Novichoks: The Dangerous Fourth Generation of Chemical Weapons
Abstract
:1. Introduction
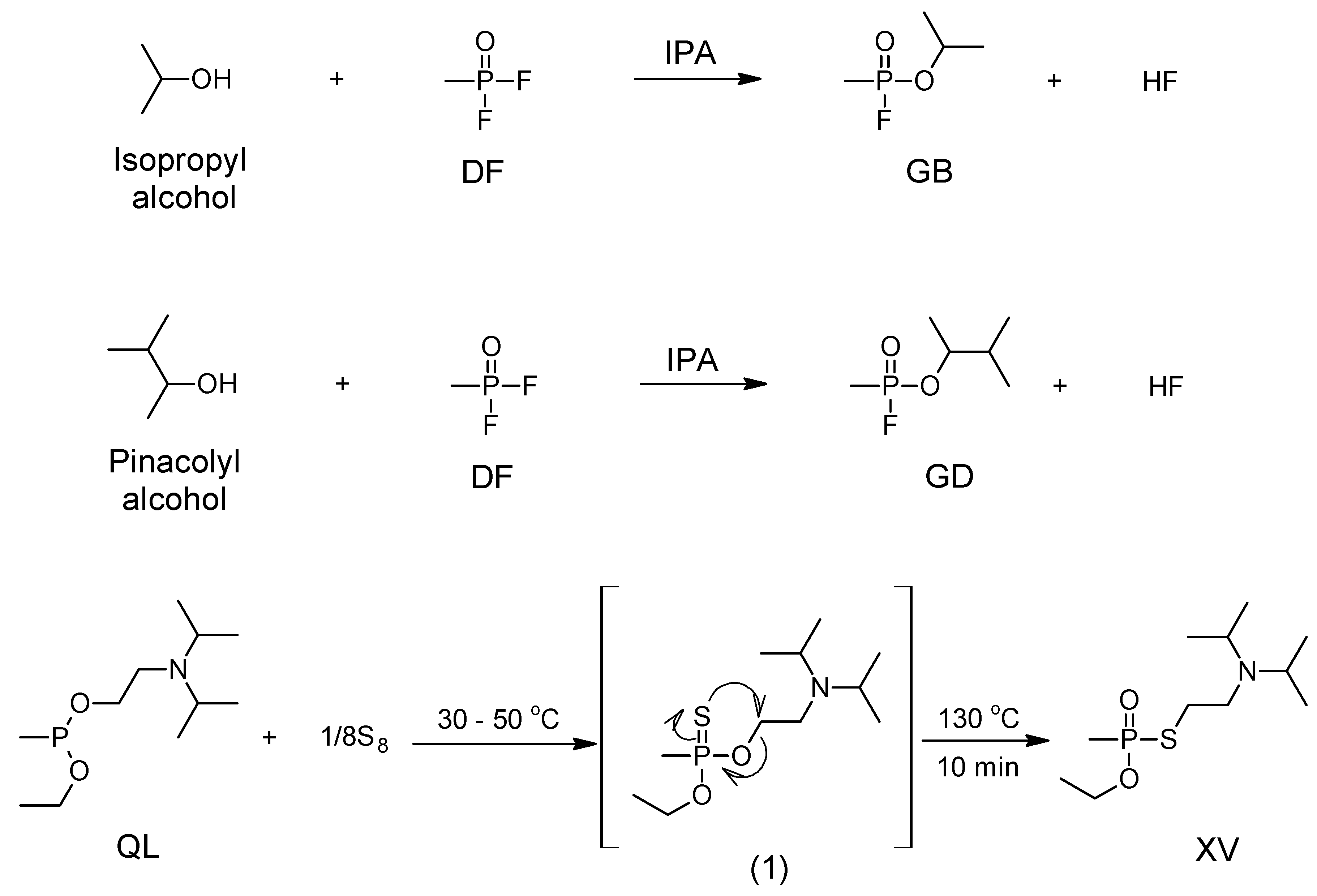
2. Development of the A-Series Nerve Agents
3. Binary Weapons
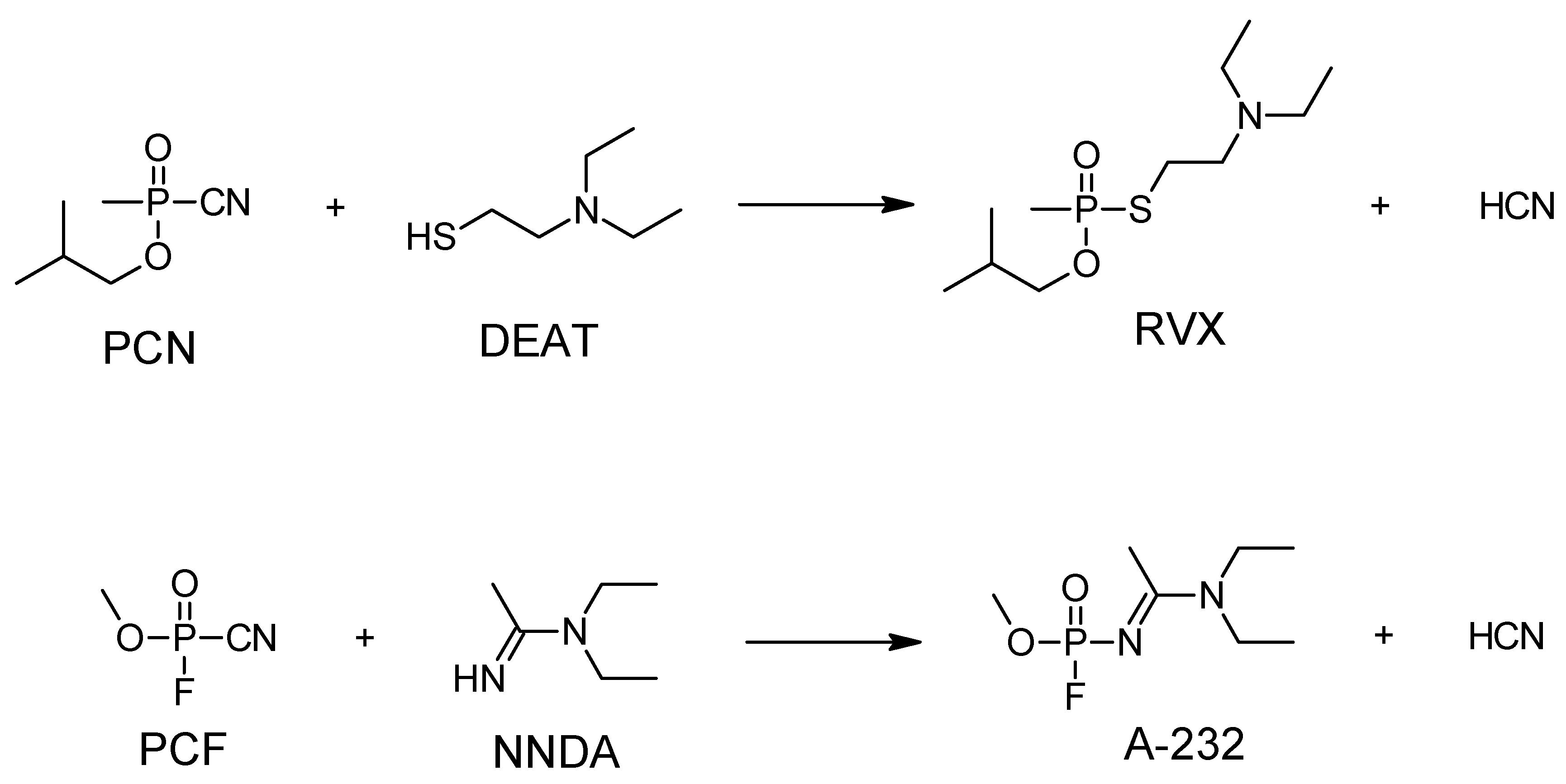
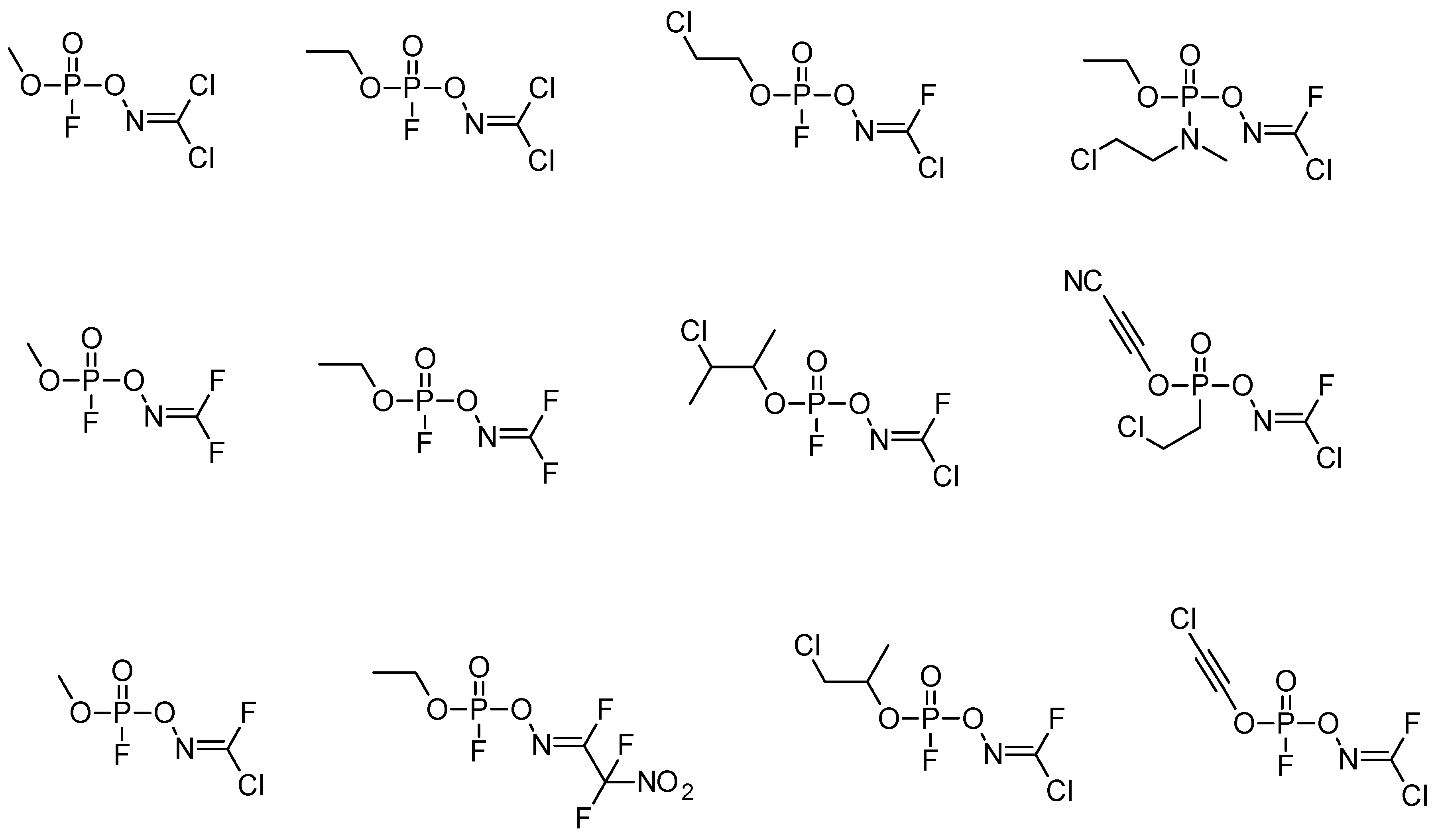
4. Other Possible Chemicals in the A-Series
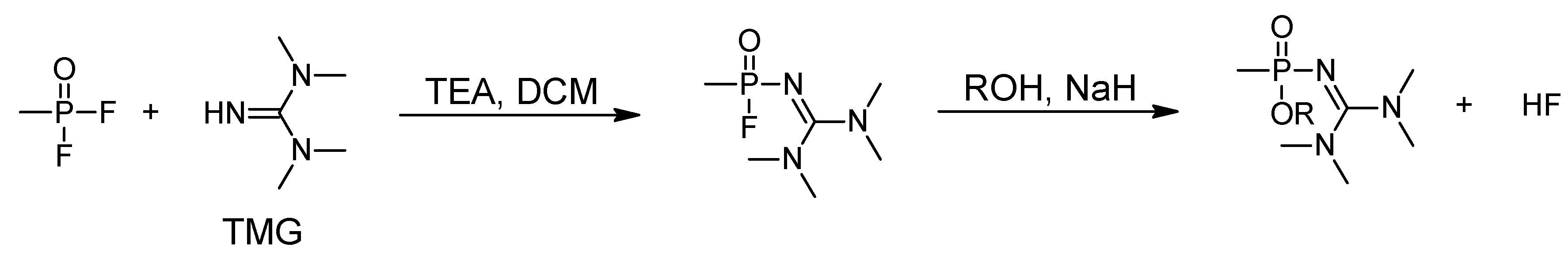
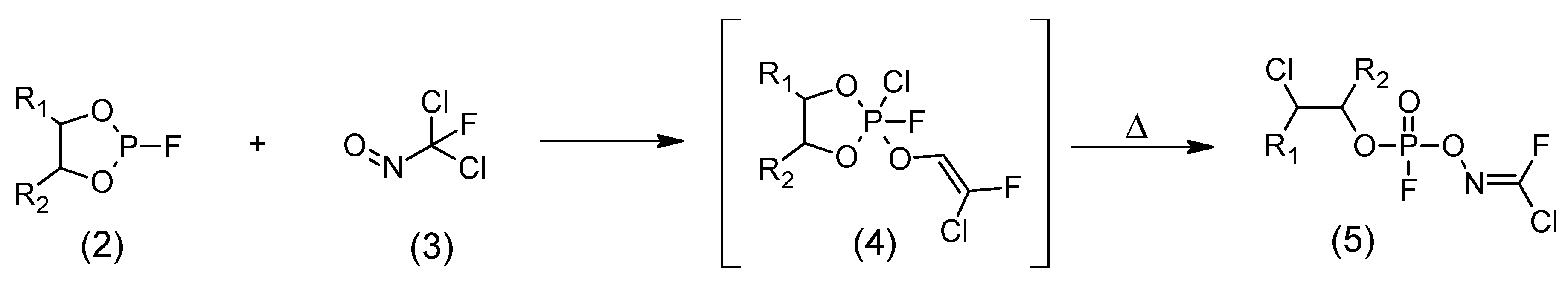
5. Synthesis of A-series Nerve Agents
6. Physico-Chemical Properties of A-series Nerve Agents
7. Toxicity of A-series Nerve Agents
8. Protection, Treatment, and Decontamination of A-Series Agents
9. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| CW | Chemical Weapons |
