5-Iodo-4-thio-2′-Deoxyuridine as a Sensitizer of X-ray Induced Cancer Cell Killing
Abstract
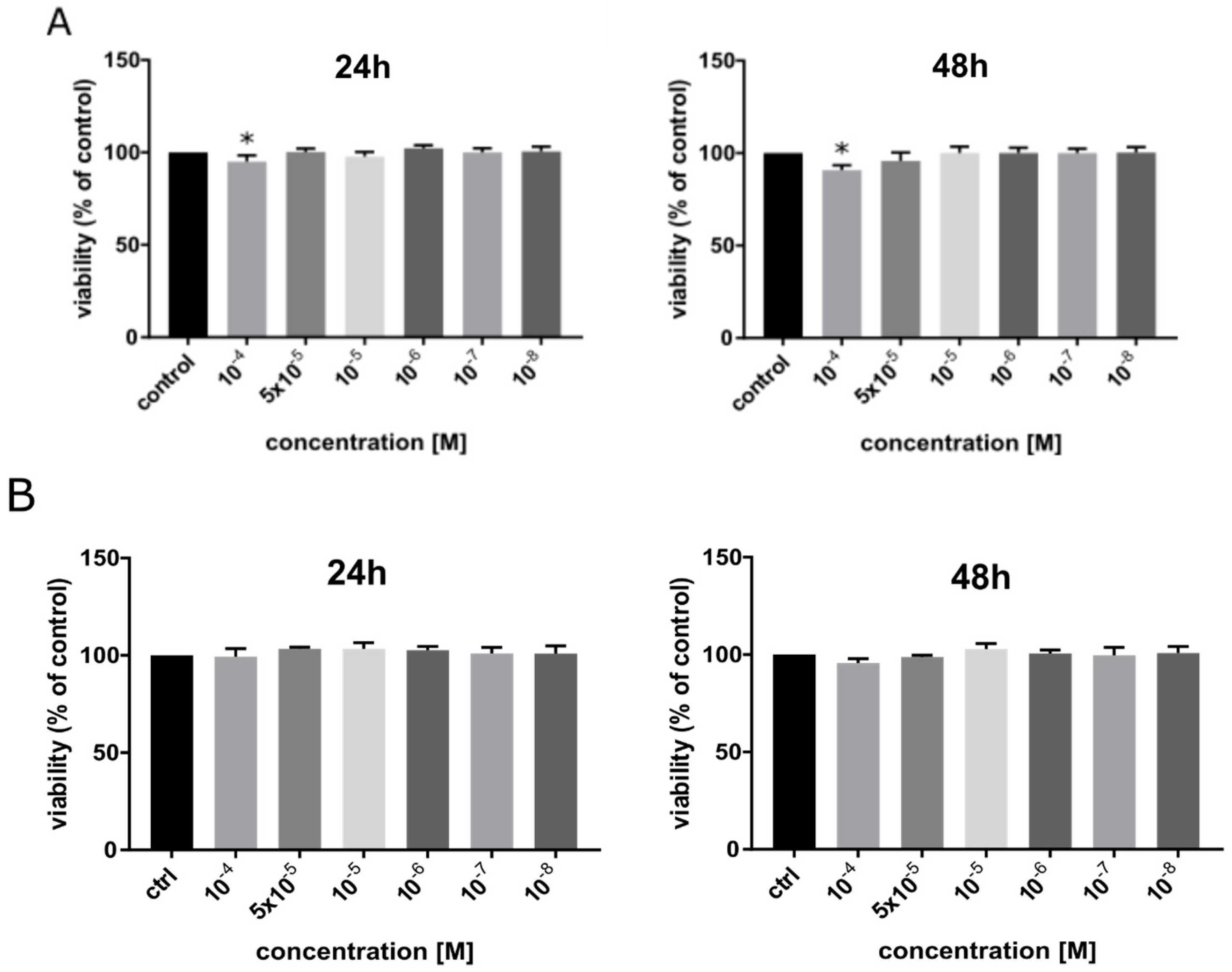
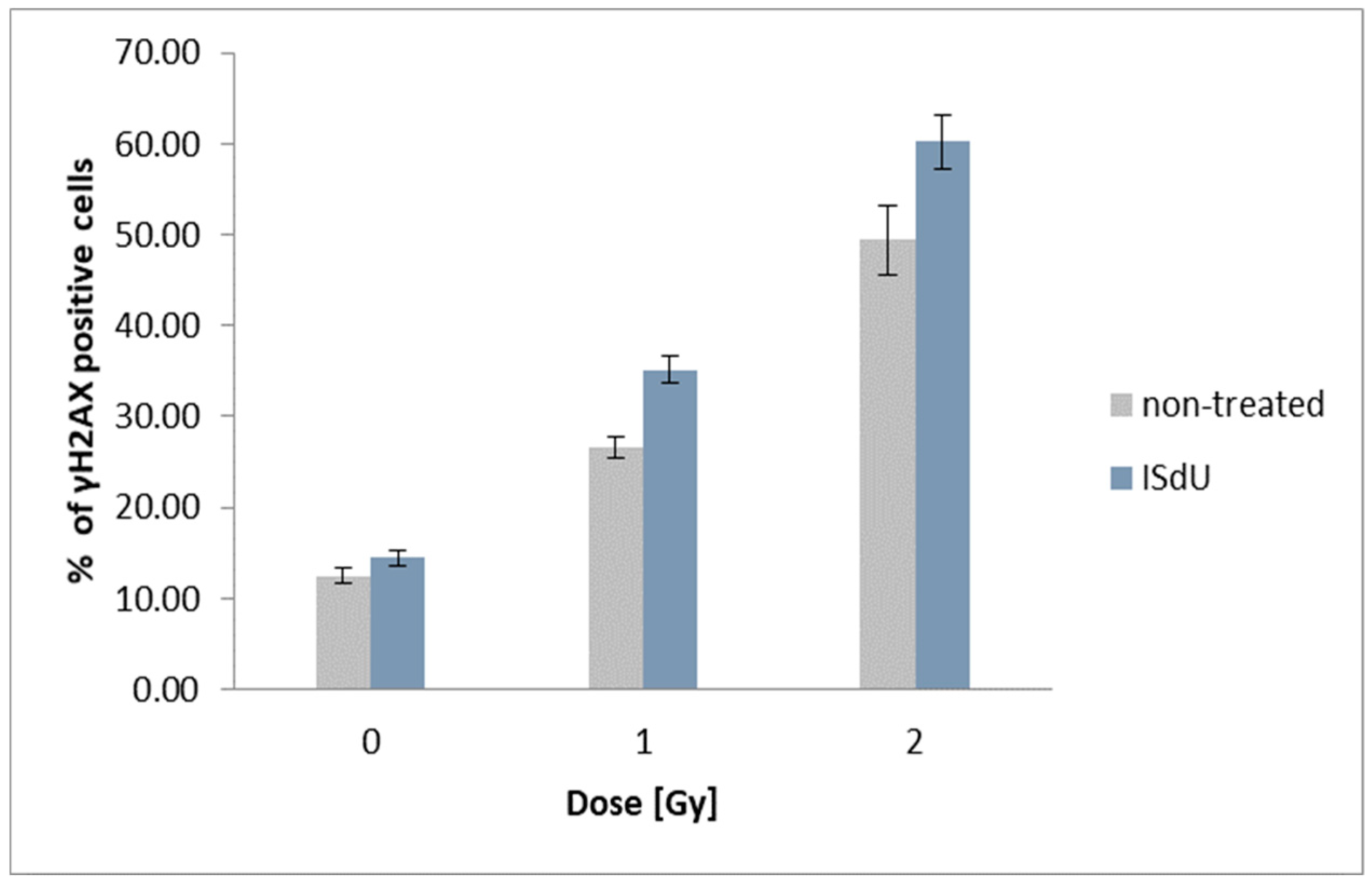
1. Introduction
2. Results and Discussion
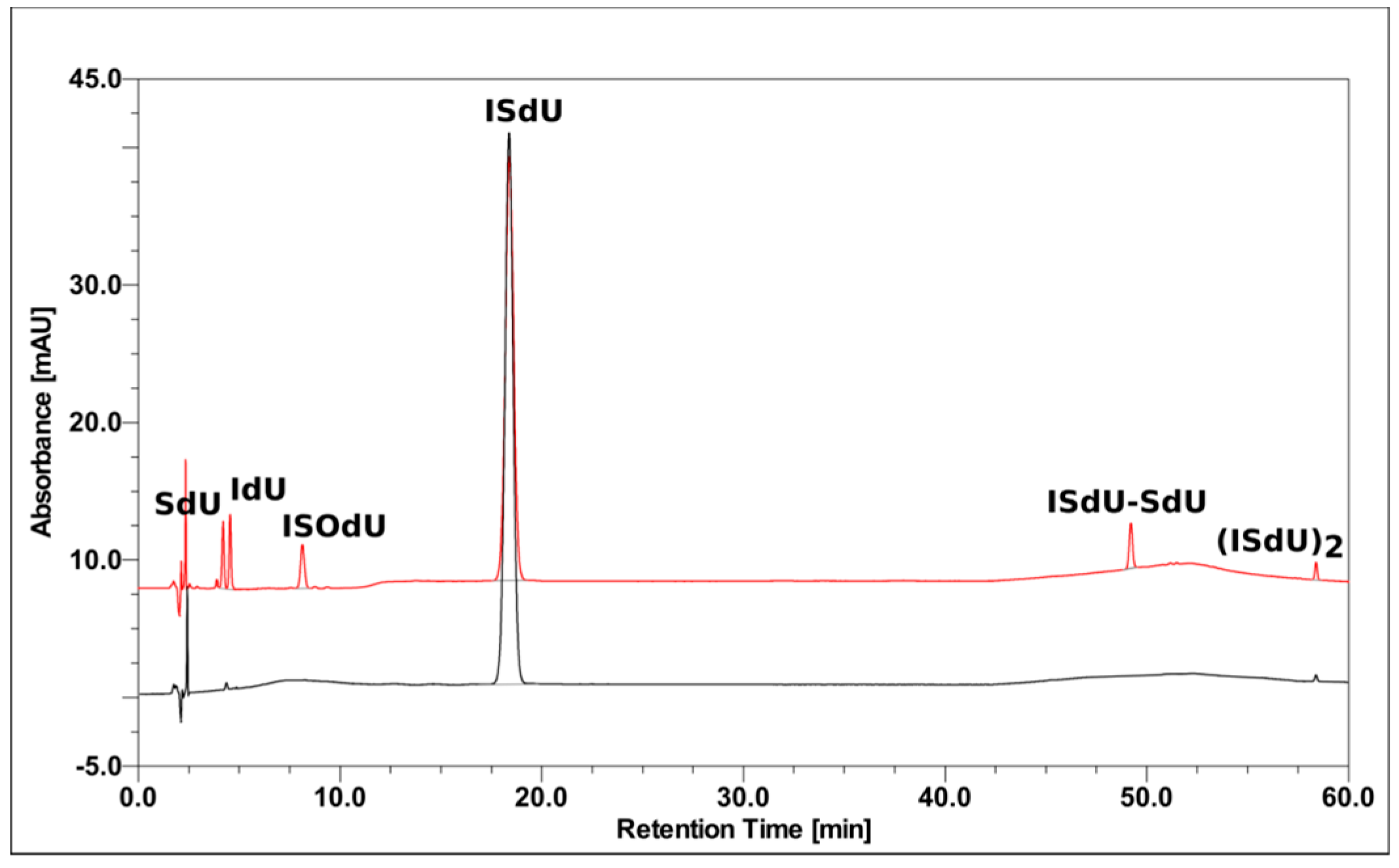
2.1. Radiolysis
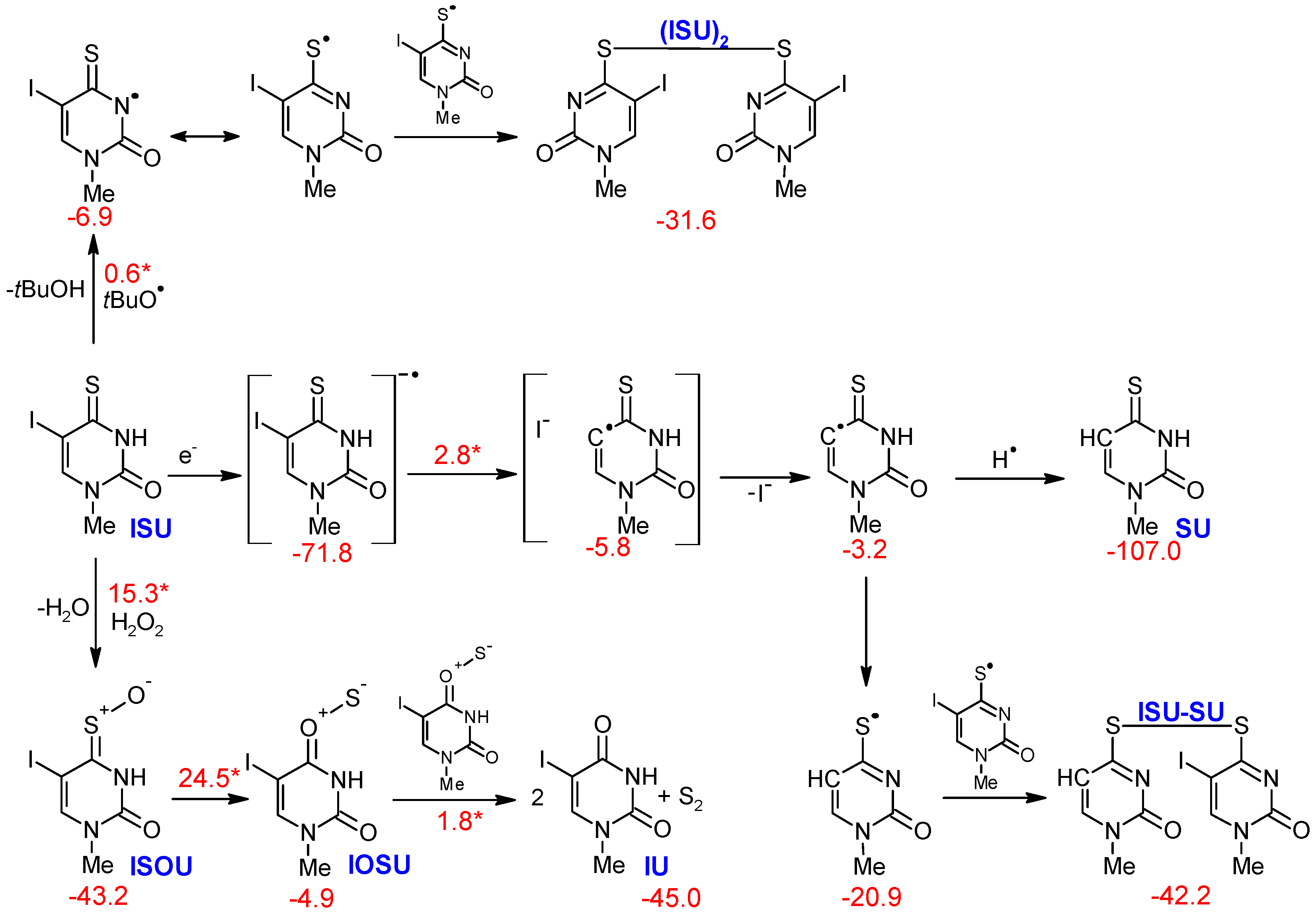
2.2. Computational
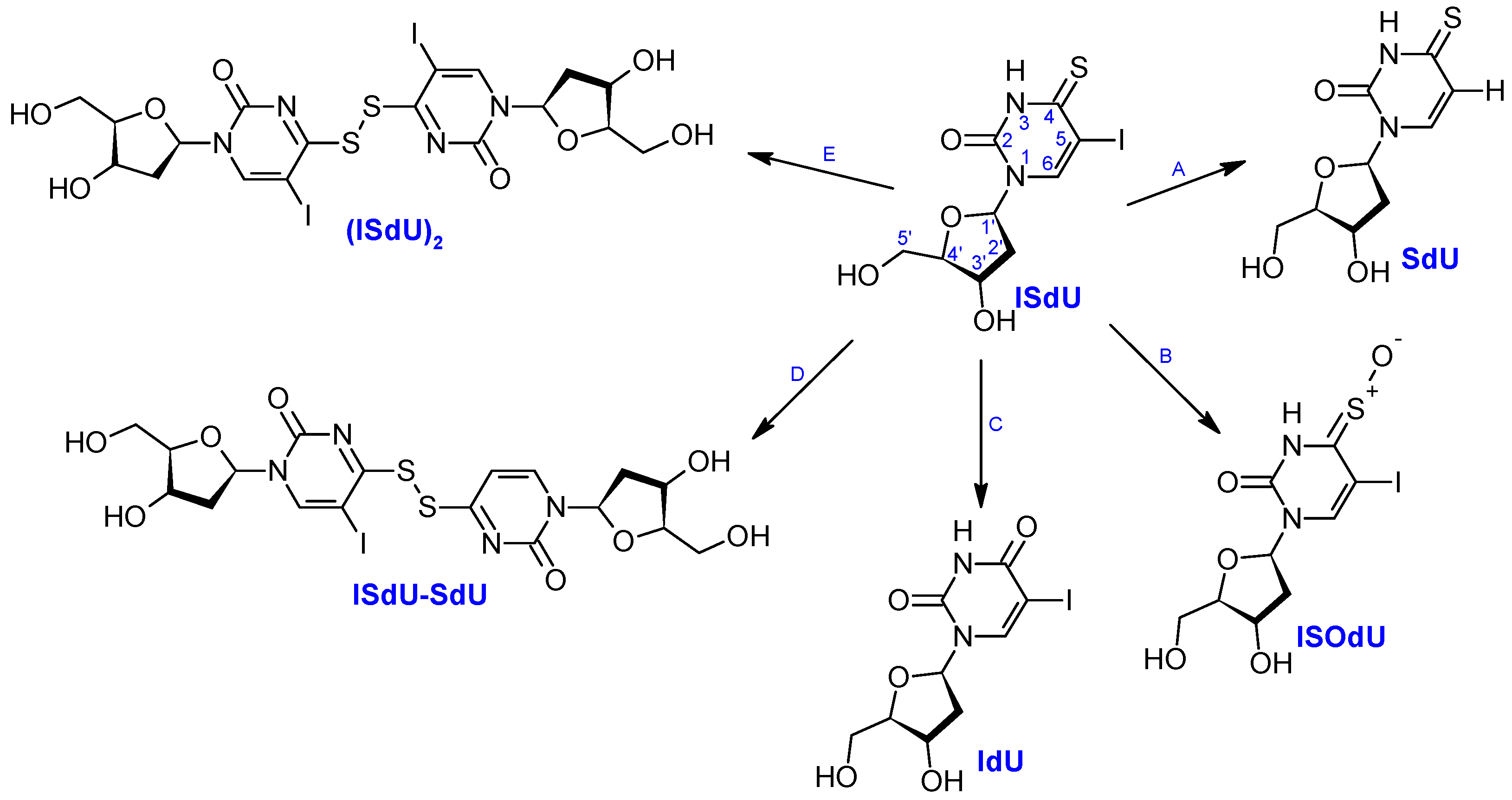
2.2.1. Products Induced by Solvated Electrons
2.2.2. Oxidation Products
2.2.3. Formation of Dimers
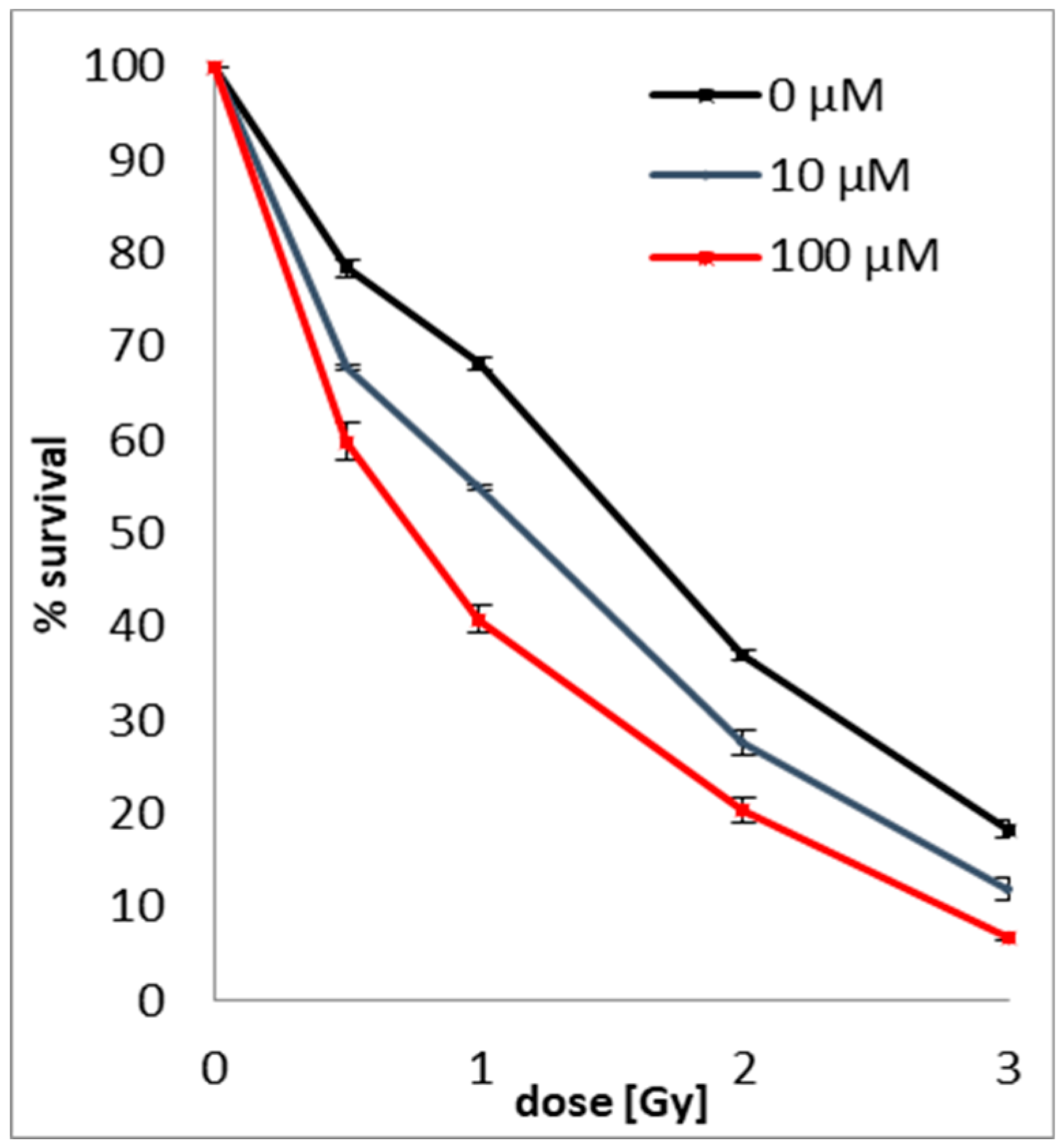
2.3. Clonogenic Assay
2.4. MTT Assay
2.5. Histone H2A.X Phosphorylation and Cell Death Assays
3. Materials and Methods
3.1. Experimental Methods
3.1.1. Synthesis of 3′,5′-di-O-acetyl-2′-Deoxyuridine
3.1.2. Synthesis of 3′,5′-di-O-acetyl-5-iodo-2′-Deoxyuridine
3.1.3. Synthesis of 3′,5′-di-O-acetyl-5-Iodo-4-Thio-2′-Deoxyuridine
3.1.4. Synthesis of 5-Iodo-4-Thio-2′-Deoxyuridine
3.1.5. Radiolysis
3.1.6. HPLC Analysis
3.1.7. LC–MS Analysis
3.1.8. Clonogenic Assay
3.1.9. Cytotoxicity Assay
3.1.10. Histone H2A.X Phosphorylation Assay
3.1.11. Cell Death Assay
3.2. Computational
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DEA | Dissociative electron attachment |
| FBS | Fetal bovine serum |
| HPLC | High-performance liquid chromatography |
| LC-MS | Liquid chromatography–mass spectrometry |
| PCM | Polarizable continuum model |
| DFT | Density functional theory |
| RPMI | Roswell Park Memorial Institute medium |
| DMEM | Dulbecco’s Modified Eagle medium |
| SSB | Single strand break |
| TLC | Thin layer chromatography |
| TS | Transition State |
References
