The Usefulness of Immunohistochemistry in the Differential Diagnosis of Lesions Originating from the Myometrium
Abstract
1. Introduction
1.1. Epidemiology and Etiopathology of Myometrial Neoplasms
1.2. Differentiation and Advanced Pathological Diagnostics in Myometrial Neoplasm
1.3. Classification of Uterine Smooth Muscle Tumors
2. Methodology of Obtaining Data and Data Analysis
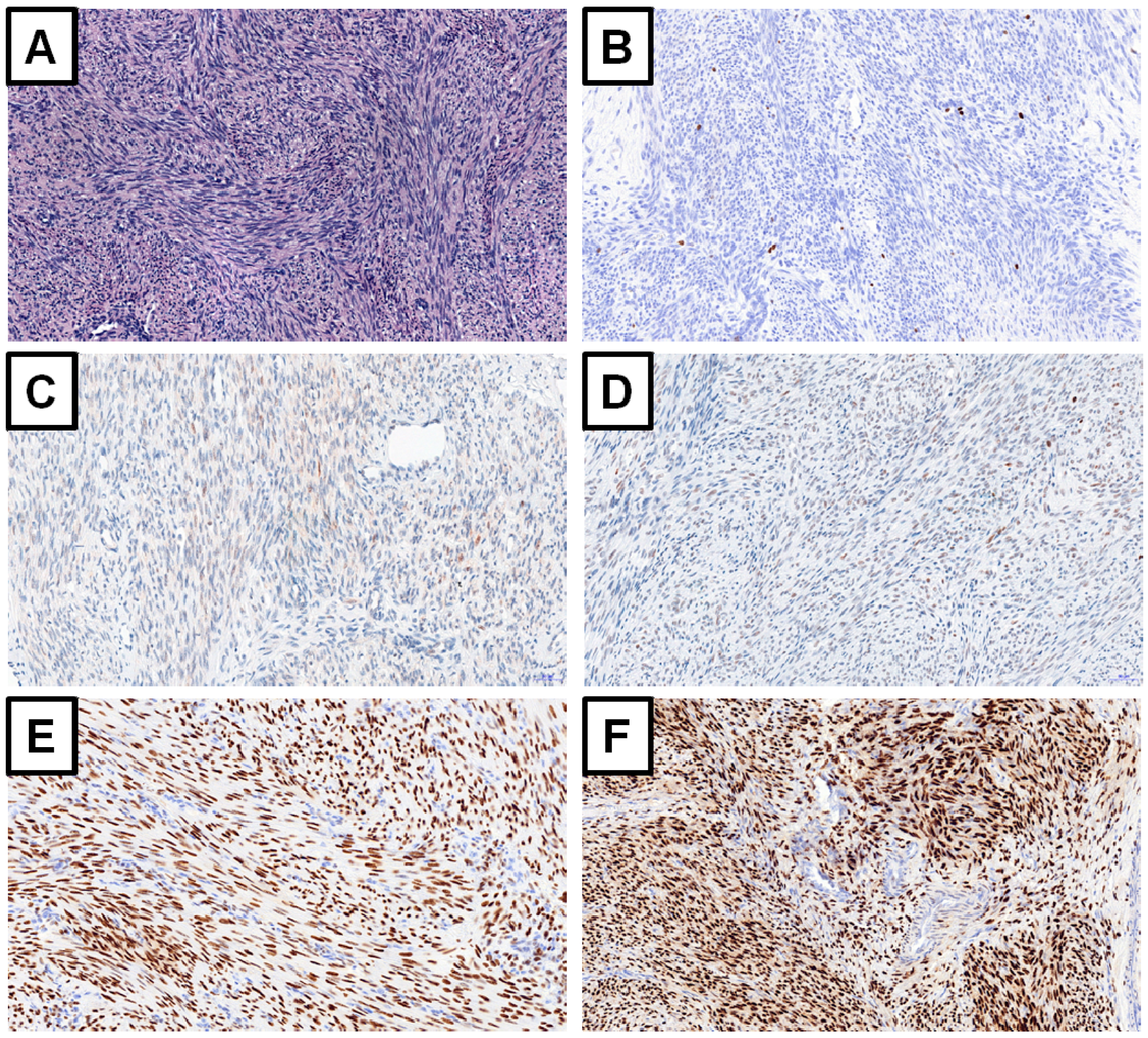
3. Available Immunohistochemical Markers
3.1. Markers with Strong Evidence
3.1.1. Ki-67
3.1.2. Tumor Protein p53 (p53, Cellular Tumor Antigen p53)
3.1.3. p16 Protein (Cyclin-Dependent Kinase Inhibitor 2A, Multiple Tumor Suppressor 1)
3.1.4. Proliferating Cell Nuclear Antigen (PCNA)
3.1.5. Bcl-2 Protein (B-Cell Lymphoma 2)
3.1.6. Other Markers
3.1.7. Non-Myometrial Tumors in Differential Diagnosis
3.1.8. Steroid Receptors
3.1.9. Future Directions
3.1.10. Summary
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AR | androgen receptor |
| Bcl-2 | B-cell lymphoma 2 |
| COMT | catechol-O-methyltransferase |
| EGCG | epigallocatechin gallate |
| EGFR | epidermal growth factor receptor |
| ER | estrogen receptor |
| ESN | endometrial stromal nodule |
| ESS | endometrial stromal sarcoma |
| GnRH | gonadotropin-releasing hormone |
| HE | hematoxylin and eosin staining |
| HG-ESS | high-grade endometrial stromal sarcoma |
| IGF | insulin-like growth factor |
| IHC | immunohistochemistry |
| LDH | lactate dehydrogenase |
| LG-ESS | low-grade endometrial stromal sarcoma |
| LM | leiomyoma |
| LMS | leiomyosarcoma |
| MCM | minichromosome maintenance protein |
| MITF | microphthalmia associated transcription factor |
| MMP | matrix metalloproteinase |
| MVP | major vault protein |
| PCNA | proliferating cell nuclear antigen |
| PEComa | perivascular epithelioid cell tumor |
| PR | progesterone receptor |
| SMA | smooth muscle actin |
| SPRM | selective progesterone receptor modulator |
| STUMP | smooth muscle tumor of uncertain malignant potential |
| TGF | transforming growth factor |
| TNF-α | tumor necrosis factor alpha |
| TOP-2A | DNA topoisomerase 2-alpha |
| TSP1 | thrombospondin 1 |
| UPA | ulipristal acetate |
| WHO | World Health Organization |
| VEGF | vascular endothelial growth factor |
References
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG 2017, 124, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Palmer, J.R.; Stewart, E.A.; Rosenberg, L. Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women’s Health Study. Obstet. Gynecol. 2005, 105, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.F.; Patel, A. The frequency of uterine leiomyomas. Am. J. Clin. Pathol. 1990, 94, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Oi, Y.; Katayama, K.; Hirata, G.; Ishidera, Y.; Yoshida, H.; Shigeta, H. Significance of postmenopausal uterine leiomyomas: Focus on variants. J. Obstet. Gynaecol. Res. 2018, 44, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Wlodarczyk, M.; Wrzosek, M.; Meczekalski, B.; Nowicka, G.; Lukaszuk, K.; Ciebiera, M.; Slabuszewska-Jozwiak, A.; Jakiel, G. Role of transforming growth factor beta in uterine fibroid biology. Int. J. Mol. Sci. 2017, 18, 2435. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Greco, S.; Janjusevic, M.; Ciavattini, A.; Giannubilo, S.R.; D’Adderio, A.; Biagini, A.; Fiorini, R.; Castellucci, M.; Ciarmela, P. Growth factors and pathogenesis. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Protic, O.; Toti, P.; Islam, M.S.; Occhini, R.; Giannubilo, S.R.; Catherino, W.H.; Cinti, S.