| CWC | Chemical Weapons Convention |
| DEAT | 2-(diethylamino)ethanethiol |
| DF | Methylphosphonic difluoride |
| GA | Tabun |
| GB | Sarin |
| GB-2 | Binary GB |
| GD | Soman |
| GD-2 | Binary GD |
| GOSNIIOKhT | Institute for Organic Chemistry and Technology in Moscow |
| IJMS | International Journal of Molecular Sciences |
| IPA | Isopropylamine |
| LCt50 | Median Lethal Concentration |
| LD50 | Median Lethal Dose |
| OCAD | OPCW Central Analytical Database |
| OP | Organophosphate |
| OPCW | Organization for Prevention of Chemical Weapons |
| PCF | Methyl phosphorocyanidofluoridate |
| PCN | 2-methylpropyl methylphosphonocyanidate |
| QL | O-Ethyl O-2-diisopropylaminoethyl methylphosphonite |
| NNDA | N,N-diethyl-2-iminopropan-1-amine |
| RVX | Russian VX |
| SLUDGEM | Salivation, Lachrymation, Urination, Defecation, Gastrointestinal, Emesis and Miosis |
| TEG | 1,1,3,3 tetraethylguanidine |
| TMG | 1,1,3,3 tetramethylguanidine |
| UK | United Kingdom |
| USA | United States of America |
| USSR | Union of Soviet Socialist Republics |
| VX-2 | Binary VX |
References
- Nepovimova, E.; Kuca, K. The History of Poisoning: From Ancient Times Until Modern ERA. Arch. Toxicol. 2019, 93, 11–24. [Google Scholar] [PubMed]
- Pitschmann, V. Vladimír Overall View of Chemical and Biochemical Weapons. Toxins 2014, 6, 1761–1784. [Google Scholar] [PubMed]
- Mirzayanov, V.S. State Secrets: An Insider’s Chronicle of the Russian Chemical Weapons Program; Outskirts Press, Inc.: Parker, CO, USA, 2009; ISBN 1432725661. [Google Scholar]
- Croddy, E.A.; Wirtz, J.J.; Larsen, J.A.; Kay, D.; Barbara, S.; Denver, C.; Oxford, C. Weapons of Mass Destruction An Encyclopedia of Worldwide Policy, Technology, and History Volume I: Chemical and Biological Weapons; ABC-CLIO: Santa Barbara, CA, USA, 2005. [Google Scholar]
- Velez-Daubon, L.I.; Benitez, F.L. CBRNE—Nerve Agents, Binary—GB2, VX2: Background, Pathophysiology, Epidemiology. Available online: https://emedicine.medscape.com/article/831901-overview (accessed on 6 January 2019).
