PD-1 Ligand Expression in Epithelial Thyroid Cancers: Potential Clinical Implications
Abstract
1. Thyroid Cancer: An Overview
2. Dysregulation of the Immune System in Thyroid Cancer
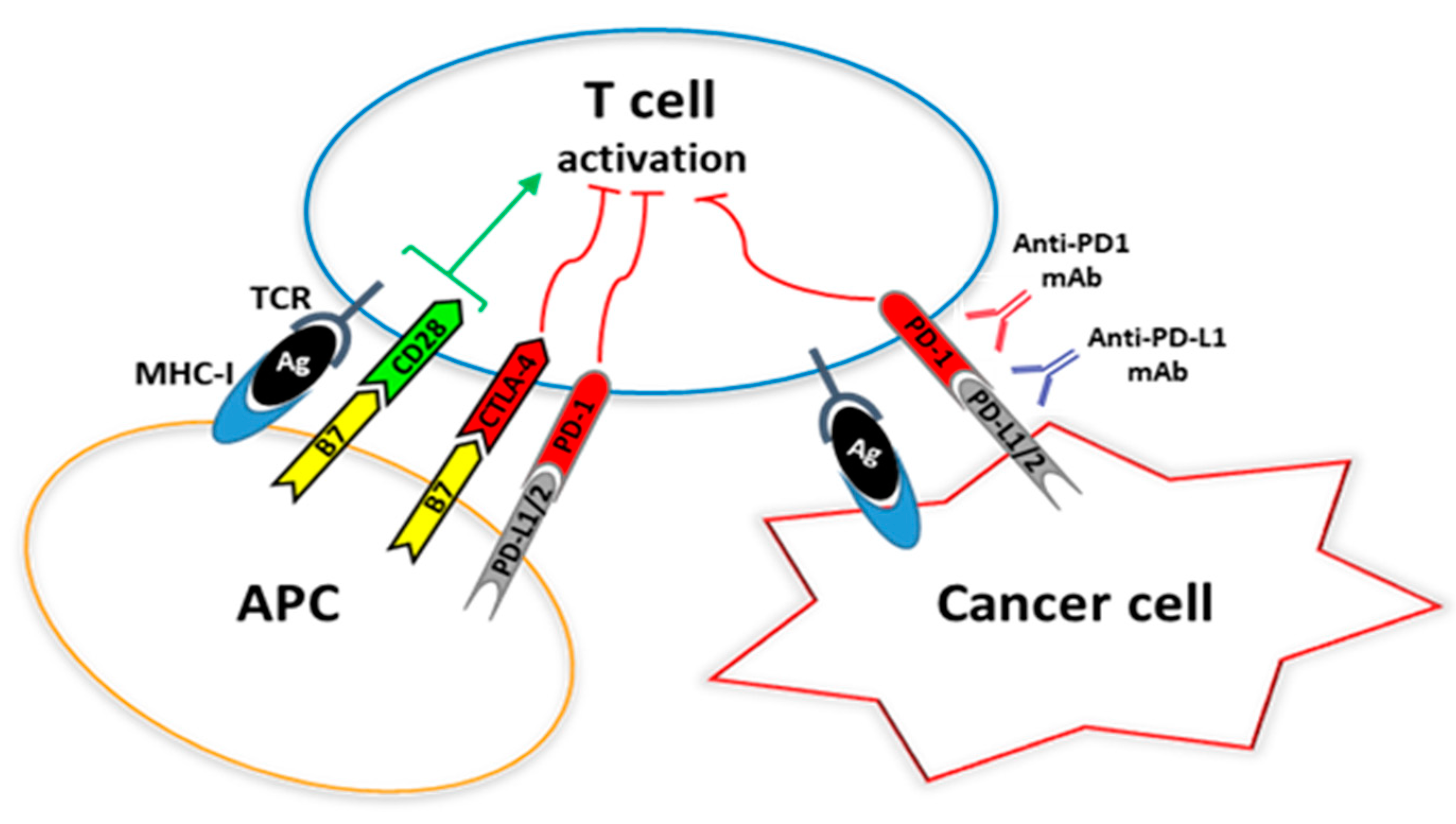
3. Programmed Cell Death 1 (PD-1) and Its Ligands
4. Expression and Clinical Utility of PD-1 Ligands in Thyroid Cancer
4.1. PD-L1 Expression and Thyroid Cancer Diagnosis
4.2. PD-1 Ligand Expression and Thyroid Cancer Prognosis
4.3. Anti-PD-1/PD-L1 Directed Therapies and Thyroid Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| WDTC | Well-Differentiated Thyroid Cancers |
| PTC | Papillary Thyroid Cancer |
| FTC | Follicular Thyroid Cancer |
| PDTC | Poorly Differentiated Thyroid Cancers |
| ATC | Anaplastic Thyroid Cancer |
| FNAC | Fine-Needle Aspiration Cytology |
| TNM | Tumor Node Metastasis |
| CTL | Cytotoxic T Lymphocytes |
| NK | Natural Killer |
| PD-1 | Programmed Cell Death 1 |
| TGF-TGF-β | Transforming Growth Factor β |
| IL-10 | Interleukin 10 |
| VEGF | Vascular Endothelial Growth Factor |
| CD | Cluster of Differentiation |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| DFS | Disease-free survival |
| DFI | Disease-free interval |
| ORR | Overall response rate |
References
- American Cancer Society, Inc. Available online: https://cancerstatisticscenter.cancer.org (accessed on 23 January 2019).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/statfacts/html/thyro.html (accessed on 23 January 2019).
