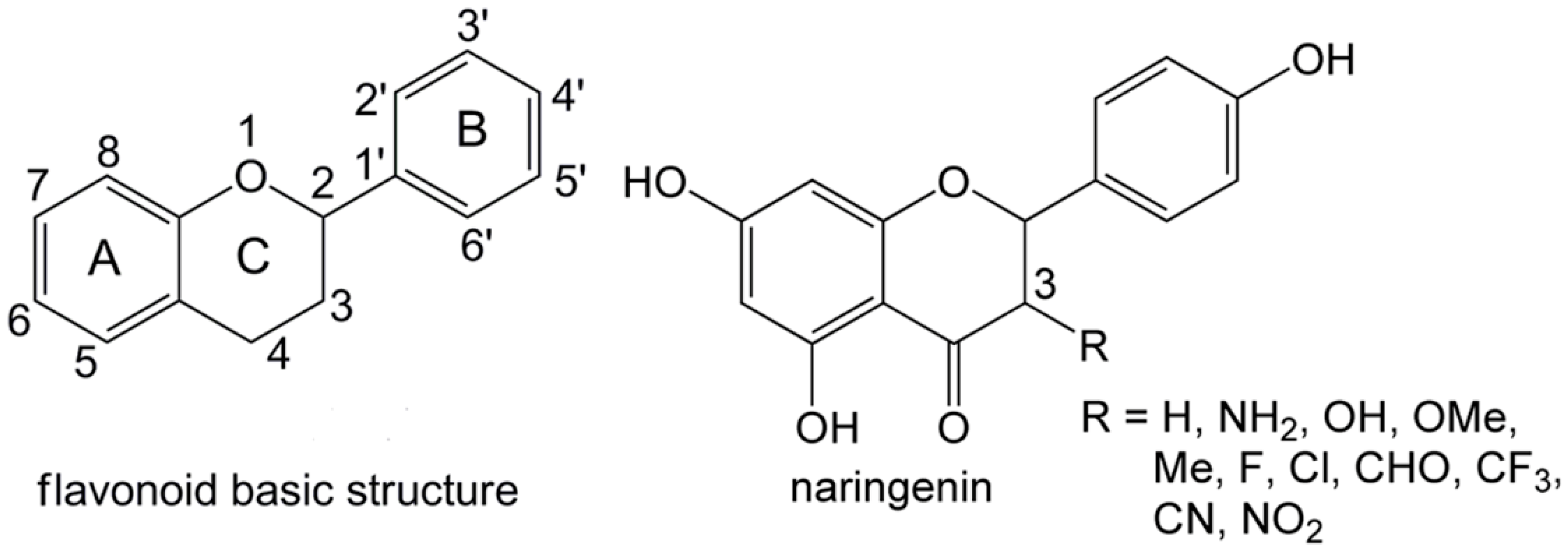
DFT Studies on the Antioxidant Activity of Naringenin and Its Derivatives: Effects of the Substituents at C3
Abstract
:1. Introduction
2. Results and Discussion
2.1. HAT Mechanism
2.1.1. Calculated BDEs of Naringenin and its C3-Substituted Derivatives
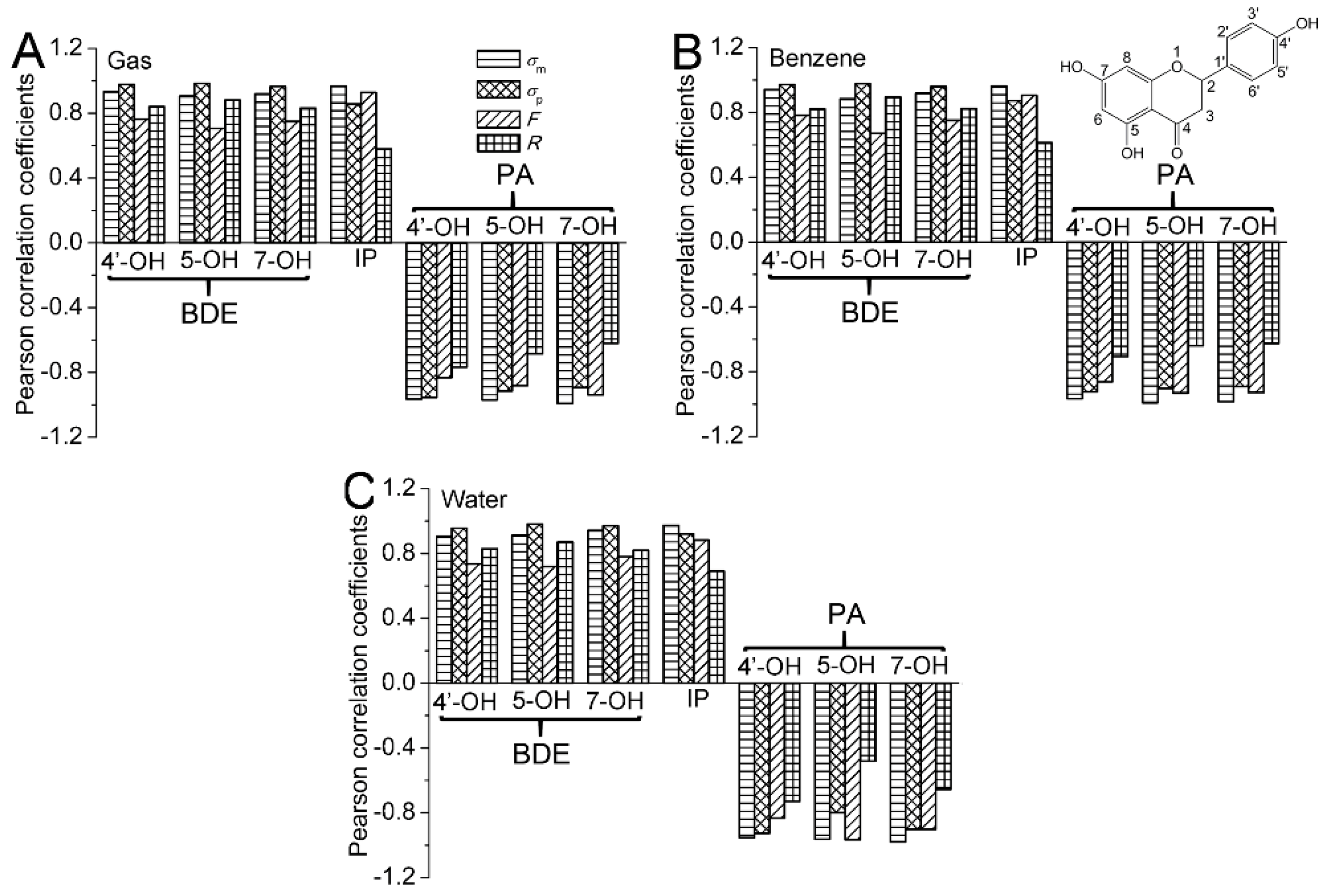
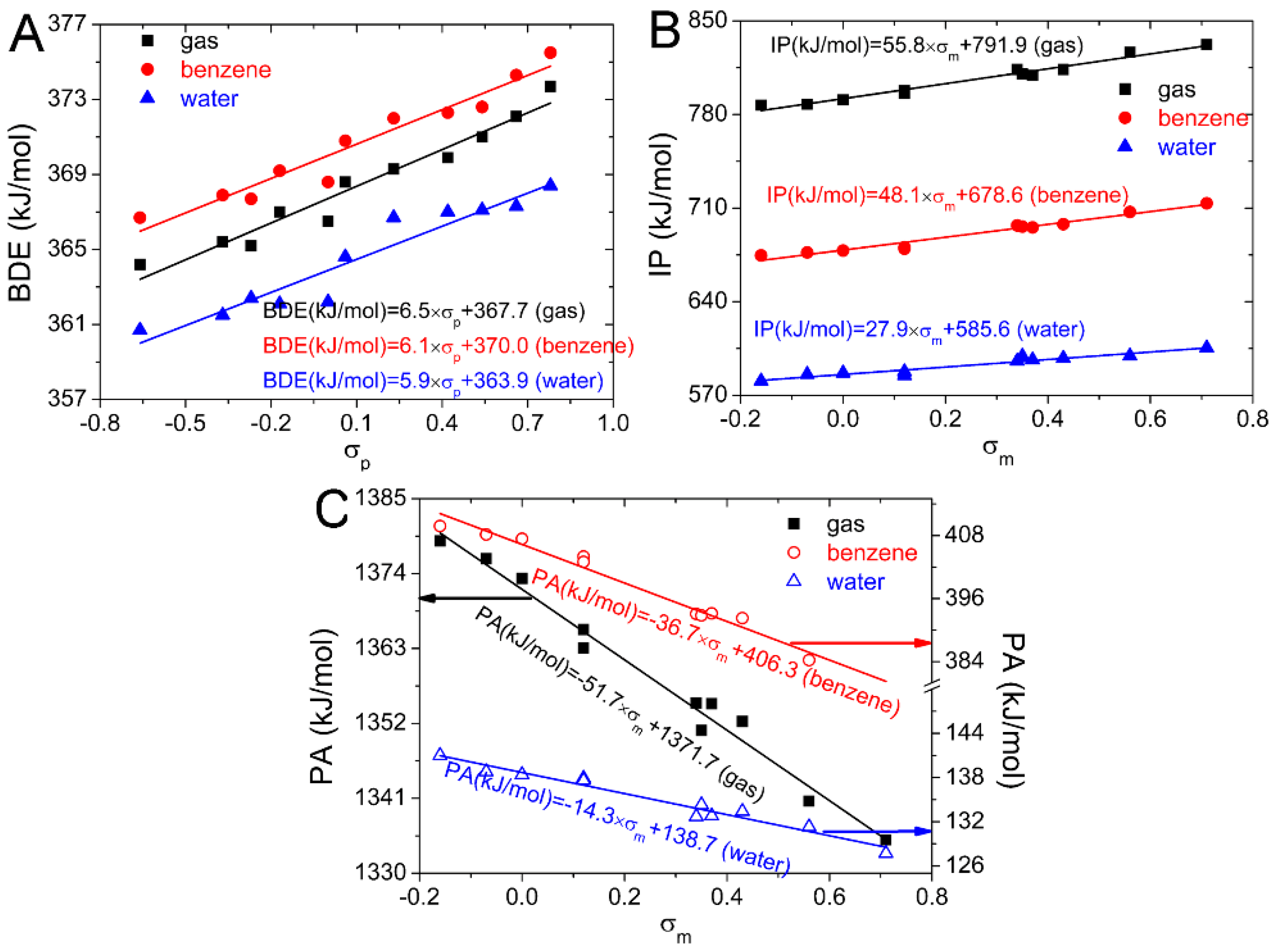
2.1.2. Dependence of BDEs on Hammett Sigma Constants
2.1.3. The Electronic Effects of the Substituents on the BDEs
2.2. SET-PT Mechanism
2.2.1. Calculated IPs of Naringenin and its C3-Substituted Derivatives
2.2.2. Dependence of IPs on Hammett Sigma Constants
2.2.3. The Electronic Effects of the Substituents on the IPs
2.3. SPLET mechanism
2.3.1. Calculated PAs of Naringenin and its C3-Substituted Derivatives
2.3.2. Dependence of PAs on Hammett Sigma Constants
2.3.3. The Electronic Effects of the Substituents on the PAs
2.4. The antioxidant Activity Strength Influenced by the Substituted Groups
3. Materials and Methods
3.1. Computational Details
3.2. Statistics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DFT | Density Functional Theory |
| HAT | Hydrogen Atom Transfer |
| SPLET | Sequential Proton Loss Electron Transfer |
| SET-PT | Single Electron Transfer Followed by Proton Transfer |
| ArOH | Flavonoid |
| BDE | Bond Dissociation Enthalpy |
| IP | Ionization Potential |
| PDE | Proton Dissociation Enthalpy |
| PA | Proton Affinity |
| ETE | Electron Transfer Enthalpy |
| ΔrG | Gibbs Free Energy |
| σ | Hammett sigma constants |
| F | field/inductive effect |
| R | resonance effect |
| P | Pearson correlation coefficient |
References
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free radical-induced damage to DNA: Mechanisms and measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A. Vitamin E and heart disease: Basic science to clinical intervention trials. Free Radic. Biol. Med. 2000, 28, 141–164. [Google Scholar] [CrossRef]