- Gupta, R.C.; Ramesh, C. Handbook of Toxicology of Chemical Warfare Agents; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780128001592. [Google Scholar]
- Halámek, E.; Kobliha, Z. Potenciální Bojové Chemické Látky. Chem. Listy 2011, 105, 323–333. [Google Scholar]
- Halamek, E. Poslední generace chemických zbraní. Vojenské Rozhledy 2008, 17, 137–146. [Google Scholar]
- Vásárhelyi, G.; Földi, L. History of Russia’s Chemical Weapons. Acad. Appl. Res. Mil. Sci. 2007, 6, 135–146. [Google Scholar]
- Costanzi, S.; Machado, J.-H.; Mitchell, M. Nerve Agents: What They Are, How They Work, How to Counter Them. ACS Chem. Neurosci. 2018, 9, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Chai, P.R.; Hayes, B.D.; Erickson, T.B.; Boyer, E.W. Novichok agents: A historical, current, and toxicological perspective. Toxicol. Commun. 2018, 2, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, S.L. Compendium of Chemical Warfare Agents; Springer: Berlin, Germany, 2007; ISBN 9780387692609. [Google Scholar]
- Patočka, J. Novichok agents—Mysterious poisonous substances from the cold war period. Mil. Med. Sci. Lett. 2018, 87, 92–94. [Google Scholar] [CrossRef]