- Nikiforov, Y.E.; Biddinger, P.W.; Thompson, L.D.R. Diagnostic Pathology and Molecular Genetics of the Thyroid; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Tavares, C.; Melo, M.; Cameselle-Teijeiro, J.M.; Soares, P.; Sobrinho-Simões, M. Endocrine Tumours: Genetic predictors of thyroid cancer outcome. Eur. J. Endocrinol. 2016, 174, R117–R126. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ghossein, R. Genomic Landscape of poorly Differentiated and Anaplastic Thyroid Carcinoma. Endocr. Pathol. 2016, 27, 205–212. [Google Scholar] [CrossRef]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Ugolini, C.; Viola, D.; Lupi, C.; Biagini, A.; Giannini, R.; Romei, C.; Miccoli, P.; Pinchera, A.; Basolo, F. BRAFV600E mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 3943–3949. [Google Scholar] [CrossRef]
- Haugen, B.R.; Sawka, A.M.; Alexander, E.K.; Bible, K.C.; Caturegli, P.; Doherty, G.M.; Mandel, S.J.; Morris, J.C.; Nassar, A.; Pacini, F.; et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 2017, 27, 481–483. [Google Scholar] [CrossRef]
- Trimboli, P.; Virili, C.; Romanelli, F.; Crescenzi, A.; Giovanella, L. Galectin-3 Performance in Histologic a Cytologic Assessment of Thyroid Nodules: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2017, 18, 1756. [Google Scholar] [CrossRef]
- Nikiforov, Y.E. Role of molecular markers in thyroid nodule management: Then and now. Endocr. Pract. 2017, 23, 979–988. [Google Scholar] [CrossRef]
- Ulisse, S.; Bosco, D.; Nardi, F.; Nesca, A.; D’Armiento, E.; Guglielmino, V.; De Vito, C.; Sorrenti, S.; Pironi, D.; Tartaglia, F.; et al. Thyroid imaging reporting and data system score combined with the new italian classification for thyroid cytology improves the clinical management of indeterminate nodules. Int. J. Endocrinol. 2017, 2017, 9692304. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Pacini, F.; Castagna, M.G.; Brilli, L.; Pentheroudakis, G. On behalf of the ESMO guidelines working group. Thyroid cancer: ESMO clinical practice guidelines for diagnosis treatment and follow-up. Ann. Oncol. 2012, 23, vii110–vii119. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer 2017, 123, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Ain, K.B.; Asa, S.L.; Bible, K.C.; Brierley, J.D.; Burman, K.D.; Kebebew, E.; Lee, N.Y.; Nikiforov, Y.E.; Rosenthal, M.S.; et al. American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012, 22, 1104–1139. [Google Scholar] [CrossRef]
- Tuccilli, C.; Baldini, E.; Arlot-Bonnemains, Y.; Chesnel, F.; Sorrenti, S.; De Vito, C.; D’Armiento, E.; Antonelli, A.; Fallahi, P.; Watutantrige, S.; et al. Expression and prognostic value of the cell polarity PAR complex members in thyroid cancer. Int. J. Oncol. 2017, 50, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.; Tuccilli, C.; Arlot-Bonnemains, Y.; Chesnel, F.; Sorrenti, S.; De Vito, C.; Catania, A.; D’Armiento, E.; Antonelli, A.; Fallahi, P.; et al. Deregulated expression of VHL mRNA variants in papillary thyroid cancer. Mol. Cell. Endocrinol. 2017, 443, 121–127. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Politti, U.; Materazzi, G.; Baldini, E.; Ulisse, S.; Miccoli, P.; Antonelli, A. Molecular Targeted Therapies of Aggressive Thyroid Cancer. Front. Endocrinol. 2015, 6, 176. [Google Scholar] [CrossRef]
- Fallahi, P.; Ruffilli, I.; Elia, G.; Ragusa, F.; Ulisse, S.; Baldini, E.; Miccoli, M.; Materazzi, G.; Antonelli, A.; Ferrari, S.M. Novel treatment options for anaplastic thyroid cancer. Expert Rev. Endocrinol. Metab. 2017, 12, 279–288. [Google Scholar] [CrossRef]
