The Therapeutic Potential of Apigenin
Abstract
1. Introduction
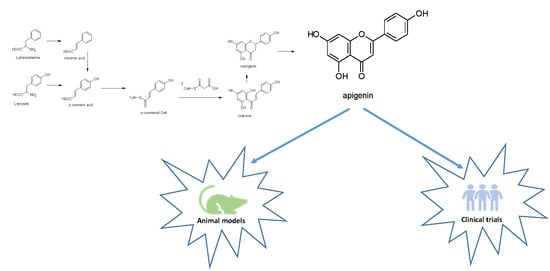
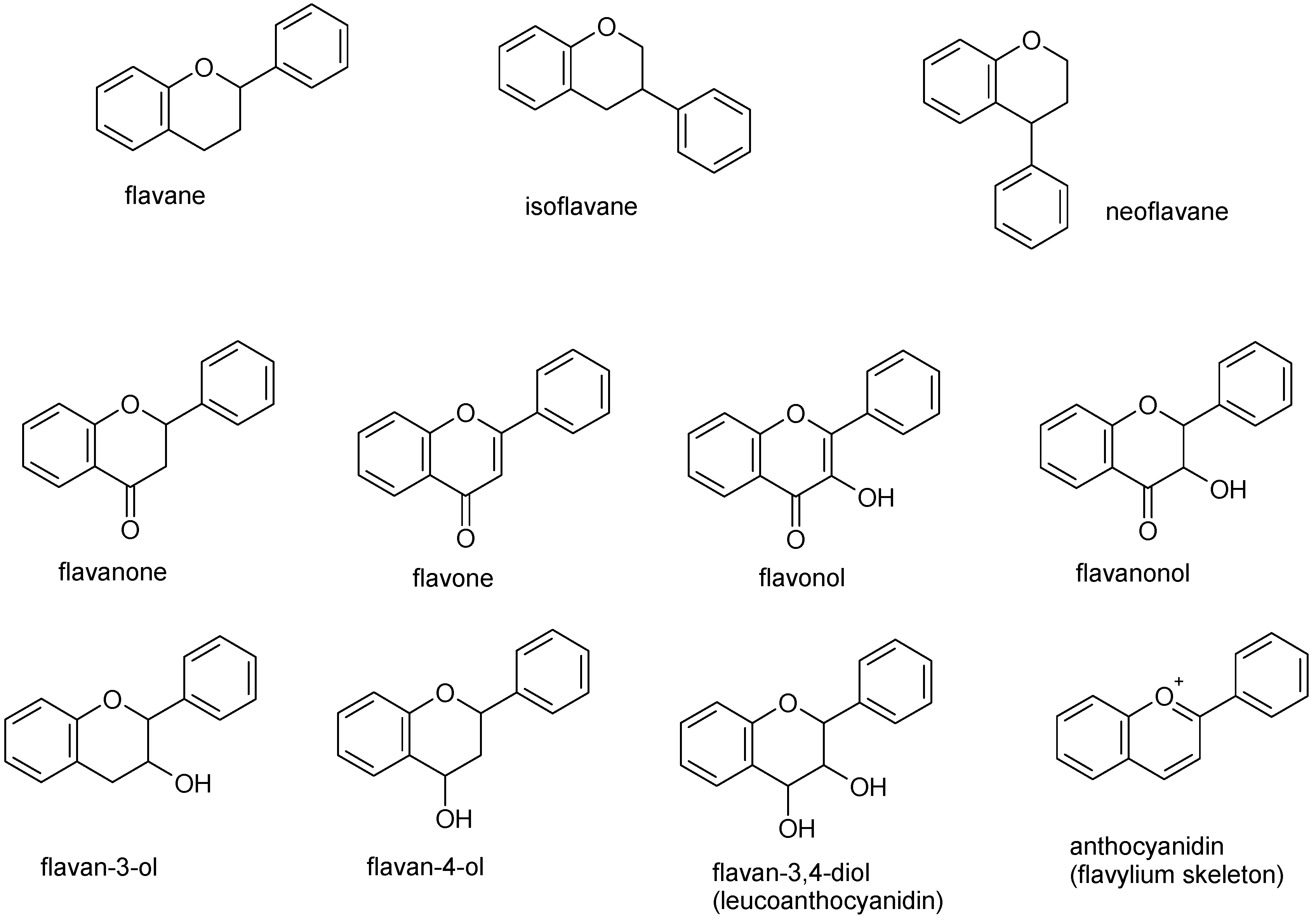
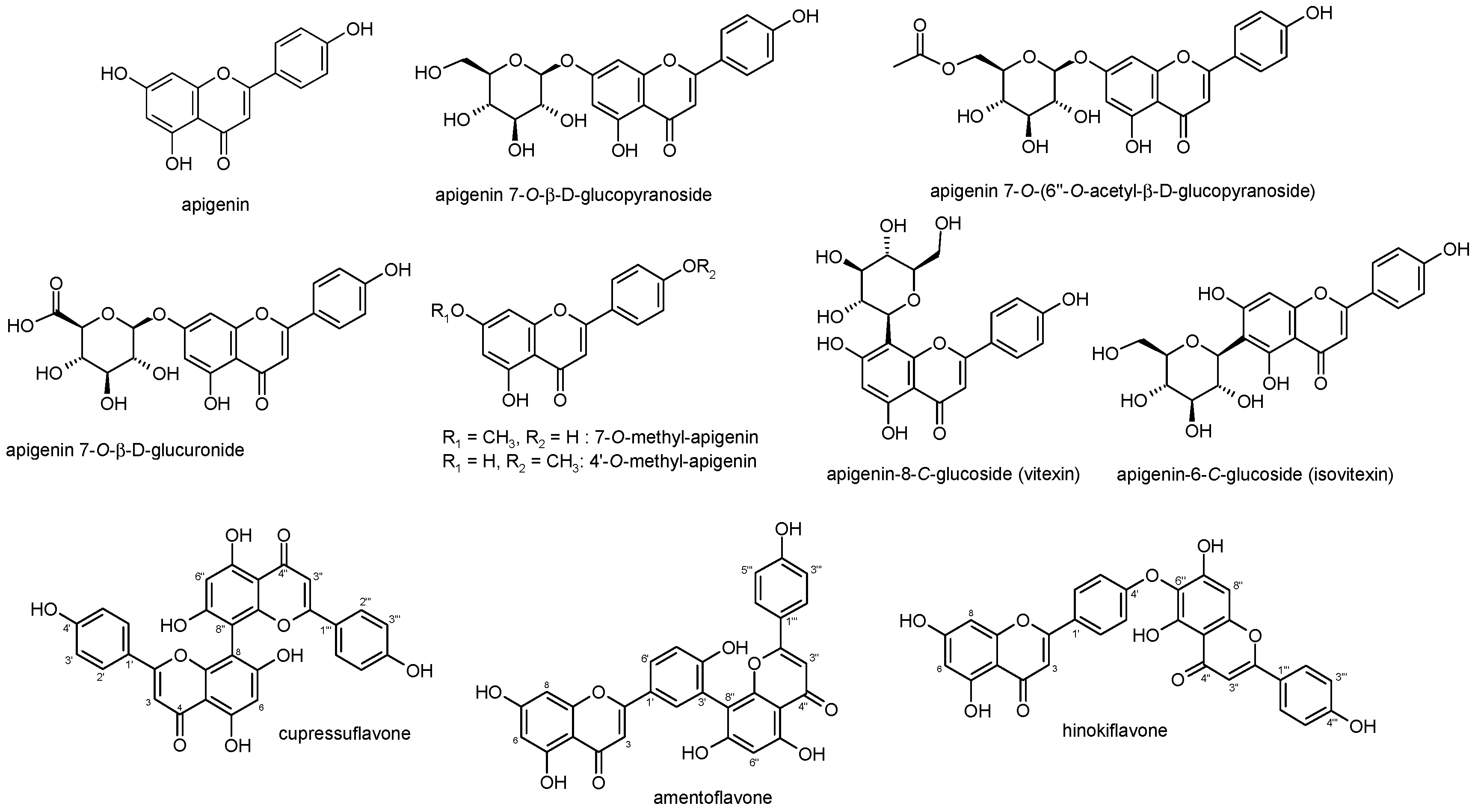
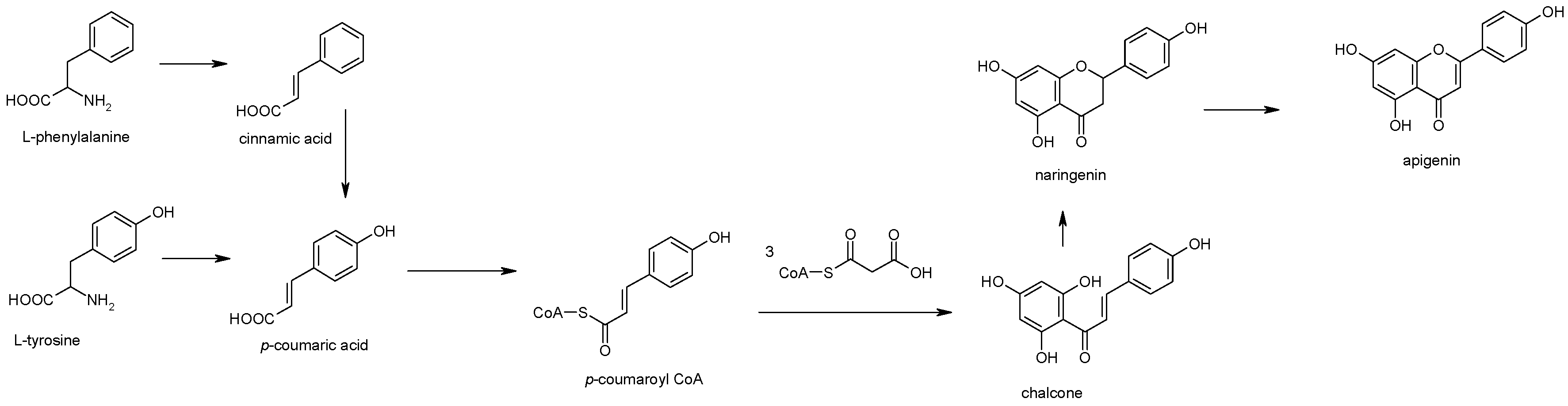
2. Apigenin Chemistry
3. Apigenin Biological Potential: from Molecular Aspects to Health Promoting Targets
3.1. Apigenin as a Nutraceutical: Introduction of the Concept and its Absorption, Distribution, Metabolism, and Excretion (ADME) Behavior
3.2. Healing Properties of Apigenin: An Overview
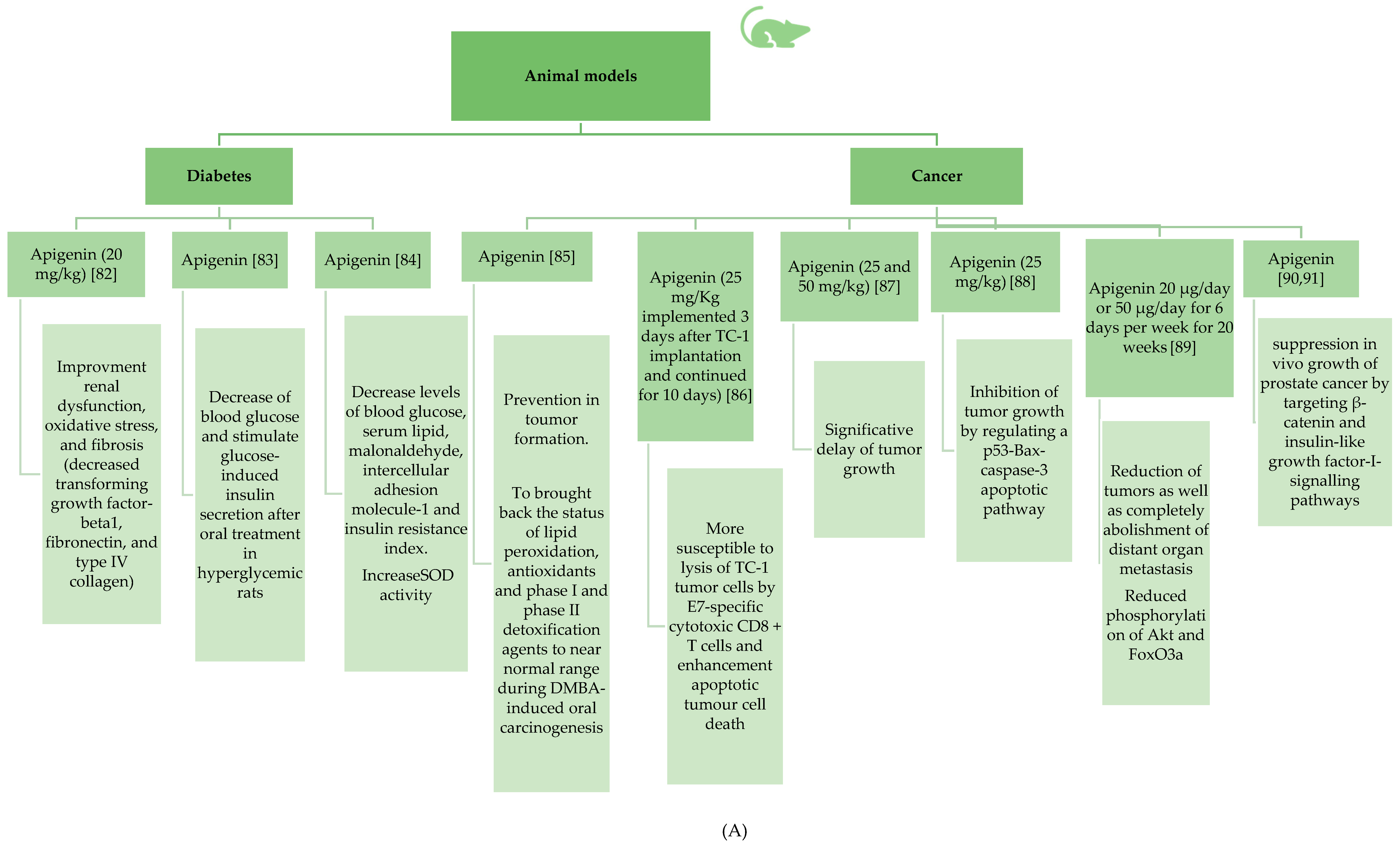
3.2.1. Antidiabetic Properties of Apigenin
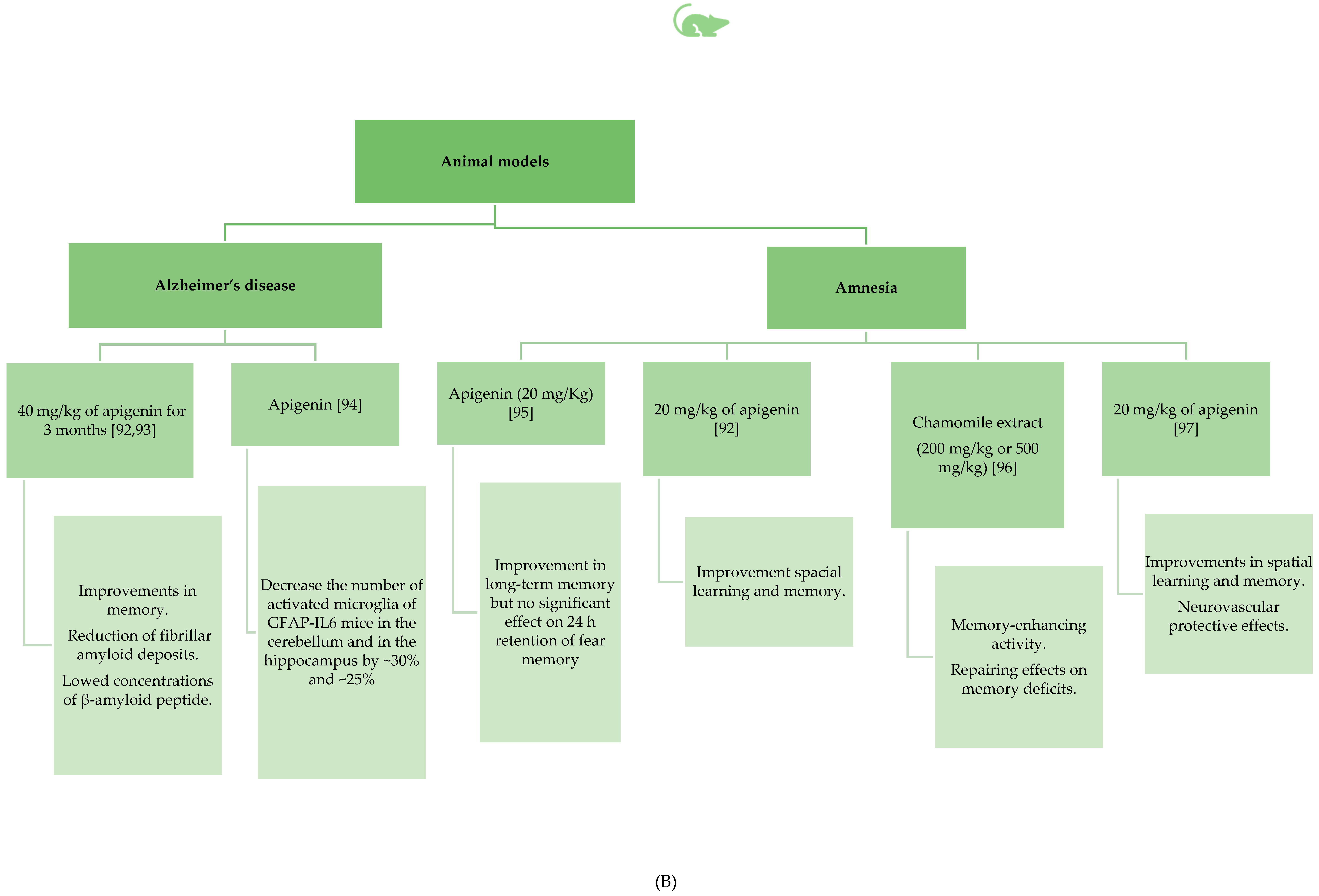
3.2.2. Apigenin’s Beneficial Role in Amnesia and Alzheimer’s Disease
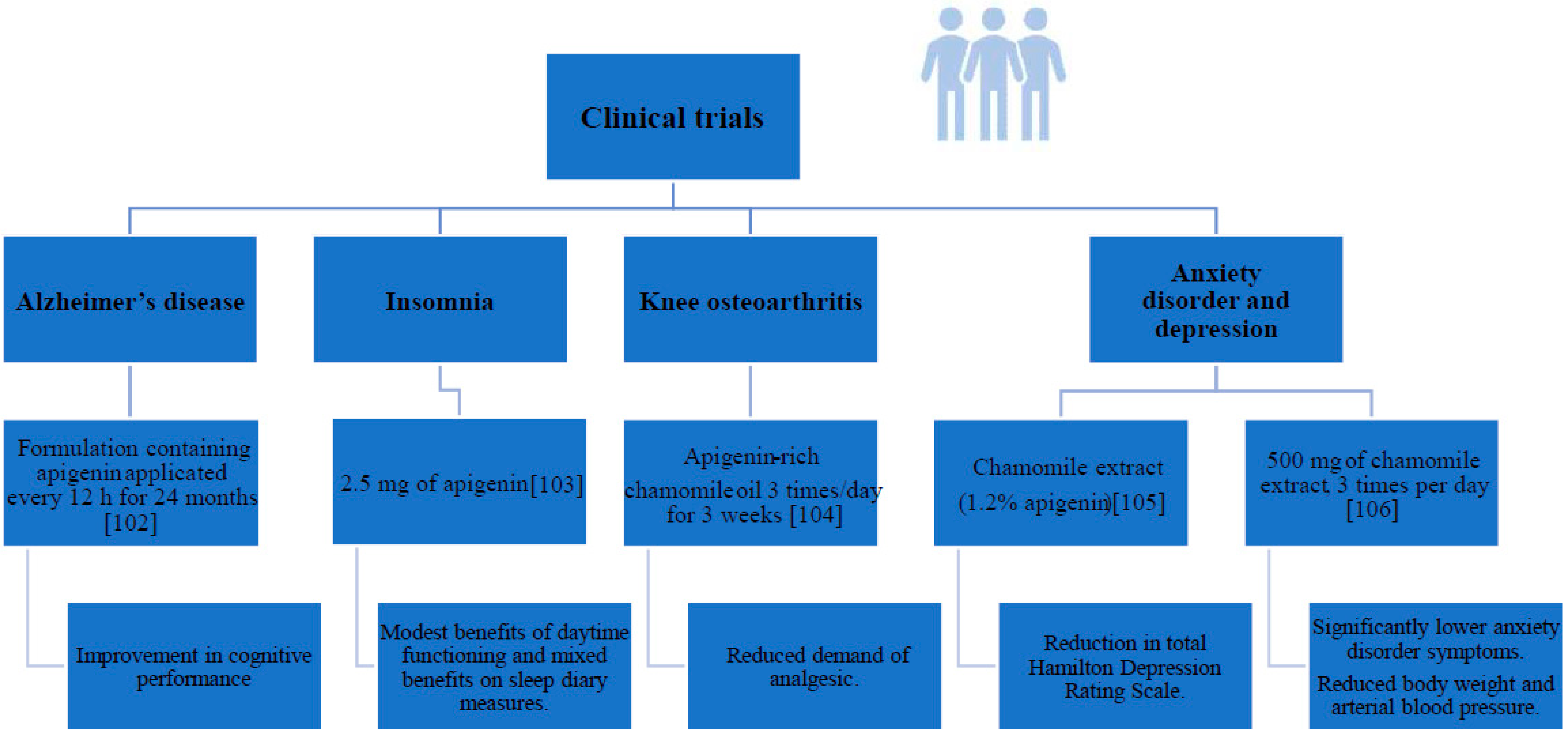
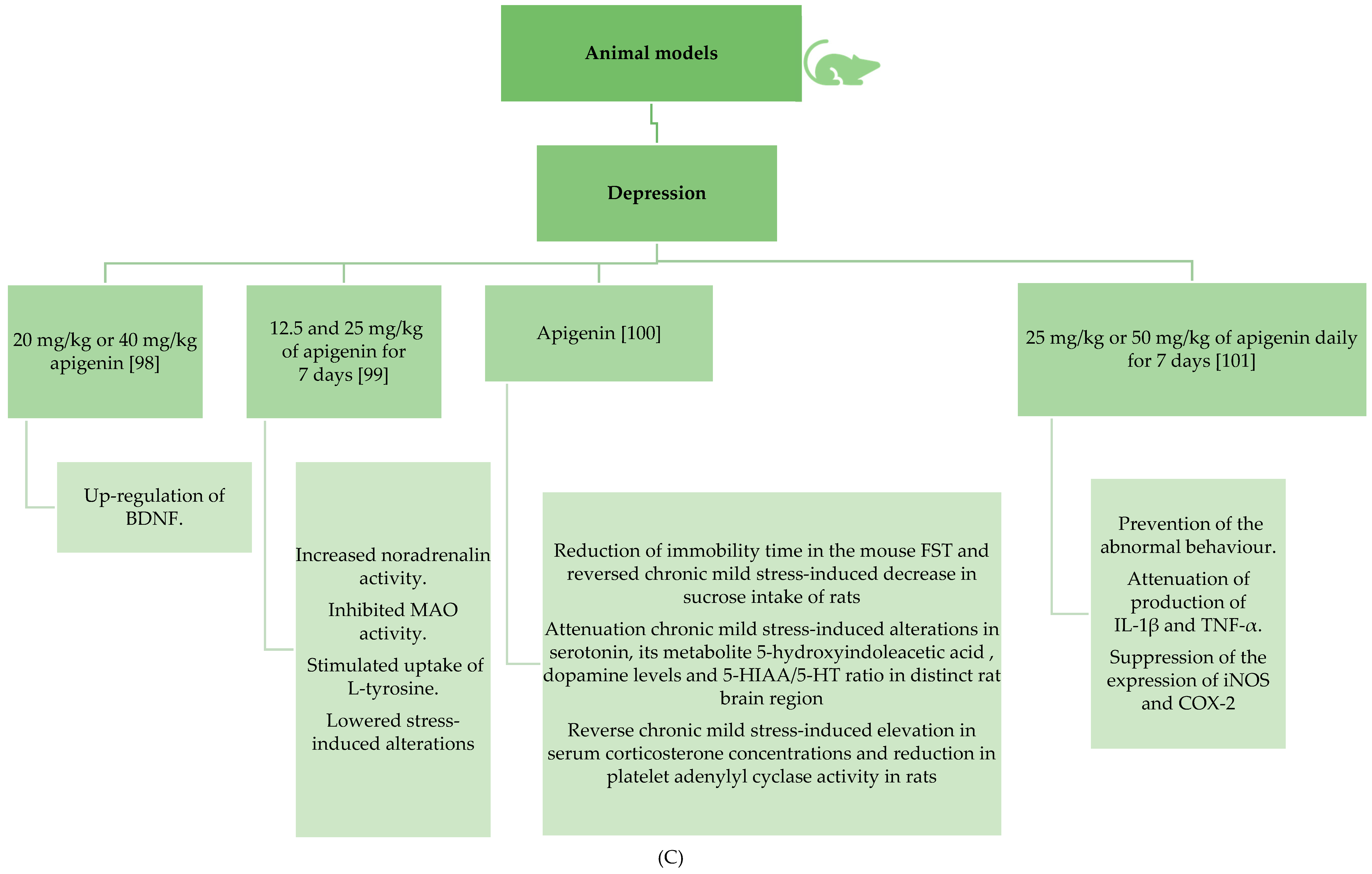
3.2.3. Apigenin’s Beneficial Effects in Depression and Insomnia
3.2.4. Anticancer Effects of Apigenin
3.2.5. Other Effects of Apigenin
3.3. Limitations and Future Perspectives
4. Apigenin in Databases
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amarowicz, R.; Carle, R.; Dongowski, G.; Durazzo, A.; Galensa, R.; Kammerer, D.; Maiani, G.; Piskula, M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53, S151–S183. [Google Scholar] [CrossRef] [PubMed]
- Cermak, R.; Durazzo, A.; Maiani, G.; Böhm, V.; Kammerer, D.R.; Carle, R.; Wiczkowski, W.; Piskula, M.K.; Galensa, R. The influence of postharvest processing and storage of foodstuffs on the bioavailability of flavonoids and phenolic acids. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S184–S193. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant. Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise (review). Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Miccadei, S.; Di Venere, D.; Cardinali, A.; Romano, R.; Durazzo, A.; Foddai, M.S.; Fraioli, R.; Mobarhan, S.; Maiani, G. Antioxidative and apoptotic properties of polyphenolic extracts from edible part of artichoke (Cynara scolymus L.) on cultured rat hepatocytes and on human hepatoma cells. Nutr. Cancer 2008, 60, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- D’Evoli, L.; Morroni, F.; Lombardi-Boccia, G.; Lucarini, M.; Hrelia, P.; Cantelli-Forti, G.; Tarozzi, A. Red chicory (Cichorium intybus L. cultivar) as a potential source of antioxidant anthocyanins for intestinal health. Oxid. Med. Cell. Longev. 2013, 2013, 704310. [Google Scholar]
- Azzini, E.; Maiani, G.; Garaguso, I.; Polito, A.; Foddai, M.S.; Venneria, E.; Durazzo, A.; Intorre, F.; Palomba, L.; Rauseo, M.L.; et al. The potential health benefits of polyphenol-rich extracts from Cichorium intybus L. studied on Caco-2 cells model. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 28, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B. The Systematic Identification of Flavonoids; Springer Verlag: Berlin, Germany, 1970. [Google Scholar]
- Dewick, P.M. Chimica, Biosintesi e Bioattività delle Sostanze Naturali; Piccin: Roma, Italy, 2001. [Google Scholar]
- Ornano, L.; Venditti, A.; Donno, Y.; Sanna, C.; Ballero, M.; Bianco, A. Phytochemical analysis of non-volatile fraction of Artemisia caerulescens subsp. densiflora (Viv.) (Asteraceae), an endemic species of La Maddalena Archipelago (Sardinia–Italy). Nat. Prod. Res. 2016, 30, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Maggi, F.; Vittori, S.; Papa, F.; Serrilli, A.M.; Di Cecco, M.; Bianco, A. Antioxidant and α-glucosidase inhibitory activities of Achillea tenorii. Pharm. Biol. 2015, 53, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Guarcini, L.; Bianco, A.; Rosselli, S.; Bruno, M.; Senatore, F. Phytochemical analysis of Achillea ligustica all. from Lipari Island (Aeolian islands). Nat. Prod. Res. 2016, 30, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; Del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Sciubba, F.; Serafini, M.; Bianco, A.; Cianfaglione, K.; Maggi, F. Volatile components, polar constituents and biological activity of tansy daisy (Tanacetum macrophyllum (Waldst. et Kit.) Schultz Bip. Ind. Crop. Prod. 2018, 118, 225–235. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Guarcini, L.; Foddai, S.; Serafini, M.; Bianco, A. Phytochemical study of a species with ethnopharmacological interest: Sideritis romana L. Eur. J. Med. Plants 2016, 12, 1–9. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Trancanella, E.; Zadeh, S.M.M.; Foddai, S.; Sciubba, F.; Bianco, A. A new natural neo-clerodane from Teucrium polium L. collected in Northern Iran. Ind. Crop. Prod. 2017, 97, 632–638. [Google Scholar] [CrossRef]