- Ellison, D.H. Handbook of Chemical and Biological Warfare Agents; CRC Press: Hoboken, NJ, USA, 2008; ISBN 9780849314346. [Google Scholar]
- Wagner, M.J.; Promes, S.B. Last Minute Emergency Medicine; McGraw-Hill: New York, NY, USA, 2007; ISBN 0071459626. [Google Scholar]
- Hosseini, S.E.; Saeidian, H.; Amozadeh, A.; Naseri, M.T.; Babri, M. Fragmentation pathways and structural characterization of organophosphorus compounds related to the Chemical Weapons Convention by electron ionization and electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Nepovimova, E.; Kuca, K. Chemical warfare agent novichok—Mini-review of available data. Food Chem. Toxicol. 2018, 121, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Korabecny, J.; Soukup, O.; Dolezal, R.; Spilovska, K.; Nepovimova, E.; Andrs, M.; Nguyen, T.; Jun, D.; Musilek, K.; Kucerova-Chlupacova, M.; et al. From Pyridinium-based to Centrally Active Acetylcholinesterase Reactivators. Mini-Rev. Med. Chem. 2014, 14, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kuca, K.; Jun, D.; Musilek, K.; Pohanka, M.; Karasova, J.; Soukup, O. Prophylaxis and Post-exposure Treatment of Intoxications Caused by Nerve Agents and Organophosphorus Pesticides. Mini-Rev. Med. Chem. 2013, 13, 2102–2115. [Google Scholar] [CrossRef] [PubMed]
