Fluvastatin Sodium Ameliorates Obesity through Brown Fat Activation
Abstract
1. Introduction
2. Results
2.1. FS Stimulates BAT Activity in Human Brown Adipocyte
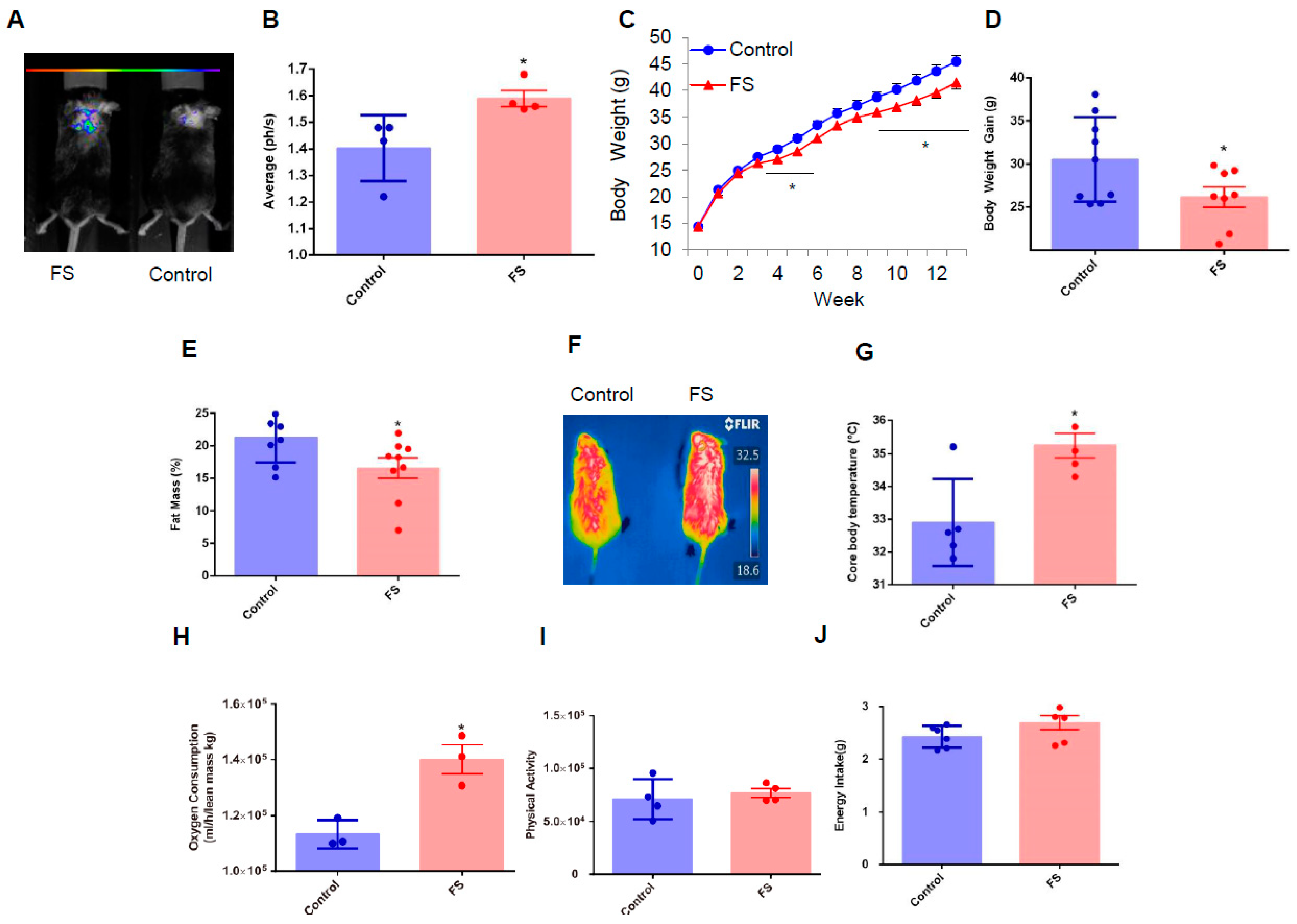
2.2. FS Reduces Adiposity, Improves Glucose Homeostasis, and Increases Energy Expenditure in HFD Mice
2.3. FS Improves Glucose Homeostasis in HFD Mice
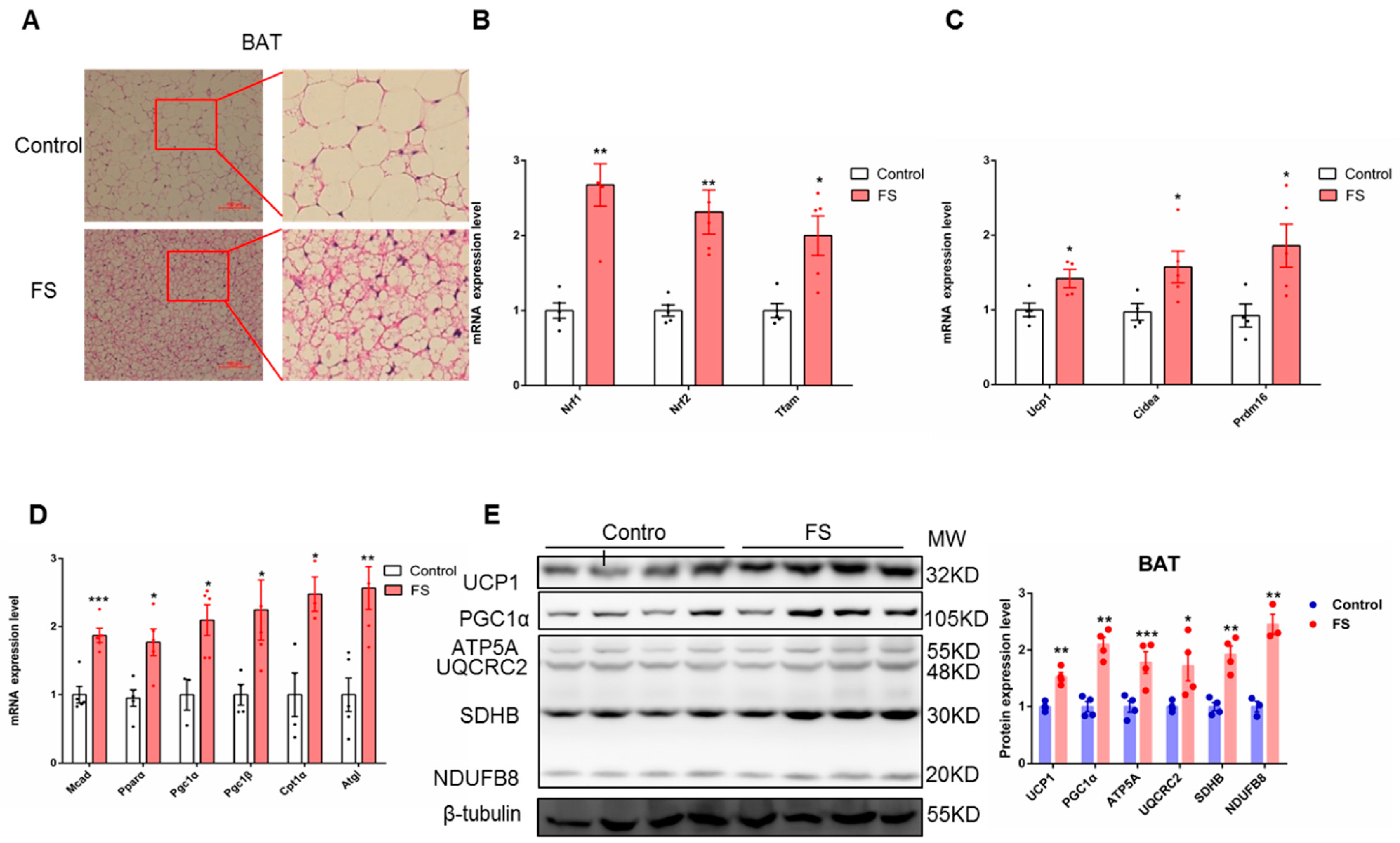
2.4. FS Increases Thermogenesis by Increasing BAT Activity
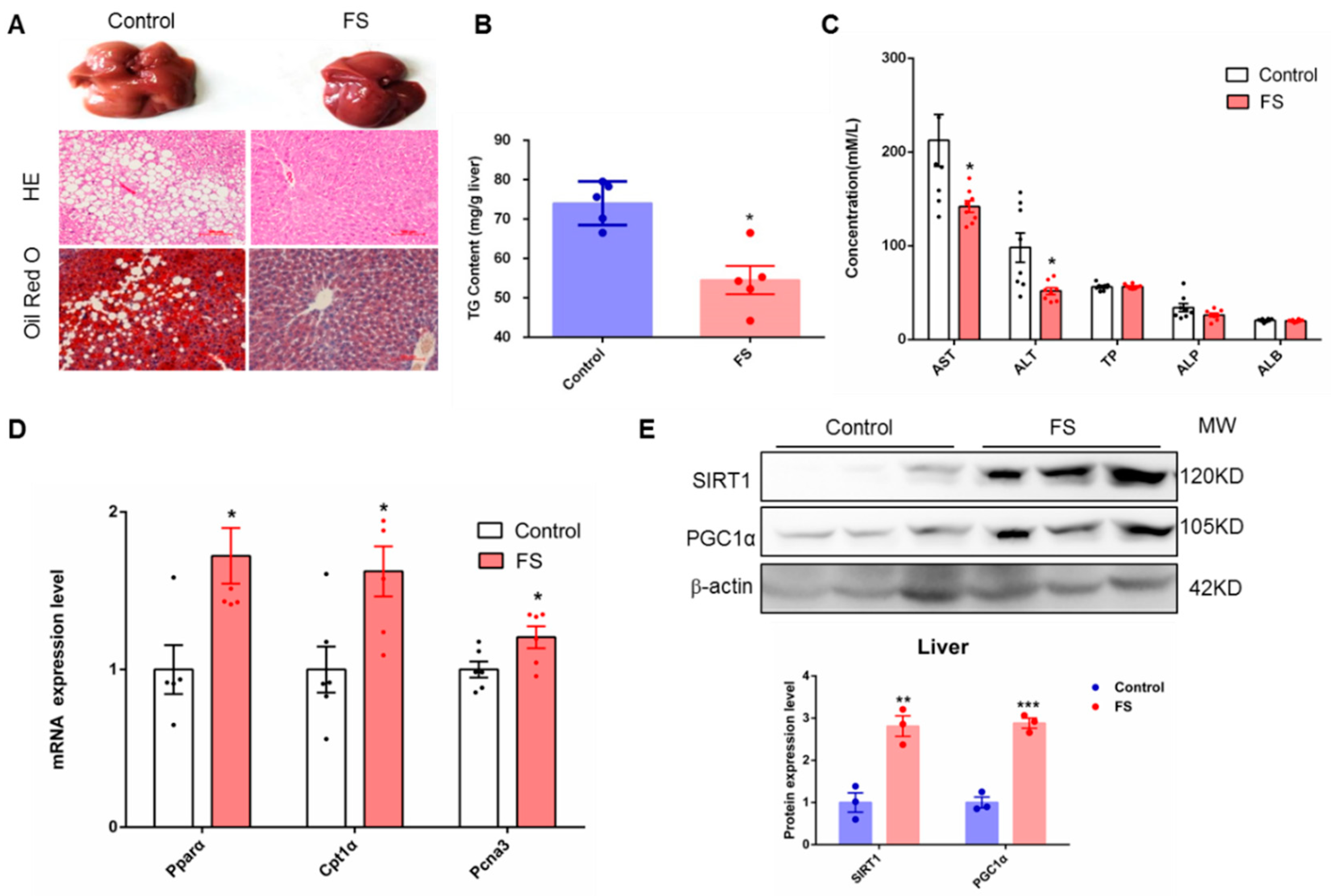
2.5. FS Relieves Hepatic Steatosis
2.6. FS Increases Browning of sWAT
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Fluvastatin Sodium Treatment
4.3. Cell Experiments
4.4. Compound Screening
4.5. Real-Time Quantitative PCR
4.6. Western Blotting
4.7. Glucose Tolerance Test and Insulin Tolerance Test
4.8. In Vivo Imaging
4.9. Cold Challenge Experiment
4.10. Hematoxylin-Eosin (H&E) Staining
4.11. Oil Red O Staining
4.12. Measurement of Mitochondrial DNA Copy Number
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jin, W.; Patti, M.-E. Genetic determinants and molecular pathways in the pathogenesis of Type 2 diabetes. Clin. Sci. 2009, 116, 99–111. [Google Scholar] [CrossRef]
- Wahba, I.M.; Mak, R.H. Obesity and Obesity-Initiated Metabolic Syndrome: Mechanistic Links to Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Colao, A.; Orio, F. Insulin-Mediated Diseases: Adrenal Mass and Polycystic Ovary Syndrome. Endocrinol. Metab. 2015, 26, 512–514. [Google Scholar] [CrossRef]
- Lindholm, Å.; Andersson, L.; Eliasson, M.; Bixo, M.; Sundström-Poromaa, I. Prevalence of symptoms associated with polycystic ovary syndrome. Int. J. Gynecol. Obstet. 2008, 102, 39–43. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Heglind, M.; Westergren, R.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Virtanen, K.A.; Lidell, M.E.; Orava, J.; Niemi, T.; Enerbäck, S.; et al. Functional Brown Adipose Tissue in Healthy Adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar]
