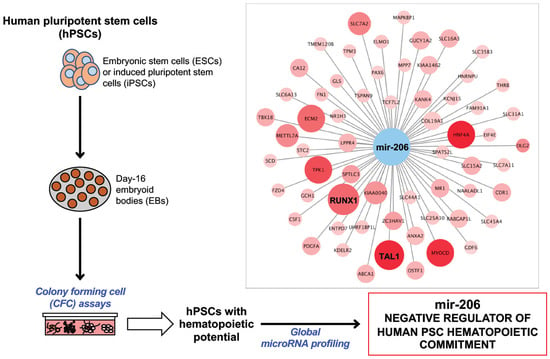
Global MicroRNA Profiling Uncovers miR-206 as a Negative Regulator of Hematopoietic Commitment in Human Pluripotent Stem Cells
Abstract
1. Introduction
2. Results
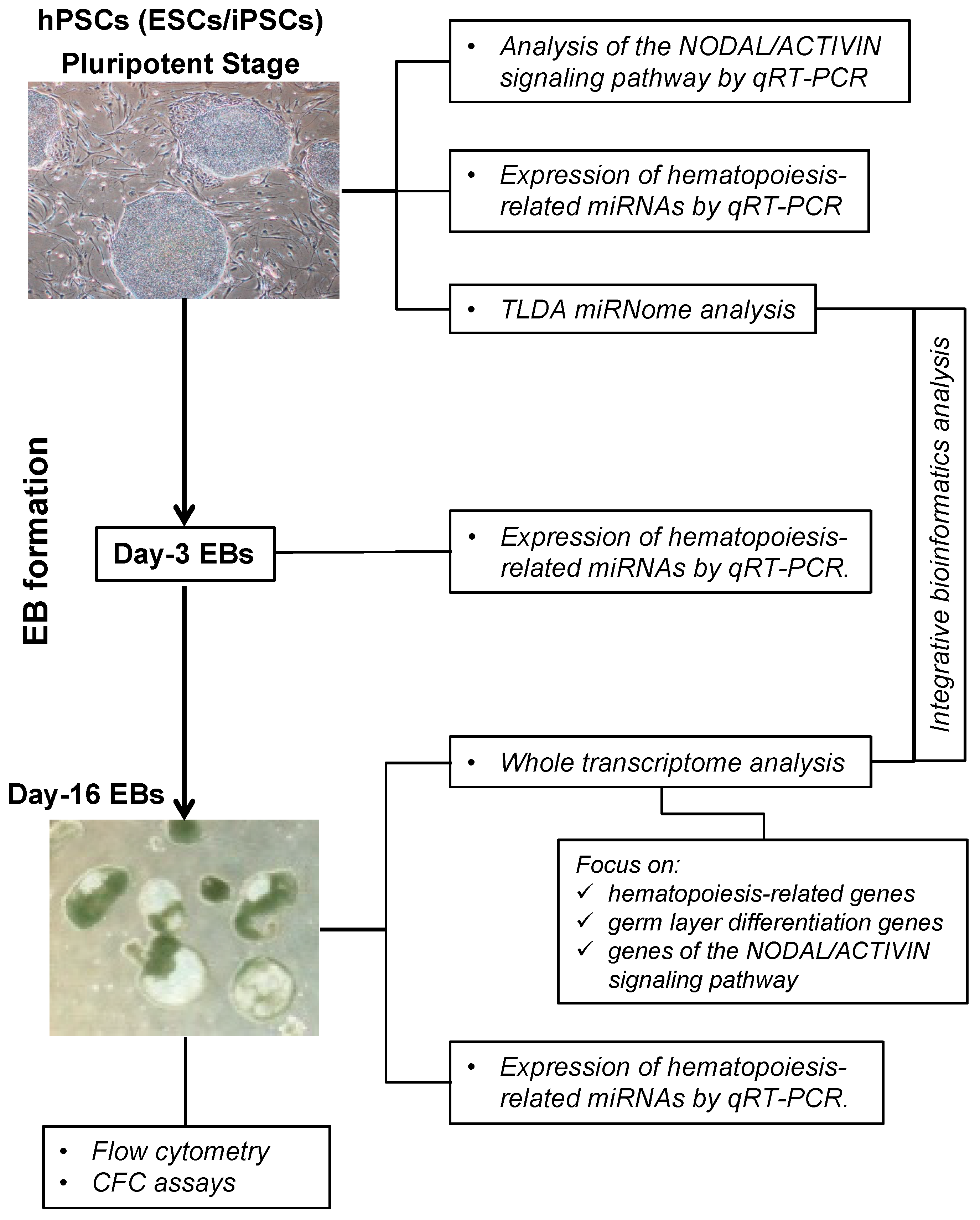
2.1. Overview of the Protocol
2.2. Characterization of the Hematopoietic Potential of hPSC Lines
2.3. Gene Expression Analysis of the NODAL/ACTIVIN Signaling Pathway
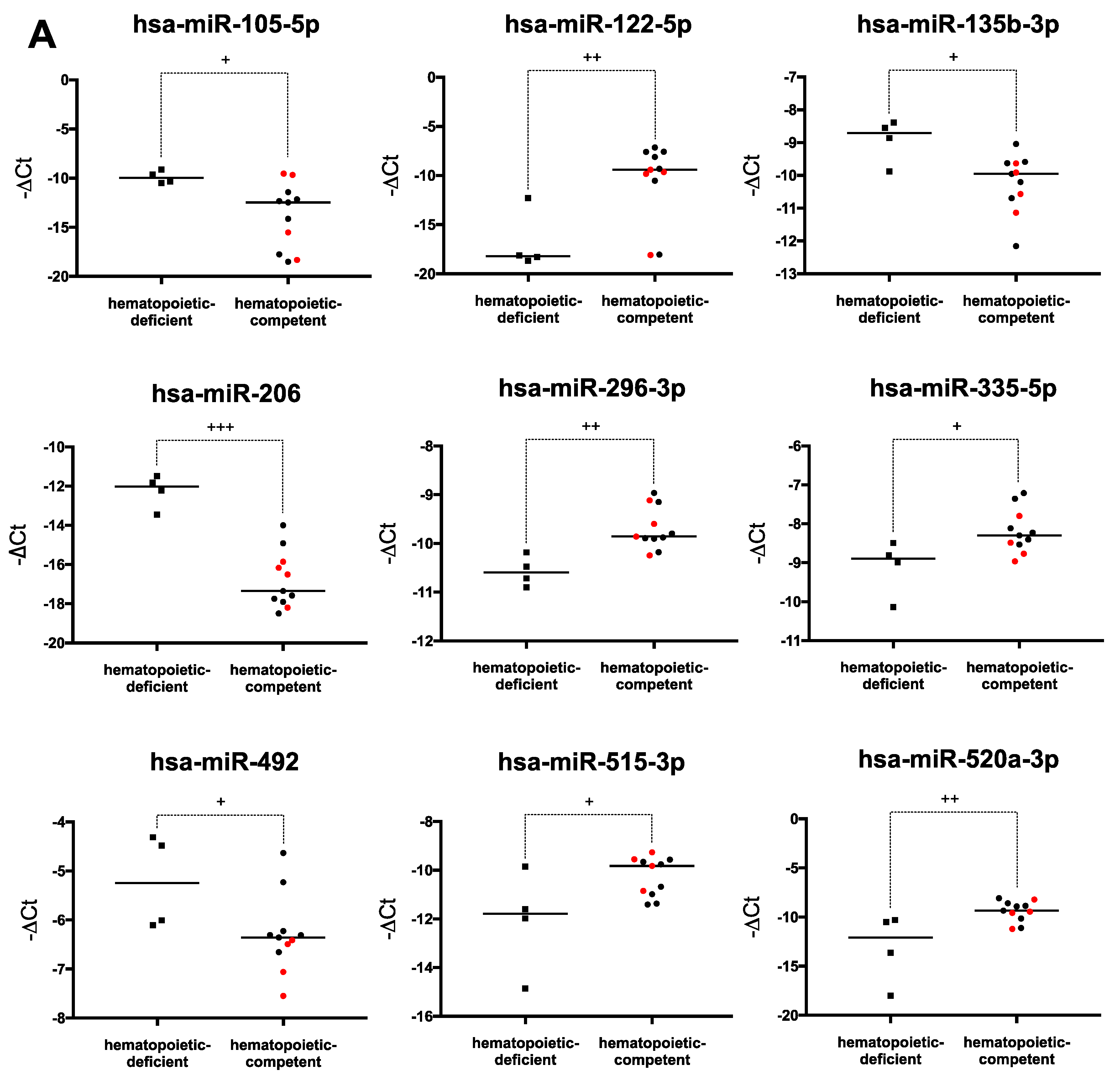
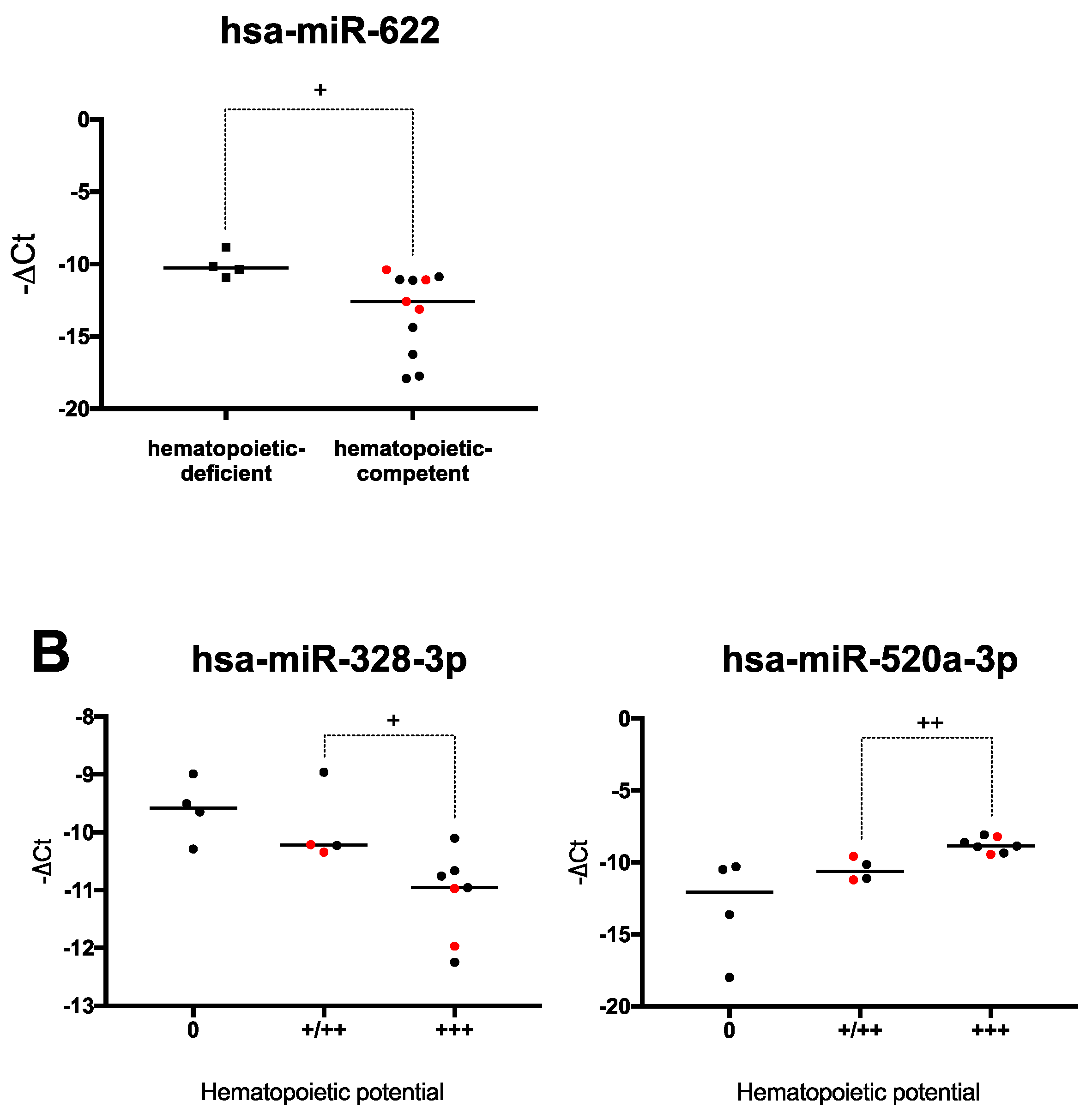
2.4. Hematopoiesis-Related miRNA Expression during Hematopoietic Differentiation
2.5. Global microRNA Expression Profiling in Human PSCs
2.6. Human PSCs Hematopoietic Potential and miRNA Expression
2.7. Integrative miRNome and Transcriptome Analysis
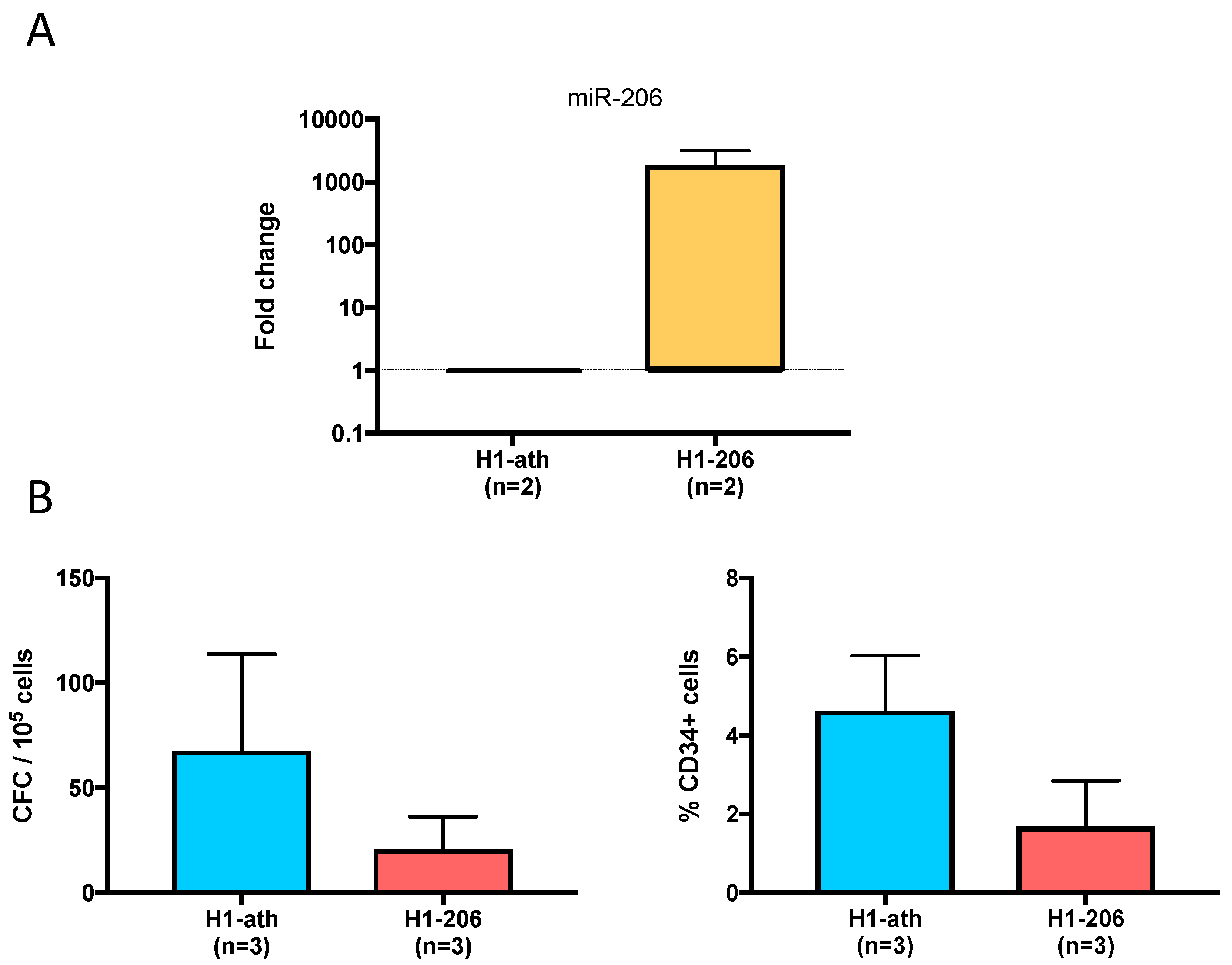
2.8. Overexpression of miR206 in the H1 ES Cell Line
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. Differentiation of hPSCs in EB Cells
4.3. Clonogenic Progenitor Assays
4.4. Flow Cytometry
4.5. Total RNA, miRNA Extraction, and cDNA Synthesis
4.6. Whole Transcriptome and qRT-PCR Analyses
4.7. Hematopoiesis-Related miRNA qRT-PCR Analysis
4.8. TLDA Analysis
4.9. Integrative miRNome and Transcriptome Analysis
4.10. Overexpression of miR-206 in a Blood-Competent ES Cell Line
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ath | Arabidopsis thaliana |
| BFU-E | Burst-forming unit-erythroid |
| CFC | Colony-forming cell |
| CFU-E | Colony-forming unit-erythroid |
| CFU-GEMM | Colony-forming unit-granulocytes erythrocytes macrophages megakaryocytes |
| CFU-GM | Colony-forming unit-granulocytes macrophages |
| CGH | Comparative genomic hybridization |
| EB | Embryoid bodies |
| ESCs | Embryonic stem cells |
| hiPSCs | Human induced pluripotent stem cells |
| hPSCs | Human pluripotent stem cells |
| LiMMa | Linear models for microarrays |
| miRNAs | Micrornas |
| PSCs | Pluripotent stem cells |
| qRT-PCR | Quantitative real-time RT-PCR |
| TLDA | TaqMan low-density array |
References