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016, 364, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Myers, E.R.; Stewart, E. Uterine fibroids: Burden and unmet medical need. Semin. Reprod. Med. 2017, 35, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Di Martino, M.; Candiani, M.; Vigano, P. Dietary components and uterine leiomyomas: A review of published data. Nutr. Cancer 2015, 67, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Lukaszuk, K.; Meczekalski, B.; Ciebiera, M.; Wojtyla, C.; Slabuszewska-Jozwiak, A.; Jakiel, G. Alternative oral agents in prophylaxis and therapy of uterine fibroids-an up-to-date review. Int. J. Mol. Sci. 2017, 18, 2586. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Uterine fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Makinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M.; et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Moroni, R.M.; Vieira, C.S.; Ferriani, R.A.; Reis, R.M.; Nogueira, A.A.; Brito, L.G. Presentation and treatment of uterine leiomyoma in adolescence: A systematic review. BMC Womens Health 2015, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Tolvanen, J.; Uimari, O.; Ryynanen, M.; Aaltonen, L.A.; Vahteristo, P. Strong family history of uterine leiomyomatosis warrants fumarate hydratase mutation screening. Hum. Reprod. 2012, 27, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Ohara, N.; Wang, J.; Matsuo, H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum. Reprod. Update 2004, 10, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Chill, H.H.; Safrai, M.; Reuveni Salzman, A.; Shushan, A. The rising phoenix-progesterone as the main target of the medical therapy for leiomyoma. Biomed. Res. Int. 2017, 2017, 4705164. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Sefton, E.C. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol. Cell. Endocrinol. 2012, 358, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Islam, M.S.; Reis, F.M.; Gray, P.C.; Bloise, E.; Petraglia, F.; Vale, W.; Castellucci, M. Growth factors and myometrium: Biological effects in uterine fibroid and possible clinical implications. Hum. Reprod. Update 2011, 17, 772–790. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.Z.; Birdsong, G.G.; Mosunjac, M.B. Recent developments in surgical pathology of the uterine corpus. Arch. Pathol. Lab. Med. 2017, 141, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Ducimetiere, F.; Lurkin, A.; Ranchere-Vince, D.; Decouvelaere, A.V.; Peoc’h, M.; Istier, L.; Chalabreysse, P.; Muller, C.; Alberti, L.; Bringuier, P.P.; et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS ONE 2011, 6, e20294. [Google Scholar] [CrossRef] [PubMed]
- Kho, K.A.; Lin, K.; Hechanova, M.; Richardson, D.L. Risk of occult uterine sarcoma in women undergoing hysterectomy for benign indications. Obstet. Gynecol. 2016, 127, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Graebe, K.; Garcia-Soto, A.; Aziz, M.; Valarezo, V.; Heller, P.B.; Tchabo, N.; Tobias, D.H.; Salamon, C.; Ramieri, J.; Dise, C.; et al. Incidental power morcellation of malignancy: A retrospective cohort study. Gynecol. Oncol. 2015, 136, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.C.; Bi, F.F.; Li, D.; Yang, Q. Incidence and clinical characteristics of unexpected uterine sarcoma after hysterectomy and myomectomy for uterine fibroids: A retrospective study of 10,248 cases. Oncol. Targets Ther. 2015, 8, 2943–2948. [Google Scholar] [CrossRef]