- Lennon, P.; Deady, S.; Healy, M.L.; Toner, M.; Kinsella, J.; Timon, C.I.; O’Neill, J.P. Anaplastic thyroid carcinoma: Failure of conventional therapy but hope of targeted therapy. Head Neck 2016, 38 (Suppl. 1), E1122–E1129. [Google Scholar] [CrossRef]
- Baldini, E.; D’Armiento, M.; Ulisse, S. A new aurora in anaplastic thyroid cancer therapy. Int. J. Endocrinol. 2014, 2014, 816430. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J. Immunol. 2009, 182, 4499–4506. [Google Scholar] [CrossRef] [PubMed]
- Mougiakakos, D.; Choudhury, A.; Kiessling, R.; Johansson, C.C. Regulatory T cells in cancer. Adv. Cancer Res. 2010, 107, 57–117. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Roberts, J.M.; Swartz, M.A. Induction of lymphoid-like stroma and immune escape by tumors that express the chemokine CCL21. Science 2010, 328, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Pang, Y.; Moses, H.L. TGF-beta and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010, 31, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Henick, B.S.; Herbst, R.S.; Goldberg, S.B. The PD-1 pathway as a therapeutic target to overcome immune escape mechanisms in cancer. Expert Opin. Ther. Targets 2014, 18, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- La-Beck, N.M.; Jean, G.W.; Huynh, C.; Alzghari, S.K.; Lowe, D.B. Immune Checkpoint Inhibitors: New Insights and Current Place in Cancer Therapy. Pharmacotherapy 2015, 35, 963–976. [Google Scholar] [CrossRef]
- Liotti, F.; Prevete, N.; Vecchio, G.; Melillo, R.M. Recent advances in understanding immune phenotypes of thyroid carcinomas: Prognostication and emerging therapies. F1000Res 2019, 28, 8. [Google Scholar] [CrossRef]
- Bruno, T.C.; French, J.D.; Jordan, K.R.; Ramirez, O.; Sippel, T.R.; Borges, V.F.; Haugen, B.R.; McCarter, M.D.; Waziri, A.; Slansky, J.E. Influence of human immune cells on cancer: Studies at the University of Colorado. Immunol. Res. 2013, 55, 22–33. [Google Scholar] [CrossRef]
- French, J.D. Revisiting immune-based therapies for aggressive follicular cell-derived thyroid cancers. Thyroid 2013, 23, 529–542. [Google Scholar] [CrossRef] [PubMed]
- French, J.D.; Weber, Z.J.; Fretwell, D.L.; Said, S.; Klopper, J.P.; Haugen, B.R. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2010, 95, 2325–2333. [Google Scholar] [CrossRef]
- Angell, T.E.; Lechner, M.G.; Jang, J.K.; Correa, A.J.; LoPresti, J.S.; Epstein, A.L. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 2014, 24, 1385–1393. [Google Scholar] [CrossRef]
- Cunha, L.L.; Marcello, M.A.; Ward, L.S. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr. Relat. Cancer 2014, 21, R85–R103. [Google Scholar] [CrossRef]
- Lewinski, A.; Sliwka, P.W.; Stasiolek, M. Dendritic cells in autoimmune disorders and cancer of the thyroid. Folia Histochem. Cytobiol. 2014, 52, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Severson, J.J.; Serracino, H.S.; Mateescu, V.; Raeburn, C.D.; McIntyre, R.C.; Sams, S.B.; Haugen, B.R.; French, B.R. PD-1+Tim-3+ CD8+ T Lymphocytes Display Varied Degrees of Functional Exhaustion in Patients with Regionally Metastatic Differentiated Thyroid Cancer. Cancer Immunol. Res. 2015, 3, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Bastman, J.J.M.; Serracinom, H.S.; Zhu, Y.; Koenig, M.R.; Mateescu, V.; Sams, S.B.; Davies, K.D.; Raeburn, C.D.; McIntyre, R.C., Jr.; Haugen, B.R.; et al. Tumor-Infiltrating T Cells and the PD-1 Checkpoint Pathway in Advanced Differentiated and Anaplastic Thyroid Cancer. J. Clin. Endocrinol. Metab. 2016, 101, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.L.; Marcello, M.A.; Morari, E.C.; Nonogaki, S.; Conte, F.F.; Gerhard, R.; Soares, F.A.; Vassallo, J.; Ward, L.S. Differentiated thyroid carcinomas may elude the immune system by B7H1 up-regulation. Endocr. Relat. Cancer 2013, 20, 103–110. [Google Scholar] [CrossRef]
