3.1. Carotenoid Supplementation and Biomarkers of Stetaosis
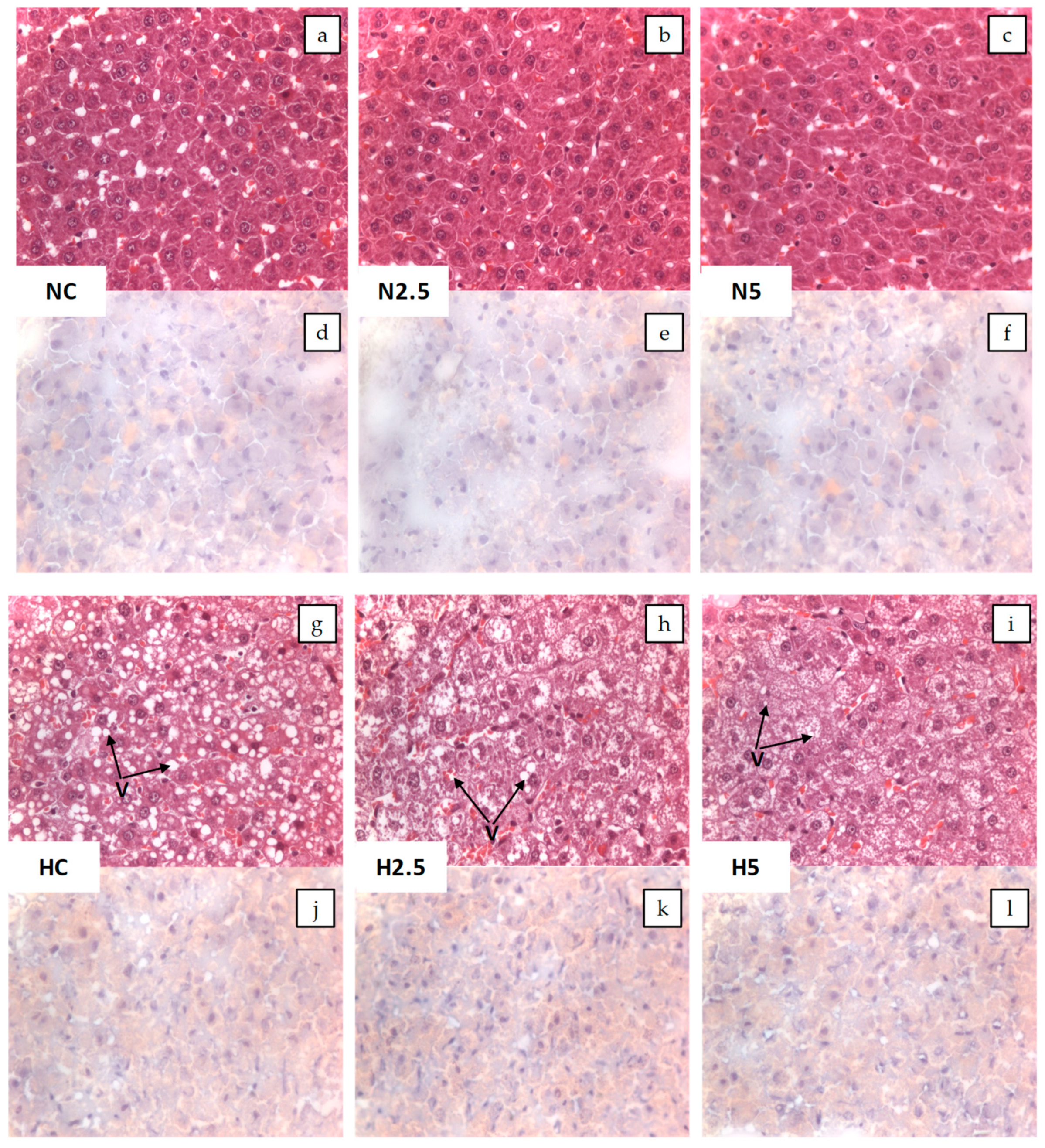
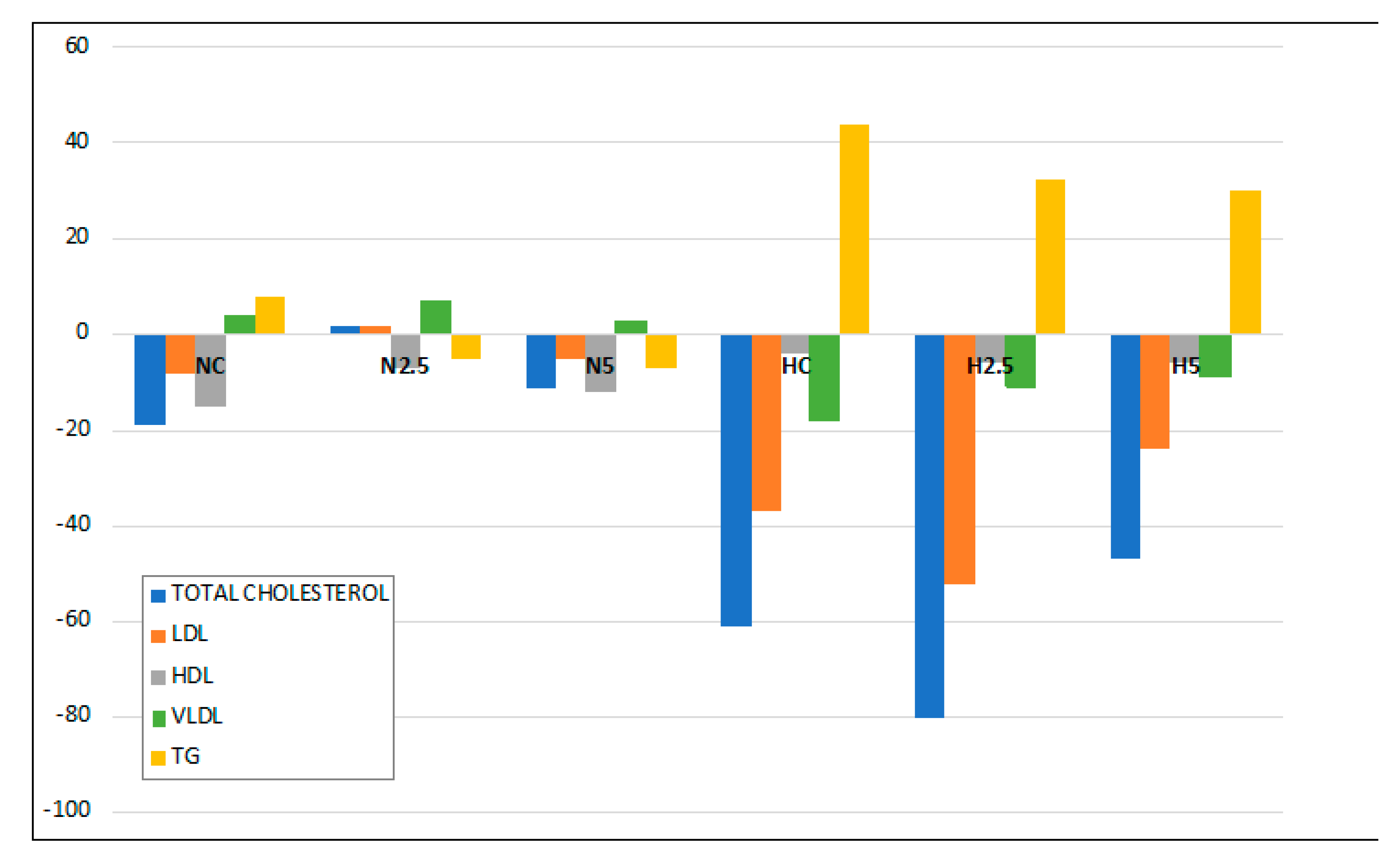
In the present study, the intake of high fat provoked steatosis in rats in the H groups, as described by different authors [
9,
14,
15]. Animals fed the high-fat diet showed a significant increase in the activities of hepatic enzymes and microvesicular steatosis. However, this hepatic disturbance was not severe, because there were no clear symptoms of lipotoxicity and steatohepatitis, as revealed by the inflammation and oxidative stress biomarkers. Although the consumption of spinach had no clear effect in the inflammation and stress biomarkers, we observed in the histological examination a reduction of the lipid size of vacuoles and a significant reduction in the total weight of the liver and plasmatic glucose levels in H2.5 and H5 groups. This effect of carotenoids on glucose metabolism has been reported in previous in vivo studies using dietary carotenoids [
16,
17], showing a beneficial effect on steatosis features by decreasing insulin resistance. Although some plasmatic parameters remained within the normal range [
18,
19], from the beginning to the end of the experiment, it was expected that the most significant changes would occur in the lipid profile of the plasma due to the alteration of lipid metabolism by the hepatic steatosis, increasing total cholesterol and TG. In contrast, a decrease in total cholesterol and lipoprotein LDL and VLDL could be explained by a decline in the synthesis of lipoprotein in the liver, so that lipids accumulated in the hepatocytes instead of being liberated into the peripheral circulation [
20], whereas TG increased in plasma. It is remarkable that the consumption of spinach significantly reduced the proportion of plasmatic TG, indicating a role in lipid metabolism.