- Gantzer, J.; Ray-Coquard, I. Gynecological sarcomas: What’s new in 2018, a brief review of published literature. Curr. Opin. Oncol. 2018, 30, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Bonafede, M.M.; Pohlman, S.K.; Miller, J.D.; Thiel, E.; Troeger, K.A.; Miller, C.E. Women with newly diagnosed uterine fibroids: Treatment patterns and cost comparison for select treatment options. Popul. Health Manag. 2018, 21, S13–S20. [Google Scholar] [CrossRef] [PubMed]
- Seagle, B.L.; Alexander, A.L.; Strohl, A.E.; Shahabi, S. Discussing sarcoma risks during informed consent for nonhysterectomy management of fibroids: An unmet need. Am. J. Obstet. Gynecol. 2018, 218, 103.e101–103.e105. [Google Scholar] [CrossRef] [PubMed]
- Tanos, V.; Berry, K.E. Benign and malignant pathology of the uterus. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, B. Immunohistochemical analysis of p16, p53, and ki-67 expression in uterine smooth muscle tumors. Int. J. Gynecol. Pathol. 2008, 27, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Demura, T.A.; Revazova, Z.V.; Kogan, E.A.; Adamyan, L.V. [The molecular mechanisms and morphological manifestations of leiomyoma reduction induced by selective progesterone receptor modulators]. Arkh. Patol. 2017, 79, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.R.; Manduch, M.; Childs, T.J. Differential immunoreactivity of p16 in leiomyosarcomas and leiomyoma variants. Int. J. Gynecol. Pathol. 2008, 27, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, I.M.; Hornick, J.L.; Sholl, L.M.; Quade, B.J.; Nucci, M.R.; Parra-Herran, C. Abnormal p53 and p16 staining patterns distinguish uterine leiomyosarcoma from inflammatory myofibroblastic tumour. Histopathology 2017, 70, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Ali, R.H.; Rouzbahman, M.; Marino-Enriquez, A.; Zhu, M.; Guo, X.; Brunner, A.L.; Chiang, S.; Leung, S.; Nelnyk, N.; et al. Cyclin D1 as a diagnostic immunomarker for endometrial stromal sarcoma with ywhae-fam22 rearrangement. Am. J. Surg. Pathol. 2012, 36, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Buza, N.; Hui, P. Immunohistochemistry in gynecologic pathology: An example-based practical update. Arch. Pathol. Lab. Med. 2017, 141, 1052–1071. [Google Scholar] [CrossRef] [PubMed]
- Courtoy, G.E.; Donnez, J.; Marbaix, E.; Barreira, M.; Luyckx, M.; Dolmans, M.M. Progesterone receptor isoforms, nuclear corepressor-1 and steroid receptor coactivator-1 and B-cell lymphoma 2 and AKT and AKT phosphorylation status in uterine myomas after ulipristal acetate treatment: A systematic immunohistochemical evaluation. Gynecol. Obstet. Investig. 2018, 83, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Zheng, W.; Xu, M. A retrospective analysis of the clinicopathologic characteristics of uterine cellular leiomyomas in China. Int. J. Gynaecol. Obstet. 2012, 118, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.M.; Albasry, A.M.; Eldosouky, M.K.; Abdelhamid, H.S. Immunoexpression of progesterone receptor, epithelial growth factor receptor and galectin-3 in uterine smooth muscle tumors. Cell. Mol. Biol. 2018, 64, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Segars, J.H. The role of angiogenic factors in fibroid pathogenesis: Potential implications for future therapy. Hum. Reprod. Update 2014, 20, 194–216. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.; He, H.; Haseman, J.K. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ. Health Perspect. 2000, 108 (Suppl. 5), 795–802. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, T.; Kuhn, C.; Mayr, D.; Pavlik, R.; Friese, K.; Scholz, C.; Jeschke, U.; Ditsch, N.; Dian, D. Expression of mucin-1, galectin-1 and galectin-3 in human leiomyosarcoma in comparison to leiomyoma and myometrium. Anticancer Res. 2011, 31, 451–457. [Google Scholar] [PubMed]
