Characterization of Stackebrandtia nassauensis GH 20 Beta-Hexosaminidase, a Versatile Biocatalyst for Chitobiose Degradation
Abstract
1. Introduction
2. Results
2.1. Gene Cloning of SnHex
2.2. Protein Expression and Purification
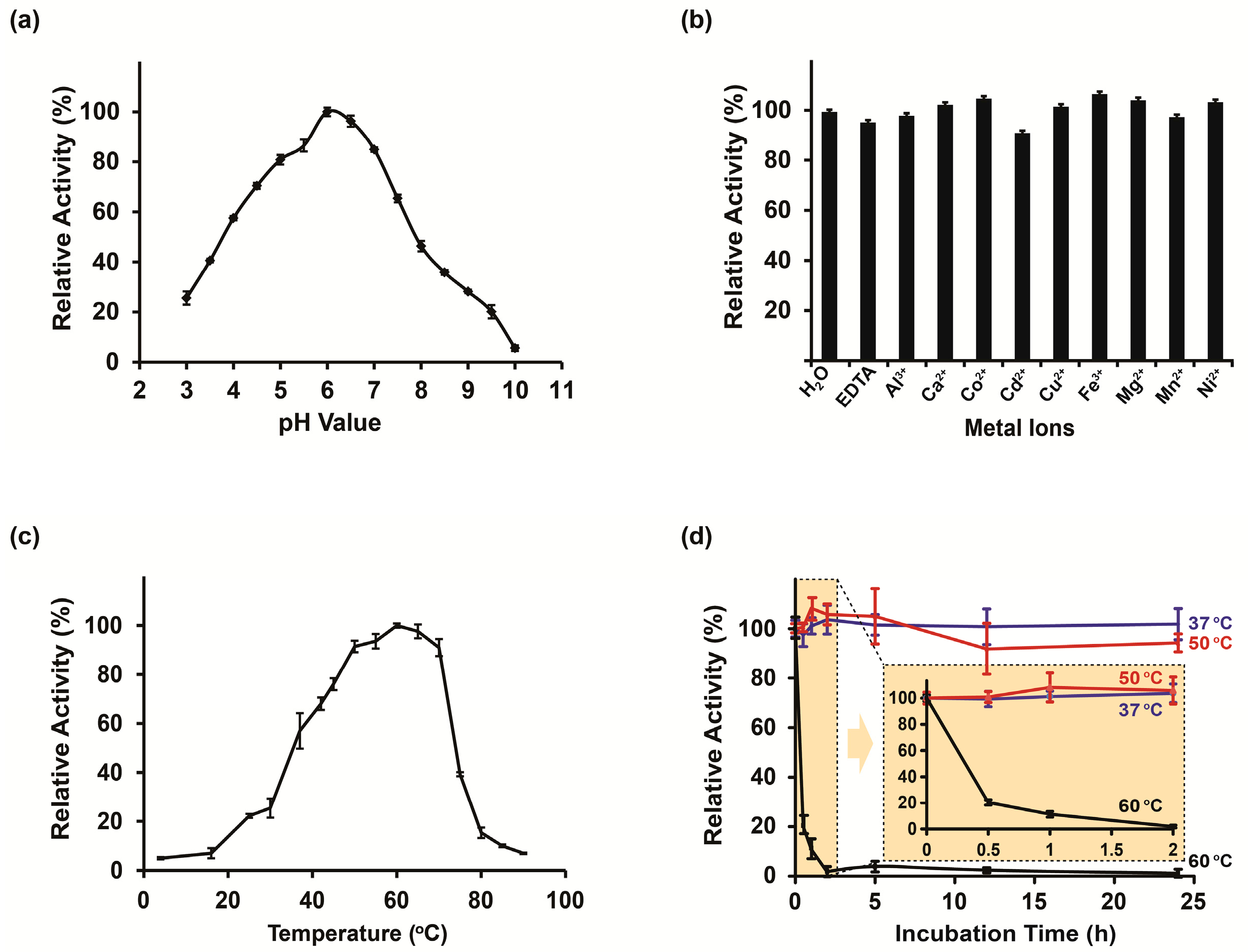
2.3. Biochemical Characterization
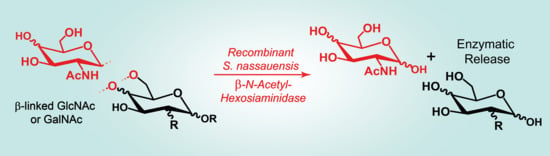
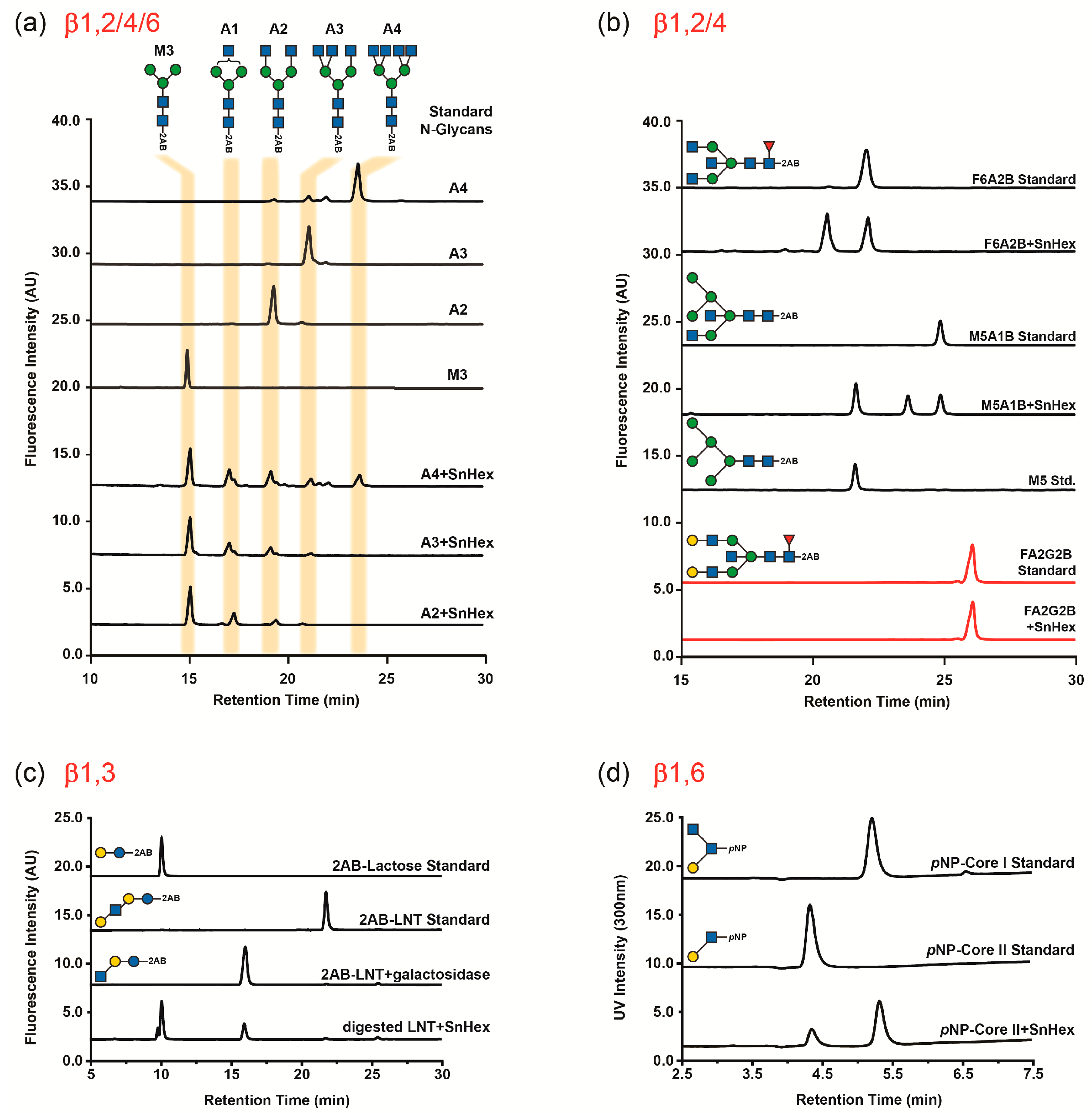
2.4. Substrate Specificity
2.5. Mutational Analysis
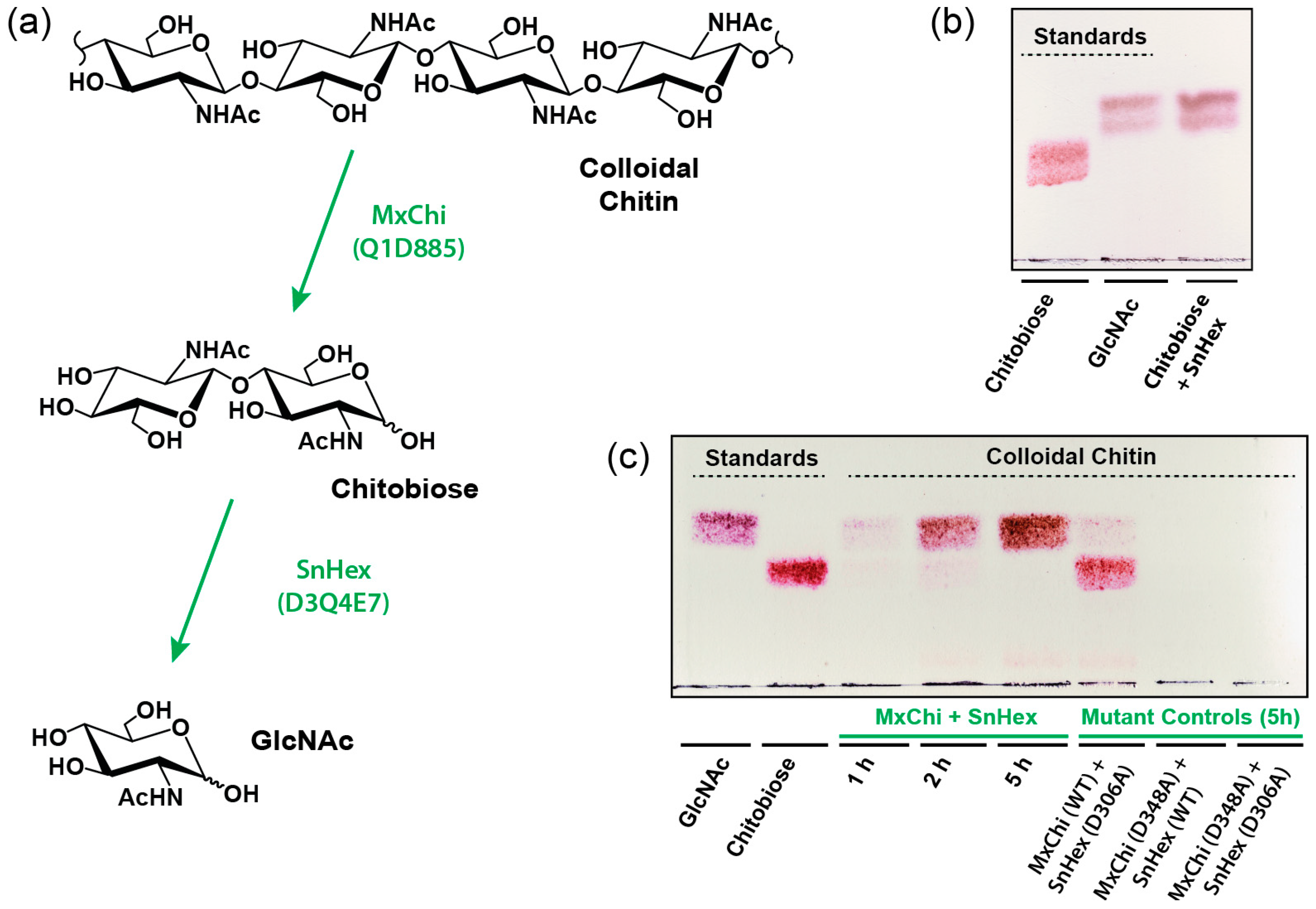
2.6. GlcNAc Generation from Colloidal Chitin
3. Discussion
3.1. Enzyme Characterization
3.2. Glycan Substrate Promiscuity
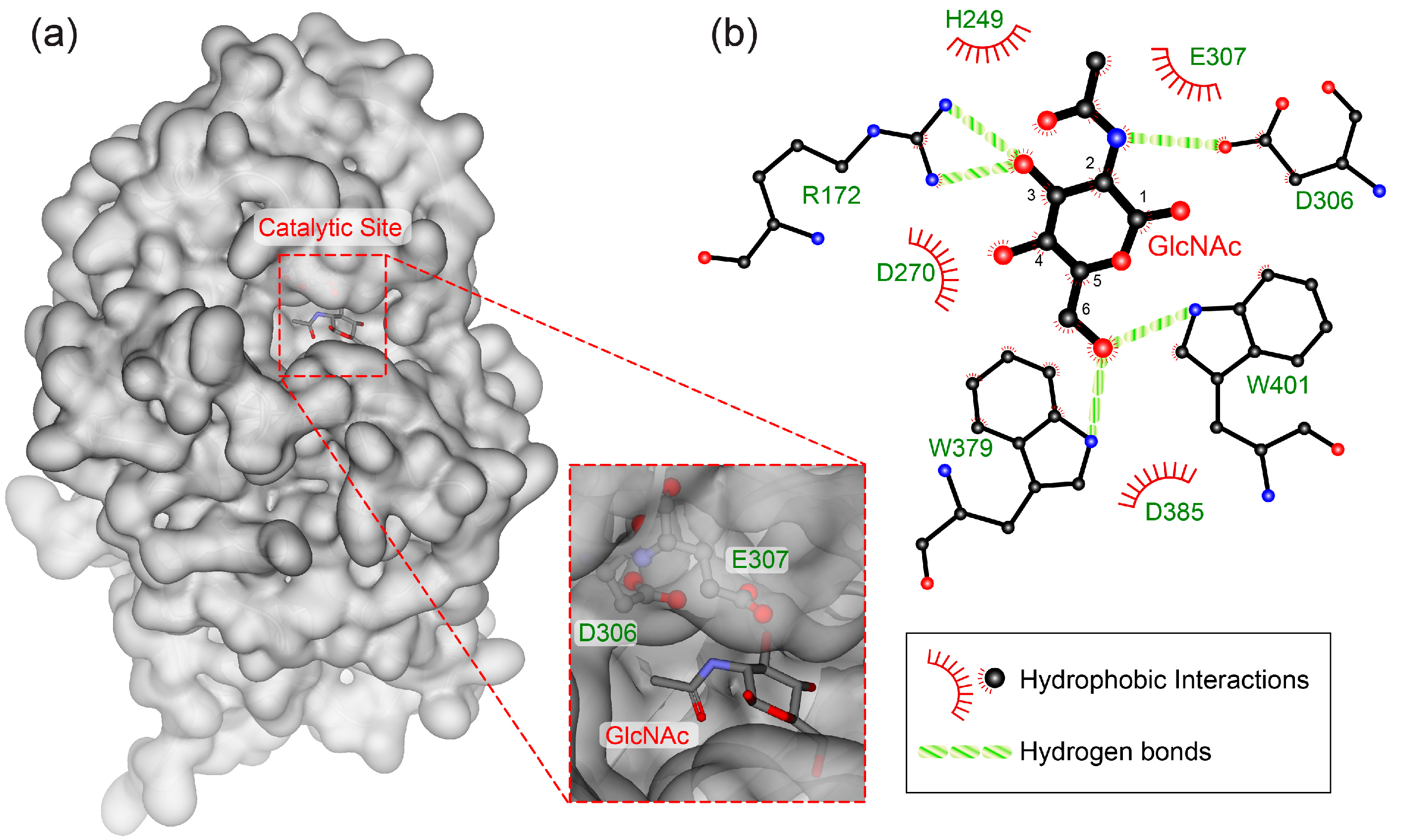
3.3. Molecular Mechanism
4. Materials and Methods
4.1. General
4.2. Gene Amplification, Construction of the Expression Vector, and Mutant Generation
4.3. Protein Expression and Purification
4.4. Activity Assays and Biochemical Characterization
4.5. Glycan Analysis
4.6. Homology Modeling Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCIP/NBT | 5-Bromo-4-chloro-3’-indolyphosphate/nitro-blue tetrazolium |
| CAZy | Carbohydrate-active enzymes database |
| dNTP | Deoxy-ribonucleotide triphosphate |
| DPA | Diphenylamine-aniline-phosphoric acid |
| DHB | 2,5-Dihydroxybenzoic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| ESI+ | Electron spray ionization positive mode |
| HPLC | High-performance liquid chromatography |
| HRMS | High-resolution mass spectrometry |
| GalNAc | N-acetyl-D-galactosamine |
| GlcNAc | N-acetyl-D-glucosamine |
| IPTG | Isopropyl-β-D-thiogalactopyranoside |
| LB | Lysogeny broth |
| LNT | Lacto-N-tetraose |
| MALDI-TOF MS | Matrix-assisted laser desorption/ ionization time of flight mass spectrometry |
| MES | 4-Morpholineethanesulfonic acid hydrate |
| OD | Optical density |
| OGT | O-GlcNAc transferase |
| PAGE | Polyacrylamide gel electrophoresis |
| PCR | Polymerase chain reaction |
| pNP | para-Nitrophenol |
| PUGNAc | O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate |
| SDS | Sodium dodecyl sulfate |
| TAMRA | 5-Carboxytetramethylrhodamine |
| UPLC | Ultra high-performance liquid chromatography |
References
