Obesity-Altered Adipose Stem Cells Promote ER+ Breast Cancer Metastasis through Estrogen Independent Pathways
Abstract
:1. Introduction
2. Results
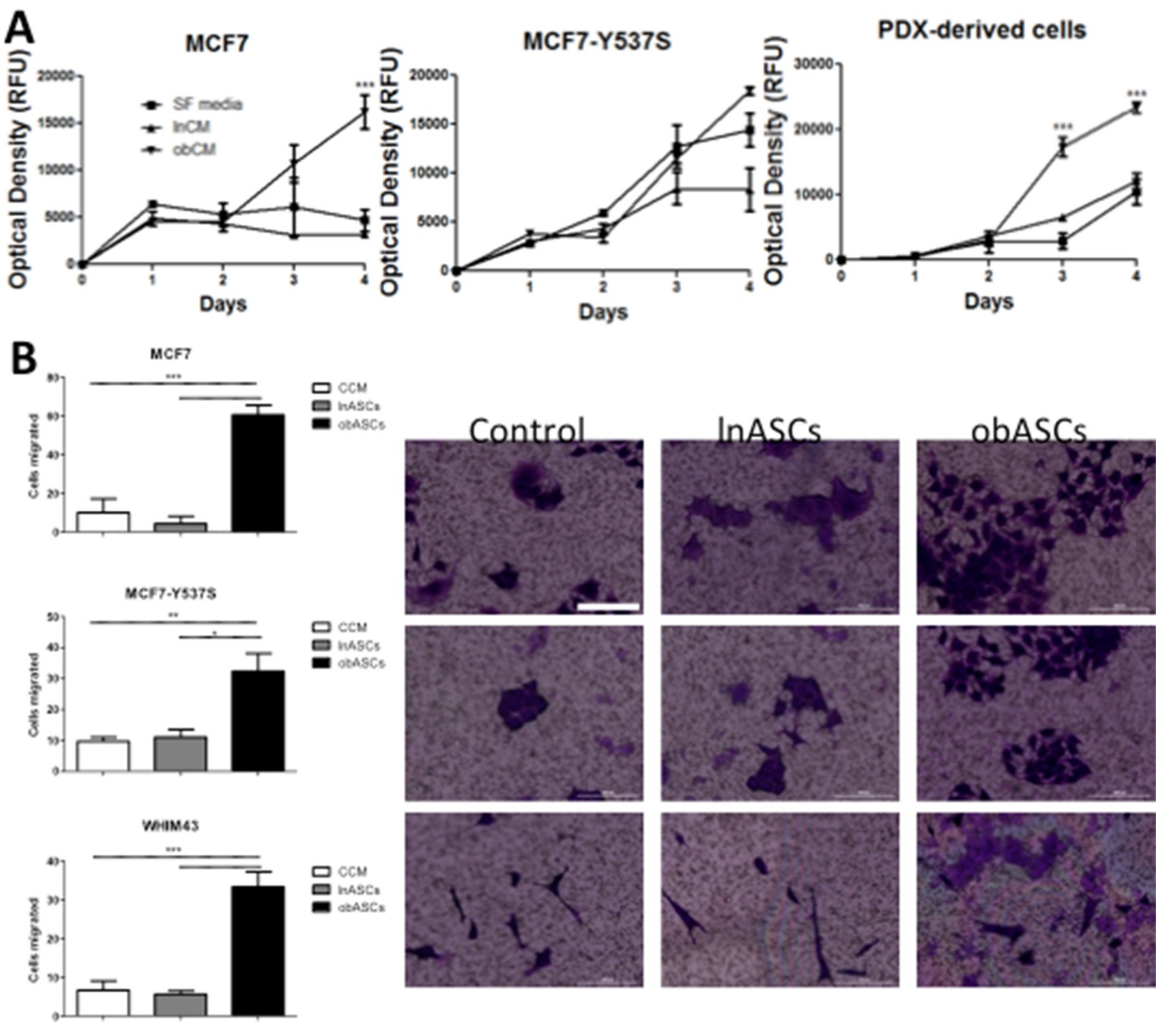
2.1. Obesity-Altered Adipose Stem Cells Promote Metastasis but Not Tumor Growth of Breast Cancer with Mutant ERα
2.2. In Vitro obASCs Promote Proliferation and Migration of ER WT and ER MUT Cells
2.3. Regulation of Breast Cancer Related Genes in ER WT and ER MUT Cells by obASCs
3. Discussion
4. Materials and Methods
4.1. Human Subjects
4.2. Cell Culture
4.3. RT-qPCR
4.4. Conditioned Media Proliferation Assay
4.5. Migration Assay
4.6. Orthotopic Xenografts
4.7. Patient-Derived Xenografts
4.8. Flow Cytometry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCs | Adipose stem cells |
| obASCs | Obesity-altered ASCs |
| lnASCs | Lean ASCs |
| ER+ | Estrogen Receptor positive |
| BC | Breast cancer |
| WT | Wild Type |
| MUT | Mutant |
| BMI | Body mass index |
| EMT | Epithelial-to-mesenchymal transition |
| CAFs | Cancer-associated fibroblasts |
| BCCs | Breast cancer cells |
| CCM | Complete culture media |
| IACUC | Institutional animal care and use committee |
| PDX | Patient-derived xenografts |
| CTCs | Circulating tumor cells |
| CM | Conditioned media |
| TME | Tumor microenvironment |
| cAMP | Cyclic adenosine monophosphate |
| PKA | Protein Kinase A |
| PTEN | Phosphatase and tensin homolog |
| PI3K | Phosphatidylinositol 3-kinase |
| AKT | Protein kinase B |
| uPA | Urokinase plasminogen activator |
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Enger, S.M.; Ross, R.K.; Paganini-Hill, A.; Carpenter, C.L.; Bernstein, L. Body size, physical activity, and breast cancer hormone receptor status: Results from two case-control studies. Cancer Epidemiol. Biomark. Prev. 2000, 9, 681–687. [Google Scholar]
- Loi, S.; Milne, R.L.; Friedlander, M.L.; McCredie, M.R.; Giles, G.G.; Hopper, J.L.; Phillips, K.A. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Expert panel on the identification, evaluation, and treatment of overweight in adults. Am. J. Clin. Nutr. 1998, 68, 899–917. [CrossRef] [PubMed]