- Allegrucci, C.; Young, L.E. Differences between human embryonic stem cell lines. Hum. Reprod. Update 2007, 13, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Diep, D.; Gore, A.; Panopoulos, A.D.; Montserrat, N.; Plongthongkum, N.; Kumar, S.; Fung, H.L.; Giorgetti, A.; Bilic, J.; et al. Identification of a specific reprogramming-associated epigenetic signature in human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 16196–16201. [Google Scholar] [CrossRef]
- Toivonen, S.; Ojala, M.; Hyysalo, A.; Ilmarinen, T.; Rajala, K.; Pekkanen-Mattila, M.; Äänismaa, R.; Lundin, K.; Palgi, J.; Weltner, J.; et al. Comparative analysis of targeted differentiation of human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells reveals variability associated with incomplete transgene silencing in retrovirally derived hiPSC lines. Stem Cells Transl. Med. 2013, 2, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Osafune, K.; Caron, L.; Borowiak, M.; Martinez, R.J.; Fitz-Gerald, C.S.; Sato, Y.; Cowan, C.A.; Chien, K.R.; Melton, D.A. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol. 2008, 26, 313–315. [Google Scholar] [CrossRef]
- Wahlster, L.; Daley, G.Q. Progress towards generation of human haematopoietic stem cells. Nat. Cell Biol. 2016, 18, 1111–1117. [Google Scholar] [CrossRef]
- Ivanovs, A.; Rybtsov, S.; Ng, E.S.; Stanley, E.G.; Elefanty, A.G.; Medvinsky, A. Human haematopoietic stem cell development: From the embryo to the dish. Development 2017, 144, 2323–2337. [Google Scholar] [CrossRef]
- Sugimura, R.; Jha, D.K.; Han, A.; Soria-Valles, C.; da Rocha, E.L.; Lu, Y.F.; Goettel, J.A.; Serrao, E.; Rowe, R.G.; Malleshaiah, M.; et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 2017, 545, 432–438. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Menendez, P.; Cerdan, C.; Bhatia, M. Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood 2005, 105, 4598–4603. [Google Scholar] [CrossRef] [PubMed]