- Holmes, D. Metabolism: Transplantation comBATs obesity. Nat. Rev. Endocrinol. 2015, 11, 317. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; You, Y.; Meng, M.; Zheng, Z.; Dong, M.; Lin, J.; Zhao, Q.; Zhang, C.; Yuan, X.; et al. Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice. Endocrinology 2015, 156, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Hu, T.; Zhao, H.; Huang, Y.; Ye, R.; Lin, J.; Zhang, C.; Zhang, H.; Wei, G.; Zhou, H.; et al. Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, 2708–2713. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Z.; Zhu, X.; Meng, M.; Li, L.; Shen, Y.; Chi, Q.; Wang, D.; Zhang, Z.; Li, C.; et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013, 23, 851–854. [Google Scholar] [CrossRef]
- Chi, Q.S.; Wang, D.H. Thermal physiology and energetics in male desert hamsters (Phodopus roborovskii) during cold acclimation. J. Comp. Physiol. B 2011, 181, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wei, G.; You, Y.; Huang, Y.; Lee, H.J.; Dong, M.; Lin, J.; Hu, T.; Zhang, H.; Zhang, C.; et al. Rutin ameliorates obesity through brown fat activation. FASEB J. 2017, 31, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Bouvy, N.D.; Teule, G.J.; Lichtenbelt, W.D.V.M.; Kemerink, G.J.; Schrauwen, P. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar]
- Cypess, A.M.; Chen, Y.-C.; Sze, C.; Wang, K.; English, J.; Chan, O.; Holman, A.R.; Tal, I.; Palmer, M.R.; Kolodny, G.M.; et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 10001–10005. [Google Scholar] [CrossRef] [PubMed]