- Rak, J.; Chomicz, L.; Wiczk, J.; Westphal, K.; Zdrowowicz, M.; Wityk, P.; Żyndul, M.; Makurat, S.; Golon, Ł. Mechanisms of Damage to DNA Labeled with Electrophilic Nucleobases Induced by Ionizing or UV Radiation. J. Phys. Chem. B 2016, 119, 8227–8238. [Google Scholar] [CrossRef] [PubMed]
- Makurat, S.; Chomicz-Mańka, L.; Rak, J. Electrophilic 5-Substituted Uracils as Potential Radiosensitizers. A DFT Study. ChemPhysChem 2016, 17, 2572–2578. [Google Scholar] [CrossRef]
- Chomicz, L.; Zdrowowicz, M.; Kasprzykowski, F.; Rak, J.; Buonaugurio, A.; Wang, Y.; Bowen, K.H. How to Find Out Whether a 5-Substituted Uracil Could Be a Potential DNA Radiosensitizer. J. Phys. Chem. Lett. 2013, 4, 2853–2857. [Google Scholar] [CrossRef]
- Westphal, K.; Zdrowowicz, M.; Żylicz-Stachula, A.; Rak, J. Chemically-Enzymatic Synthesis of Photosensitive DNA. J. Photochem. Photobiol. B Biol. 2017, 167, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Zdrowowicz, M.; Wityk, P.; Michalska, B.; Rak, J. 5-Bromo-2′-deoxycytidine—A Potential DNA Photosensitizer. Org. Biomol. Chem. 2016, 14, 9312–9321. [Google Scholar] [CrossRef]
- Park, Y.; Polska, K.; Rak, J.; Wagner, J.R.; Sanche, L. Fundamental Mechanisms of DNA Radiosensitization: Damage Induced by Low-Energy Electrons in Brominated Oligonucleotide Trimers. J. Phys. Chem. B 2012, 116, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Iliakis, G.; Kurtzman, S.; Pantelias, G.; Okayasu, R. Mechanism of Radiosensitization by Halogenated Pyrimidines: Effect of BrdU on Radiation Induction of DNA and Chromosome Damage and its Correlation with Cell Killing. Radiat. Res. 1989, 119, 286–304. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Chemical Radiosensitizers for Use in Radiotherapy. Clin. Oncol. 