- Hahn, M.; Baierle, M.; Charão, M.F.; Bubols, G.B.; Gravina, F.S.; Zielinsky, P.; Arbo, M.D.; Cristina Garcia, S. Polyphenol-rich food general and on pregnancy effects: A review. Drug Chem. Toxicol. 2017, 40, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, D.; Hubert, J.; Lalun, N.; Renault, J.H.; Bobichon, H.; Nour, M.; Voutquenne-Nazabadioko, L. Isolation of flavonoids and triterpenoids from the fruits of Alphitonia neocaledonica and evaluation of their anti-oxidant, anti-tyrosinase and cytotoxic activities. Phytochem. Anal. 2015, 26, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Trimech, I.; Weiss, E.K.; Chedea, V.S.; Marin, D.; Detsi, A.; Ioannou, E.; Roussis, V.; Kefalas, P. Evaluation of anti-oxidant and acetylcholinesterase activity and identification of polyphenolics of the invasive weed Dittrichia viscosa. Phytochem. Anal. 2015, 25, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Tapas, A.R.; Sakarkar, D.M.; Kakde, R.B. Flavonoids as nutraceuticals: A review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Bandy, B. Mechanisms of flavonoid protection against myocardial ischemia–reperfusion injury. J. Mol. Cell. Cardiol. 2009, 46, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.T.; Stafford, G.I.; van Staden, J.; Christensen, S.B.; Jäger, A.K. Isolation of the MAOinhibitor naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008, 117, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic. Res. 2005, 39, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006, 147, S287–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Song, J.; Guo, P.; Cao, W.; Bian, J. Exploring a possible way to synthesize novel better antioxidants based on vitamin E: A DFT study. Bioorg. Med. Chem. Lett. 2006, 16, 5874–5877. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Lukeš, V.; Ilčin, M. DFT/B3LYP study of tocopherols and chromans antioxidant action energetics. Chem. Phys. 2007, 336, 51–57. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Stȩpień, B.T. Sigma-and pi-electron delocalization: Focus on substituent effects. Chem. Rev. 2005, 105, 3482–3512. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Štefanič-Petek, A.; Krbavčič, A.; Šolmajer, T. QSAR of flavonoids: 4. Differential inhibition of aldose reductase and p56 lck protein tyrosine kinase. Croat. Chem. Acta 2002, 75, 517–529. [Google Scholar]