- Van Marken Lichtenbelt, W. Brown adipose tissue and the regulation of nonshivering thermogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Rohla, M.; Haberfeld, H.; Tscharre, M.; Huber, K.; Weiss, T.W. Awareness, treatment, and control of hypertension in Austria: A multicentre cross-sectional study. J. Hypertens. 2016, 34, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Rohla, M.; Haberfeld, H.; Sinzinger, H.; Kritz, H.; Tscharre, M.; Freynhofer, M.K.; Huber, K.; Weiss, T.W. Systematic screening for cardiovascular risk at pharmacies. Open Heart 2016, 3, 000497. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.P.; Sekhon, S.S.; Tsang, M.; Wright, J.M. Fluvastatin for lowering lipids. Cochrane Database Syst. Rev. 2018, 3, CD012282. [Google Scholar] [CrossRef] [PubMed]
- Galmozzi, A.; Sonne, S.B.; Keylin, S.; Hasegawa, Y.; Shinoda, K.; Luijten, I.H.N.; Chang, J.W.; Sharp, L.Z.; Cravatt, B.F.; Saez, E.; et al. ThermoMouse: An in vivo model to identify modulators of UCP1 expression in brown adipose tissue. Cell Rep. 2014, 9, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Zhang, H.; Liu, S.; Lee, H.J.; Jin, W.; Cheng, J. Hepatitis C virus core protein induces hepatic steatosis via Sirt1-dependent pathway. Liver Int. 2018, 38, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef]
- Pfannenberg, C.; Werner, M.K.; Ripkens, S.; Stef, I.; Deckert, A.; Schmadl, M.; Reimold, M.; Häring, H.-U.; Claussen, C.D.; Stefan, N. Impact of Age on the Relationships of Brown Adipose Tissue with Sex and Adiposity in Humans. Diabetes 2010, 59, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Abletshauser, C.; Hoffmann, M.M.; Friedrich, I.; Baumstark, M.W.; Wieland, H.; März, W. Effect of Fluvastatin Slow-Release on Low Density Lipoprotein (LDL) Subfractions in Patients with Type 2 Diabetes Mellitus: Baseline LDL Profile Determines Specific Mode of Action. J. Clin. Endocrinol. Metab. 2002, 87, 5485–5490. [Google Scholar] [CrossRef]