2007, 19, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Rieber, M.; Strasberg Rieber, M. Sensitization to Radiation-Induced DNA Damage Accelerates Loss of Bcl-2 and Increases Apoptosis and Autophagy. Cancer Biol. Ther. 2008, 7, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Seo, Y.; Schupp, J.E.; Zeng, X.; Desai, A.B.; Kinsella, T.J. Methoxyamine Potentiates Iododeoxyuridine-Induced Radiosensitization by Altering Cell Cycle Kinetics and Enhancing Senescence. Mol. Cancer Ther. 2006, 5, 893–902. [Google Scholar] [CrossRef]
- Semenenko, V.A.; Stewart, R.D. Fast Monte Carlo simulation of DNA damage formed by electrons and light ions. Phys. Med. Biol. 2006, 51, 1693–1706. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; O’Neill, P.; Stewart, R.D. Induction and Repair of Clustered DNA Lesions: What Do We Know So Far? Radiat. Res. 2013, 180, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, A.G.; Bennett, P.V.; Wilson, D.M., III; Sutherland, B.M. Processing of bistranded abasic DNA clusters in gamma-irradiated human hematopoietic cells. Nucleic Acids Res. 2004, 32, 5609–5620. [Google Scholar] [CrossRef]
- Gulston, M.; de Lara, C.; Jenner, T.; Davis, E.; O’Neill, P. Processing of clustered DNA damage generates additional double-strand breaks in mammalian cells post-irradiation. Nucleic Acids Res. 2004, 32, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Westphal, K.; Wiczk, J.; Miloch, J.; Kciuk, G.; Bobrowski, K.; Rak, J. Irreversible Electron Attachment—A Key to DNA Damage by Solvated Electrons in Aqueous Solution. Org. Biomol. Chem. 2015, 13, 10362–10369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.-Z. NMR and UV Studies of 4-Thio-2′-deoxyuridine and its Derivatives. Molecules 2011, 16, 5655–5664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Yin, H.-Y.; Trigiante, G.; Brem, R.; Karran, P.; Pitak, M.B.; Coles, S.J.; Xu, Y.-Z. 5-Iodo-4-thio-2′-deoxyuridine: Synthesis, Structure, and Cytotoxic Activity. Chem. Lett. 2014, 44, 147–149. [Google Scholar] [CrossRef]