- Zambidis, E.T.; Peault, B.; Park, T.S.; Bunz, F.; Civin, C.I. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood 2005, 106, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Mejia, V.; Melen, G.J.; Sanchez, L.; Gutierrez-Aranda, I.; Ligero, G.; Cortes, J.L.; Real, P.J.; Bueno, C.; Menendez, P. Nodal/Activin signaling predicts human pluripotent stem cell lines prone to differentiate toward the hematopoietic lineage. Mol. Ther. 2010, 18, 2173–2181. [Google Scholar] [CrossRef]
- Shen, M.M. Nodal signaling: Developmental roles and regulation. Development 2007, 134, 1023–1034. [Google Scholar] [CrossRef]
- Chen, C.Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Baltimore, D. MicroRNAs and hematopoietic cell development. Curr. Top. Dev. Biol. 2012, 99, 145–174. [Google Scholar]
- Mallanna, S.K.; Rizzino, A. Emerging roles of microRNAs in the control of embryonic stem cells and the generation of induced pluripotent stem cells. Dev. Biol. 2010, 344, 16–25. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Calin, G.A.; Picanço-Castro, V.; Kashima, S.; Covas, D.T.; de Castro, F.A. Hematopoietic stem cells from induced pluripotent stem cells—Considering the role of microRNA as a cell differentiation regulator. J. Cell Sci. 2018, 131, 1–8. [Google Scholar] [CrossRef]
- Féraud, O.; Valogne, Y.; Melkus, M.W.; Zhang, Y.; Oudrhiri, N.; Haddad, R.; Daury, A.; Rocher, C.; Larbi, A.; Duquesnoy, P.; et al. Donor Dependent Variations in Hematopoietic Differentiation among Embryonic and Induced Pluripotent Stem Cell Lines. PLoS ONE 2016, 11, 1–22. [Google Scholar] [CrossRef][Green Version]
- Seiler, K.; Soroush Noghabi, M.; Karjalainen, K.; Hummel, M.; Melchers, F.; Tsuneto, M. Induced pluripotent stem cells expressing elevated levels of sox-2, oct-4, and klf-4 are severely reduced in their differentiation from mesodermal to hematopoietic progenitor cells. Stem Cells Dev. 2011, 20, 1131–1142. [Google Scholar] [CrossRef]
- Roy, A.; Cowan, G.; Mead, A.J.; Filippi, S.; Bohn, G.; Chaidos, A.; Tunstall, O.; Chan, J.K.; Choolani, M.; Bennett, P.; et al. Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proc. Natl. Acad. Sci. USA 2012, 109, 17579–17584. [Google Scholar] [CrossRef]