- Aparicio, S. A systematic computational study on flavonoids. Int. J. Mol. Sci. 2010, 11, 2017–2038. [Google Scholar] [CrossRef]
- Trouillas, P.; Fagnère, C.; Lazzaroni, R.; Calliste, C.; Marfak, A.; Duroux, J.L. A theoretical study of the conformational behavior and electronic structure of taxifolin correlated with the free radical-scavenging activity. Food Chem. 2004, 88, 571–582. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanisms of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Stepanić, V.; Trošelj, K.G.; Lučić, B.; Marković, Z.; Amić, D. Bond dissociation free energy as a general parameter for flavonoid radical scavenging activity. Food Chem. 2013, 141, 1562–1570. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Siskos, D. Radical scavenging activity characterization of synthetic isochroman-derivatives of hydroxytyrosol: A gas-phase DFT approach. Food Res. Int. 2015, 76, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Vagánek, A.; Rimarčík, J.; Dropková, K.; Lengyel, J.; Klein, E. Reaction enthalpies of O–H bonds splitting-off in flavonoids: The role of non-polar and polar solvent. Comput. Theor. Chem. 2014, 1050, 31–38. [Google Scholar] [CrossRef]
- Vargas-Sánchez, R.D.; Mendoza-Wilson, A.M.; Balandrán-Quintana, R.R.; Torrescano-Urrutia, G.R.; Sánchez-Escalante, A. Study of the molecular structure and chemical reactivity of pinocembrin by DFT calculations. Comput. Theor. Chem. 2015, 1058, 21–27. [Google Scholar] [CrossRef]
- Wang, G.; Xue, Y.; An, L.; Zheng, Y.; Dou, Y.; Zhang, L.; Liu, Y. Theoretical study on the structural and antioxidant properties of some recently synthesised 2, 4, 5-trimethoxy chalcones. Food Chem. 2015, 171, 89–97. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Chen, D.F.; Liang, Q.; Guo, R.; Fu, Z.M. Theoretical studies on the antioxidant activity of pinobanksin and its ester derivatives: Effects of the chain length and solvent. Food Chem. 2018, 240, 323–329. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Siri, D.; Lazzaroni, R.; Duroux, J.L. A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: The specificity of the 3-OH site. Food Chem. 2006, 97, 679–688. [Google Scholar] [CrossRef]