- Mitchell, N.S.; Catenacci, V.A.; Wyatt, H.R.; Hill, J.O. Obesity: Overview of an epidemic. Psychiatr. Clin. N. Am. 2011, 34, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, N.C.D. Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Zahid, H.; Subbaramaiah, K.; Iyengar, N.M.; Zhou, X.K.; Chen, I.C.; Bhardwaj, P.; Gucalp, A.; Morrow, M.; Hudis, C.A.; Dannenberg, A.J.; et al. Leptin regulation of the p53-hif1alpha/pkm2-aromatase axis in breast adipose stromal cells: A novel mechanism for the obesity-breast cancer link. Int. J. Obes. 2018, 42, 711–720. [Google Scholar] [CrossRef]
- Pettinelli, P.; Videla, L.A. Up-regulation of ppar-gamma mrna expression in the liver of obese patients: An additional reinforcing lipogenic mechanism to srebp-1c induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Oberkofler, H.; Fukushima, N.; Esterbauer, H.; Krempler, F.; Patsch, W. Sterol regulatory element binding proteins: Relationship of adipose tissue gene expression with obesity in humans. Biochim. Biophys. Acta 2002, 1575, 75–81. [Google Scholar] [CrossRef]
- Bao, J.; Zhu, L.; Zhu, Q.; Su, J.; Liu, M.; Huang, W. Srebp-1 is an independent prognostic marker and promotes invasion and migration in breast cancer. Oncol. Lett. 2016, 12, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Fujii, N.; Narita, T.; Higami, Y. Srebp-1c-dependent metabolic remodeling of white adipose tissue by caloric restriction. Int. J. Mol. Sci. 2018, 19, 3335. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, Y.; Liu, S.; Cao, Y.; Wang, X.; Tao, Y. Pkm2: The thread linking energy metabolism reprogramming with epigenetics in cancer. Int. J. Mol. Sci. 2014, 15, 11435–11445. [Google Scholar] [CrossRef]
- Bojkova, B.; Garajova, M.; Kajo, K.; Pec, M.; Kubatka, P.; Kassayova, M.; Kiskova, T.; Orendas, P.; Ahlersova, E.; Ahlers, I. Pioglitazone in chemically induced mammary carcinogenesis in rats. Eur. J. Cancer Prev. 2010, 19, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Stollarova, N.; Skarda, J.; Zihlavnikova, K.; Kajo, K.; Kapinova, A.; Adamicova, K.; Pec, M.; Dobrota, D.; Bojkova, B.; et al. Preventive effects of fluvastatin in rat mammary carcinogenesis. Eur. J. Cancer Prev. 2013, 22, 352–357. [Google Scholar] [CrossRef]
- Eterno, V.; Zambelli, A.; Pavesi, L.; Villani, L.; Zanini, V.; Petrolo, G.; Manera, S.; Tuscano, A.; Amato, A. Adipose-derived mesenchymal stem cells (ascs) may favour breast cancer recurrence via hgf/c-met signaling. Oncotarget 2014, 5, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Muehlberg, F.L.; Song, Y.H.; Krohn, A.; Pinilla, S.P.; Droll, L.H.; Leng, X.; Seidensticker, M.; Ricke, J.; Altman, A.M.; Devarajan, E.; et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis 2009, 30, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated sdf-1/cxcl12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Devarajan, E.; Song, Y.H.; Krishnappa, S.; Alt, E. Epithelial-mesenchymal transition in breast cancer lines is mediated through pdgf-d released by tissue-resident stem cells. Int. J. Cancer 2012, 131, 1023–1031. [Google Scholar] [CrossRef]
- Goto, H.; Shimono, Y.; Funakoshi, Y.; Imamura, Y.; Toyoda, M.; Kiyota, N.; Kono, S.; Takao, S.; Mukohara, T.; Minami, H. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene 2019, 38, 767–779. [Google Scholar] [CrossRef]
- Yamamura, Y.; Asai, N.; Enomoto, A.; Kato, T.; Mii, S.; Kondo, Y.; Ushida, K.; Niimi, K.; Tsunoda, N.; Nagino, M.; et al. Akt-girdin signaling in cancer-associated fibroblasts contributes to tumor progression. Cancer Res. 2015, 75, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Pei, D.T.; Hurst, C.G.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Obesity enhances the conversion of adipose-derived stromal/stem cells into carcinoma-associated fibroblast leading to cancer cell proliferation and progression to an invasive phenotype. Stem Cells Int. 2017, 2017, 9216502. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Ohlstein, J.F.; Biagas, B.A.; Rhodes, L.V.; Pei, D.T.; Tucker, H.A.; Llamas, C.; Bowles, A.C.; Dutreil, M.F.; Zhang, S.; et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015, 17, 112. [Google Scholar] [CrossRef]
- Strong, A.L.; Strong, T.A.; Rhodes, L.V.; Semon, J.A.; Zhang, X.; Shi, Z.; Zhang, S.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Obesity associated alteration in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Res. 2013, 5, R102. [Google Scholar] [CrossRef]
- Harrod, A.; Fulton, J.; Nguyen, V.T.M.; Periyasamy, M.; Ramos-Garcia, L.; Lai, C.F.; Metodieva, G.; de Giorgio, A.; Williams, R.L.; Santos, D.B.; et al. Genomic modelling of the esr1 y537s mutation for evaluating function and new therapeutic approaches for metastatic breast cancer. Oncogene 2017, 36, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Toy, W.; Shen, Y.; Won, H.; Green, B.; Sakr, R.A.; Will, M.; Li, Z.; Gala, K.; Fanning, S.; King, T.A.; et al. Esr1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013, 45, 1439–1445. [Google Scholar] [CrossRef]