- Chou, S.T.; Byrska-Bishop, M.; Tober, J.M.; Yao, Y.; Vandorn, D.; Opalinska, J.B.; Mills, J.A.; Choi, J.K.; Speck, N.A.; Gadue, P.; et al. Trisomy 21-associated defects in human primitive hematopoiesis revealed through induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 17573–17578. [Google Scholar] [CrossRef]
- Maclean, G.A.; Menne, T.F.; Guo, G.; Sanchez, D.J.; Park, I.H.; Daley, G.Q.; Orkin, S.H. Altered hematopoiesis in trisomy 21 as revealed through in vitro differentiation of isogenic human pluripotent cells. Proc. Natl. Acad. Sci. USA 2012, 109, 17567–17572. [Google Scholar] [CrossRef]
- Bissels, U.; Bosio, A.; Wagner, W. MicroRNAs are shaping the hematopoietic landscape. Haematologica 2012, 97, 160–167. [Google Scholar] [CrossRef]
- Lu, S.J.; Feng, Q.; Caballero, S.; Chen, Y.; Moore, M.A.; Grant, M.B.; Lanza, R. Generation of functional hemangioblasts from human embryonic stem cells. Nat. Methods 2007, 4, 501–509. [Google Scholar] [CrossRef]
- Suh, M.R.; Lee, Y.; Kim, J.Y.; Kim, S.K.; Moon, S.H.; Lee, J.Y.; Cha, K.Y.; Chung, H.M.; Yoon, H.S.; Moon, S.Y.; et al. Human embryonic stem cells express a unique set of microRNAs. Dev. Biol. 2004, 270, 488–498. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Chaudhuri, A.A.; Rao, D.S.; Gibson, W.S.; Balazs, A.B.; Baltimore, D. MicroRNAs enriched in hematopoietic stem cells differentially regulate long-term hematopoietic output. Proc. Natl. Acad. Sci. USA 2010, 107, 14235–14240. [Google Scholar] [CrossRef]
- Johnnidis, J.B.; Harris, M.H.; Wheeler, R.T.; Stehling-Sun, S.; Lam, M.H.; Kirak, O.; Brummelkamp, T.R.; Fleming, M.D.; Camargo, F.D. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 2008, 451, 1125–1129. [Google Scholar] [CrossRef]
- Nishiyama, T.; Kaneda, R.; Ono, T.; Tohyama, S.; Hashimoto, H.; Endo, J.; Tsuruta, H.; Yuasa, S.; Ieda, M.; Makino, S.; et al. miR-142-3p is essential for hematopoiesis and affects cardiac cell fate in zebrafish. Biochem. Biophys. Res. Commun. 2012, 425, 755–761. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; He, Q.; Gao, J.; Gao, Y.; Liu, B.; Liu, F. miR-142-3p regulates the formation and differentiation of hematopoietic stem cells in vertebrates. Cell Res. 2013, 23, 1356–1368. [Google Scholar] [CrossRef][Green Version]