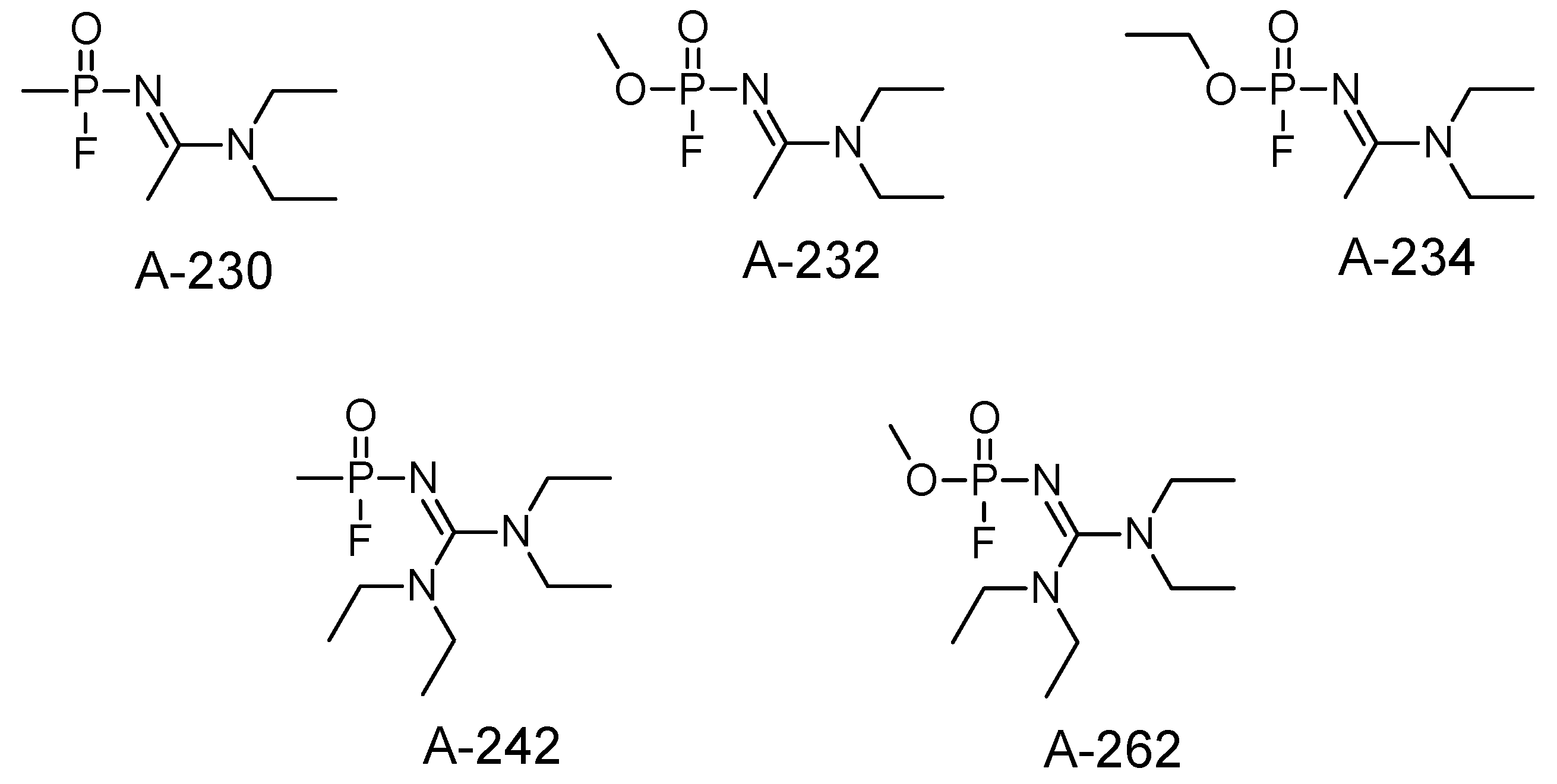
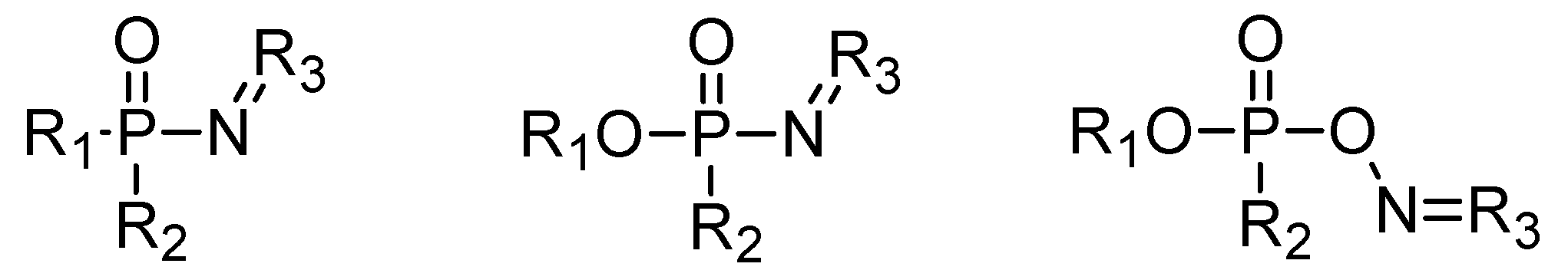
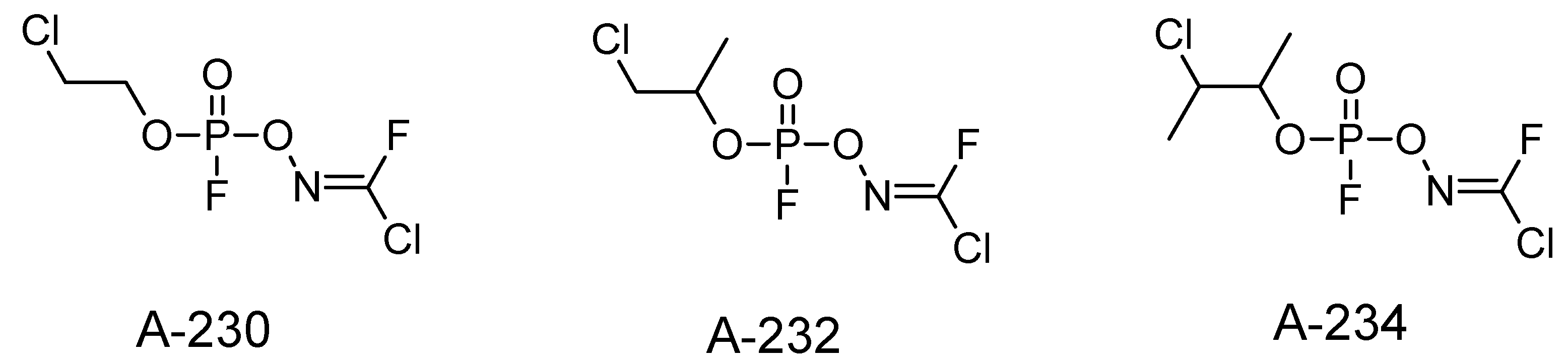
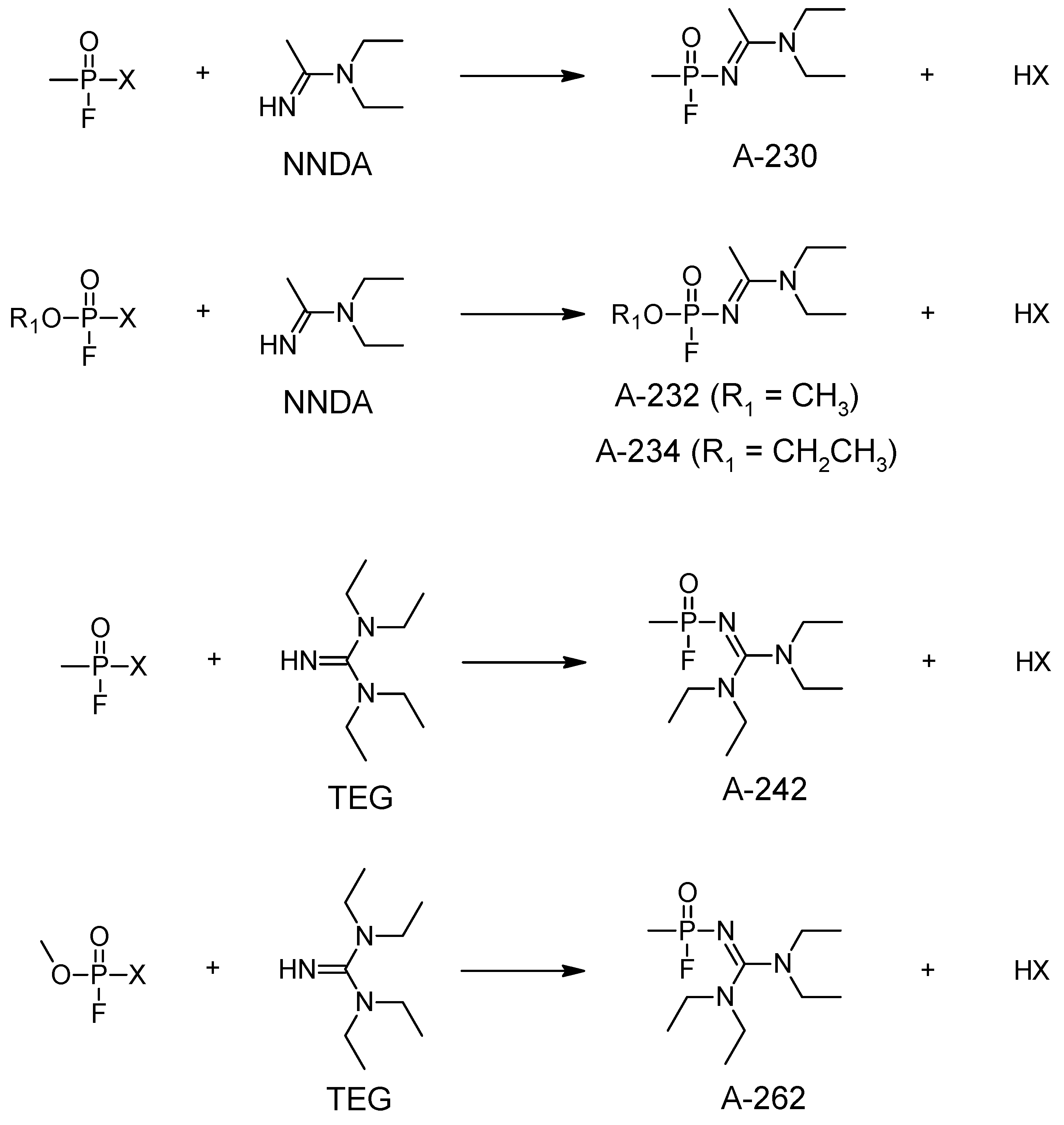
| Nerve Agent | Melting Point (°C) | Boiling Point (°C) | Vapor Pressure (Pa) | Solubility at 25 °C (g/L) | Log P |
|---|---|---|---|---|---|
| Tabun | −50 | 240 | 0.057 | 9.8 | − |
| Sarin | −57 | 147 | 2.9 | Miscible | − |
| Soman | −42 | 167–200 | 0.4 | 2.1 | − |
| VX | Below −51 | 298 | 0.0007 | Miscible <2.4 °C | − |
| A−230 | 5.56 | 259.92 | 2.13 | 4.826 | 2.14 |
| A−232 | 5.65 | 266.59 | 1.48 | 1.775 | 2.55 |
| A−234 | 3.06 | 264.11 | 1.7 | 0.6512 | 2.97 |
| A−242 | 21.46 | 284.85 | 0.579 | 1000 | 0.45 |
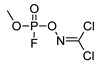 | −12.33 | 218.93 | 18.5 | 16.74 | 1.76 |
 | −35.05 | 191.37 | 73.8 | 37.64 | 1.47 |
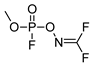 | −58.28 | 162.02 | 304 | 84.02 | 1.18 |
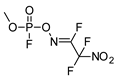 | 48.34 | 256.81 | 1.53 | 9.940 | 1.34 |
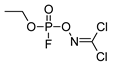 | −1.27 | 237.51 | 7.04 | 5.374 | 2.12 |
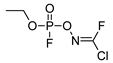 | −23.2 | 211.21 | 27.4 | 12.15 | 1.83 |
 | −46.48 | 183.14 | 110 | 27.29 | 1.54 |