- Fanning, S.W.; Mayne, C.G.; Dharmarajan, V.; Carlson, K.E.; Martin, T.A.; Novick, S.J.; Toy, W.; Green, B.; Panchamukhi, S.; Katzenellenbogen, B.S.; et al. Estrogen receptor alpha somatic mutations y537s and d538g confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. eLife 2016, 5, e12792. [Google Scholar] [CrossRef]
- Haakinson, D.J.; Leeds, S.G.; Dueck, A.C.; Gray, R.J.; Wasif, N.; Stucky, C.C.; Northfelt, D.W.; Apsey, H.A.; Pockaj, B. The impact of obesity on breast cancer: A retrospective review. Ann. Surg. Oncol. 2012, 19, 3012–3018. [Google Scholar] [CrossRef]
- Strong, A.L.; Burow, M.E.; Gimble, J.M.; Bunnell, B.A. Concise review: The obesity cancer paradigm: Exploration of the interactions and crosstalk with adipose stem cells. Stem Cells 2015, 33, 318–326. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Jiang, Q.; Deng, J.; Xu, F.; Chen, X.; Cheng, F.; Zhang, Y.; Yao, Y.; Xia, Z.; et al. Human adipose-derived mesenchymal stem cell-secreted cxcl1 and cxcl8 facilitate breast tumor growth by promoting angiogenesis. Stem Cells 2017, 35, 2060–2070. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Zhao, H.; Wang, J.; Zhang, Q. Cxcl5 secreted from adipose tissue-derived stem cells promotes cancer cell proliferation. Oncol. Lett. 2018, 15, 1403–1410. [Google Scholar] [PubMed]
- Koellensperger, E.; Bonnert, L.C.; Zoernig, I.; Marme, F.; Sandmann, S.; Germann, G.; Gramley, F.; Leimer, U. The impact of human adipose tissue-derived stem cells on breast cancer cells: Implications for cell-assisted lipotransfers in breast reconstruction. Stem Cell Res. Ther. 2017, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Gros, P.; Croop, J.; Housman, D. Mammalian multidrug resistance gene: Complete cdna sequence indicates strong homology to bacterial transport proteins. Cell 1986, 47, 371–380. [Google Scholar] [CrossRef]
- Higgins, C.F. Abc transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef] [PubMed]
- Sekine, I.; Shimizu, C.; Nishio, K.; Saijo, N.; Tamura, T. A literature review of molecular markers predictive of clinical response to cytotoxic chemotherapy in patients with breast cancer. Int. J. Clin. Oncol. 2009, 14, 112–119. [Google Scholar] [CrossRef]
- Nakagami, H.; Soukupova, H.; Schikora, A.; Zarsky, V.; Hirt, H. A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in arabidopsis. J. Biol. Chem. 2006, 281, 38697–38704. [Google Scholar] [CrossRef]