- Brem, R.; Zhang, X.; Xu, Y.Z.; Karran, P. UVA Photoactivation of DNA Containing Halogenated Thiopyrimidines Induces Cytotoxic DNA Lesions. J. Photochem. Photobiol. B Biol. 2015, 145, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brem, R.; Guven, M.; Karran, P. Oxidatively-Generated Damage to DNA and Proteins Mediated by Photosensitized UVA. Free Radic. Biol. Med. 2017, 107, 101–109. [Google Scholar] [CrossRef]
- Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair: A Chemical Perspective; Springer Science & Business Media: Heidelberg, Germany, 2010. [Google Scholar]
- Greer, S. Studies on Ultraviolet Irradiation of Escherichia Coli Containing 5-Bromouracil in its DNA. J. Gen. Microbiol. 1960, 22, 618–634. [Google Scholar] [CrossRef]
- Djordjevic, B.; Szybalski, W. Genetics of Human Cell Lines III. Incorporation of 5-Bromo- and 5-Iododeoxyuridine into the Deoxyribonucleic Acid of Human Cells and its Effect on Radiation Sensitivity. J. Exp. Med. 1960, 112, 509–531. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, H.; Orlowski, C.; Georgiadis, G.T.; Ververis, K.; El-Osta, A.; Karagiannis, T.C. Clonogenic Assay: Adherent Cells. J. Vis. Exp. 2011, 49, 2573–2576. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, S.D.; Boyd, R.J.; Eriksson, L.A. A Theoretical Study of 5-Halouracils: Electron Affinities, Ionization Potentials and Dissociation of the Related Anions. Chem. Phys. Lett. 2001, 343, 151–158. [Google Scholar] [CrossRef]