- Venditti, A. Secondary metabolites from Teucrium polium L. collected in Southern Iran. AJMAP 2017, 3, 108–123. [Google Scholar]
- Venditti, A.; Frezza, C.; Foddai, S.; Serafini, M.; Bianco, A. A rare bis-rhamnopyranosyl-aromadendrin derivative and other flavonoids from the flowers of Genista cilentina Vals. an endemic species of Southern Italy. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Fatma, W.; Taufeeq, H.M.; Shaida, W.A.; Rahman, W. Biflavanoids from Juniperus macropoda Boiss and Juniperus phoenicea Linn. (Cupressaceae). Indian J. Chem. B Org. 1979, 17, 193–194. [Google Scholar]
- Stassi, V.; Verykokidou, E.; Loukis, A.; Harvala, C. Polyphenolic compounds from the leaves of Juniperus oxycedrus L. subsp. macrocarpa (Sm.) Ball. Pharm. Acta Helv. 1998, 72, 311–312. [Google Scholar] [CrossRef]
- Alquasoumi, S.I.; Farraj, A.I.; Abdel-Kader, M.S. Study of the hepatoprotective effect of Juniperus phoenicea constituents. Pak. J. Pharm. Sci. 2013, 26, 999–1008. [Google Scholar]
- Venditti, A.; Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Ornano, L.; Bianco, A. Bioactive Constituents of Juniperus turbinata Guss. from La Maddalena Archipelago. Chem. Biodivers. 2018, 15, e1800148. [Google Scholar] [CrossRef] [PubMed]
- Forkmann, G. Flavonoids as Flower Pigments: The Formation of the Natural Spectrum and its Extension by Genetic Engineering. Plant. Breed. 1991, 106, 1–26. [Google Scholar] [CrossRef]
- Herrmann, K.M. The shikimate pathway as an entry to aromatic secondary metabolism. Plant. Physiol. 1995, 107, 7–12. [Google Scholar] [CrossRef]
- Martens, S.; Forkmann, G.; Matern, U.; Lukacin, R. Cloning of parsley flavone synthase I. Phytochemistry 2001, 58, 43–46. [Google Scholar] [CrossRef]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef]
- Leonard, E.; Yan, Y.; Lim, K.H.; Koffas, M.A. Investigation of two distinct flavone synthases for plant-specific flavone biosynthesis in Saccharomyces cerevisiae. Appl Environ. Microbiol. 2005, 71, 8241–8248. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.G.; Kim, M.; Ahn, J.H. Biosynthesis of Two Flavones, Apigenin and Genkwanin, in Escherichia coli. J. Microbiol. Biotechnol. 2015, 25, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, Q.C.; Faraone, I.; Russo, D.; Aragão Catunda, F.E., Jr.; Vignola, L.; de Carvalho, M.G.; de Tommasi, N.; Milella, L. Chemical and Biological insights of Ouratea hexasperma (A. St.-Hil.) Baill.: A source of bioactive compounds with multifunctional properties. Nat. Prod. Res. 2018, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodriguez, J.A.; Kerimi, A.; Abranko, L.; Tumova, S.; Ford, L.; Blackburn, R.S.; Rayner, C.; Williamson, G. Acute metabolic actions of the major polyphenols in chamomile: An in vitro mechanistic study on their potential to attenuate postprandial hyperglycaemia. Sci. Rep. 2018, 3, 5471. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Barker, G.; Wall, C.A.; Lappas, M. Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Mol. Hum. Reprod. 2013, 19, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, Y.; Lin, L.; Zhou, J. Apigenin suppresses the apoptosis of H9C2 rat cardiomyocytes subjected to myocardial ischemia-reperfusion injury via upregulation of the PI3K/Akt pathway. Mol. Med. Rep. 2018, 18, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017, 41, e12376. [Google Scholar] [CrossRef]
- Pereira, S.V.; Reis, R.A.S.P.; Garbuio, D.C.; de Freitas, L.A.P. Dynamic maceration of Matricaria chamomilla inflorescences: Optimal conditions for flavonoids and antioxidant activity. Rev. Bras. Farmacogn. 2018, 28, 111–117. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Kaleem, M.; Ahmad, A. Therapeutic, Probiotic and Unconventional Foods; Chapter 8, Flavonoids as Nutraceuticals; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press, Elsevier: Amsterdam, The Netherland, 2018; pp. 137–155. [Google Scholar]