- Nimmo, R.; Ciau-Uitz, A.; Ruiz-Herguido, C.; Soneji, S.; Bigas, A.; Patient, R.; Enver, T. MiR-142-3p controls the specification of definitive hemangioblasts during ontogeny. Dev. Cell 2013, 26, 237–249. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Hsu, S.H.; Wang, B.; Kota, J.; Yu, J.; Costinean, S.; Kutay, H.; Yu, L.; Bai, S.; La Perle, K.; Chivukula, R.R.; et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J. Clin. Investig. 2012, 122, 2871–2883. [Google Scholar] [CrossRef]
- Tsai, W.C.; Hsu, S.D.; Hsu, C.S.; Lai, T.C.; Chen, S.J.; Shen, R.; Huang, Y.; Chen, H.C.; Lee, C.H.; Tsai, T.F.; et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J. Clin. Investig. 2012, 122, 2884–2897. [Google Scholar] [CrossRef]
- Doddapaneni, R.; Chawla, Y.K.; Das, A.; Kalra, J.K.; Ghosh, S.; Chakraborti, A. Overexpression of microRNA-122 enhances in vitro hepatic differentiation of fetal liver-derived stem/progenitor cells. J. Cell Biochem. 2013, 114, 1575–1583. [Google Scholar] [CrossRef]
- Ema, H.; Nakauchi, H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 2000, 95, 2284–2288. [Google Scholar]
- Laurent, L.C.; Chen, J.; Ulitsky, I.; Mueller, F.J.; Lu, C.; Shamir, R.; Fan, J.B.; Loring, J.F. Comprehensive microRNA profiling reveals a unique human embryonic stem cell signature dominated by a single seed sequence. Stem Cells 2008, 26, 1506–1516. [Google Scholar] [CrossRef]
- Morin, R.D.; O’Connor, M.D.; Griffith, M.; Kuchenbauer, F.; Delaney, A.; Prabhu, A.L.; Zhao, Y.; McDonald, H.; Zeng, T.; Hirst, M.; et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 2008, 18, 610–621. [Google Scholar] [CrossRef]
- Shu, M.; Zheng, X.; Wu, S.; Lu, H.; Leng, T.; Zhu, W.; Zhou, Y.; Ou, Y.; Lin, X.; Lin, Y.; et al. Targeting oncogenic miR-335 inhibits growth and invasion of malignant astrocytoma cells. Mol. Cancer 2011, 10, 59. [Google Scholar] [CrossRef]