- Garg, G.; Mohanty, S.K. Uterine angioleiomyoma: A rare variant of uterine leiomyoma. Arch. Pathol. Lab. Med. 2014, 138, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Oliva, E.; Young, R.H.; Amin, M.B.; Clement, P.B. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: A study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am. J. Surg. Pathol. 2002, 26, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Shi, Y.F.; Cheng, X.D.; Wu, Y.Z. [The differential diagnosis between uterine leiomyosarcoma and the special subtypes of leiomyoma]. Zhonghua Yi Xue Za Zhi 2003, 83, 1419–1421. [Google Scholar] [PubMed]
- Sarlomo-Rikala, M.; Tsujimura, T.; Lendahl, U.; Miettinen, M. Patterns of nestin and other intermediate filament expression distinguish between gastrointestinal stromal tumors, leiomyomas and schwannomas. APMIS 2002, 110, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Abeler, V.M.; Nenodovic, M. Diagnostic immunohistochemistry in uterine sarcomas: A study of 397 cases. Int. J. Gynecol. Pathol. 2011, 30, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Laury, A.L.; Nucci, M.R.; Quade, B.J. Predictors of adverse outcome in uterine smooth muscle tumours of uncertain malignant potential (STUMP): A clinicopathological analysis of 22 cases with a proposal for the inclusion of additional histological parameters. Histopathology 2018, 73, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Kefeli, M.; Caliskan, S.; Kurtoglu, E.; Yildiz, L.; Kokcu, A. Leiomyoma with bizarre nuclei: Clinical and pathologic features of 30 patients. Int. J. Gynecol. Pathol. 2018, 37, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Juhasz-Boss, I.; Jungmann, P.; Radosa, J.; von Heesen, A.; Stroder, R.; Juhasz-Boss, S.; Meyberg-Solomayer, G.; Solomayer, E. Two novel classification systems for uterine fibroids and subsequent uterine reconstruction after myomectomy. Arch. Gynecol. Obstet. 2017, 295, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R. WHO classification of tumours of female reproductive organs. In World Health Organization Classification of Tumours, 6th ed.; Kurman, R., Ed.; WHO Press: Geneva, Switzerland, 2014. [Google Scholar]
- Flake, G.P.; Moore, A.B.; Sutton, D.; Kissling, G.E.; Horton, J.; Wicker, B.; Walmer, D.; Robboy, S.J.; Dixon, D. The natural history of uterine leiomyomas: Light and electron microscopic studies of fibroid phases, interstitial ischemia, inanosis, and reclamation. Obstet. Gynecol. Int. 2013, 2013, 528376. [Google Scholar] [CrossRef] [PubMed]
- Quade, B.J. Uterine smooth muscle tumors. In Pathology of the Female Reproductive Tract, 2nd ed.; Robboy, S., Ed.; Churchill Livingstone: London, UK, 2009; p. 474. [Google Scholar]
- Bell, S.W.; Kempson, R.L.; Hendrickson, M.R. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am. J. Surg. Pathol. 1994, 18, 535–558. [Google Scholar] [CrossRef] [PubMed]
- Stanford University. Leiomyosarcoma of Deep Soft Tissue, Retroperitoneum, Mesentery and Omentum. Available online: http://surgpathcriteria.stanford.edu/softsmoothmuscle/soft_tissue_leiomyosarcoma/differentialdiagnosis.html (accessed on 8 October 2018).