- Chowdhury, S.; Veyhl, J.; Jessa, F.; Polyakova, O.; Alenzi, A.; MacMillan, C.; Ralhan, R.; Walfish, P.G. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 2016, 7, 32318–32328. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, T.H.; Kim, S.W.; Ki, C.S.; Jang, H.W.; Kim, J.S.; Kim, J.H.; Choe, J.H.; Shin, J.H.; Hahn, S.Y.; et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr. Relat. Cancer 2017, 24, 97–106. [Google Scholar] [CrossRef]
- Shi, R.L.; Qu, N.; Luo, T.X.; Xiang, J.; Liao, T.; Sun, G.H.; Wang, Y.; Wang, Y.L.; Huang, C.P.; Ji, Q.H. Programmed death-ligand 1 expression in papillary thyroid cancer and its correlation with clinicopathologic factors and recurrence. Thyroid 2017, 27, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Zwaenepoel, K.; Jacobs, J.; De Meulenaere, A.; Silence, K.; Smits, E.; Siozopoulou, V.; Hauben, E.; Rolfo, C.; Rottey, S.; Pauwels, P. CD70 and PD-L1 in anaplastic thyroid cancer—Promising targets for immunotherapy. Histopathology 2017, 71, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Lahat, N.; Rahat, M.A.; Sadeh, O.; Kinarty, A.; Kraiem, Z. Regulation of HLA-DR and costimulatory B7 molecules in human thyroid carcinoma cells: Differential binding of transcription factors to the HLA-DRalpha promoter. Thyroid 1998, 8, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Battifora, M.; Pesce, G.; Paolieri, F.; Fiorino, N.; Giordano, C.; Riccio, A.M.; Torre, G.; Olive, D.; Bagnasco, M. B7.1 costimulatory molecule is expressed on thyroid follicular cells in Hashimoto’s thyroiditis, but not in Graves’ disease. J. Clin. Endocrinol. Metab. 1998, 83, 4130–4139. [Google Scholar] [CrossRef]
- Shah, R.; Banks, K.; Patel, A.; Dogra, S.; Terrell, R.; Powers, P.A.; Fenton, C.; Dinauer, C.A.; Tuttle, R.M.; Francis, G.L. Intense expression of the b7-2 antigen presentation coactivator is an unfavorable prognostic indicator for differentiated thyroid carcinoma of children and adolescents. J. Clin. Endocrinol. Metab. 2002, 87, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.C.; Li, Z.B.; Chen, Y.R.; Li, X.T.; Huang, J.X.; Li, Y.G.; Chen, S.R. Expression and distribution of S-100, CD83, and costimulatory molecules (CD80 and CD86) in tissues of thyroid papillary carcinoma. Cancer Investig. 2011, 29, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Di, G.; Bian, M. Dysfunction of natural killer cells mediated by PD-1 and Tim-3 pathway in anaplastic thyroid cancer. Int. Immunopharmacol. 2018, 64, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Na, K.J.; Choi, H. Immune landscape of papillary thyroid cancer and immunotherapeutic implications. Endocr. Relat. Cancer 2018, 25, 523–531. [Google Scholar] [CrossRef]
- Shinohara, T.; Taniwaki, M.; Ishida, Y.; Kawaichi, M.; Honjo, T. Structure and chromosomal localization of the human PD-1 gene (PDCD1). Genomics 1994, 23, 704–706. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of PD-1 pathway in the immune response. Am. J. Transplant. 2012, 12, 2575–2587. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.R.; Aslani, S.; Salmaninejad, A.; Javan, M.R.; Rezaei, N. PD-1/PD-L and autoimmunity: A growing relationship. Cell. Immunol. 