technol. 2007, 67, 1813–1822. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Yang, J.; Li, J.; Wan, L. Functionalization of graphene using trimethoxysilanes and its reinforcement on polypropylene nanocomposites. Chem.Phys. Lett. 2013, 570, 125–131. [Google Scholar] [CrossRef]
- Dugas, V.; Chevalier, Y. Surface hydroxylation and silane grafting on fumed and thermal silica. J. Colloid Interface Sci. 2003, 264, 354–361. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, T.-J.; Gao, H.; Jin, Y. High density silanization of nano-silica particles using γ-aminopropyltriethoxysilane (APTES). Appl. Surf. Sci. 2015, 351, 646–654. [Google Scholar] [CrossRef]
- Dugas, V.; Chevalier, Y. Chemical Reactions in Dense Monolayers: In situ thermal cleavage of grafted esters for preparation of solid surfaces functionalized with carboxylic acids. Langmuir 2011, 27, 14188–14200. [Google Scholar] [CrossRef] [PubMed]
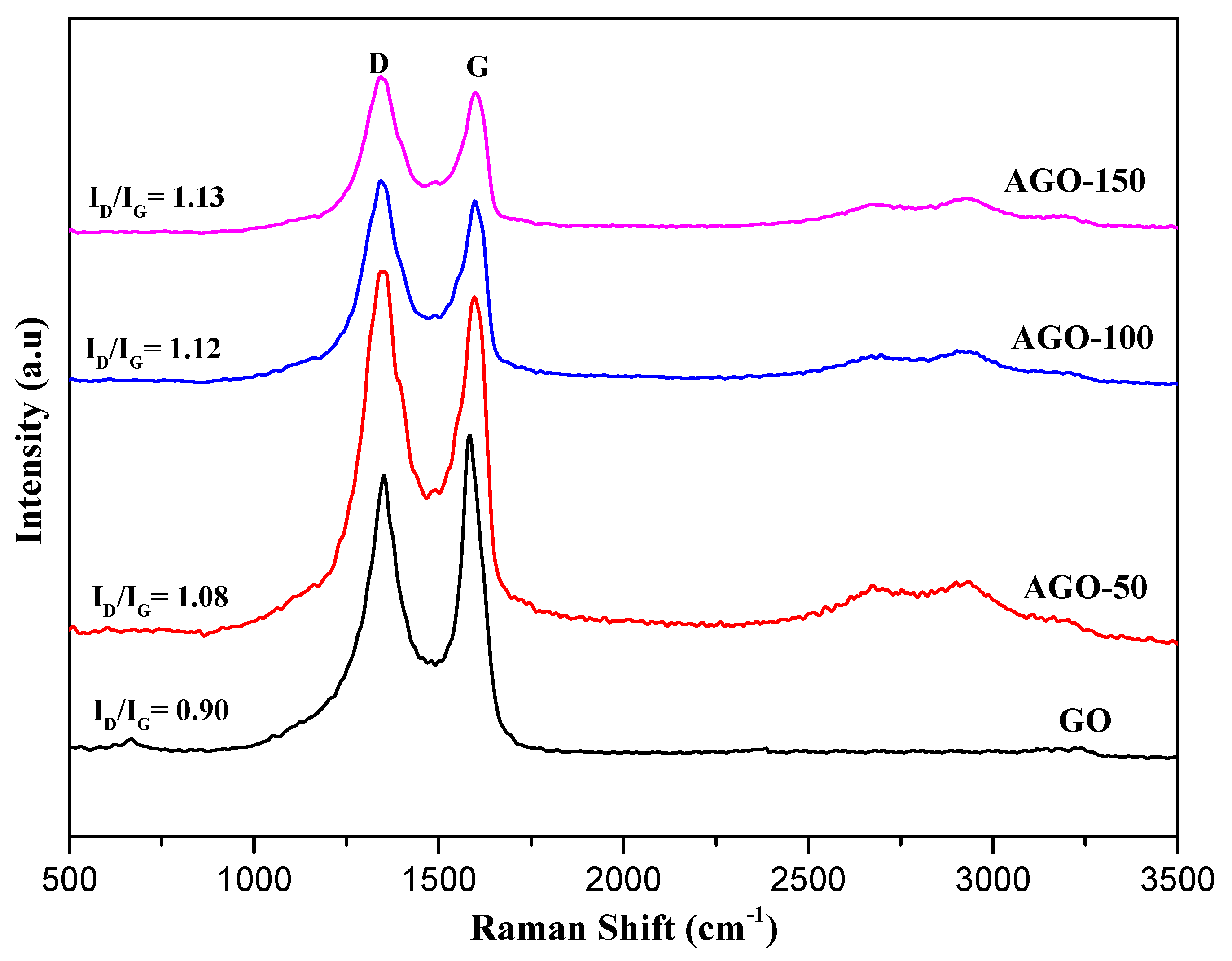
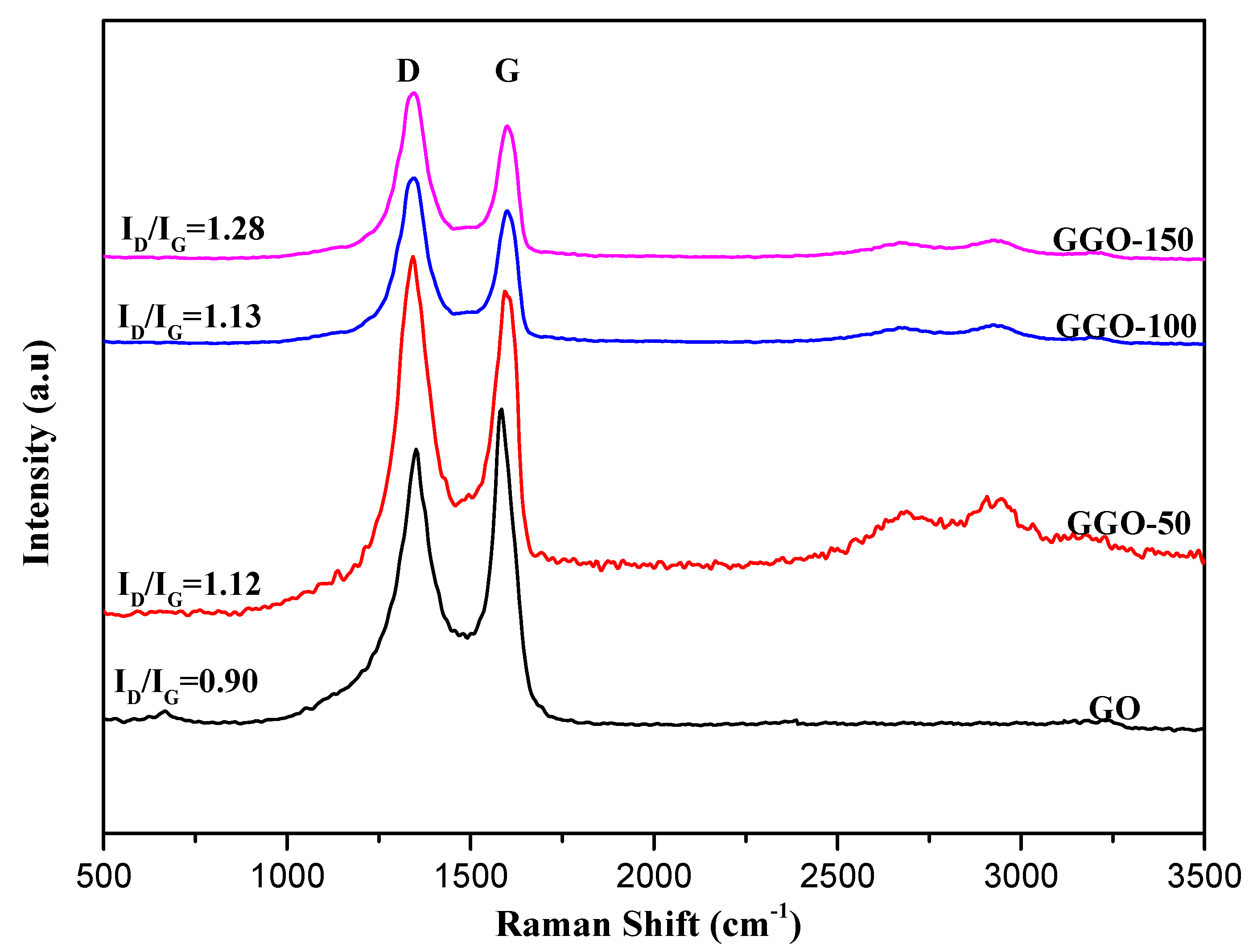
| Samples | Raman Shift of D Band (cm−1) | Raman Shift of D Band (cm−1) | ID/IG |
|---|---|---|---|
| GO | 1345.77 | 1583.33 | 0.90 |
| AGO-50 | 1345.77 | 1589.55 | 1.08 |
| AGO-100 | 1345.77 | 1594.52 | 1.12 |
| AGO-150 | 1345.77 | 1599.50 | 1.13 |
| GGO-50 | 1345.77 | 1597.01 | 1.12 |
| GGO-100 | 1345.77 | 1597.01 | 1.13 |
| GGO-150 | 1345.77 | 1601.99 | 1.28 |