- Leopoldini, M.; Rondinelli, F.; Russo, N.; Toscano, M. Pyranoanthocyanins: A theoretical investigation on their antioxidant activity. J. Agric. Food Chem. 2010, 58, 8862–8871. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.; Milenković, D.; Đorović, J.; Marković, J.M.D.; Stepanić, V.; Lučić, B.; Amić, D. PM6 and DFT study of free radical scavenging activity of morin. Food Chem. 2012, 134, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, J.; Rimarčík, J.; Vagánek, A.; Klein, E. On the radical scavenging activity of isoflavones: Thermodynamics of O–H bond cleavage. Phys. Chem. Chem. Phys. 2013, 15, 10895–10903. [Google Scholar] [CrossRef] [PubMed]
- Anouar, E.; Košinová, P.; Kozlowski, D.; Mokrini, R.; Duroux, J.L.; Trouillas, P. New aspects of the antioxidant properties of phenolic acids: A combined theoretical and experimental approach. Phys. Chem. Chem. Phys. 2009, 11, 7659–7668. [Google Scholar] [CrossRef]
- Silva, M.M.; Santos, M.R.; Caroço, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant activity relationships of flavonoids: A re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Sun, Y.M.; Wang, X.L. Electronic effects on O–H proton dissociation energies of phenolic cation radicals: A DFT Study. J. Org. Chem. 2002, 67, 2709–2712. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Sun, Y.M.; Chen, D.Z. O–H bond dissociation energies of phenolic compounds are determined by field/inductive effect or resonance effect? A DFT study and its implication. Mol. Inform. 2001, 20, 148–152. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H. Gaussian 09 (Revision B.01); Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Bartmess, J.E. Thermodynamics of the electron and the proton. J. Phys. Chem. 1994, 98, 6420–6424. [Google Scholar] [CrossRef]