2016, 310, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Tang, H.Y.; Davis, F.B.; Davis, P.J. Resveratrol and apoptosis. Ann. N. Y. Acad. Sci. 2011, 1215, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.T.; Wei, P.L.; Ho, Y.; Nana, A.W.; Changou, C.A.; Chen, Y.R.; Yang, Y.S.; Hsieh, M.T.; Hercbergs, A.; Davis, P.J.; et al. Thyroxine inhibits resveratrol-caused apoptosis by PD-L1 in ovarian cancer cells. Endocr. Relat. Cancer 2018, 25, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chin, Y.T.; Nana, A.W.; Shih, Y.J.; Lai, H.Y.; Tang, H.Y.; Leinung, M.; Mousa, S.A.; Davis, P.J. Actions of l-thyroxine and Nano-diamino-tetrac (Nanotetrac) on PD-L1 in cancer cells. Steroids 2016, 114, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sun, Y.; Ye, H.; Yang, S.; Lee, S.L.; de las Morenas, A. Anaplastic thyroid cancer: Outcome and the mutation/expression profiles of potential targets. Pathol. Oncol. Res. 2015, 21, 695–701. [Google Scholar] [CrossRef]
- Bai, Y.; Niu, D.; Huang, X.; Jia, L.; Kang, Q.; Dou, F.; Ji, X.; Xue, W.; Liu, Y.; Li, Z.; et al. PD-L1 and PD-1 expression are correlated with distinctive clinicopathological features in papillary thyroid carcinoma. Diagn. Pathol. 2017, 12, 72. [Google Scholar] [CrossRef]
- cBioportal. Available online: http://www.cbioportal.org (accessed on 14 February 2019).
- Tuccilli, C.; Baldini, E.; Sorrenti, S.; Catania, A.; Antonelli, A.; Fallahi, P.; Tartaglia, F.; Barollo, S.; Mian, C.; Palmieri, A.; et al. CTLA-4 and PD-1 Ligand Gene Expression in Epithelial Thyroid Cancers. Int. J. Endocrinol. 2018, 2018, 1742951. [Google Scholar] [CrossRef]
- Rosenbaum, M.W.; Gigliotti, B.J.; Pai, S.I.; Parangi, S.; Wachtel, H.; Mino-Kenudson, M.; Gunda, V.; Faquin, W.C. PD-L1 and IDO1 Are Expressed in Poorly Differentiated Thyroid Carcinoma. Endocr. Pathol. 2018, 29, 59–67. [Google Scholar] [CrossRef]
- Aghajani, M.J.; Yang, T.; McCafferty, C.E.; Graham, S.; Wu, X.; Niles, N. Predictive relevance of programmed cell death protein 1 and tumor-infiltrating lymphocyte expression in papillary thyroid cancer. Surgery 2018, 163, 130–136. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, T.; Huang, X.; Wu, Q.; Niu, D.; Ji, X.; Feng, Q.; Li, Z.; Kakudo, K. In papillary thyroid carcinoma, expression by immunohistochemistry of BRAF V600E, PD-L1, and PD-1 is closely related. Virchows Arch. 2018, 472, 779–787. [Google Scholar] [CrossRef]
- Chintakuntlawar, A.V.; Rumilla, K.M.; Smith, C.Y.; Jenkins, S.M.; Foote, R.L.; Kasperbauer, J.L.; Morris, J.C.; Ryder, M.; Alsidawi, S.; Hilger, C.; et al. Expression of PD-1 and PD-L1 in Anaplastic Thyroid Cancer Patients Treated With Multimodal Therapy: Results From a Retrospective Study. J. Clin. Endocrinol. Metab. 2017, 102, 1943–1950. [Google Scholar] [CrossRef]
- Fu, G.; Polyakova, O.; MacMillan, C.; Ralhan, R.; Walfish, P.G. Programmed Death—Ligand 1 Expression Distinguishes Invasive Encapsulated Follicular Variant of Papillary Thyroid Carcinoma from Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features. EBioMedicine 2017, 18, 50–55. [Google Scholar] [CrossRef]
- Rossi, E.D.; Martini, M. New Insight in a New Entity: NIFTPS and Valuable Role of Ancillary Techniques. The Role of PD-L1. EBioMedicine 2017, 18, 11–12. [Google Scholar] [CrossRef]
- Hsieh, A.M.; Polyakova, O.; Fu, G.; Chazen, R.S.; MacMillan, C.; Witterick, I.J.; Ralhan, R.; Walfish, P.G. Programmed death-ligand 1 expression by digital image analysis advances thyroid cancer diagnosis among encapsulated follicular lesions. Oncotarget 2018, 9, 19767–19782. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef]
- Maletta, F.; Massa, F.; Torregrossa, L.; Duregon, E.; Casadei, G.P.; Basolo, F.; Tallini, G.; Volante, M.; Nikiforov, Y.E.; Papotti, M. Cytological features of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum. Pathol. 2016, 54, 134–142. [Google Scholar] [CrossRef]