- Slámová, K.; Bojarová, P.; Petrásková, L.; Křen, V. β-N-Acetylhexosaminidase: What’s in a name…? Biotechnol. Adv. 2010, 28, 682–693. [Google Scholar] [CrossRef]
- Alteen, M.G.; Oehler, V.; Nemčovičová, I.; Wilson, I.B.H.; Vocadlo, D.J.; Gloster, T.M. Mechanism of human nucleocytoplasmic hexosaminidase D. Biochemistry 2016, 55, 2735–2747. [Google Scholar] [CrossRef]
- Chang, C.-T.; Young, F.-P.; Chang, M.-H.; Sung, H.-Y. Purification and properties of β-N-acetylhexosaminidase from cabbage. Biochem. Mol. Biol. Int. 1998, 45, 371–380. [Google Scholar] [CrossRef]
- Yang, S.; Song, S.; Yan, Q.; Fu, X.; Jiang, Z.; Yang, X. Biochemical characterization of the first fungal glycoside hydrolyase family 3 β-N-Acetylglucosaminidase from Rhizomucor miehei. J. Agric. Food Chem. 2014, 62, 5181–5190. [Google Scholar] [CrossRef]
- Léonard, R.; Rendić, D.; Rabouille, C.; Wilson, I.B.H.; Préat, T.; Altmann, F. The Drosophila fused lobes gene encodes an N-acetylglucosaminidase involved in N-glycan processing. J. Biol. Chem. 2006, 281, 4867–4875. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.-Y.; Guo, R.-R.; Cai, Z.-P.; Hu, X.-C.; Chen, H.; Wei, S.; Voglmeir, J.; Liu, L. Cloning, purification and biochemical characterization of two β-N-acetylhexosaminidases from the mucin-degrading gut bacterium Akkermansia muciniphila. Carbohydr. Res. 2018, 457, 1–7. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Jin, L.; Sun, B.; Gu, G.; Lu, L.; Xiao, M. Efficient and regioselective synthesis of β-GalNAc/GlcNAc-Lactose by a bifunctional transglycosylating β-N-Acetylhexosaminidase from Bifidobacterium bifidum. Appl. Environ. Microbiol. 2016, 82, 5642–5652. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Nguyen, T.-H.; Křen, V.; Eijsink, V.G.H.; Haltrich, D.; Peterbauer, C.K. Heterologous expression and characterization of an N-Acetyl-β-D-hexosaminidase from Lactococcus lactis ssp. lactis IL1403. J. Agric. Food Chem. 2012, 60, 3275–3281. [Google Scholar] [CrossRef]
- Mayer, C.; Vocadlo David, J.; Mah, M.; Rupitz, K.; Stoll, D.; Warren, R.A.J.; Withers Stephen, G. Characterization of a β-N-acetylhexosaminidase and a β-N-acetylglucosaminidase/β-glucosidase from Cellulomonas fimi. FEBS J. 2006, 273, 2929–2941. [Google Scholar] [CrossRef]