- De Felice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Santini, A.; Novellino, E. To Nutraceuticals and Back: Rethinking a Concept. Foods 2017, 6, 74. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals: Beyond the Diet Before the Drugs. Curr. Bioact. Compd. 2014, 10, 1–12. [Google Scholar] [CrossRef]
- Santini, A. Nutraceuticals: An Healthy Bet for the Future. J. Food Res. 2014, 3, 1–2. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An Overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals: Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2878. [Google Scholar] [CrossRef]
- Angelino, D.; Berhow, M.; Ninfali, P.; Jeffery, E.H. Caecal absorption of vitexin-2-O-xyloside and its aglycone apigenin, in the rat. Food Funct. 2013, 4, 1339–1345. [Google Scholar] [CrossRef]
- Ali, F.; Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Lotha, R.; Sivasubramanian, A. Flavonoids nutraceuticals in prevention and treatment of cancer: A review. Asian J. Pharm. Clin. Res. 2018, 11, 42–47. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, D.P.; Huang, Y.T.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.Y.; Chen, H.; Wang, C.; Zhai, Y.J.; Zhai, G.X. Preparation and in-vitro evaluation of apigenin loaded lipid nanocapsules. J. Nanosci. Nanotechnol. 2013, 13, 6546–6552. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.J.; Guo, S.S.; Liu, C.H.; Yang, C.F.; Dou, J.F.; Li, L.B.; Zhai, G.X. Preparation and in-vitro evaluation of apigenin-loaded polymeric micelles. Colloid Surf. A 2013, 429, 24–30. [Google Scholar] [CrossRef]
- Al Shaal, L.; Shegokar, R.; Muller, R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. 2011, 420, 133–140. [Google Scholar] [CrossRef]
- Le-Ngoc Vo, C.; Park, C.; Lee, B.J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar]
- Karim, R.; Palazzo, C.; Laloy, J.; Delvigne, A.S.; Vanslambroul, S.; Jerome, C.; Lepeltier, E.; Orage, F.; Dogne, J.M.; Evrard, B.; et al. Development and evaluation of injectable nanosized drug delivery systems for apigenin. Int. J. Pharm. 2017, 532, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Giacometti, J.; Russo, G.L. Antioxidant Phytochemicals at the Pharma-Nutrition Interface. Oxid. Med. Cell Longev. 2017, 6986143. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Garg, V.K.; Buttar, H.S.; Setzer, W.N.; Sethi, G. Apigenin: A natural bioactive flavone-type molecule with promising therapeutic function. J. Funct. Foods 2018, 48, 457–471. [Google Scholar] [CrossRef]
- Takagaki, N.; Sowa, Y.; Oki, T.; Nakanishi, R.; Yogosawa, S.; Sakai, T. Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. Int. J. Oncol. 2005, 26, 185–189. [Google Scholar] [CrossRef]
- Maggioni, D.; Garavello, W.; Rigolio, R.; Pignataro, L.; Gaini, R.; Nicolini, G. Apigenin impairs oral squamous cell carcinoma growth in vitro inducing cell cycle arrestandapoptosis. Int. J. Oncol. 2013, 43, 1675–1682. [Google Scholar] [CrossRef]
- Iizumi, Y.; Oishi, M.; Taniguchi, T.; Goi, W.; Sowa, Y.; Sakai, T. The flavonoid apigenin downregulates CDK1 by directly targeting ribosomal protein S9. PLoS ONE 2013, 8, e73219. [Google Scholar] [CrossRef]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Woo, J.K.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Induction of caspase-dependent apoptosis by apigenin by inhibiting STAT3 signaling in HER2-overexpressing MDA-MB-453 breast cancer cells. Anticancer Res. 2014, 34, 2869–2882. [Google Scholar]
- Seo, H.S.; Choi, H.S.; Kim, S.R.; Choi, Y.K.; Woo, S.M.; Shin, I.; Woo, J.K.; Park, S.Y.; Shin, Y.C.; Ko, S.K. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012, 366, 319–334. [Google Scholar] [CrossRef]
- Karmakar, S.; Davis, K.A.; Choudhury, S.R.; Deeconda, A.; Banik, N.L.; Ray, S.K. Bcl-2 inhibitor and apigenin worked synergistically in human malignant neuroblastoma cell lines and increased apoptosis with activation of extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 2009, 388, 705–710. [Google Scholar] [CrossRef]