- Wei, N.; Sun, H.; Wang, F.; Liu, G. H1, a novel derivative of tetrandrine reverse p-glycoprotein-mediated multidrug resistance by inhibiting transport function and expression of p-glycoprotein. Cancer Chemother. Pharmacol. 2011, 67, 1017–1025. [Google Scholar] [CrossRef]
- Bark, H.; Choi, C.H. Psc833, cyclosporine analogue, downregulates mdr1 expression by activating jnk/c-jun/ap-1 and suppressing nf-kappab. Cancer Chemother. Pharmacol. 2010, 65, 1131–1136. [Google Scholar] [CrossRef]
- Rohlff, C.; Glazer, R.I. Regulation of multidrug resistance through the camp and egf signalling pathways. Cell Signal. 1995, 7, 431–443. [Google Scholar] [CrossRef]
- Ziemann, C.; Riecke, A.; Rudell, G.; Oetjen, E.; Steinfelder, H.J.; Lass, C.; Kahl, G.F.; Hirsch-Ernst, K.I. The role of prostaglandin e receptor-dependent signaling via camp in mdr1b gene activation in primary rat hepatocyte cultures. J. Pharmacol. Exp. Ther. 2006, 317, 378–386. [Google Scholar] [CrossRef]
- Fine, R.L.; Patel, J.; Chabner, B.A. Phorbol esters induce multidrug resistance in human breast cancer cells. Proc. Natl. Acad. Sci. USA 1988, 85, 582–586. [Google Scholar] [CrossRef]
- Blobe, G.C.; Sachs, C.W.; Khan, W.A.; Fabbro, D.; Stabel, S.; Wetsel, W.C.; Obeid, L.M.; Fine, R.L.; Hannun, Y.A. Selective regulation of expression of protein kinase c (pkc) isoenzymes in multidrug-resistant mcf-7 cells. Functional significance of enhanced expression of pkc alpha. J. Biol. Chem. 1993, 268, 658–664. [Google Scholar]
- Keniry, M.; Parsons, R. The role of pten signaling perturbations in cancer and in targeted therapy. Oncogene 2008, 27, 5477–5485. [Google Scholar] [CrossRef]
- Maehama, T.; Dixon, J.E. The tumor suppressor, pten/mmac1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Pacold, M.E.; Suire, S.; Perisic, O.; Lara-Gonzalez, S.; Davis, C.T.; Walker, E.H.; Hawkins, P.T.; Stephens, L.; Eccleston, J.F.; Williams, R.L. Crystal structure and functional analysis of ras binding to its effector phosphoinositide 3-kinase gamma. Cell 2000, 103, 931–943. [Google Scholar] [CrossRef]
- Kuo, M.T.; Liu, Z.; Wei, Y.; Lin-Lee, Y.C.; Tatebe, S.; Mills, G.B.; Unate, H. Induction of human mdr1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate nf-kappab signaling. Oncogene 2002, 21, 1945–1954. [Google Scholar] [CrossRef]