- Li, X.; Sanche, L.; Sevilla, M.D. Dehalogenation of 5- Halouracils after Low Energy Electron Attachment: A Density Functional Theory Investigation. J. Phys. Chem. A 2002, 106, 11248–11253. [Google Scholar] [CrossRef]
- Ramnath, N.; Ramesh, V.; Ramamurthy, V. Photochemical Oxidation of Thioketones: Steric and Electronic Aspects. J. Org. Chem. 1983, 48, 214–222. [Google Scholar] [CrossRef]
- Zou, X.; Dai, X.; Liu, K.; Zhao, H.; Song, D.; Su, H. Photophysical and Photochemical Properties of 4-Thiouracil: Time-Resolved IR Spectroscopy and DFT Studies. J. Phys. Chem. B 2014, 118, 5864–5872. [Google Scholar] [CrossRef]
- Sánchez-Arroyo, A.J.; Pardo, Z.D.; Moreno-Jiménez, F.; Herrera, A.; Martín, N.; García-Fresnadillo, D. Photochemical Oxidation of Thioketones by Singlet Molecular Oxygen Revisited: Insights into Photoproducts, Kinetics, and Reaction Mechanism. J. Org. Chem. 2015, 80, 10575–10584. [Google Scholar] [CrossRef]
- Gara, P.M.D.; Garabano, N.I.; Llansola-Portoles, M.J.; Moreno, M.S.; Dodat, D.; Casas, O.R.; Gonzalez, M.C.; Kotler, M.L. ROS Enhancement by Silicon Nanoparticles in X-ray Irradiated Aqueous Suspensions and in Glioma C6 Cells. J. Nanopart. Res. 2012, 14, 741. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.; Tajik, M. Trimethylsilyl Chloride Promoted Selective Desulfurization of Thiocarbonyls to Carbonyls with Hydrogen Peroxide. Synthesis 2010, 24, 4282–4286. [Google Scholar] [CrossRef]
- Chu, J.W.; Trout, B.L. On the Mechanisms of Oxidation of Organic Sulfides by H2O2 in Aqueous Solutions. J. Am. Chem. Soc. 2004, 126, 900–908. [Google Scholar] [CrossRef]