- Lee, J.-H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Antiinflammatory mechanisms of apigenin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharmacal Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Boitano, P.D. Effect of the pleiotropic drug CNB-001 on tissue plasminogen activator (tPA) protease activity in vitro: Support for combination therapy to treat acute ischemic stroke. J. Neurol. Neurophysiol. 2014, 5, 214. [Google Scholar]
- Huang, C.-H.; Kuo, P.-L.; Hsu, Y.-L.; Chang, T.-T.; Tseng, H.-I.; Chu, Y.-T.; Kuo, C.-H.; Chen, H.-N.; Hung, C.-H. The natural flavonoid apigenin suppresses Th1- and Th2-related chemokine production by human monocyte THP-1 cells through mitogen-activated protein kinase pathways. J. Med. Food 2010, 13, 391–398. [Google Scholar] [CrossRef]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J. Immunol. 2007, 179, 7121–7127. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Carlsen, H.; Nordström, O.; Blomhoff, R.; Moskaug, J.Ø. Flavonoids increase the intracellular glutathione level by transactivation of the γ-glutamylcysteine synthetase catalytical subunit promoter. Free Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
- Huang, C.S.; Lii, C.K.; Lin, A.H.; Yeh, Y.W.; Yao, H.T.; Li, C.C.; Wang, T.S.; Chen, H.W. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch. Toxicol. 2013, 87, 167–178. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Jeffery, S.; Saw, C.L.L.; Shu, L.; Su, Z.Y.; Kong, A.N.T. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015, 36, 440–451. [Google Scholar] [CrossRef]
- Peng, Q.; Deng, Z.; Pan, H.; Gu, L.; Liu, O.; Tang, Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol. Lett. 2017, 15, 1379–1388. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Ehrhart, J.; Bai, Y.; Sanberg, P.R.; Bickford, P.; Tan, J.; Douglas, R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflamm. 2008, 5, 41–51. [Google Scholar] [CrossRef]
- Jäger, A.K.; Krydsfeldt, K.; Rasmussen, H.B. Bioassay-guided isolation of apigenin with GABAbenzodiazepine activity from Tanacetum parthenium. Phytother. Res. 2009, 23, 1642–1644. [Google Scholar] [CrossRef]
- Wasowski, C.; Marder, M.; Viola, H.; Medina, J.H.; Paladini, A.C. Isolation and identification of 6-methylapigenin, a competitive ligand for the brain GABA(A) receptors, from Valeriana wallichii. Planta Med. 2002, 68, 934–936. [Google Scholar] [CrossRef]
- Campbell, E.L.; Chebib, M.; Johnston, G.A.R. The dietary flavonoids apigenin and (-)- epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABA(A) receptors. Biochem. Pharmacol. 2004, 68, 1631–1638. [Google Scholar] [CrossRef]
- Sloley, B.D.; Urichuk, L.J.; Morley, P.; Durkin, J.; Shan, J.J.; Pang, P.K.T.; Coutts, R.T. Identification of kaempferol as a monoamine oxidase inhibitor and potential neuroprotectant in extracts of Ginkgo biloba leaves. J. Pharm. Pharmacol. 2000, 52, 451–459. [Google Scholar] [CrossRef]
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-kappaB-TNF-alpha and TGF-beta1-MAPK-fibronectin pathways. Am. J. Physiol. Ren. Physiol. 2017, 313, F414–F422. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Folador, P.; Moresco, H.H.; Brighente, I.M.; Pizzolatti, M.G.; Silva, F.R. Mechanism of action of the stimulatory effect of apigenin-6-C-(2’’-O-alpha-l-rhamnopyranosyl)-beta-L-fucopyranoside on 14C-glucose uptake. Chem. Biol. Interact. 2009, 179, 407–412. [Google Scholar] [CrossRef]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharm. 2016, 773, 13–23. [Google Scholar] [CrossRef]
- Silvan, S.; Manoharan, S.; Baskaran, N.; Anusuya, C.; Karthikeyan, S.; Prabhakar, M.M. Chemopreventive potential of apigenin in 7,12-dimethylbenz(a)anthracene induced experimental oral carcinogenesis. Eur. J. Pharmacol. 2011, 670, 571–577. [Google Scholar] [CrossRef]
- Chuang, C.-M.; Monie, A.; Wu, A.; Hung, C.-F. Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. J. Biomed. Sci. 2009, 16, 49. [Google Scholar] [CrossRef]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.O.; Natali, P.G.; Brunetti, M.; Aiello, F.B.; Piantelli, M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer 2000, 87, 595–600. [Google Scholar] [CrossRef]