The role of carotenoids in the regulation of specific functions and in the prevention of disease is determined by their bioavailability. Carotenoids are absorbed through the mucosa of the small intestine by passive diffusion, lodging inside the chylomicrons, which are rich in triglycerides, due to their lipophilic nature, and are transported in the lymph to the liver [
21]. Later, carotenoids are transported by the LDL and are incorporated into the inner body of the lipoproteins, whereas xanthophylls become attached to their surface; for this reason, xanthophylls are transported at a greater rate to other organs [
22]. The accumulation of total carotenoids in the liver (carotenes and lutein) was significantly correlated with the amount of spinach provided and the type of diet, since the fat content of the feed facilitated the absorption of carotenoids, as has been mentioned in other studies [
9]; for this reason, in rats in group N2.5, carotenoids were not detected in the liver.
Although carotenoids were absorbed and accumulated in the liver of rats of N5, H2.5 and H5 groups, these antioxidants did not appear to have a significant effect on the biomarkers of oxidative stress and inflammation in plasma. Ko et al. [
23] reported that the oxidative stress caused by hyperlipidemia can be partly prevented by the antioxidant activities of spinach administrated at 5%, together with a fat-rich diet. However, these authors described a decline in thiobarbituric acid reactive substances (TBARs) in the liver, but not in plasma. Other researchers have reported that several dietary carotenoids—such as lycopene from tomato juice administered ad libitum, lutein (100 mg/g of diet), zeaxanthin (0.25 mg/g), and astaxanthin (0.2 mg/g)—can diminish the oxidative stress and/or levels of biomarkers of cellular inflammation in rats [
6,
9,
10,
16]. These concentrations are higher than those assayed in this study. Hence, to evaluate the beneficial effect of spinach on these biomarkers, a higher concentration in the diet could be required. Although no changes were observed for plasma adiponectin and TNF-α, the modulation of the inflammatory response by carotenoids depends on different factors, such as specific compounds and their concentrations, but also on the level of oxidative stress [
24]. Carotenoids have been used to reduce the inflammatory response through effects on the transcription system of nuclear factor-κB (NF-κB). However, the results are contradictory: in different cell cultures, lycopene was found to repress NF-κB, while β-carotene stimulated it [
25]. Changes in the genes related to the inflammatory response were studied for the NC, H5, and N5 groups, and will be discussed later.