- Zeida, A.; Babbush, R.; Gonzalez Lebrero, M.C.; Trujillo, M.; Radi, R.; Estrin, D.A. Molecular Basis of the Mechanism of Thiol Oxidation by Hydrogen Peroxide in Aqueous Solution: Challenging the SN2 Paradigm. Chem. Res. Toxicol. 2012, 25, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.J.; Montilla, F.; Álvarez, E.; Mealli, C.; Manca, G.; Galindo, A. Experimental and Theoretical Insights into the Oxodiperoxomolybdenum-Catalysed Sulphide Oxidation using Hydrogen Peroxide in Ionic Liquids. Dalton Trans. 2014, 43, 13711–13730. [Google Scholar] [CrossRef]
- van Bergen, L.A.; Roos, G.; De Proft, F. From Thiol to Sulfonic Acid: Modeling the Oxidation Pathway of Protein Thiols by Hydrogen Peroxide. J. Phys. Chem. A 2014, 118, 6078–6084. [Google Scholar] [CrossRef]
- McCaw, P.G.; Buckley, N.M.; Collins, S.G.; Maguire, A.R. Generation, Reactivity and Uses of Sulfines in Organic Synthesis. Eur. J. Org. Chem. 2016, 9, 1630–1650. [Google Scholar] [CrossRef]
- Reisenauer, H.P.; Mloston, G.; Romanski, J.; Schreiner, P.R. Photochemical Formation and Reactivities of Substituted Oxathiiranes in Low-Temperature Argon Matrices. Eur. J. Org. Chem. 2011, 31, 6269–6275. [Google Scholar] [CrossRef]
- Williams, C.R.; Harpp, D.N. Sulfur Extrusion Reactions—Scope and Mechanistic Aspects. J. Sulfur Chem. 1990, 10, 103–191. [Google Scholar] [CrossRef]
- Steudel, Y.; Steudel, R.; Wong, M.W. The Thermal Decomposition of Thiirane: A Mmechanistic Study by Aab Iinitio MO Theory. Chem. Eur. J. 2002, 8, 217–228. [Google Scholar] [CrossRef]
- Mulder, P.; Mozenson, O.; Lin, S.; Ingold, K.U. The Unexpected Desulfurization of 4-Aminothiophenols. J. Org. Chem. 2007, 72, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Adam, W.; Bargon, R.M. Synthesis of Thiiranes by Direct Sulfur Transfer: The Challenge of Developing Effective Sulfur Donors and