- Torkin, R.; Lavoie, J.F.; Kaplan, D.R.; Yeger, H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol. Cancer 2005, 4, 1–11. [Google Scholar]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; Maclennan, G.T.; Gupta, S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef]
- Shukla, S.; MacLennan, G.T.; Flask, C.A.; Fu, P.; Mishra, A.; Resnick, M.I.; Gupta, S. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007, 67, 6925–6935. [Google Scholar] [CrossRef]
- Shukla, S.; MacLennan, G.T.; Fu, P.; Gupta, S. Apigenin attenuates insulin-like growth factor-I signaling in an autochthonous mouse prostate cancer model. Pharma Res. 2012, 29, 1506–1517. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Liu, R.; Li, X.X.; Li, J.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Wang, Y.R.; Fa, X.Z. Apigenin attenuates copper-mediated β-amyloid neurotoxicity through antioxidation, mitochondrion protection and MAPK signal inactivation in an AD cell model. Brain Res. 2013, 1492, 33–45. [Google Scholar] [CrossRef]
- Liang, H.; Sonego, S.; Gyengesi, E.; Rangel, A.; Niedermayer, G.; Karl, T.; Münch, G. Anti-Inflammatory and neuroprotective effect of apigenin: Studies in the GFAP-IL6 mouse model of chronic neuroinflammation. Free Radic. Biol. Med. 2017, 108, S4–S13. [Google Scholar] [CrossRef]
- Popović, M.; Caballero-Bleda, M.; Benavente-García, O.; Castillo, J. The flavonoid apigenin delays forgetting of passive avoidance conditioning in rats. J. Psychopharmacol. 2014, 28, 498–501. [Google Scholar] [CrossRef]
- Alibabaei, Z.; Rabiei, Z.; Rahnama, S.; Mokhtari, S.; Rafieian-kopaei, M. Matricaria chamomilla extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Biomed. Aging Pathol. 2014, 4, 355–360. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.; Yang, H.; Lan, X.; Ying, J.; Du, G. The flavonoid apigenin protects brain neurovascular c8upling against amyloid-beta(2) (5)(-)(3)(5)-induced toxicity in mice. J. Alzheimers Dis. 2011, 24, 85–100. [Google Scholar] [CrossRef]
- Weng, L.; Guo, X.; Li, Y.; Yang, X.; Han, Y. Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur. J. Pharmacol. 2016, 774, 50–54. [Google Scholar] [CrossRef]
- Nakazawa, T.; Yasuda, T.; Ueda, J.; Ohsawa, K. Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003, 26, 474–480. [Google Scholar] [CrossRef]
- Yi, L.T.; Li, J.M.; Li, Y.C.; Pan, Y.; Xu, Q.; Kong, L. Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin. Life Sci. 2008, 82, 741–751. [Google Scholar] [CrossRef]
- Li, R.; Zhao, D.; Qu, R.; Fu, Q.; Ma, S. The effects of apigenin on lipopolysaccharide-induced depressive-like behavior in mice. Neurosci. Lett. 2015, 594, 17–22. [Google Scholar] [CrossRef]
- De Font-Reaulx Rojas, E.; Dorazco-Barragan, G. Clinical stabilisation in neurodegenerative diseases: Clinical study in phase II. Rev. De Neurol. 2010, 50, 520–528. [Google Scholar]
- Zick, S.M.; Wright, B.D.; Sen, A.; Arnedt, J.T. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: A randomized placebo-controlled pilot study. BMC Complement. Altern. Med. 2011, 11, 78. [Google Scholar] [CrossRef]
- Shoara, R.; Hashempur, M.H.; Ashraf, A.; Salehi, A.; Dehshahri, S.; Habibagahi, Z. Efficacy and safety of topical Matricaria chamomilla L. (chamomile) oil for knee osteoarthritis: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2015, 21, 181–187. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Shults, J.; Soeller, I.; Mao, J.J.; Rockwell, K.; Newberg, A.B. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: An exploratory study. Altern. Ther. Health Med. 2012, 18, 44–49. [Google Scholar] [PubMed]
- Mao, J.J.; Xie, S.X.; Keefe, J.R.; Soeller, I.; Li, Q.S.; Amsterdam, J.D. Long-term chamomile (Matricaria chamomilla L.) treatment for generalized anxiety disorder: A randomized clinical trial. Phytomedicine 2016, 23, 1735–1742. [Google Scholar] [CrossRef]