- Bizzarro, T.; Martini, M.; Capodimonti, S.; Straccia, P.; Lombardi, C.P.; Pontecorvi, A.; Larocca, L.M.; Rossi, E.D. Young investigator challenge: The morphologic analysis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on liquid-based cytology: Some insights into their identification. Cancer Cytopathol. 2016, 124, 699–710. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Hui, R.; Garon, E.B.; Goldman, J.W.; Leighl, N.B.; Hellmann, M.D.; Patnaik, A.; Gandhi, L.; Eder, J.P.; Ahn, M.J.; Horn, L.; et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: A phase 1 trial. Ann. Oncol. 2017, 28, 874–881. [Google Scholar] [CrossRef]
- Brauner, E.; Gunda, V.; Vanden Borre, P.; Zurakowski, D.; Kim, Y.S.; Dennett, K.V.; Amin, S.; Freeman, G.J.; Parangi, S. Combining BRAF inhibitor and anti PD-L1 antibody dramatically improves tumor regression and anti tumor immunity in an immunocompetent murine model of anaplastic thyroid cancer. Oncotarget 2016, 7, 17194–17211. [Google Scholar] [CrossRef]
- Aghajani, M.; Graham, S.; McCafferty, C.; Shaheed, C.A.; Roberts, T.; DeSouza, P.; Yang, T.; Niles, N. Clinicopathologic and Prognostic Significance of Programmed Cell Death Ligand 1 Expression in Patients with Non-Medullary Thyroid Cancer: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 349–361. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P. Current and future immunotherapies for thyroid cancer. Expert Rev. Anticancer Ther. 2018, 18, 149–159. [Google Scholar] [CrossRef]
- Constantinidou, A.; Alifieris, C.; Trafalis, D.T. Targeting programmed cell death-1(PD-1) and ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol. Ther. 2018, 194, 84–106. [Google Scholar] [CrossRef]
- Romano, E.; Romero, P. The therapeutic promise of disrupting the PD-1/PD-L1 immune checkpoint in cancer: Unleashing the CD8 T cell mediated anti-tumor activity results in significant, unprecedented clinical efficacy in various solid tumors. J. Immunother. Cancer 2015, 3, 15. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Powles, T.; Eder, J.P.; Fine, G.D.; Braiteh, F.S.; Loriot, Y.; Cruz, C.; Bellmunt, J.; Burris, H.A.; Petrylak, D.P.; Teng, S.L.; et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014, 515, 558–562. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jeon, S.; Kim, T.M.; Jung, C.K. Immune Gene Signature Delineates a Subclass of Papillary Thyroid Cancer with Unfavorable Clinical Outcomes. Cancers 2018, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Gunda, V.; Gigliotti, B.; Ashry, T.; Ndishabandi, D.; McCarthy, M.; Zhou, Z.; Amin, S.; Lee, K.E.; Stork, T.; Wirth, L.; et al. Anti-PD-1/PD-L1 therapy augments lenvatinib’s efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int. J. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Cantara, S.; Bertelli, E.; Occhini, R.; Regoli, M.; Brilli, L.; Pacini, F.; Castagna, M.G.; Toti, P. Blockade of the programmed death ligand 1 (PD-L1) as potential therapy for anaplastic thyroid cancer. Endocrine 2019. [Google Scholar] [CrossRef] [PubMed]
- Kollipara, R.; Schneider, B.; Radovich, M.; Babu, S.; Kiel, P.J. Exceptional Response with Immunotherapy in a Patient with Anaplastic Thyroid Cancer. Oncologist 2017, 22, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Dadu, R.; Gule-Monroe, M.; Busaidy, N.L.; Ferrarotto, R.; Habra, M.A.; Zafereo, M.; Williams, M.D.; Gunn, B.; Grosu, H.; et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J. Immunother. Cancer 2018, 6, 68. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Varga, A.; Brose, M.S.; Aggarwal, R.R.; Lin, C.C.; Prawira, A.; de Braud, F.; Tamura, K.; Doi, T.; Piha-Paul, S.A.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer 2019, 19, 196. [Google Scholar] [CrossRef]
- Clinical Trials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02688608 (accessed on 18 February 2019).