- Winkler, K.; Abletshauser, C.; Friedrich, I.; Hoffmann, M.M.; Wieland, H.; März, W. Fluvastatin Slow-Release Lowers Platelet-Activating Factor Acetyl Hydrolase Activity: A Placebo-Controlled Trial in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Scuffham, P.A.; Chaplin, S. A cost-effectiveness analysis of fluvastatin in patients with diabetes after successful percutaneous coronary intervention. Clin. Ther. 2005, 27, 1467–1477. [Google Scholar] [CrossRef]
- Singh, R.; Krishan, P. Modulation of impact of high fat diet in pathological and physiological left ventricular cardiac hypertrophy by fluvastatin. Biomed. Pharmacother. 2010, 64, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, A.; Hattori, Y.; Suzuki, K.; Okayasu, T.; Kase, H.; Satoh, H.; Kasai, K. Effects of statins on vascular endothelial function in hypercholesterolemic patients with type 2 diabetes mellitus: Fluvastatin vs. rosuvastatin. Int. J. Cardiol. 2010, 144, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Savić, V.; Janić, M.; Lunder, M.; Kanc, K.; Janez, A.; Eržen, B.; Sabovic, M. Long-term improvement of arterial wall characteristics in patients with diabetes mellitus type 1 using cyclic, intermittent treatment with a low-dose fluvastatin and valsartan combination. Exp. Ther. Med. 2015, 10, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, P.; Rioja, J.; García-Arias, C.; Sánchez-Chaparro, M.A.; Gonzalez-Santos, P. Omega 3 fatty acids induce a marked reduction of apolipoprotein B48 when added to fluvastatin in patients with type 2 diabetes and mixed hyperlipidemia: A preliminary report. Cardiovasc. Diabetol. 2009, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, M.; Guazzini, B.; Righini, V.; Barrella, M.; Toscano, R.; Chebat, E. Metabolic effects of fluvastatin extended release 80 mg and atorvastatin 20 mg in patients with type 2 diabetes mellitus and low serum high-density lipoprotein cholesterol levels: A 4-month, prospective, open-label, randomized, blinded-end point (probe) trial. Curr. Ther. Res. Clin. Exp. 2004, 65, 330–344. [Google Scholar] [PubMed]