- Harlow, B.L.; Weiss, N.S.; Lofton, S. The epidemiology of sarcomas of the uterus. J. Natl. Cancer Inst. 1986, 76, 399–402. [Google Scholar] [PubMed]
- Berchuck, A.; Rubin, S.C.; Hoskins, W.J.; Saigo, P.E.; Pierce, V.K.; Lewis, J.L., Jr. Treatment of uterine leiomyosarcoma. Obstet. Gynecol. 1988, 71, 845–850. [Google Scholar] [PubMed]
- Duffaud, F.; Ray-Coquard, I.; Salas, S.; Pautier, P. Recent advances in understanding and managing leiomyosarcomas. F1000Prime Rep. 2015, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Xiao, Z.; Lv, F.; Liu, Y.; Zou, C.; Shen, Y. Utility of clinical parameters and multiparametric mri as predictive factors for differentiating uterine sarcoma from atypical leiomyoma. Acad. Radiol. 2018, 25, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Wlodarczyk, M.; Wrzosek, M.; Wojtyla, C.; Meczekalski, B.; Nowicka, G.; Lukaszuk, K.; Jakiel, G. TNF-alpha serum levels are elevated in women with clinically symptomatic uterine fibroids. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418779461. [Google Scholar] [CrossRef] [PubMed]
- Croce, S.; Ducoulombier, A.; Ribeiro, A.; Lesluyes, T.; Noel, J.C.; Amant, F.; Guillou, L.; Stoeckle, E.; Devouassoux-Shisheboran, M.; Penel, N.; et al. Genome profiling is an efficient tool to avoid the STUMP classification of uterine smooth muscle lesions: A comprehensive array-genomic hybridization analysis of 77 tumors. Mod. Pathol. 2018, 31, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, A.; Gizzo, S.; Musaro, A.; Quaranta, M.; Noventa, M.; Migliavacca, C.; Sozzi, G.; Monica, M.; Mautone, D.; Berretta, R. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): Pathology, follow-up and recurrence. Int. J. Clin. Exp. Pathol. 2014, 7, 8136–8142. [Google Scholar] [PubMed]
- Ip, P.P.; Cheung, A.N.; Clement, P.B. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): A clinicopathologic analysis of 16 cases. Am. J. Surg. Pathol. 2009, 33, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Prat, J. Uterine sarcomas: A review. Gynecol. Oncol. 2010, 116, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Ayhan, A. Diagnostic immunohistochemistry in gynaecological neoplasia: A brief survey of the most common scenarios. J. Clin. Pathol. 2018, 71, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.; Schwab, U.; Lemke, H.; Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 1983, 31, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Key, G.; Becker, M.H.; Baron, B.; Duchrow, M.; Schluter, C.; Flad, H.D.; Gerdes, J. New Ki-67-equivalent murine monoclonal antibodies (MIB 1-3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab. Investig. 1993, 68, 629–636. [Google Scholar] [PubMed]
- Mayerhofer, K.; Lozanov, P.; Bodner, K.; Bodner-Adler, B.; Kimberger, O.; Czerwenka, K. Ki-67 expression in patients with uterine leiomyomas, uterine smooth muscle tumors of uncertain malignant potential (STUMP) and uterine leiomyosarcomas (LMS). Acta Obstet. Gynecol. Scand. 2004, 83, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Demopoulos, R.I. MIB-1 (Ki-67), p53, estrogen receptor, and progesterone receptor expression in uterine smooth muscle tumors. Hum. Pathol. 2001, 32, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, D.; Babic, D.; Forko, J.I.; Martinac, I. Expression of Ki-67, p53 and progesterone receptors in uterine smooth muscle tumors. Diagnostic value. Coll. Antropol. 2010, 34, 93–97. [Google Scholar] [PubMed]
- Lee, C.H.; Turbin, D.A.; Sung, Y.C.; Espinosa, I.; Montgomery, K.; van de Rijn, M.; Gilks, C.B. A panel of antibodies to determine site of origin and malignancy in smooth muscle tumors. Mod. Pathol. 2009, 22, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Akhan, S.E.; Yavuz, E.; Tecer, A.; Iyibozkurt, C.A.; Topuz, S.; Tuzlali, S.; Bengisu, E.; Berkman, S. The expression of Ki-67, p53, estrogen and progesterone receptors affecting survival in uterine leiomyosarcomas. A clinicopathologic study. Gynecol. Oncol. 2005, 99, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, K.; Lozanov, P.; Bodner, K.; Bodner-Adler, B.; Obermair, A.; Kimberger, O.; Czerwenka, K. Ki-67 and vascular endothelial growth factor expression in uterine leiomyosarcoma. Gynecol. Oncol. 2004, 92, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Lusby, K.; Savannah, K.B.; Demicco, E.G.; Zhang, Y.; Ghadimi, M.P.; Young, E.D.; Colombo, C.; Lam, R.; Dogan, T.E.; Hornick, J.L.; et al. Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: A single institution’s experience. Ann. Surg. Oncol. 2013, 20, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Espinosa, I.; Ali, R.; Gilks, C.B.; Rijn, M.; Lee, C.H.; Prat, J. Uterine leiomyosarcomas: Tumor size, mitotic index, and biomarkers Ki-67, and Bcl-2 identify two groups with different prognosis. Gynecol. Oncol. 2011, 121, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Surget, S.; Khoury, M.P.; Bourdon, J.C. Uncovering the role of p53 splice variants in human malignancy: A clinical perspective. Oncol. Targets Ther. 2013, 7, 57–68. [Google Scholar] [CrossRef]