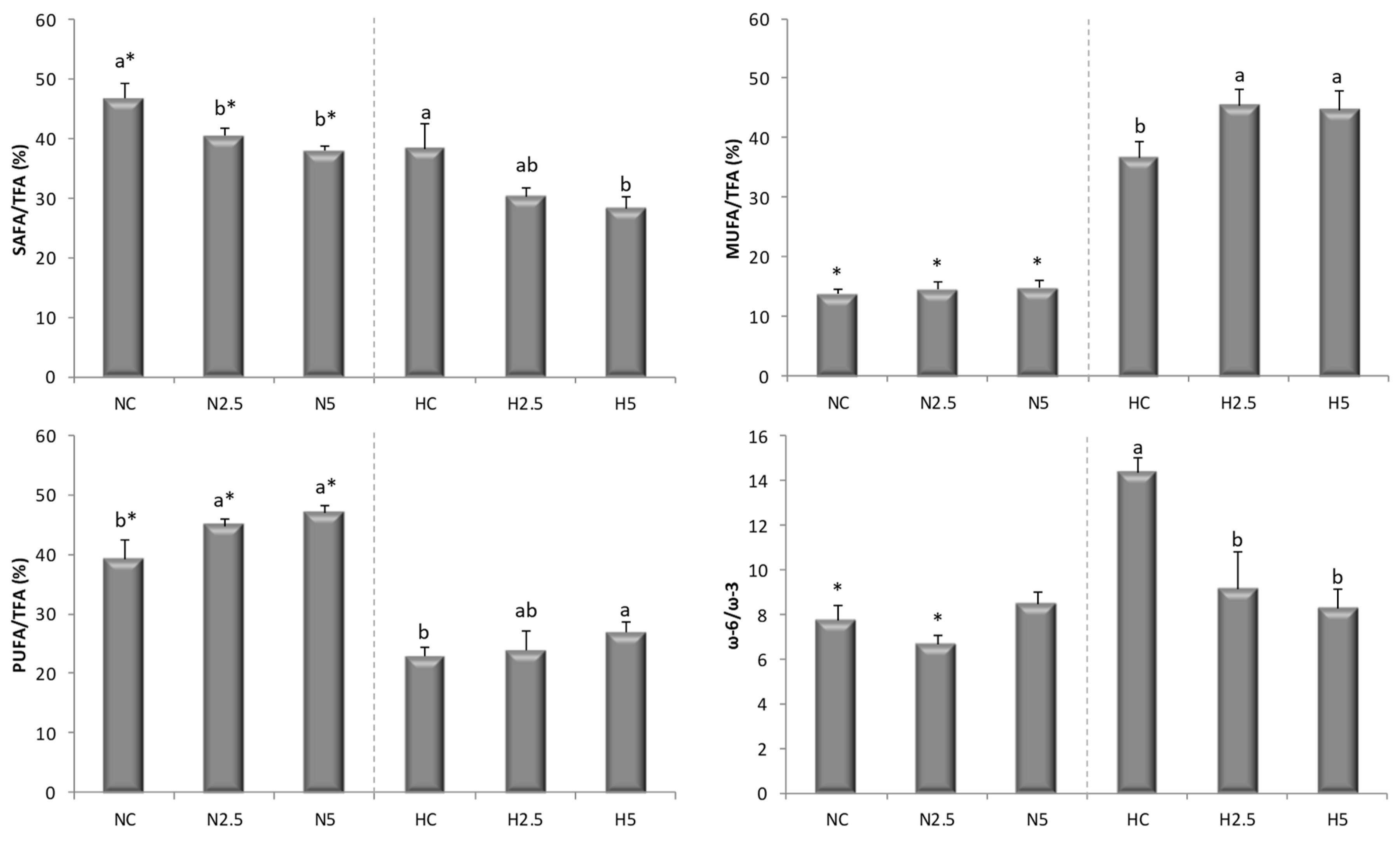
The depletion of PUFA in the H groups indicates a decline in fatty acid oxidation and triglyceride release from the liver, with a consequent increase in triglyceride synthesis that may have contributed significantly to the triglyceride accumulation in hepatocytes [
26]. The consumption of spinach led to significant changes in the liver fatty acid profile, which became healthier, with increases in the proportions of MUFA and PUFA and decreases in SAFA and the n-6/n-3 ratio. These changes could be considered beneficial with respect to the inhibition of stress-related kinases and apoptosis [
27], since it has been reported that MUFA decreases intracellular lipid levels and the markers of inflammation, increasing fatty acid oxidation and triglycerides synthesis [
28]. Moreover, this behavior suggests that the accumulation of carotenoids stimulated the conversion of fatty acids into long-chain unsaturated products, yielding α-linolenic acid (C18:3 n-3, ALA) and eicosadienoic acid (C20:2 n-6, EDA), as described by Bell et al. [
29] for the flesh of salmon. In our study, when the high-fat diet was supplemented with spinach, the MUFA and PUFA concentrations increased significantly, reducing the SAFA contribution (
Figure 2). The most plausible explanation is a higher activity of both desaturases and elongase enzymes, as described by other authors [
30]. These changes reduce or inhibit the de novo synthesis of fatty acids and activate their β-oxidation by the stimulation of MUFA and PUFA. The decrease in the n-6/n-3 ratio indicates an increase in the concentration of n-3 fatty acids, which limits the storage of triglycerides in the liver, reducing the risk of the development of NAFLD [
31]. In addition, n-3 PUFAs are precursors of anti-inflammatory eicosanoids, as opposed to n-6 PUFAs, which produce pro-inflammatory eicosanoids [
32]. When carotenoids were present in the liver, the concentrations of the ALA (C18:3 n-3 α), EDA, EPA (C20:5 n-3), and DHA (C22:6 n-3) fatty acids increased, improving the liver fatty acid profile in health terms.
In addition, carotenoids also influenced the reduction of the cholesterol level in the liver of rats with steatosis, as has been described in the scientific literature for different carotenoids. Kim et al. [
10] reported a significant reduction in the percentage distribution of free cholesterol in the liver of rats after the administration of 0.1 g/100 g of lutein. Nicolle et al. [