- Hu, M.-Y.; Li, Y.-L.; Jiang, C.-H.; Liu, Z.-Q.; Qu, S.-L.; Huang, Y.-M. Comparison of lycopene and fluvastatin effects on atherosclerosis induced by a high-fat diet in rabbits. Nutrition 2008, 24, 1030–1038. [Google Scholar] [CrossRef]
- Svetek, M.B.; Eržen, B.; Kanc, K.; Šabovič, M. Impaired endothelial function and arterial stiffness in patients with type 2 diabetes—The effect of a very low-dose combination of fluvastatin and valsartan. J. Diabetes Complicat. 2017, 31, 544–550. [Google Scholar] [CrossRef]
- DeRosa, G.; Cicero, A.F.G.; Murdolo, G.; Ciccarelli, L.; Fogari, R. Comparison of metabolic effects of orlistat and sibutramine treatment in Type 2 diabetic obese patients. Diabetes Nutr. Metab. 2004, 17, 222–229. [Google Scholar] [PubMed]
- Mnafgui, K.; Derbali, A.; Sayadi, S.; Gharsallah, N.; Elfeki, A.; Allouche, N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet-induced obese rats. J. Food Sci. Technol. 2015, 52, 4369–4377. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, S.-P.; Hu, M.; Li, J.-X. Fluvastatin blunts the effect of a high-fat meal on plasma triglyceride and high-sensitivity C-reactive protein concentrations in patients at high risk for cardiovascular events. Coron. Dis. 2007, 18, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Arampatzis, C.A.; Goedhart, D.; Serruys, P.W.; Saia, F.; Lemos, P.A.; De Feyter, P. Fluvastatin reduces the impact of diabetes on long-term outcome after coronary intervention—A Lescol Intervention Prevention Study (LIPS) substudy. Am. Heart J. 2005, 149, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Procino, G.; Milano, S.; Carmosino, M.; Barbieri, C.; Nicoletti, M.C.; Li, J.H.; Wess, J.; Svelto, M. Combination of secretin and fluvastatin ameliorates the polyuria associated with X-linked nephrogenic diabetes insipidus in mice. Kidney Int. 2014, 86, 127–138. [Google Scholar] [CrossRef]