- Ashcroft, M.; Kubbutat, M.H.; Vousden, K.H. Regulation of p53 function and stability by phosphorylation. Mol. Cell. Biol. 1999, 19, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Oren, M. The first 30 years of p53: Growing ever more complex. Nat. Rev. Cancer 2009, 9, 749–758. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.J.; McBride, H.A.; Connolly, L.E.; McCluggage, W.G. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB-1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology 2007, 50, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Dastranj Tabrizi, A.; Ghojazadeh, M.; Thagizadeh Anvar, H.; Vahedi, A.; Naji, S.; Mostafidi, E.; Berenjian, S. Immunohistochemical profile of uterine leiomyoma with bizarre nuclei; comparison with conventional leiomyoma, smooth muscle tumors of uncertain malignant potential and leiomyosarcoma. Adv. Pharm. Bull. 2015, 5, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Azimpouran, M.; Vazifekhah, S.; Moslemi, F.; Piri, R.; Naghavi-Behzad, M. Immunohistochemical profile of uterine leiomyomas; a comparison between different subtypes. Niger Med. J. 2016, 57, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, H.; Yuan, L.J.; Xiong, Y.; Huang, X.; Lin, J.X.; Zheng, M. CD146 as an adverse prognostic factor in uterine sarcoma. Eur. J. Med. Res. 2015, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Layfield, L.J.; Liu, K.; Dodge, R.; Barsky, S.H. Uterine smooth muscle tumors: Utility of classification by proliferation, ploidy, and prognostic markers versus traditional histopathology. Arch. Pathol. Lab. Med. 2000, 124, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Blom, R.; Guerrieri, C.; Stal, O.; Malmstrom, H.; Simonsen, E. Leiomyosarcoma of the uterus: A clinicopathologic, DNA flow cytometric, p53, and mdm-2 analysis of 49 cases. Gynecol. Oncol. 1998, 68, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Maltese, G.; Fontanella, C.; Lepori, S.; Scaffa, C.; Fuca, G.; Bogani, G.; Provenzano, S.; Carcangiu, M.L.; Raspagliesi, F.; Lorusso, D. Atypical uterine smooth muscle tumors: A retrospective evaluation of clinical and pathologic features. Oncology 2018, 94, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, A.D.; Nistor, E.; Sajin, M.; Stepan, A.E. Immunohistochemical analysis in the diagnosis of uterine myometrial smooth muscle tumors. Rom. J. Morphol. Embryol. 2014, 55, 1129–1136. [Google Scholar] [PubMed]
- Nobori, T.; Miura, K.; Wu, D.J.; Lois, A.; Takabayashi, K.; Carson, D.A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 1994, 368, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Yamakoshi, K.; Takahashi, A.; Hara, E. The p16ink4a-rb pathway: Molecular link between cellular senescence and tumor suppression. J. Med. Investig. 2004, 51, 146–153. [Google Scholar] [CrossRef]
- Serra, S.; Chetty, R. P16. J. Clin. Pathol. 2018, 71, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Skubitz, K.M.; Skubitz, A.P. Differential gene expression in leiomyosarcoma. Cancer 2003, 98, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Missaoui, N.; Mestiri, S.; Bdioui, A.; Zahmoul, T.; Hamchi, H.; Mokni, M.; Hmissa, S. Hpv infection and p16(ink4a) and tp53 expression in rare cancers of the uterine cervix. Pathol. Res. Pract. 2018, 214, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Bodner-Adler, B.; Bodner, K.; Czerwenka, K.; Kimberger, O.; Leodolter, S.; Mayerhofer, K. Expression of p16 protein in patients with uterine smooth muscle tumors: An immunohistochemical analysis. Gynecol. Oncol. 2005, 96, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Hakverdi, S.; Gungoren, A.; Yaldiz, M.; Hakverdi, A.U.; Toprak, S. Immunohistochemical analysis of p16 expression in uterine smooth muscle tumors. Eur. J. Gynaecol. Oncol. 2011, 32, 513–515. [Google Scholar] [PubMed]
- Liang, Y.; Zhang, X.; Chen, X.; Lu, W. Diagnostic value of progesterone receptor, p16, p53 and PHH3 expression in uterine atypical leiomyoma. Int. J. Clin. Exp. Pathol. 2015, 8, 7196–7202. [Google Scholar] [PubMed]
- Moldovan, G.L.; Pfander, B.; Jentsch, S. Pcna, the maestro of the replication fork. Cell 2007, 129, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Mailand, N.; Gibbs-Seymour, I.; Bekker-Jensen, S. Regulation of pcna-protein interactions for genome stability. Nat. Rev. Mol. Cell Biol. 2013, 14, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Greenspan, D.L.; Wu, T.C.; Zacur, H.A.; Kurman, R.J. Cellular proliferation, estrogen receptor, progesterone receptor, and Bcl-2 expression in gnrh agonist-treated uterine leiomyomas. Hum. Pathol. 1998, 29, 359–363. [Google Scholar] [CrossRef]