- Tanaka, T.; Fukui, T.; Atomi, H.; Imanaka, T. Characterization of an exo-beta-D-glucosaminidase involved in a novel chitinolytic pathway from the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 2003, 185, 5175–5181. [Google Scholar] [CrossRef]
- Alves, D.R.; Gaudion, A.; Bean, J.E.; Perez Esteban, P.; Arnot, T.C.; Harper, D.R.; Kot, W.; Hansen, L.H.; Enright, M.C.; Jenkins, A.T.A. Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation. Appl. Environ. Microbiol. 2014, 80, 6694–6703. [Google Scholar] [CrossRef]
- Okada, T.; Ishiyama, S.; Sezutsu, H.; Usami, A.; Tamura, T.; Mita, K.; Fujiyama, K.; Seki, T. Molecular cloning and expression of two Novel β-N-Acetylglucosaminidases from silkworm Bombyx mori. Biosci. Biotechnol. Biochem. 2007, 71, 1626–1635. [Google Scholar] [CrossRef]
- Katta, S.; Ankati, S.; Podile, A.R. Chitooligosaccharides are converted to N-acetylglucosamine by N-acetyl-β-hexosaminidase from Stenotrophomonas maltophilia. FEMS Microbiol. Lett. 2013, 348, 19–25. [Google Scholar] [CrossRef]
- Mahuran, D.J. Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim. Biophys. Acta Mol. Basis Dis. 1999, 1455, 105–138. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, H.; Merdek, K.; Park, J.T. Molecular characterization of the beta-N-acetylglucosaminidase of Escherichia coli and its role in cell wall recycling. J. Bacteriol. 2000, 182, 4836–4840. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, C.; Su, S.; Luo, C.; Han, M. The role of β-hexosaminidase in peach (Prunus persica) fruit softening. Sci. Hortic. 2014, 169, 226–233. [Google Scholar] [CrossRef]
- Li, S.C.; Li, Y.T. Studies on the glycosidases of jack bean meal. J. Biol. Chem. 1970, 245, 5153. [Google Scholar]
- Plíhal, O.; Sklenár, J.; Kmonícková, J.; Man, P.; Pompach, P.; Havlícek, V.; Kren, V.; Bezouska, K. N-glycosylated catalytic unit meets O-glycosylated propeptide: Complex protein architecture in a fungal hexosaminidase. Biochem. Soc. Trans. 2004, 32, 764–765. [Google Scholar] [CrossRef]