- Henriksbo, B.D.; Lau, T.C.; Cavallari, J.F.; Denou, E.; Chi, W.; Lally, J.S.; Crane, J.; Duggan, B.M.; Foley, K.P.; Fullerton, M.D.; et al. Fluvastatin Causes NLRP3 Inflammasome-Mediated Adipose Insulin Resistance. Diabetes 2014, 63, 3742–3747. [Google Scholar] [CrossRef]
- Aguirre, L.; Hijona, E.; Macarulla, M.T.; Gracia, A.; Larrechi, I.; Bujanda, L.; Hijona, L.; Portillo, M.P. Several statins increase body and liver fat accumulation in a model of metabolic syndrome. J. Physiol. Pharmacol. 2013, 64, 281–288. [Google Scholar]
- Hu, T.; Yuan, X.; Ye, R.; Zhou, H.; Lin, J.; Zhang, C.; Zhang, H.; Wei, G.; Dong, M.; Huang, Y.; et al. Brown adipose tissue activation by rutin ameliorates polycystic ovary syndrome in rat. J. Nutr. Biochem. 2017, 47, 21–28. [Google Scholar] [CrossRef]
- Wu, N.-N.; Zhang, C.-H.; Lee, H.-J.; Ma, Y.; Wang, X.; Ma, X.-J.; Ma, W.; Zhao, D.; Feng, Y.-M. Brown adipogenic potential of brown adipocytes and peri-renal adipocytes from human embryo. Sci. Rep. 2016, 6, 39193. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.D.; Lynes, M.D.; Dreyfuss, J.M.; Shamsi, F.; Schulz, T.J.; Zhang, H.B.; Huang, T.L.; Townsend, K.L.; Li, Y.M.; Takahashi, H.; et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat. Med. 2015, 21, 760. [Google Scholar] [CrossRef]
- Lasar, D.; Rosenwald, M.; Kiehlmann, E.; Baláž, M.; Tall, B.; Opitz, L.; Lidell, M.E.; Zamboni, N.; Krznar, P.; Sun, W.; et al. Peroxisome Proliferator Activated Receptor Gamma Controls Mature Brown Adipocyte Inducibility through Glycerol Kinase. Cell Rep. 2018, 22, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Masand, R.; Paulo, E.; Wu, D.; Wang, Y.; Swaney, D.L.; Jimenez-Morales, D.; Krogan, N.J.; Wang, B. Proteome Imbalance of Mitochondrial Electron Transport Chain in Brown Adipocytes Leads to Metabolic Benefits. Cell Metab. 2018, 27, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Villegas, L.A.; Perino, A.; Lemos, V.; Ziętak, M.; Nomura, M.; Pols, T.W.H.; Schoonjans, K. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Kaneko, T.; Li, J.S.; Liu, A.-D.; Voss, C.; Li, S.S.C. A phosphorylation switch controls the spatiotemporal activation of Rho GTPases in directional cell migration. Nat. Commun. 2015, 6, 7721. [Google Scholar] [CrossRef]
- Vazquez, M.J.; Toro, C.A.; Castellano, J.M.; Ruiz-Pino, F.; Roa, J.; Beiroa, D.; Heras, V.; Velasco, I.; Dieguez, C.; Pinilla, L.; et al. SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nat. Commun. 2018, 9, 4194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, H.; Liu, Z.; Ding, Y.; LeDoux, S.P.; Wilson, G.L.; Voellmy, R.; Lin, Y.; Lin, W.; Nahta, R.; et al. Overcoming Trastuzumab Resistance in Breast Cancer by Targeting Dysregulated Glucose Metabolism. Cancer Res. 2011, 71, 4585–4597. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| Human-Cyclophilin | TAAAGCATACGGGTCCTGGC | GACTGAGTGGTTGGATGGCA |
| Human-UCP1 | GCAGGGAAAGAAACAGCACC | CCCGTGTAGCGAGGTTTGAT |
| Human-PGC1α | CAGGCAGTAGATCCTCTTCAAG | TCCTCGTAGCTGTCATACCTG |
| Human-PGC1β | GCCCAGATACACTGACTACG | CTCGAGGGTTAAGGCTGTTATC |
| Human-PPARα | GCTATCATTACGGAGTCCACG | TCGCACTTGTCATACACCAG |
| Human-PPARγ | ATACATAAAGTCCTTCCCGCTG | GGGTGATGTGTTTGAACTTGATT |
| Human-PRDM16 | TTCGGATGGGAGCAAATACTG | CACGGATGTACTTGAGCCAG |
| Human-CPT1β | ATCCTACTCCTATGACCCCG | TCTGCATTGAGACCCAACTG |
| Human-CPT1α | CCTCCAGTTGGCTTATCGTG | TTCTTCGTCTGGCTGGACAT |
| Human-CEBP/α | ACTAGGAGATTCCGGTGCCT | GAATTCTCCCCTCCTCGCAG |
| Human-CEBP/β | GCACAGCGACGAGTACAAGA | TTGAACAAGTTCCGCAGGGT |
| Mouse-Cyclophilin | CAAATGCTGGACCAAACACAA | GCCATCCAGCCATTCAGTCT |
| Mouse-Ucp1 | GGCAAAAACAGAAGGATTGC | TAAGCCGGCTGAGATCTTGT |
| Mouse-Cidea | TGCTCTTCTGTATCGCCCAGT | GCCGTGTTAAGGAATCTGCTG |
| Mouse-Prdm16 | GAAGTCACAGGAGGACACGG | CTCGCTCCTCAACACACCTC |
| Mouse-Cpt1α | GACTCCGCTCGCTCATTCC | GACTGTGAACTGGAAGGCCA |
| Mouse-Mcad | ACTCGAAAGCGGCTCACAA | ACGGGGATAATCTCCTCTCTGG |