- Lee, K.H.; Lin, F.C.; Hsu, T.I.; Lin, J.T.; Guo, J.H.; Tsai, C.H.; Lee, Y.C.; Lee, Y.C.; Chen, C.L.; Hsiao, M.; et al. MicroRNA-296-5p (miR-296-5p) functions as a tumor suppressor in prostate cancer by directly targeting Pin1. Biochim. Biophys. Acta 2014, 1843, 2055–2066. [Google Scholar] [CrossRef]
- Kamat, V.; Paluru, P.; Myint, M.; French, D.L.; Gadue, P.; Diamond, S.L. MicroRNA screen of human embryonic stem cell differentiation reveals miR-105 as an enhancer of megakaryopoiesis from adult CD34+ cells. Stem Cells 2014, 32, 1337–1346. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Faggioni, A.; Trivedi, P.; Slack, F.J. The Nefarious Nexus of Noncoding RNAs in Cancer. Int. J. Mol. Sci. 2018, 19, E2072. [Google Scholar] [CrossRef]
- Samaeekia, R.; Adorno-Cruz, V.; Bockhorn, J.; Chang, Y.F.; Huang, S.; Prat, A.; Ha, N.; Kibria, G.; Huo, D.; Zheng, H.; et al. miR-206 Inhibits Stemness and Metastasis of Breast Cancer by Targeting MKL1/IL11 Pathway. Clin. Cancer Res. 2017, 23, 1091–1103. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, Y.S.; Sivaprasad, U.; Malhotra, A.; Dutta, A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J. Cell Biol. 2006, 174, 677–687. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a key modulator of skeletal muscle development and disease. Int. J. Biol. Sci. 2015, 11, 345–352. [Google Scholar] [CrossRef]
- Mitchelson, K.R.; Qin, W.Y. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J. Biol. Chem. 2015, 6, 162–208. [Google Scholar] [CrossRef]
- Ivey, K.N.; Muth, A.; Arnold, J.; King, F.W.; Yeh, R.F.; Fish, J.E.; Hsiao, E.C.; Schwartz, R.J.; Conklin, B.R.; Bernstein, H.S.; et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell 2008, 2, 219–229. [Google Scholar] [CrossRef]
- Imperato, M.R.; Cauchy, P.; Obier, N.; Bonifer, C. The RUNX1-PU.1 axis in the control of hematopoiesis. Int. J. Hematol. 2015, 101, 319–329. [Google Scholar] [CrossRef]
- Hoang, T.; Lambert, J.A.; Martin, R. SCL/TAL1 in Hematopoiesis and Cellular Reprogramming. Curr. Top. Dev. Biol. 2016, 118, 163–204. [Google Scholar]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, 1–38. [Google Scholar] [CrossRef]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef]