- Pamunuwa, G.; Karunaratne, D.N.; Waisundara, V.Y. Antidiabetic Properties, Bioactive Constituents, and Other Therapeutic Effects of Scoparia dulcis. Evid. Based Complement. Alternat. Med. 2016, 2016, 8243215. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Huttemann, M. Molecular Mechanisms and Therapeutic Effects of (-)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Cheng, N.; Yi, W.B.; Peng, S.M.; Zou, X.Q. Synthesis, nitric oxide release, and alpha-glucosidase inhibition of nitric oxide donating apigenin and chrysin derivatives. Bioorg. Med. Chem. 2014, 22, 1515–1521. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Apigenin (4’,5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan-induced diabetic mice. J. Pharm. Pharmacol. 2007, 59, 1543–1548. [Google Scholar] [CrossRef]
- Liu, H.J.; Fan, Y.L.; Liao, H.H.; Liu, Y.; Chen, S.; Ma, Z.G.; Zhang, N.; Yang, Z.; Deng, W.; Tang, Q.Z. Apigenin alleviates STZ-induced diabetic cardiomyopathy. Mol. Cell. Biochem. 2017, 428, 9–21. [Google Scholar] [CrossRef]
- Mahajan, U.B.; Chandrayan, G.; Patil, C.R.; Arya, D.S.; Suchal, K.; Agrawal, Y.O.; Ojha, S.; Goyal, S.N. The Protective Effect of Apigenin on Myocardial Injury in Diabetic Rats mediating Activation of the PPAR-gamma Pathway. Int. J. Mol. Sci. 2017, 18, 756. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; More, S.V.; Han, S.-D.; Choi, J.-Y.; Choi, D.-K. Promising Therapeutics with Natural Bioactive Compounds for Improving Learning and Memory—A Review of Randomized Trials. Molecules 2012, 17, 10503–10539. [Google Scholar] [CrossRef] [PubMed]
- Stella, F.; Radanovic, M.; Canineu, P.R.; de Paula, V.J.R.; Forlenza, O.V. Anti-dementia medications: Current prescriptions in clinical practice and new agents in progress. Adv. Drug Saf. 2015, 6, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Millington, C.; Sonego, S.; Karunaweera, N.; Rangel, A.; Aldrich-Wright, J.; Campbell, I.; Gyengesi, E.; Münch, G. Chronic neuroinflammation in Alzheimer’s disease: New perspectives on animal models and promising candidate drugs. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as neuroprotective agent: Of mice and men. Pharm. Res. 2018, 128, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Boskabady, M.H. A review of the relaxant effect of various medicinal plants on tracheal smooth muscle, their possible mechanism(s) and potency. J. Ethnopharmacol. 2015, 175, 528–548. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, M.; Gyengesim, E.; Munch, G. Curcumin and Apigenin—Novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Nat. Reagen Res. 2015, 10, 1181–1185. [Google Scholar]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef]
- Zhang, F.; Li, F.; Chen, G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol. Sci. 2014, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, P.; Avallone, R.; Baraldi, M. Behavioural characterization of the flavonoids apigenin and crysin. Fitoterapia 2000, 71, S117–S123. [Google Scholar] [CrossRef]
- Avallone, R.; Zanoli, P.; Puia, G.; Kleinschnitz, M.; Shreiner, P.; Baraldi, M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol. 2000, 59, 1387–1394. [Google Scholar] [CrossRef]
- Han, X.H.; Hong, S.S.; Hwang, J.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Monoamine oxidase inhibitory components from Cayratia japonica. Arch. Pharmacal Res. 2007, 30, 13. [Google Scholar] [CrossRef]
- Chaurasiya, N.; Ibrahim, M.; Muhammad, I.; Walker, L.; Tekwani, B. Monoamine Oxidase Inhibitory Constituents of Propolis: Kinetics and Mechanism of Inhibition of Recombinant Human MAO-A and MAO-B. Molecules 2014, 19, 18936–18952. [Google Scholar] [CrossRef]
- Lorenzo, P.S.; Rubio, M.C.; Medina, J.H.; Adler-Graschinsky, E. Involvement of monoamine oxidase and noradrenaline uptake in the positive chronotropic effects of apigenin in rat atria. Eur. J. Pharmacol. 1996, 312, 203–207. [Google Scholar] [CrossRef]
- Morita, K.; Hamano, S.; Oka, M.; Teraoka, K. Stimulatory actions of bioflavonoids on tyrosine uptake into cultured bovine adrenal chromaffin cells. Biochem. Biophys. Res. Commun. 1990, 171, 1199–1204. [Google Scholar] [CrossRef]
- Leach, M.J.; Page, A.T. Herbal medicine for insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2015, 24, 1–12. [Google Scholar] [CrossRef]