- Oka, M.; Kounoura, K.; Narasaki, F.; Sakamoto, A.; Fukuda, M.; Matsuo, I.; Ikeda, K.; Tsurutani, J.; Ikuno, N.; Omagari, K.; et al. P-glycoprotein is positively correlated with p53 protein accumulation in human colorectal cancers. Jpn. J. Cancer Res. 1997, 88, 738–742. [Google Scholar] [CrossRef]
- Matsuhashi, N.; Saio, M.; Matsuo, A.; Sugiyama, Y.; Saji, S. The evaluation of gastric cancer sensitivity to 5-fu/cddp in terms of induction of apoptosis: Time- and p53 expression-dependency of anti-cancer drugs. Oncol. Rep. 2005, 14, 609–615. [Google Scholar] [CrossRef]
- Dhanda, J.; Triantafyllou, A.; Liloglou, T.; Kalirai, H.; Lloyd, B.; Hanlon, R.; Shaw, R.J.; Sibson, D.R.; Risk, J.M. Serpine1 and sma expression at the invasive front predict extracapsular spread and survival in oral squamous cell carcinoma. Br. J. Cancer 2014, 111, 2114–2121. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Cell surface remodeling by plasmin: A new function for an old enzyme. J. Biomed. Biotechnol. 2012, 2012, 564259. [Google Scholar] [CrossRef]
- Beaufort, N.; Plaza, K.; Utzschneider, D.; Schwarz, A.; Burkhart, J.M.; Creutzburg, S.; Debela, M.; Schmitt, M.; Ries, C.; Magdolen, V. Interdependence of kallikrein-related peptidases in proteolytic networks. Biol. Chem. 2010, 391, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Hattori, N.; Senoo, T.; Akita, S.; Ishikawa, N.; Fujitaka, K.; Haruta, Y.; Murai, H.; Kohno, N. Sk-216, an inhibitor of plasminogen activator inhibitor-1, limits tumor progression and angiogenesis. Mol. Cancer Ther. 2013, 12, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Bajou, K.; Noel, A.; Gerard, R.D.; Masson, V.; Brunner, N.; Holst-Hansen, C.; Skobe, M.; Fusenig, N.E.; Carmeliet, P.; Collen, D.; et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 1998, 4, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Simone, T.M.; Higgins, C.E.; Czekay, R.P.; Law, B.K.; Higgins, S.P.; Archambeault, J.; Kutz, S.M.; Higgins, P.J. Serpine1: A molecular switch in the proliferation-migration dichotomy in wound-“activated” keratinocytes. Adv. Wound Care (New Rochelle) 2014, 3, 281–290. [Google Scholar] [CrossRef] [PubMed]

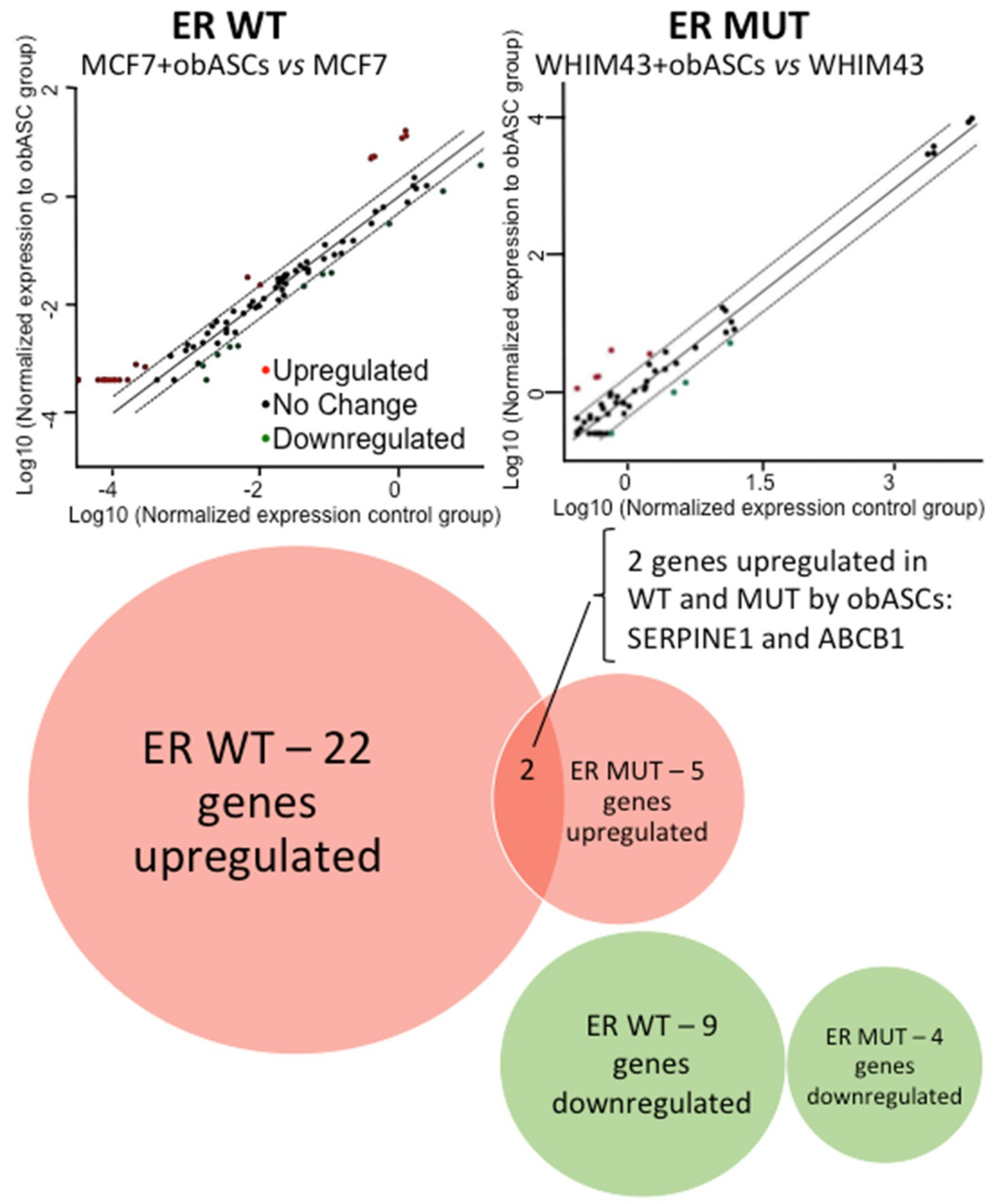
| Gene Expression Changes after Transwell Co-Culture with obASCs | |||||
|---|---|---|---|---|---|
| ER WT | ER MUT | ||||
| Gene Name | Fold Change | Gene Name | Fold Change | ||
| ABCB1 | 6.48 | ↑ | ABCB1 | 4.99 | ↑ |
| ADAM23 | 3.93 | ↑ | CTNNB1 | 4.29 | ↑ |
| ATM | 0.3 | ↓ | CTSD | 0.41 | ↓ |
| CCNA1 | 0.2 | ↓ | MKI67 | 0.35 | ↓ |