- Loft, K.J.; Bojarová, P.; Slámová, K.; Křen, V.; Williams, S.J. Synthesis of sulfated glucosaminides for profiling substrate specificities of sulfatases and fungal β-N-acetylhexosaminidases. ChemBioChem 2009, 10, 565–576. [Google Scholar] [CrossRef]
- Robbins, P.W.; Overbye, K.; Albright, C.; Benfield, B.; Pero, J. Cloning and high-level expression of chitinase-encoding gene of Streptomyces plicatus. Gene 1992, 111, 69–76. [Google Scholar] [CrossRef]
- Yamashita, K.; Ohkura, T.; Yoshima, H.; Kobata, A. Substrate specificity of diplococcal β-N-acetylhexosaminidase, a useful enzyme for the structural studies of complex type asparagine-linked sugar chains. Biochem. Biophys. Res. Commun. 1981, 100, 226–232. [Google Scholar] [CrossRef]
- Labeda, D.P.; Kroppenstedt, R.M. Stackebrandtia nassauensis gen. nov., sp. nov. and emended description of the family Glycomycetaceae. Int. J. Syst. Evol. Microbiol. 2005, 55, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Munk, C.; Lapidus, A.; Copeland, A.; Jando, M.; Mayilraj, S.; Glavina Del Rio, T.; Nolan, M.; Chen, F.; Lucas, S.; Tice, H.; et al. Complete genome sequence of Stackebrandtia nassauensis type strain (LLR-40K-21T). Stand. Genomic Sci. 2009, 1, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Jungmann, N.A.; van Herwerden, E.F.; Hügelland, M.; Süssmuth, R.D. The Supersized Class III Lanthipeptide Stackepeptin Displays Motif Multiplication in the Core Peptide. ACS Chem. Biol. 2016, 11, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, P.; Xu, J.-H.; Zheng, G.-W. Identification of an imine reductase for asymmetric reduction of bulky dihydroisoquinolines. Org. Lett. 2017, 19, 3151–3154. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Wani, S.I.; Kumar, R.; Chhabra, R.; Chimni, S.S.; Sareen, D. Trigger factor assisted folding of the recombinant epoxide hydrolases identified from C. pelagibacter and S. nassauensis. Protein Expr. Purif. 2014, 104, 71–84. [Google Scholar] [CrossRef]
- Sumida, T.; Ishii, R.; Yanagisawa, T.; Yokoyama, S.; Ito, M. Molecular cloning and crystal structural analysis of a novel β-N-acetylhexosaminidase from Paenibacillus sp. TS12 capable of degrading glycosphingolipids. J. Mol. Biol. 2009, 392, 87–99. [Google Scholar] [CrossRef]