- Culhane, A.C.; Thioulouse, J.; Perrière, G.; Higgins, D.G. MADE4: An R package for multivariate analysis of gene expression data. Bioinformatics 2005, 21, 2789–2790. [Google Scholar] [CrossRef]
- Cline, M.S.; Smoot, M.; Cerami, E.; Kuchinsky, A.; Landys, N.; Workman, C.; Christmas, R.; Avila-Campilo, I.; Creech, M.; Gross, B.; et al. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2007, 2, 2366–2382. [Google Scholar] [CrossRef]

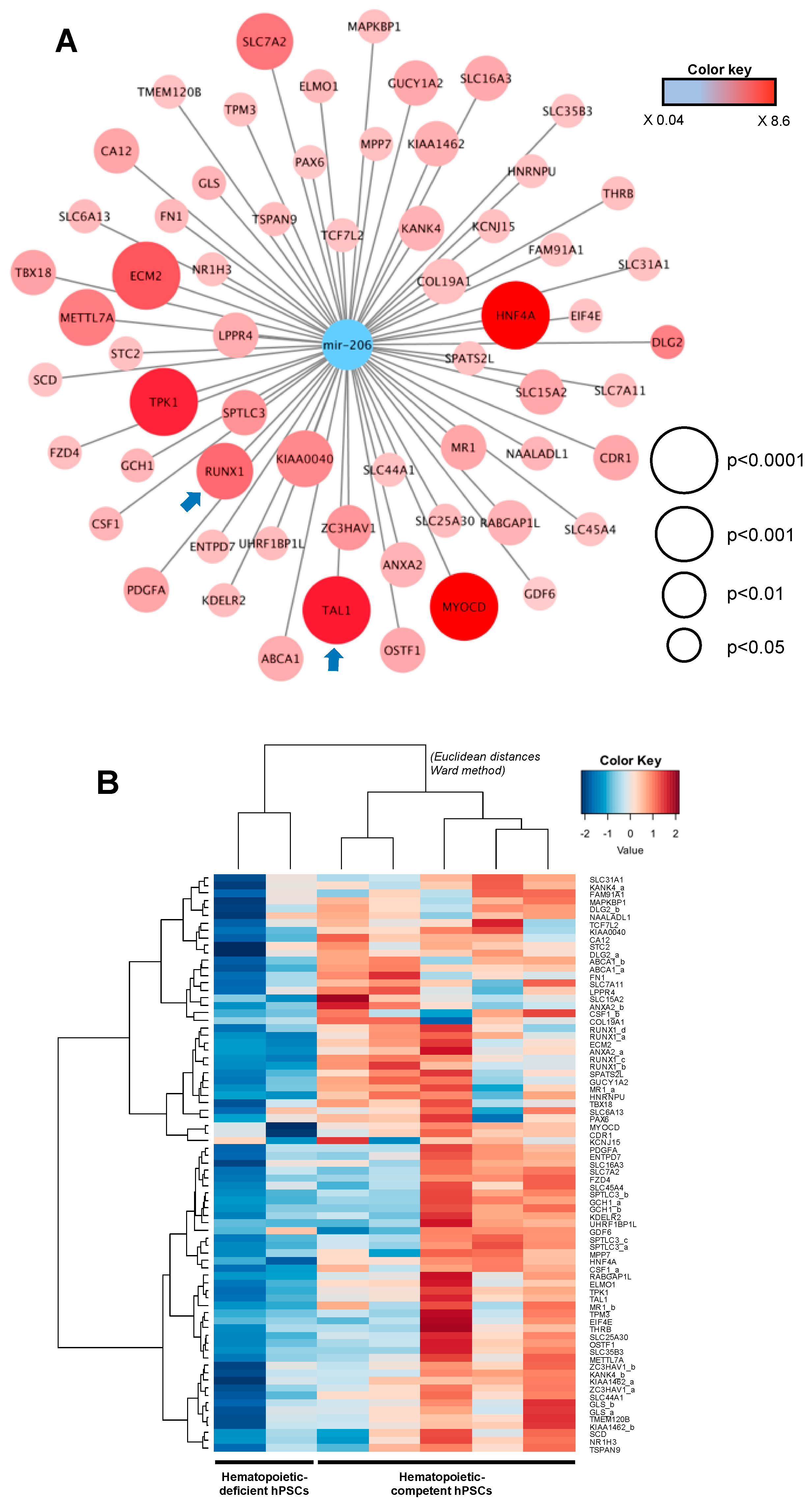
| Cell Line | Passage | Karyotype/CGH | Origin |
|---|---|---|---|
| iPSC lines | |||
| PB3 | 43 | 46, XX | Normal amniocyte |
| PB4 | 26 | 46, XY | Amniocyte, sickle cell anemia |
| PB6 | 23 | 47, XY, +21 | Amniocyte, Down syndrome |
| PB6.1 | 14 | 47, XY, +21 | Amniocyte, Down syndrome |
| PB7 | 63 | 46, XY | Normal amniocyte |
| PB9 | 17 | 46, XY | Normal amniocyte |
| PB10 | 16 | 46, XY | Normal amniocyte |
| PB12.1 | 33 | 47, XY, +21 | Amniocyte, Down syndrome |
| PB13 | 28 | 46, XY | Endothelial progenitor cell from peripheral blood |
| PB14.3 | 41 | 47, XX, +21 | Amniocyte, Down syndrome |
| PB17 | 38 | 46, XY | Endothelial progenitor cell from peripheral blood |
| ESC lines | |||
| SA01 | 22+4+7 | 46, XY | Cellartis AB, Sweden |
| H1 | 67 | 46, XY | WiCell, Wisconsin, USA |
| H9 | 31 | 46, XX | WiCell, Wisconsin, USA |
| CL01 | 16 | 46, XY, der21, t(1;21) | Paris Sud University, France |
| Cell line | CFC/105 Cells | Distribution of Hematopoietic Colony Subtypes (/105 cells) | %CD34+ Cells | Hematopoietic Potential | ||||
|---|---|---|---|---|---|---|---|---|
| CFU-E | BFU-E | CFU-GM | CFU-GEMM | |||||
| iPSCs | PB3 | 140 | 0 | 1 | 139 | 0 | 35 ± 10 | +++ |
| PB4 | 80 | 0 | 1 | 79 | 0 | 13 ± 3 | ++ | |
| PB6 | 2 | 1 | 0 | 1 | 0 | 2 ± 1 | 0 | |
| PB6.1 | 120 | 41 | 46 | 30 | 3 | 17 ± 7 | +++ | |
| PB7 | 144 | 52 | 33 | 58 | 1 | 13 ± 6 | +++ | |
| PB9 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0 | |