33] reported that supplementation of the diet with 0.25% cholesterol and 20% lyophilized carrots significantly reduced the cholesterol levels in plasma and in the liver of C57BL/6J mice. Qiu et al. [
34] fed Sprague–Dawley rats with a high-fat diet supplemented with 50 mg of lutein/kg body weight/day and found a reduction in the cholesterol content in the liver, which correlated with the concentration of lutein in the diet. In other animal studies involving a high-fat diet supplemented with 20 mg of lycopene/kg body weight/day or 30 mg of astaxanthin/kg body weight/day, the administration of these carotenoids reduced the concentration of cholesterol in the liver to levels similar to the control [
35,
36]. In general, these authors used carotenoid-rich plant material or pure compounds at a dose 4–8 times higher than that used in our experiment. Although we used a lower dose, liver cholesterol decreased in rats with steatosis after the intake of spinach, as well in healthy animals when taking in around 50 μg/day of total carotenoids. Different mechanisms have been proposed for the reduction of cholesterol accumulation in the liver; one is the inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) activity, as has been described for other carotenoids such as lycopene [
37] and β-carotene [
38].
3.2. Bioactivity of Diets Supplemented with Spinach: Modulation of Gene Expression
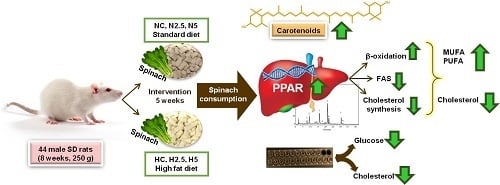
Although the available evidence regarding the potential use of non-provitamin A carotenoids in the prevention and treatment of NAFLD suggests that these compounds are effective in decreasing lipid accumulation, insulin resistance, oxidative stress, and inflammation in hepatic tissue, more complex pathways related to gene expression have not been elucidated completely [
6]. In this research, we observed that the intake of a fatty diet and spinach led to changes in the expression of genes related to fatty liver disease. In the N5 group—the healthy animals that consumed spinach—there was an over-expression of a great number of genes in comparison with the control group (NC). The over-expression of genes related to β-oxidation, Acyl-Coenzyme A dehydrogenase, long-chain (
ACADL), Carnitine palmitoyltransferase 2 (
CPT2), and peroxisome proliferator activated receptor alpha (
PPARA) was observed.