- Mark, B.L.; Vocadlo, D.J.; Knapp, S.; Triggs-Raine, B.L.; Withers, S.G.; James, M.N. Crystallographic evidence for substrate-assisted catalysis in a bacterial beta-hexosaminidase. J. Biol. Chem. 2001, 276, 10330. [Google Scholar] [CrossRef]
- Ramasubbu, N.; Thomas, L.; Ragunath, C.; Kaplan, J.B. Structural analysis of dispersin B, a biofilm-releasing glycoside hydrolase from the periodontopathogen Actinobacillus actinomycetemcomitans. J. Mol. Biol. 2005, 349, 475–486. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, H.; Liu, F.; Wu, Q.; Shen, X.; Yang, Q. Structural determinants of an insect beta-N-Acetyl-D-hexosaminidase specialized as a chitinolytic enzyme. J. Biol. Chem. 2011, 286, 4049–4058. [Google Scholar] [CrossRef]
- Rath, A.; Glibowicka, M.; Nadeau, V.G.; Chen, G.; Deber, C.M. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 1760–1765. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, X.; Kodaira, R.; Zhou, Z.; Shimosaka, M. Gene cloning, expression, and characterization of a second β-N-acetylglucosaminidase from the chitinolytic bacterium Aeromonas hydrophila strain SUWA-9. Biosci. Biotechnol. Biochem. 2008, 72, 492–498. [Google Scholar] [CrossRef]
- Sakai, K.; Narihara, M.; Kasama, Y.; Wakayama, M.; Moriguchi, M. Purification and characterization of thermostable beta-N-acetylhexosaminidase of Bacillus stearothermophilus CH-4 isolated from chitin-containing compost. Appl. Environ. Microbiol. 1994, 60, 2911–2915. [Google Scholar]
- Liu, F.; Liu, T.; Qu, M.; Yang, Q. Molecular and biochemical characterization of a novel β-N-acetyl-D-hexosaminidase with broad substrate-spectrum from the Aisan corn borer, Ostrinia furnacalis. Int. J. Biol. Sci. 2012, 8, 1085–1096. [Google Scholar] [CrossRef]
- Lisboa De Marco, J.; Valadares-Inglis, M.C.; Felix, C.R. Purification and characterization of an N-acetylglucosaminidase produced by a Trichoderma harzianum strain which controls Crinipellis perniciosa. Appl. Microbiol. Biotechnol. 2004, 64, 70–75. [Google Scholar] [CrossRef]