| PB10 | 24 | 0 | 4 | 20 | 0 | 3 ± 0.3 | + | |
| PB12.1 | 0 | 0 | 0 | 0 | 0 | 0.4 | 0 | |
| PB13 | 249 | 115 | 134 | 0 | 0 | 16 ± 10 | +++ | |
| PB14.3 | 5 | 0 | 1 | 4 | 0 | 3 ± 0.5 | 0 | |
| PB17 | 235 | 47 | 0 | 188 | 0 | 17 ± 4 | +++ | |
| ESCs | SA01 | 402 | 145 | 84 | 161 | 12 | 23 ± 9 | +++ |
| H1 | 318 | 216 | 29 | 70 | 3 | 33 ± 16 | +++ | |
| H9 | 38 | 2 | 1 | 35 | 0 | 9 ± 2 | ++ | |
| CL01 | 41 | 2 | 1 | 37 | 1 | 8 ± 2 | ++ | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flamant, S.; Chomel, J.-C.; Desterke, C.; Féraud, O.; Gobbo, E.; Mitjavila-Garcia, M.-T.; Foudi, A.; Griscelli, F.; Turhan, A.G.; Bennaceur-Griscelli, A. Global MicroRNA Profiling Uncovers miR-206 as a Negative Regulator of Hematopoietic Commitment in Human Pluripotent Stem Cells. Int. J. Mol. Sci. 2019, 20, 1737. https://doi.org/10.3390/ijms20071737
Flamant S, Chomel J-C, Desterke C, Féraud O, Gobbo E, Mitjavila-Garcia M-T, Foudi A, Griscelli F, Turhan AG, Bennaceur-Griscelli A. Global MicroRNA Profiling Uncovers miR-206 as a Negative Regulator of Hematopoietic Commitment in Human Pluripotent Stem Cells. International Journal of Molecular Sciences. 2019; 20(7):1737. https://doi.org/10.3390/ijms20071737
Chicago/Turabian StyleFlamant, Stéphane, Jean-Claude Chomel, Christophe Desterke, Olivier Féraud, Emilie Gobbo, Maria-Teresa Mitjavila-Garcia, Adlen Foudi, Frank Griscelli, Ali G. Turhan, and Annelise Bennaceur-Griscelli. 2019. "Global MicroRNA Profiling Uncovers miR-206 as a Negative Regulator of Hematopoietic Commitment in Human Pluripotent Stem Cells" International Journal of Molecular Sciences 20, no. 7: 1737. https://doi.org/10.3390/ijms20071737
APA StyleFlamant, S., Chomel, J.-C., Desterke, C., Féraud, O., Gobbo, E., Mitjavila-Garcia, M.-T., Foudi, A., Griscelli, F., Turhan, A. G., & Bennaceur-Griscelli, A. (2019). Global MicroRNA Profiling Uncovers miR-206 as a Negative Regulator of Hematopoietic Commitment in Human Pluripotent Stem Cells. International Journal of Molecular Sciences, 20(7), 1737. https://doi.org/10.3390/ijms20071737