- Rimarčík, J.; Lukeš, V.; Klein, E.; Ilčin, M. Study of the solvent effect on the enthalpies of homolytic and heterolytic N–H bond cleavage in pphenylenediamine and tetracyano- p-phenylenediamine. J. Mol. Struct. Theochem. 2010, 925, 25–30. [Google Scholar] [CrossRef]
- Parker, V.D. Homolytic bond (H–A) dissociation free energies in solution. Applications of the standard potential of the (H+/H. bul.) couple. J. Am. Chem. Soc. 1992, 114, 7458–7462. [Google Scholar] [CrossRef]
- Rodriguez-Lujan, I.; Huerta, R.; Elkan, C.; Cruz, C.S. Quadratic programming feature selection. J. Mach. Learn. Res. 2010, 11, 1491–1516. [Google Scholar]
| Substituents | Gas | Benzene | Water | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 4′−OH | 5−OH | 7−OH | 4′−OH | 5−OH | 7−OH | 4′−OH | 5−OH | 7−OH | |
| H | 366.5 | 428 | 391.1 | 368.6 | 423.2 | 395.2 | 362.2 | 391.2 | 393.4 |
| NH2 | 364.2 | 425.3 | 389.7 | 366.7 | 421.0 | 393.6 | 360.7 | 389.9 | 391.0 |
| OH | 365.4 | 426.1 | 390.1 | 367.9 | 421.5 | 393.8 | 361.5 | 390.1 | 391.6 |
| OMe | 365.2 | 425.8 | 390.4 | 367.7 | 421.3 | 394.2 | 362.4 | 390.2 | 392.1 |
| Me | 367.0 | 427.2 | 390.9 | 369.2 | 422.7 | 394.5 | 362.1 | 390.6 | 392.5 |
| F | 368.6 | 429.1 | 391.5 | 370.8 | 423.8 | 396.1 | 364.6 | 391.6 | 394.8 |
| Cl | 369.3 | 430.1 | 393.9 | 372.0 | 423.9 | 396.6 | 366.7 | 392.1 | 395.5 |
| CHO | 369.9 | 430.9 | 394.3 | 372.3 | 424.1 | 398.4 | 367.0 | 392.7 | 396.0 |
| CF3 | 371.0 | 431.2 | 394.8 | 372.6 | 424.7 | 399.3 | 367.1 | 393.6 | 396.9 |
| CN | 372.1 | 431.9 | 396.2 | 374.3 | 425.5 | 400.9 | 367.3 | 393.9 | 398.5 |
| NO2 | 373.7 | 432.4 | 397.3 | 375.5 | 426.1 | 402.3 | 368.4 | 394.3 | 400.5 |
| Substituents | Gas | Benzene | Water |
|---|---|---|---|
| H | 791.0 | 678.2 | 586.6 |
| NH2 | 786.9 | 674.6 | 580.6 |
| OH | 797.8 | 679.6 | 587.6 |
| OMe | 795.7 | 680.8 | 584.7 |
| Me | 787.7 | 676.8 | 585.6 |
| F | 813.7 | 697.1 | 595.5 |
| Cl | 809.3 | 695.6 | 596.4 |
| CHO | 810.4 | 696.2 | 599.2 |
| CF3 | 813.8 | 698.0 | 597.7 |
| CN | 826.5 | 707.1 | 599.4 |
| NO2 | 832.4 | 713.6 | 605.4 |
| Substituents | Gas | Benzene | Water | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 4′−OH | 5−OH | 7−OH | 4′−OH | 5−OH | 7−OH | 4′−OH | 5−OH | 7−OH | |