- Campos-Bedolla, P.; Walter, F.R.; Veszelka, S.; Deli, M.A. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014, 45, 610–638. [Google Scholar] [CrossRef]
- Végh, K.; Riethmüller, E.; Hosszú, L.; Darcsi, A.; Müller, J.; Alberti, Á.; Tóth, A.; Béni, S.; Könczöl, Á.; Balogh, G.T.; et al. Three newly identified lipophilic flavonoids in Tanacetum parthenium supercritical fluid extract penetrating the Blood-Brain Barrier. J. Pharm. Biomed. Anal. 2018, 149, 488–493. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, L.; Li, X.; Xiong, J.; Xu, P.; Guo, C.; Xue, M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: An evaluation using the blood-brain barrier cell and Caco-2 cell models. Toxicol. In Vitro 2014, 28, 388–396. [Google Scholar] [CrossRef]
- Knekt, P.; Järvinen, R.; Seppänen, R.; Hellövaara, M.; Teppo, L.; Pukkala, E.; Aromaa, A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997, 146, 223–230. [Google Scholar] [CrossRef]
- Rossi, M.; Negri, E.; Lagiou, P.; Talamini, R.; Dal Maso, L.; Montella, M.; Franceschi, S.; La Vecchia, C. Flavonoids and ovarian cancer risk: A case-control study in Italy. Int. J. Cancer 2008, 123, 895–898. [Google Scholar] [CrossRef]
- Bosetti, C.; Spertini, L.; Parpinel, M.; Gnagnarella, P.; Lagiou, P.; Negri, E.; Franceschi, S.; Montella, M.; Peterson, J.; Dwyer, J.; et al. Flavonoids and Breast Cancer Risk in Italy. Cancer Epidemiol. Biomark. Prev. 2005, 14, 805–808. [Google Scholar] [CrossRef]
- Hoensch, H.; Groh, B.; Edler, L.; Kirch, W. Prospective cohortcomparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J. Gastroenterol. 2008, 14, 2187–2193. [Google Scholar] [CrossRef]
- Moore, P.; Ginwala, R.; Revuri, N.; Kranz, V.A.; Houle, J.D.; Khan, Z.K.; Jain, P. Nutraceutical Apigenin: Mechanism of action associated with its anti-inflammatory activity and regulation of dendritic cell metabolism. J. Immunol. 2017, 198 (Suppl. 1), 219-12. [Google Scholar]
- Kim, M.K.; Yun, K.J.; Lim, D.H.; Kim, J.; Jang, Y.P. Anti-Inflammatory Properties of Flavone di-C-Glycosides as Active Principles of Camellia Mistletoe, Korthalsella japonica. Biomol. Ther. 2016, 24, 630–637. [Google Scholar] [CrossRef]
- Tapas, A.R.; Sakerkar, D.M.; Kalde, R.B. Flavonoids as nutraceuticals: A review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Young, J.F.; Daneshvar, B.; Lauridsen, S.T.; Knuthsen, P.; Sandström, B.; Dragsted, L.O. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br. J. Nutr. 1999, 81, 447–455. [Google Scholar] [CrossRef]
- Sui, H.; Yu, Q.; Zhi, Y.; Geng, G.; Liu, H.; Xu, H. Effects of apigenin on the expression of angiotensin-converting enzyme 2 in kidney in spontaneously hypertensive rats. Wei Sheng Yan Jiu 2010, 39, 693–696. [Google Scholar]
- Tamayose, C.I.; Romoff, P.; Toyama, D.O.; Gaeta, H.H.; Costa, C.R.C.; Belchor, M.N.; Ortolan, B.D.; Velozo, L.S.M.; Kaplan, M.A.C.; Ferreira, M.J.P.; et al. Non-Clinical Studies for Evaluation of 8-C-Rhamnosyl Apigenin Purified from Peperomia obtusifolia against Acute Edema. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zu, Y.; Wang, L.; Deng, Y.; Wu, M.; Wang, H. Enhanced Solubility and Bioavailabitity of Apigenin via Preparation of Solid Dispersions of Mesoporous Silica Nanoparticles IRANIAN. J. Pharm. Res. 2019, 18, 168–182. [Google Scholar]
- El Shoubaky, G.A.; Abdel-Daim, M.M.; Mansour, M.H.; Salem, E.A. Salem Isolation and Identification of a Flavone Apigenin from Marine Red Alga Acanthophora spicifera with Antinociceptive and Anti-Inflammatory Activities. J. Exp. Neurosci. 2016, 10, 21–29. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.O.; Mendes, M.A. Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; an overview. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Nguyen, V.T. Potential uses and future perspectives of agricultural wastes. In Recovering Bioactive Compounds from Agricultural Wastes; Nguyen, V.T., Ed.; Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; p. 262. [Google Scholar]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-Based Compounds from Grape Seeds: A Biorefinery Approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef] [PubMed]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on merging Approaches. In Chemistry and Chemical Technologies in Waste Valorization; Topics in Current Chemistry Collections, Lin, C., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; D’Addezio, L.; Camilli, E.; Piccinelli, R.; Turrini, A.; Marletta, L.; Marconi, S.; Lucarini, M.; Lisciani, S.; Gabrielli, P.; et al. From Plant Compounds to Botanicals and Back: A Current Snapshot. Molecules 2018, 23, 1844. [Google Scholar] [CrossRef] [PubMed]
- USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 8 January 2019).
- USDA Database for the Flavonoid Content of Selected Foods Release 3.3; U.S. Department of Agriculture, Agricultural Service. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 10 January 2019).
- Phenol-Explorer—Database on Polyphenol Content in Foods. Available online: http://phenol-explorer.eu/ (accessed on 10 January 2019).
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- eBASIS—Bioactive Substances in Food Information System. Available online: http://ebasis.eurofir.org/Default.asp (accessed on 29 October 2018).
- Kiely, M.; Black, L.J.; Plumb, J.; Kroon, P.A.; Hollman, P.C.; Larsen, J.C.; Speijers, G.J.; Kapsokefalou, M.; Sheehan, D.; Gry, J.; et al. EuroFIR consortium. EuroFIR eBASIS: Application for health claims submissionsand evaluations. Eur. J. Clin. Nutr. 2010, 3, S101. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.; Pigat, S.; Bompola, F.; Cushen, M.; Pinchen, H.; Nørby, E.; Astley, S.; Lyons, J.; Kiely, M.; Finglas, P. eBASIS (Bioactive Substances in Food Information Systems) and Bioactive Intakes: Major Updates of the Bioactive Compound Composition and Beneficial Bioeffects Database and the Development of a Probabilistic Model to Assess Intakes in Europe. Nutrients 2017, 9, 320. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordonez, M.; Knox, C.; Llorach, R.; Eisner, R.; Cruz, J.; Neveu, V.; Wishart, D.; Manach, C.; et al. Phenol-Explorer 2.0: A major update of the Phenol-Explorer database integrating data on polyphenol metabolism and pharmacokinetics in humans and experimental animals. Database 2012, 2012, bas031. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Dragsted, L.O.; Gao, Q.; Praticò, G.; Manach, C.; Wishart, D.S.; Scalbert, A.; Feskens, E.J.M. Dietary and health biomarkers—Time for an update. Genes Nutr. 2017, 12, 24. [Google Scholar] [CrossRef]
- HMDB—Human Metabolome Database. Available online: www.hmdb.ca (accessed on 1 January 2019).
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0—The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- PhytoHub Database. Available online: www.phytohub.eu (accessed on 8 January 2019).
- Bento da Silva, A.; Giacomoni, F.; Pavot, B.; Fillâtre, Y.; Rothwell, J.A.; Sualdea, B.B.; Veyrat, C.; Garcia-Villalba, R.; Gladine, C.; Kopec, R.; et al. PhytoHub V1.4: A new release for the online database dedicated to food phytochemicals and their human metabolites. In Proceedings of the 1st International Conference on Food Bioactivities & Health, Norwich, UK, 13–15 September 2016. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. https://doi.org/10.3390/ijms20061305
Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, Lucarini M, Santini A, Souto EB, Novellino E, et al. The Therapeutic Potential of Apigenin. International Journal of Molecular Sciences. 2019; 20(6):1305. https://doi.org/10.3390/ijms20061305
Chicago/Turabian StyleSalehi, Bahare, Alessandro Venditti, Mehdi Sharifi-Rad, Dorota Kręgiel, Javad Sharifi-Rad, Alessandra Durazzo, Massimo Lucarini, Antonello Santini, Eliana B. Souto, Ettore Novellino, and et al. 2019. "The Therapeutic Potential of Apigenin" International Journal of Molecular Sciences 20, no. 6: 1305. https://doi.org/10.3390/ijms20061305
APA StyleSalehi, B., Venditti, A., Sharifi-Rad, M., Kręgiel, D., Sharifi-Rad, J., Durazzo, A., Lucarini, M., Santini, A., Souto, E. B., Novellino, E., Antolak, H., Azzini, E., Setzer, W. N., & Martins, N. (2019). The Therapeutic Potential of Apigenin. International Journal of Molecular Sciences, 20(6), 1305. https://doi.org/10.3390/ijms20061305