| CCND2 | 12.51 | ↑ | MUC1 | 0.45 | ↓ |
| CDH13 | 5.41 | ↑ | NME1 | 0.34 | ↓ |
| CDKN1C | 0.41 | ↓ | PTEN | 2.36 | ↑ |
| CDKN2A | 12.51 | ↑ | SERPINE1 | 4.47 | ↑ |
| CSF1 | 3.72 | ↑ | VEGFA | 7.41 | ↑ |
| CST6 | 5.6 | ↑ | |||
| ESR2 | 4.98 | ↑ | |||
| GLI1 | 2.47 | ↑ | |||
| GSTP1 | 2.51 | ↑ | |||
| HIC1 | 3.31 | ↑ | |||
| IGF1 | 5.22 | ↑ | |||
| IGFBP3 | 4.18 | ↑ | |||
| IL6 | 4.42 | ↑ | |||
| KRT5 | 12.51 | ↑ | |||
| MMP2 | 12.51 | ↑ | |||
| PGR | 0.34 | ↓ | |||
| PLAU | 3.82 | ↑ | |||
| PTGS2 | 12.51 | ↑ | |||
| PYCARD | 0.42 | ↓ | |||
| RARB | 0.38 | ↓ | |||
| SERPINE1 | 2.51 | ↑ | |||
| SFRP1 | 12.51 | ↑ | |||
| SCL39A6 | 0.42 | ↓ | |||
| SNAI2 | 0.41 | ↓ | |||
| TFF3 | 0.29 | ↓ | |||
| TGFB1 | 2.01 | ↑ | |||
| TWIST1 | 12.51 | ↑ | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabol, R.A.; Beighley, A.; Giacomelli, P.; Wise, R.M.; Harrison, M.A.A.; O’Donnnell, B.A.; Sullivan, B.N.; Lampenfeld, J.D.; Matossian, M.D.; Bratton, M.R.; et al. Obesity-Altered Adipose Stem Cells Promote ER+ Breast Cancer Metastasis through Estrogen Independent Pathways. Int. J. Mol. Sci. 2019, 20, 1419. https://doi.org/10.3390/ijms20061419
Sabol RA, Beighley A, Giacomelli P, Wise RM, Harrison MAA, O’Donnnell BA, Sullivan BN, Lampenfeld JD, Matossian MD, Bratton MR, et al. Obesity-Altered Adipose Stem Cells Promote ER+ Breast Cancer Metastasis through Estrogen Independent Pathways. International Journal of Molecular Sciences. 2019; 20(6):1419. https://doi.org/10.3390/ijms20061419
Chicago/Turabian StyleSabol, Rachel A., Adam Beighley, Paulina Giacomelli, Rachel M. Wise, Mark A. A. Harrison, Ben A. O’Donnnell, Brianne N. Sullivan, Jacob D. Lampenfeld, Margarite D. Matossian, Melyssa R. Bratton, and et al. 2019. "Obesity-Altered Adipose Stem Cells Promote ER+ Breast Cancer Metastasis through Estrogen Independent Pathways" International Journal of Molecular Sciences 20, no. 6: 1419. https://doi.org/10.3390/ijms20061419
APA StyleSabol, R. A., Beighley, A., Giacomelli, P., Wise, R. M., Harrison, M. A. A., O’Donnnell, B. A., Sullivan, B. N., Lampenfeld, J. D., Matossian, M. D., Bratton, M. R., Wang, G., Collins-Burow, B. M., Burow, M. E., & Bunnell, B. A. (2019). Obesity-Altered Adipose Stem Cells Promote ER+ Breast Cancer Metastasis through Estrogen Independent Pathways. International Journal of Molecular Sciences, 20(6), 1419. https://doi.org/10.3390/ijms20061419