- Ferrara, M.C.; Cobucci-Ponzano, B.; Carpentieri, A.; Henrissat, B.; Rossi, M.; Amoresano, A.; Moracci, M. The identification and molecular characterization of the first archaeal bifunctional exo-β-glucosidase/N-acetyl-β-glucosaminidase demonstrate that family GH116 is made of three functionally distinct subfamilies. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 367–377. [Google Scholar] [CrossRef]
- Shigeta, S.; Matsuda, A.; Oka, S. Purification and characterization of a β-N-acetylhexosaminidase of Sea-Squirt. J. Biochem. 1982, 92, 163–172. [Google Scholar] [CrossRef]
- Souza, D.S.L.; Grossi-de-Sa, M.F.; Silva, L.P.; Franco, O.L.; Gomes-Junior, J.E.; Oliveira, G.R.; Rocha, T.L.; Magalhães, C.P.; Marra, B.M.; Grossi-de-Sa, M.; et al. Identification of a novel β-N-acetylhexosaminidase (Pcb-NAHA1) from marine Zoanthid Palythoa caribaeorum (Cnidaria, Anthozoa, Zoanthidea). Protein Expr. Purif. 2008, 58, 61–69. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Ryšlavá, H.; Kalendová, A.; Doubnerová, V.; Skočdopol, P.; Kumar, V.; Kukačka, Z.; Pompach, P.; Vaněk, O.; Slámová, K.; Bojarová, P.; et al. Enzymatic characterization and molecular modeling of an evolutionarily interesting fungal β-N-acetylhexosaminidase. FEBS J. 2011, 278, 2469–2484. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, T.; Liu, F.; Qu, M.; Qian, X. A novel β-N-acetyl-D-hexosaminidase from the insect Ostrinia furnacalis (Guenée). FEBS J. 2008, 275, 5690–5702. [Google Scholar] [CrossRef] [PubMed]
- Tomiya, N.; Narang, S.; Park, J.; Abdul-Rahman, B.; Choi, O.; Singh, S.; Hiratake, J.; Sakata, K.; Betenbaugh, M.J.; Palter, K.B.; et al. Purification, characterization, and cloning of a Spodoptera frugiperda Sf9 β-N-acetylhexosaminidase that hydrolyzes terminal N-acetylglucosamine on the N-Glycan core. J. Biol. Chem. 2006, 281, 19545–19560. [Google Scholar] [CrossRef]
- Litzinger, S.; Fischer, S.; Polzer, P.; Diederichs, K.; Welte, W.; Mayer, C. Structural and kinetic analysis of Bacillus subtilis N-acetylglucosaminidase reveals a unique Asp-His dyad mechanism. J. Biol. Chem. 2010, 285, 35675–35684. [Google Scholar] [CrossRef] [PubMed]