Metal Catalysts. Chem. Rev. 2004, 104, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wenska, G.; Taras-Goślińska, K.; Filipiak, P.; Hug, G.L.; Marciniak, B. Photochemical Reactions of 4-Thiouridine Disulfide and 4-Benzylthiouridine—The Involvement of the 4-Pyrimidinylthiyl Radical. Photochem. Photobiol. Sci. 2008, 7, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Wenska, G.; Taras-Goślińska, K.; Skalski, B.; Hug, G.L.; Carmichael, I.; Marciniak, B. Generation of Thiyl Radicals by the Photolysis of 5-Iodo-4-thiouridine. J. Org. Chem. 2005, 70, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Taneja, N.; Davis, M.; Choy, J.S.; Beckett, M.A.; Singh, R.; Kron, S.J.; Weichselbaum, R.R. Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J. Biol. Chem. 2004, 279, 2273–2280. [Google Scholar] [CrossRef]
- Essmann, F.; Engels, I.H.; Totzke, G.; Schulze-Osthoff, K.; Jänicke, R.U. Apoptosis resistance of MCF-7 breast carcinoma cells to ionizing radiation is independent of p53 and cell cycle control but caused by the lack of caspase-3 and a caffeine-inhibitable event. Cancer Res. 2004, 64, 7065–7072. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon, D.A. A Local Density Functional Study of the Structure and Vibrational Frequencies of Molecular Transition-Metal Compounds. J. Phys. Chem. 1992, 96, 6630–6636. [Google Scholar] [CrossRef]
- Yurieva, A.G.; Poleshchuk, O.K.; Filimonov, V.D. Comparative Analysis of a Full-electron Basis Set and Pseudopotential for the Iodine Atom in DFT Quantum-Chemical Calculations of Iodine-Containing Compounds. J. Struct. Chem. 2008, 49, 548–552. [Google Scholar] [CrossRef]