| Samples | Weight Loss (%) 30–120 °C | Weight Loss (%) 120–300 °C | Weight Loss (%) 300–650 °C |
|---|---|---|---|
| GO | 18.7 | 39.7 | 0 |
| AGO-50 | 12.0 | 18.3 | 20.5 |
| AGO-100 | 12.8 | 16.2 | 21.6 |
| AGO-150 | 11.8 | 15.6 | 23.4 |
| GGO-50 | 11.0 | 19.8 | 23.0 |
| GGO-100 | 8.4 | 13.7 | 29.0 |
| GGO-150 | 7.0 | 9.8 | 31.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aujara, K.M.; Chieng, B.W.; Ibrahim, N.A.; Zainuddin, N.; Thevy Ratnam, C. Gamma-Irradiation Induced Functionalization of Graphene Oxide with Organosilanes. Int. J. Mol. Sci. 2019, 20, 1910. https://doi.org/10.3390/ijms20081910
Aujara KM, Chieng BW, Ibrahim NA, Zainuddin N, Thevy Ratnam C. Gamma-Irradiation Induced Functionalization of Graphene Oxide with Organosilanes. International Journal of Molecular Sciences. 2019; 20(8):1910. https://doi.org/10.3390/ijms20081910
Chicago/Turabian StyleAujara, Kabiru Musa, Buong Woei Chieng, Nor Azowa Ibrahim, Norhazlin Zainuddin, and Chantara Thevy Ratnam. 2019. "Gamma-Irradiation Induced Functionalization of Graphene Oxide with Organosilanes" International Journal of Molecular Sciences 20, no. 8: 1910. https://doi.org/10.3390/ijms20081910
APA StyleAujara, K. M., Chieng, B. W., Ibrahim, N. A., Zainuddin, N., & Thevy Ratnam, C. (2019). Gamma-Irradiation Induced Functionalization of Graphene Oxide with Organosilanes. International Journal of Molecular Sciences, 20(8), 1910. https://doi.org/10.3390/ijms20081910