- Meekrathok, P.; Bürger, M.; Porfetye, A.T.; Vetter, I.R.; Suginta, W. Expression, purification, crystallization and preliminary crystallographic analysis of a GH20 β-N-acetylglucosaminidase from the marine bacterium Vibrio harveyi. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, M.M.; Kulinich, A.; Yao, H.L.; Ma, H.Y.; Martínez, J.E.R.; Duan, X.C.; Chen, H.; Cai, Z.P.; Flitsch, S.L.; et al. Biochemical characterisation of the neuraminidase pool of the human gut symbiont Akkermansia muciniphila. Carbohydr. Res. 2015, 415, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Laborda, P.; Lu, A.-M.; Duan, X.-C.; Ma, H.-Y.; Liu, L.; Voglmeir, J. N-acetylglucosamine 2-epimerase from Pedobacter heparinus: First experimental evidence of a deprotonation/reprotonation Mechanism. Catalysts 2016, 6, 212. [Google Scholar] [CrossRef]
- Mahuku, G.S. A simple extraction method suitable for PCR-based analysis of plant, fungal, and bacterial DNA. Plant Mol. Biol. Rep. 2004, 22, 71–81. [Google Scholar] [CrossRef]
- Wattam, A.R.; Abraham, D.; Dalay, O.; Disz, T.L.; Driscoll, T.; Gabbard, J.L.; Gillespie, J.J.; Gough, R.; Hix, D.; Kenyon, R.; et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 2014, 42, D581–D591. [Google Scholar] [CrossRef]
- Guo, B.-S.; Zheng, F.; Crouch, L.; Cai, Z.-P.; Wang, M.; Bolam, D.N.; Liu, L.; Voglmeir, J. Cloning, purification and biochemical characterisation of a GH35 beta-1,3/beta-1,6-galactosidase from the mucin-degrading gut bacterium Akkermansia muciniphila. Glycoconj. J. 2018, 35, 255–263. [Google Scholar] [CrossRef]
- Du, Y.-M.; Zheng, S.-L.; Liu, L.; Voglmeir, J.; Yedid, G. Analysis of N-glycans from Raphanus sativus cultivars using PNGase H+. JoVE 2018, e57979. [Google Scholar] [CrossRef]
- Wang, T.; Hu, X.C.; Cai, Z.P.; Voglmeir, J.; Liu, L. Qualitative and quantitative analysis of carbohydrate modification on glycoproteins from seeds of Ginkgo biloba. J. Agric. Food Chem. 2017, 65, 7669–7679. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Laborda, P.; Lu, A.-M.; Wang, M.; Duan, X.-C.; Liu, L.; Voglmeir, J. Chemo-enzymatic approach to access diastereopure α-substituted GlcNAc derivatives. J. Carbohydr. Chem. 2016, 35, 423–434. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.S.; Eramian, D.; Shen, M.-Y.; Pieper, U.; Sali, A. Comparative protein structure modeling using modeller. Curr. Protoc. Bioinform. 2006, 15, 5.6.1–5.6.30. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]