- Naoum, G.E.; Morkos, M.; Kim, B.; Arafat, W. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol. Cancer 2018, 17, 51. [Google Scholar] [CrossRef]
- Chen, M.L.; Yan, B.S.; Lu, W.C.; Chen, M.H.; Yu, S.L.; Yang, P.C.; Cheng, A.L. Sorafenib relieves cell-intrinsic and cell-extrinsic inhibitions of effector T cells in tumor microenvironment to augment antitumor immunity. Int. J. Cancer 2014, 134, 319–331. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Bocci, G.; Di Desidero, T.; Elia, G.; Ruffilli, I.; Ragusa, F.; Orlandi, P.; Paparo, S.R.; Patrizio, A.; Piaggi, S.; et al. Lenvatinib exhibits antineoplastic activity in anaplastic thyroid cancer in vitro and in vivo. Oncol. Rep. 2018, 39, 2225–2234. [Google Scholar] [CrossRef]
- Clinical Trials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02501096 (accessed on 6 March 2019).
| Correlations/Associations | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibodies | Age | Gender | T | N | M | Stage | Multifocality | ETI | DFS | BRAFV600E | ||||
| Ref. No. | Case Study | PD-L1 Expression | Host-Type | Clone (Source) | ||||||||||
| [42] | 407 patients, Including 293 DTC | Increased levels in DTC vs. benign lesions | Rabbit PAB | Ab82059 (Abcam) | No | No | No | No | No | No | No | No | -- | -- |
| [37] | 33 PTC | Increased levels in BRAFV600E vs. BRAFwt PTC | Rabbit PAB | 4059 (ProSci) | -- | -- | -- | -- | -- | -- | -- | -- | -- | Yes |
| [61] | 13 ATC | Positive in 23% of ATC patients | Mouse MAB | 5H1 (non-commercial) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| [43] | 251 patients including 185 PTC | Increased in PTC vs. benign lesions | Rabbit MAB | E1L3N (Cell Signaling) | -- | -- | -- | -- | -- | No | No | No | Yes | -- |
| [41] | 92 DTC; 22 patients with advanced DTC/ATC | Positive in 64% of DTC and 59.1% of advanced DTC/ATC | Rabbit MAB | SP142 (Spring Bioscience) | -- | -- | No | Yes | -- | -- | -- | -- | -- | No |
| [44] | 407 thyroid cancers | Positive in 6.1% of PTC, 7.6% of FTC, 22.2% of ATC | Rabbit MAB | SP142 (Spring Bioscience) | No | No | No | No | No | No | No | No | No | No |
| [45] | 260 PTC and normal matched tissues | Increased in 52.3% of PTC vs. normal tissue | Rabbit MAB | Ab174838 (Abcam) | No | No | No | No | -- | -- | Yes | Yes | Yes | -- |
| [62] | 126 PTC | Positive in 53.2% of PTC | Rabbit MAB | SP142 (Spring Bioscience) | No | Yes | No | No | -- | No | No | -- | -- | No |
| [46] | 49 ATC | Positive in 28.6% of ATC | Rabbit MAB | E1L3N (Cell Signaling) | -- | -- | -- | -- | -- | -- | -- | -- | -- | No |
| [68] | 16 ATC | Positive in 81.3% of ATC | Rabbit MAB | E1L3N (Cell Signaling) | No | No | -- | -- | -- | No | -- | -- | No | -- |
| [66] | 75 PTC | Positive in 66.7% of PTC | Mouse MAB | 22C3 (DAKO) | No | No | No | No | -- | No | No | Yes | No | -- |
| [65] | 28 PDTC | Positive in 25% of PDTC | Rabbit MAB | E1L3N (Cell Signaling) | No | No | Yes | -- | No | No | Yes | No | No | -- |
| [67] | 110 PTC | Positive in 46% of PTC | Rabbit MAB | SP142 (Spring Bioscience) | No | No | No | No | -- | No | No | -- | -- | Yes |
| Correlations/Associations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | T | N | M | Stage | Multifocality | ETI | DFS | BRAFV600E | |||