ACADL encodes a dehydrogenase enzyme that catalyzes the initial step in each cycle of fatty acid β-oxidation; this is one of a class of enzymes that are important due to their role in the metabolism of fatty acids present in the diet [
39]. In addition,
CPT2, together with carnitine palmitoyltransferase 1A, liver (
CPT1A), oxidizes long-chain fatty acids in the mitochondria, which allows the linkage of acyl-CoA derivatives to a polar molecule of carnitine, resulting in the formation of acylcarnitine molecules that are transported into the mitochondria [
40]. In this study, only
CPT2 was overexpressed, but in a previous study, we reported that the intake of lycopene from tomato juice increased the mRNA abundance of
CPT1A, together with the carnitine content, in the liver of rats with steatosis [
8,
9].
The peroxisome proliferator activated receptors (PPARs) consist of three members—PPARA, peroxisome proliferator-activated receptor gamma (PPARG), and peroxisome proliferator-activated receptor delta (PPARD)—which form obligate heterodimers with the retinoid X receptor (RXR). Carotenoids and their metabolites are activators of these receptors [
25,
41], which are involved in the transcriptional regulation of several pathways of lipid metabolism.
PPARA is expressed in the liver and has been considered to exert a critical role in the prevention of fat-related oxidative stress, inflammation and NAFLD [
42].
PPARD and
PPARG were also over-expressed in N5 rats, showing the effect of carotenoids from spinach on the activation of other types of PPAR receptors, expressed in other tissues [
43]. In addition, the acyl-CoA synthetase medium-chain family member 3 gene (
ACSM3), which encodes a protein that participates in the synthesis of medium-chain fatty acids, was over-expressed, showing an enhancement of lipid metabolism. The activity of this protein leads to a higher influx of fatty acids into the mitochondria, and in particular facilitates the oxidation of medium-chain fatty acids (from C4 to C11), since small- and medium-chain acyl-CoA derivatives have the ability to cross the inner mitochondrial membrane by diffusion [
40]. For these reasons, rats fed with spinach (groups N5 and H5) had a lower SAFA/TFA ratio in the liver (
Table 6 and
Figure 2).
Related to cholesterol transport and metabolism, the over-expression of apolipoproteins (
APOA1,
APOB, and
APOE) and membrane receptors such as low-density lipoprotein receptor (
LDLR) and
ABCG1 was observed, which could have led to an increase in the activity of proteins involved in cholesterol transport.
ABCG1 was over-expressed, increasing the efflux of cholesterol and phospholipids to lipid-poor apolipoproteins. This transporter is a major regulator of cellular cholesterol and phospholipid homeostasis, since cholesterol taken up by the liver can be recycled back through the ABCG1 pathway, being secreted into bile—as either free cholesterol or bile acids—or assembled into lipoprotein particles that are secreted back into the circulation [
44]. The overexpression of
ABCG1 was accompanied by the overexpression of the mRNA of apolipoproteins A, B, and E, and also that of the LDL receptor, which indicates an increase in lipoprotein metabolism. We also found an over-expression of the mRNA of genes involved in the repression of the synthesis of different lipids, such as nuclear receptor subfamily 1, group H, member 3 (
NR1H3), nuclear receptor subfamily 1, group H, member 4 (
NR1H4), CCHC-type zinc finger, nucleic acid binding protein (
CNBP) and sterol regulatory element binding transcription factor 2 (
SREBF2), which are responsible for the inhibition of the synthesis of cholesterol and bile acids. LXR (NR1H3) is involved in liver lipid metabolism [
45] and exhibits a homeostatic effect at the transcriptional level. This receptor regulates the synthesis of lipids via SREBF2 and the excretion of bile acids, by activating cytochrome P450, family 7, subfamily A, polypeptide 1 (
CYP7A1), thereby reaching a balance in the content of hepatic cholesterol [
46]. This effect has been described after the consumption of other carotenoids, such as lycopene from tomato, by rats with NAFLD induced by a fatty diet [
47].