| pNP-β-GlcNAc | pNP-β-GalNAc | ||||||
|---|---|---|---|---|---|---|---|
| Vmax (mM·min−1·mg−1) | KM (mM) | kcat (min−1) | kcat/KM (min−1mM−1) | Vmax (mM·min−1·mg−1) | KM (mM) | kcat (min−1) | kcat/KM (min−1mM−1) |
| 0.057 ± 0.004 | 2.47 ± 0.05 | 55.05 ± 3.69 | 22.3 ± 2.0 | 0.044 ± 0.003 | 3.29 ± 0.32 | 42.72 ± 2.86 | 13.0 ± 2.3 |
| SnHex Variant | Relative Activity (%) | |
|---|---|---|
| pNP-β-GlcNAc | A2 N-Glycan Standard | |
| Wild-Type | 100 ± 2.1 | 100 |
| D306A | ND | ND |
| D306E | 4.2 ± 0.5 | ND |
| D306N | ND | ND |
| E307A | 2.0 ± 0.2 | ND |
| E307D | 8.6 ± 0.2 | ND |
| E307Q | 1.5 ± 0.2 | ND |
| D306E/E307D | 2.7 ± 0.3 | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zheng, F.; Wang, T.; Lyu, Y.-M.; Alteen, M.G.; Cai, Z.-P.; Cui, Z.-L.; Liu, L.; Voglmeir, J. Characterization of Stackebrandtia nassauensis GH 20 Beta-Hexosaminidase, a Versatile Biocatalyst for Chitobiose Degradation. Int. J. Mol. Sci. 2019, 20, 1243. https://doi.org/10.3390/ijms20051243
Wang M, Zheng F, Wang T, Lyu Y-M, Alteen MG, Cai Z-P, Cui Z-L, Liu L, Voglmeir J. Characterization of Stackebrandtia nassauensis GH 20 Beta-Hexosaminidase, a Versatile Biocatalyst for Chitobiose Degradation. International Journal of Molecular Sciences. 2019; 20(5):1243. https://doi.org/10.3390/ijms20051243
Chicago/Turabian StyleWang, Meng, Feng Zheng, Ting Wang, Yong-Mei Lyu, Matthew G. Alteen, Zhi-Peng Cai, Zhong-Li Cui, Li Liu, and Josef Voglmeir. 2019. "Characterization of Stackebrandtia nassauensis GH 20 Beta-Hexosaminidase, a Versatile Biocatalyst for Chitobiose Degradation" International Journal of Molecular Sciences 20, no. 5: 1243. https://doi.org/10.3390/ijms20051243
APA StyleWang, M., Zheng, F., Wang, T., Lyu, Y.-M., Alteen, M. G., Cai, Z.-P., Cui, Z.-L., Liu, L., & Voglmeir, J. (2019). Characterization of Stackebrandtia nassauensis GH 20 Beta-Hexosaminidase, a Versatile Biocatalyst for Chitobiose Degradation. International Journal of Molecular Sciences, 20(5), 1243. https://doi.org/10.3390/ijms20051243