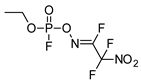 | 58.85 | 273.49 | 0.509 | 3.157 | 1.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franca, T.C.C.; Kitagawa, D.A.S.; Cavalcante, S.F.d.A.; da Silva, J.A.V.; Nepovimova, E.; Kuca, K. Novichoks: The Dangerous Fourth Generation of Chemical Weapons. Int. J. Mol. Sci. 2019, 20, 1222. https://doi.org/10.3390/ijms20051222
Franca TCC, Kitagawa DAS, Cavalcante SFdA, da Silva JAV, Nepovimova E, Kuca K. Novichoks: The Dangerous Fourth Generation of Chemical Weapons. International Journal of Molecular Sciences. 2019; 20(5):1222. https://doi.org/10.3390/ijms20051222
Chicago/Turabian StyleFranca, Tanos C. C., Daniel A. S. Kitagawa, Samir F. de A. Cavalcante, Jorge A. V. da Silva, Eugenie Nepovimova, and Kamil Kuca. 2019. "Novichoks: The Dangerous Fourth Generation of Chemical Weapons" International Journal of Molecular Sciences 20, no. 5: 1222. https://doi.org/10.3390/ijms20051222
APA StyleFranca, T. C. C., Kitagawa, D. A. S., Cavalcante, S. F. d. A., da Silva, J. A. V., Nepovimova, E., & Kuca, K. (2019). Novichoks: The Dangerous Fourth Generation of Chemical Weapons. International Journal of Molecular Sciences, 20(5), 1222. https://doi.org/10.3390/ijms20051222