- Garnock-Jones, K.P.; Duggan, S.T. Ulipristal acetate: A review in symptomatic uterine fibroids. Drugs 2017, 77, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.S.; Seong, S.J.; Cha, D.H.; Kim, J.Y.; Kim, M.L.; Shim, J.Y.; Park, J.E. Changes in proliferating and apoptotic markers of leiomyoma following treatment with a selective progesterone receptor modulator or gonadotropin-releasing hormone agonist. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yin, P.; Coon, V.J.; Cheng, Y.H.; Wiehle, R.D.; Bulun, S.E. The selective progesterone receptor modulator cdb4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil. Steril. 2010, 93, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Urban, J.; Engelhardt, U.; Baumann, G.; Stangl, K.; Stangl, V. Green and black tea are equally potent stimuli of no production and vasodilation: New insights into tea ingredients involved. Basic Res. Cardiol. 2009, 104, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Rajaratnam, V.; Al-Hendy, A. Antiproliferative and proapoptotic effects of epigallocatechin gallate on human leiomyoma cells. Fertil. Steril. 2010, 94, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.M.; Soane, L. Multiple functions of bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Otake, Y.; Soundararajan, S.; Sengupta, T.K.; Kio, E.A.; Smith, J.C.; Pineda-Roman, M.; Stuart, R.K.; Spicer, E.K.; Fernandes, D.J. Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mrna. Blood 2007, 109, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Blanck, A.; Olovsson, M.; Henriksen, R.; Lindblom, B. Expression of Bcl-2, Bcl-x, mcl-1, Bax and Bak in human uterine leiomyomas and myometrium during the menstrual cycle and after menopause. J. Steroid Biochem. Mol. Biol. 2002, 80, 77–83. [Google Scholar] [CrossRef]
- Reed, J.C.; Talwar, H.S.; Cuddy, M.; Baffy, G.; Williamson, J.; Rapp, U.R.; Fisher, G.J. Mitochondrial protein p26 Bcl2 reduces growth factor requirements of nih3t3 fibroblasts. Exp. Cell Res. 1991, 195, 277–283. [Google Scholar] [CrossRef]
- Matsuo, H.; Maruo, T.; Samoto, T. Increased expression of bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J. Clin. Endocrinol. Metab. 1997, 82, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Khurana, K.K.; Singh, S.B.; Tatum, A.H.; Schulz, V.; Badawy, S.Z. Maintenance of increased Bcl-2 expression in uterine leiomyomas after gnrh agonist therapy. J. Reprod. Med. 1999, 44, 487–492. [Google Scholar] [PubMed]
- Bodner-Adler, B.; Bodner, K.; Kimberger, O.; Czerwenka, K.; Leodolter, S.; Mayerhofer, K. Expression of matrix metalloproteinases in patients with uterine smooth muscle tumors: An immunohistochemical analysis of mmp-1 and mmp-2 protein expression in leiomyoma, uterine smooth muscle tumor of uncertain malignant potential, and leiomyosarcoma. J. Soc. Gynecol. Investig. 2004, 11, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.L.; Nikaido, T.; Toki, T.; Shiozawa, A.; Orii, A.; Fujii, S. Prognostic significance of Bcl-2 expression in leiomyosarcoma of the uterus. Br. J. Cancer 1999, 80, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Banas, T.; Pitynski, K.; Okon, K.; Czerw, A. DNA fragmentation factors 40 and 45 (dff40/dff45) and B-cell lymphoma 2 (Bcl-2) protein are underexpressed in uterine leiomyosarcomas and may predict survival. Oncol. Targets Ther. 2017, 10, 4579–4589. [Google Scholar] [CrossRef] [PubMed]
- Conconi, D.; Chiappa, V.; Perego, P.; Redaelli, S.; Bovo, G.; Lavitrano, M.; Milani, R.; Dalpra, L.; Lissoni, A.A. Potential role of Bcl2 in the recurrence of uterine smooth muscle tumors of uncertain malignant potential. Oncol. Rep. 2017, 37, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bodner, K.; Bodner-Adler, B.; Kimberger, O.; Czerwenka, K.; Mayerhofer, K. Bcl-2 receptor expression in patients with uterine smooth muscle tumors: An immunohistochemical analysis comparing leiomyoma, uterine smooth muscle tumor of uncertain malignant potential, and leiomyosarcoma. J. Soc. Gynecol. Investig. 2004, 11, 187–191. [Google Scholar] [CrossRef] [PubMed]