| H | 1406.3 | 1445.3 | 1373.3 | 439.2 | 468.8 | 407.4 | 162.3 | 160.2 | 138.4 |
| NH2 | 1409.5 | 1447.7 | 1378.8 | 442.2 | 472.0 | 409.8 | 164.2 | 161.9 | 141.0 |
| OH | 1405.7 | 1442.6 | 1363.1 | 437.9 | 464.1 | 404.0 | 162.0 | 153.8 | 137.9 |
| OMe | 1405.5 | 1435.4 | 1365.8 | 437.8 | 462.3 | 403.0 | 161.9 | 157.5 | 137.6 |
| Me | 1406.9 | 1447.3 | 1376.2 | 440.4 | 471.2 | 408.2 | 162.6 | 161.6 | 138.8 |
| F | 1404.0 | 1423.6 | 1355.0 | 437.8 | 450.1 | 393.1 | 161.5 | 150.0 | 132.7 |
| Cl | 1404.6 | 1424.0 | 1354.9 | 437.4 | 451.2 | 393.2 | 161.2 | 152.1 | 132.8 |
| CHO | 1402.9 | 1418.9 | 1351.0 | 436.4 | 450.0 | 392.8 | 161.5 | 152.7 | 134.3 |
| CF3 | 1401.1 | 1419.0 | 1352.3 | 436.2 | 448.5 | 392.3 | 160.2 | 151.8 | 133.4 |
| CN | 1399.6 | 1407.0 | 1340.6 | 433.6 | 439.4 | 384.3 | 159.3 | 148.9 | 131.3 |
| NO2 | 1397.8 | 1399.6 | 1334.9 | 431.8 | 433.0 | 379.6 | 158.6 | 142.2 | 127.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.-Z.; Deng, G.; Guo, R.; Chen, D.-F.; Fu, Z.-M. DFT Studies on the Antioxidant Activity of Naringenin and Its Derivatives: Effects of the Substituents at C3. Int. J. Mol. Sci. 2019, 20, 1450. https://doi.org/10.3390/ijms20061450
Zheng Y-Z, Deng G, Guo R, Chen D-F, Fu Z-M. DFT Studies on the Antioxidant Activity of Naringenin and Its Derivatives: Effects of the Substituents at C3. International Journal of Molecular Sciences. 2019; 20(6):1450. https://doi.org/10.3390/ijms20061450
Chicago/Turabian StyleZheng, Yan-Zhen, Geng Deng, Rui Guo, Da-Fu Chen, and Zhong-Min Fu. 2019. "DFT Studies on the Antioxidant Activity of Naringenin and Its Derivatives: Effects of the Substituents at C3" International Journal of Molecular Sciences 20, no. 6: 1450. https://doi.org/10.3390/ijms20061450
APA StyleZheng, Y.-Z., Deng, G., Guo, R., Chen, D.-F., & Fu, Z.-M. (2019). DFT Studies on the Antioxidant Activity of Naringenin and Its Derivatives: Effects of the Substituents at C3. International Journal of Molecular Sciences, 20(6), 1450. https://doi.org/10.3390/ijms20061450