| Ref. No. | Case Study | PD-L1 mRNA Expression | ||||||||||
| [42] | 407 patients, Including 293 DTC | Increased levels in DTC vs. benign lesions | Yes | No | No | No | No | Yes | No | No | -- | -- |
| [63] | 482 PTC and 58 normal tissues | Unvaried in PTC vs. normal tissues | No | Yes | No | Yes | No | No | -- | Yes | Yes | Yes |
| [41] | 92 DTC; 22 advanced DTC/ATC | Positivity in 64% of DTC and 59.1% of advanced DTC/ATC | -- | -- | No | Yes | -- | -- | -- | -- | -- | No |
| [64] | 94 PTC and normal matched tissues, 11 ATC | Increased in 46.8% of PTC and 27.3% of ATC | No | No | No | No | -- | No | -- | -- | Yes | Yes |
| Name | Commercial Name (Company) | IgG Isotype | Target | FDA Approval | |
|---|---|---|---|---|---|
| Year | Cancer Type | ||||
| Pembrolizumab | Keytruda (Merck) | IgG4 | PD-1 | 2014 | Melanoma |
| 2016 | HNSCC, NSCLC | ||||
| 2017 | Gastric/gastroesophageal adenocarcinoma, cHL, urothelial carcinoma, MSI-H cancers | ||||
| 2018 | Merkel cell carcinoma, PMBCL, HCC, cervical cancer | ||||
| Nivolumab | Opdivo (Bristol-Myers Squibb) | IgG4 | PD-1 | 2014 | Melanoma, NSCLC |
| 2016 | SCCHN, cHL | ||||
| 2017 | Urothelial carcinoma, HCC, MSI-H colorectal cancer | ||||
| 2018 | RCC, SCLC | ||||
| Cemiplimab-rwlc | Libtayo (Regeneron Pharmaceuticals) | IgG4 | PD-1 | 2018 | CSCC |
| Atezolizumab | Tecentriq (Genentech Oncology) | IgG1 | PD-L1 | 2016 | NSCLC, urothelial carcinoma |
| Avelumab | Bavencio (EMD Serono) | IgG1 | PD-L1 | 2017 | Merkel cell carcinoma, urothelial carcinoma |
| Durvalumab | Imfinzi (AstraZeneca) | IgG1k | PD-L1 | 2017 | Urothelial carcinoma |
| 2018 | NSCLC | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulisse, S.; Tuccilli, C.; Sorrenti, S.; Antonelli, A.; Fallahi, P.; D’Armiento, E.; Catania, A.; Tartaglia, F.; Amabile, M.I.; Giacomelli, L.; et al. PD-1 Ligand Expression in Epithelial Thyroid Cancers: Potential Clinical Implications. Int. J. Mol. Sci. 2019, 20, 1405. https://doi.org/10.3390/ijms20061405
Ulisse S, Tuccilli C, Sorrenti S, Antonelli A, Fallahi P, D’Armiento E, Catania A, Tartaglia F, Amabile MI, Giacomelli L, et al. PD-1 Ligand Expression in Epithelial Thyroid Cancers: Potential Clinical Implications. International Journal of Molecular Sciences. 2019; 20(6):1405. https://doi.org/10.3390/ijms20061405
Chicago/Turabian StyleUlisse, Salvatore, Chiara Tuccilli, Salvatore Sorrenti, Alessandro Antonelli, Poupak Fallahi, Eleonora D’Armiento, Antonio Catania, Francesco Tartaglia, Maria Ida Amabile, Laura Giacomelli, and et al. 2019. "PD-1 Ligand Expression in Epithelial Thyroid Cancers: Potential Clinical Implications" International Journal of Molecular Sciences 20, no. 6: 1405. https://doi.org/10.3390/ijms20061405
APA StyleUlisse, S., Tuccilli, C., Sorrenti, S., Antonelli, A., Fallahi, P., D’Armiento, E., Catania, A., Tartaglia, F., Amabile, M. I., Giacomelli, L., Metere, A., Cornacchini, N., Pironi, D., Carbotta, G., Vergine, M., Monti, M., & Baldini, E. (2019). PD-1 Ligand Expression in Epithelial Thyroid Cancers: Potential Clinical Implications. International Journal of Molecular Sciences, 20(6), 1405. https://doi.org/10.3390/ijms20061405