| Mouse-Pgc1α | ACAGCTTTCTGGGTGGATTG | TGAGGACCGCTAGCAAGTTT |
| Mouse-Pgc1β | CGTATTTGAGGACAGCAGCA | TACTGGGTGGGCTCTGGTAG |
| Mouse-Pparα | AGCCTCAGCCAAGTTGAAGT | TGGGGAGAGAGGACAGATGG |
| Mouse-Tbx1 | GGCAGGCAGACGAATGTTC | TTGTCATCTACGGGCACAAAG |
| Mouse-Tmem26 | ACCCTGTCATCCCACAGAG | TGTTTGGTGGAGTCCTAAGGTC |
| Mouse-Cd137 | CGTGCAGAACTCCTGTGATAAC | GTCCACCTATGCTGGAGAAGG |
| Mouse-Atgl | GCGCCAGGACTGGAAAGAAT | TGAGAACGCTGAGGCTTTGAT |
| Mouse-Tfam | GTCCATAGGCACCGTATTGC | CCCATGCTGGAAAAACACTT |
| Mouse-Nrf1 | CAACAGGGAAGAAACGGAAA | GCACCACATTCTCCAAAGGT |
| Mouse-Nrf2 | CCCCCGAGGACACTTCTTATG | AGCAGCCAGATGGGCAGTTA |
| Mouse-Pcna3 | CACGTCTCCTTGGTACAGCTTACTC | CACGCCCATGGCTAGGTT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, N.; Zhang, H.; Ye, R.; Dong, M.; Lin, J.; Zhou, H.; Huang, Y.; Chen, L.; Jiang, X.; Nagaoka, K.; et al. Fluvastatin Sodium Ameliorates Obesity through Brown Fat Activation. Int. J. Mol. Sci. 2019, 20, 1622. https://doi.org/10.3390/ijms20071622
Yin N, Zhang H, Ye R, Dong M, Lin J, Zhou H, Huang Y, Chen L, Jiang X, Nagaoka K, et al. Fluvastatin Sodium Ameliorates Obesity through Brown Fat Activation. International Journal of Molecular Sciences. 2019; 20(7):1622. https://doi.org/10.3390/ijms20071622
Chicago/Turabian StyleYin, Na, Hanlin Zhang, Rongcai Ye, Meng Dong, Jun Lin, Huiqiao Zhou, Yuanyuan Huang, Li Chen, Xiaoxiao Jiang, Kentaro Nagaoka, and et al. 2019. "Fluvastatin Sodium Ameliorates Obesity through Brown Fat Activation" International Journal of Molecular Sciences 20, no. 7: 1622. https://doi.org/10.3390/ijms20071622
APA StyleYin, N., Zhang, H., Ye, R., Dong, M., Lin, J., Zhou, H., Huang, Y., Chen, L., Jiang, X., Nagaoka, K., Zhang, C., & Jin, W. (2019). Fluvastatin Sodium Ameliorates Obesity through Brown Fat Activation. International Journal of Molecular Sciences, 20(7), 1622. https://doi.org/10.3390/ijms20071622