Additionally, the genes involved in the inflammatory response increased their activity, as demonstrated by overexpression of the genes suppressor of cytokine signaling 3 (
SOCS3), caspase 3 (
CASP3), and mitogen-activated protein kinase 8 (
MAPK8), which participate in anti-inflammatory and apoptotic processes. Other carotenoids, such as lycopene, can prevent oxidative stress in hepatocytes and also modulate the transcriptome response of genes related to apoptosis and regulation of the cell cycle, particularly by the over-expression of the tumor-suppressor protein (TP53), which acts as a major defense against cancer through the differential activation of target genes [
48]. However, it is important to highlight that the adiponectin receptor 1 (
ADIPOR1) and nuclear factor (
NFKB1) genes were over-expressed, which also could be considered beneficial for the liver. Adiponectin has been shown to have cytoprotective properties, improving both hepatic and peripheral insulin sensitivity and preventing steatosis, inflammation, and necrosis, whereas the inhibition of
NFKB1 induces non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma (HCC) by sensitizing hepatocytes to undergo spontaneous apoptosis [
49]. Also, in the N5 group, the over-expression of
IL1B, considered an inflammatory cytokine, was observed, but no negative effect was detected in the rats.
In the HC group (rats with steatosis induced by the fatty diet), genes such as
ABCG1 and
LPL were over-expressed. These genes encode the proteins that facilitate the extracellular transport of lipids and the hydrolysis of TG in free fatty acids, respectively. The gene expression of
LPL is higher in obese subjects with NAFLD than in subjects without NAFLD, suggesting that free fatty acids released by the lipolysis of circulating triglycerides also contribute to hepatocellular fatty acid accumulation and steatosis [
50]. In addition, there was an increase in the expression of the transcription factor
PPARG; this is responsible for increasing insulin sensitivity and for promoting the entry of fatty acids and storage of triglycerides in adipocytes [
51]. Additionally, the
IL1B and
TNFR (
FAS) genes were overexpressed, revealing inflammation in the adipocytes—a result of the accumulation of fat. These changes are in concordance with the disturbance of lipid metabolism associated with NAFLD and with lipotoxicity [
51].
Unlike the HC group, the H5 group showed some similarities with the N5 group, which indicates that spinach consumption had a positive effect on the amelioration of the fatty liver condition. For this reason, a positive modulation of the genes involved in the β-oxidation of fatty acids was observed, associated with the overexpression of
CPT1A,
CPT2, and the nuclear factor
PPARA. With regard to cholesterol transport and metabolism, the
APOE gene, which encodes lipoproteins, was also over-expressed, but to a lesser extent in comparison to the N5 group. Hence, spinach intake promoted the expression of these genes, so that liver cholesterol declined due to its efflux from the liver through the more abundant VLDL and LDL apoliproteins. The cholesterol catabolic activity was enhanced by the over-expression of the cytochrome P450, family 2, subfamily E, polypeptide 1 (
CYP2E1) and
CYP7A1 genes, responsible for the augmented synthesis of bile acids through the overexpression of
NR1H4.
FXR (
NR1H4) stimulated the expression of
CYP7A1 in H5 rats, keeping the synthesis of bile acids active and thus leading to a decline in hepatic cholesterol [
45]. PPARG is required for adipocyte differentiation and for the maintenance of differentiated adipocyte, and is considered as an adipogenic factor, since it is an activator of fatty acid synthesis and storage. However, it plays divergent roles in the metabolism, and these effects in the metabolism are controversial. Thus,
PPARG’s activation by thiazolidinediones, the most investigated synthetic agonist, results in the increased production of adipokines, including adiponectin, which enhances hepatic fatty acid oxidation. In addition, it also promotes fat storage in adipocytes and decreases adipose tissue lipolysis, thereby decreasing the concentration of fatty acids stored in the liver. Moreover, its activation also exhibits an anti-inflammatory role. This effect is associated with the increase of insulin sensitivity, improving the resistance of insulin associated with the steatosis and metabolic syndrome [
52]. In this study, we do not analyze the resistance of insulin, but the supplementation of spinach and the accumulation of carotenoids in the liver (H5 group) exhibited a significant effect on the reduction of plasmatic glucose, total cholesterol and TG, as well as cholesterol in the liver. Finally, the
ADIPOR1 and
CASP3 genes were over-expressed, as described above for rats of the N5 group.