- Rak, J.; Gutowski, M.; Dokurno, P.; Thanh, H.V.; Błażejowski, J. Theoretical Studies on Structure, Thermochemistry, Vibrational Spectroscopy, and Other Features of ZrX2−6 (X=F, Cl, Br, I): Coulombic Energy in Inorganic and Organic Hexahalogenozirconates. J. Chem. Phys. 1994, 100, 5810–5820. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab Initio Study of Solvated Molecules: A New Implementation of the Polarizable Continuum Model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic Interaction of a Solute with a Continuum. A Direct Utilization of Ab Initio Molecular Potentials for the Prevision of Solvent Effects. J. Chem. Phys. 1981, 55, 117–129. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Rev. D.01); Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makurat, S.; Spisz, P.; Kozak, W.; Rak, J.; Zdrowowicz, M. 5-Iodo-4-thio-2′-Deoxyuridine as a Sensitizer of X-ray Induced Cancer Cell Killing. Int. J. Mol. Sci. 2019, 20, 1308. https://doi.org/10.3390/ijms20061308
Makurat S, Spisz P, Kozak W, Rak J, Zdrowowicz M. 5-Iodo-4-thio-2′-Deoxyuridine as a Sensitizer of X-ray Induced Cancer Cell Killing. International Journal of Molecular Sciences. 2019; 20(6):1308. https://doi.org/10.3390/ijms20061308
Chicago/Turabian StyleMakurat, Samanta, Paulina Spisz, Witold Kozak, Janusz Rak, and Magdalena Zdrowowicz. 2019. "5-Iodo-4-thio-2′-Deoxyuridine as a Sensitizer of X-ray Induced Cancer Cell Killing" International Journal of Molecular Sciences 20, no. 6: 1308. https://doi.org/10.3390/ijms20061308
APA StyleMakurat, S., Spisz, P., Kozak, W., Rak, J., & Zdrowowicz, M. (2019). 5-Iodo-4-thio-2′-Deoxyuridine as a Sensitizer of X-ray Induced Cancer Cell Killing. International Journal of Molecular Sciences, 20(6), 1308. https://doi.org/10.3390/ijms20061308