- de Graaff, M.A.; de Rooij, M.A.; van den Akker, B.E.; Gelderblom, H.; Chibon, F.; Coindre, J.M.; Marino-Enriquez, A.; Fletcher, J.A.; Cleton-Jansen, A.M.; Bovee, J.V. Inhibition of Bcl-2 family members sensitises soft tissue leiomyosarcomas to chemotherapy. Br. J. Cancer 2016, 114, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Bodner-Adler, B.; Bodner, K.; Kimberger, O.; Czerwenka, K.; Leodolter, S.; Mayerhofer, K. MMP-1 and MMP-2 expression in uterine leiomyosarcoma and correlation with different clinicopathologic parameters. J. Soc. Gynecol. Investig. 2003, 10, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Bodner-Adler, B.; Nather, A.; Bodner, K.; Czerwenka, K.; Kimberger, O.; Leodolter, S.; Mayerhofer, K. Expression of thrombospondin 1 (TSP 1) in patients with uterine smooth muscle tumors: An immunohistochemical study. Gynecol. Oncol. 2006, 103, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan shell proteins: Primary structure, origin, and evolution. Curr. Top. Dev. Biol. 2008, 80, 209–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins: Regulators of acute and chronic inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Matsuoka, Y.; Funahashi, A.; Kitano, H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 2005, 1, 2005.0010. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.D.; Luo, X.; Panda, H.; Chegini, N. Mir-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target f3 and il-8. Mol. Endocrinol. 2012, 26, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Nowinska, K.; Dziegiel, P. [the role of mcm proteins in cell proliferation and tumorigenesis]. Postepy Hig. Med. Dosw. (Online) 2010, 64, 627–635. [Google Scholar] [PubMed]
- Lintel, N.J.; Luebker, S.A.; Lele, S.M.; Koepsell, S.A. MVP immunohistochemistry is a useful adjunct in distinguishing leiomyosarcoma from leiomyoma and leiomyoma with bizarre nuclei. Hum. Pathol. 2018, 73, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Sharan, C.; Halder, S.K.; Thota, C.; Jaleel, T.; Nair, S.; Al-Hendy, A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-o-methyltransferase. Fertil. Steril. 2011, 95, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Rajaratnam, V.; Al-Hendy, O.; Halder, S.; Al-Hendy, A. Green tea extract inhibition of human leiomyoma cell proliferation is mediated via catechol-O-methyltransferase. Gynecol. Obstet. Investig. 2014, 78, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L. Functions of intracellular retinoid binding-proteins. Subcell. Biochem. 2016, 81, 21–76. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, A.; Ferlosio, A.; Ciucci, A.; Sesti, F.; Lifschitz-Mercer, B.; Gabbiani, G.; Spagnoli, L.G.; Czernobilsky, B. Cellular retinol-binding protein-1 expression in endometrial stromal cells: Physiopathological and diagnostic implications. Histopathology 2004, 45, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, A.; Francesconi, A.; Clement, S.; Ropraz, P.; Spagnoli, L.G.; Gabbiani, G. High levels of cellular retinol binding protein-1 expression in leiomyosarcoma: Possible implications for diagnostic evaluation. Virchows Arch. 2002, 441, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, M.; Vollenhoven, B.J.; Rogers, P.A. Retinoic acid pathway genes show significantly altered expression in uterine fibroids when compared with normal myometrium. Mol. Hum. Reprod. 2007, 13, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Song, K.J.; Yu, X.N.; Lv, T.; Chen, Y.L.; Diao, Y.C.; Liu, S.L.; Wang, Y.K.; Yao, Q. Expression and prognostic value of lactate dehydrogenase-A and -A subunits in human uterine myoma and uterine sarcoma. Medicine (Baltimore) 2018, 97, e0268. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.; Poliseli, F.L.; De Brot, L.; Mantoan, H.; Schiavon, B.N.; Faloppa, C.C.; Vassallo, J.; Soares, F.A.; Cunha, I.W. TOP2A copy number and TOP2A expression in uterine benign smooth muscle tumours and leiomyosarcoma. J. Clin. Pathol. 2016, 69, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Cossu, A.; Paliogiannis, P.; Tanda, F.; Dessole, S.; Palmieri, G.; Capobianco, G. Uterine perivascular epithelioid cell neoplasms (PEComas): Report of two cases and literature review. Eur. J. Gynaecol. Oncol. 2014, 35, 309–312. [Google Scholar] [PubMed]
- Bennett, J.A.; Braga, A.C.; Pinto, A.; Van de Vijver, K.; Cornejo, K.; Pesci, A.; Zhang, L.; Morales-Oyarvide, V.; Kiyokawa, T.; Zannoni, G.F.; et al. Uterine PEComas: A morphologic, immunohistochemical, and molecular analysis of 32 tumors. Am. J. Surg. Pathol. 2018, 42, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Vang, R.; Kempson, R.L. Perivascular epithelioid cell tumor (‘PEComa’) of the uterus: A subset of hmb-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am. J. Surg. Pathol. 2002, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Musella, A.; De Felice, F.; Kyriacou, A.K.; Barletta, F.; Di Matteo, F.M.; Marchetti, C.; Izzo, L.; Monti, M.; Benedetti Panici, P.; Redler, A.; et al. Perivascular epithelioid cell neoplasm (PEComa) of the uterus: A systematic review. Int. J. Surg. 2015, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Conklin, C.M.; Longacre, T.A. Endometrial stromal tumors: The new WHO classification. Adv. Anat. Pathol. 2014, 21, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.R.; O’Connell, J.T.; Huettner, P.C.; Cviko, A.; Sun, D.; Quade, B.J. H-caldesmon expression effectively distinguishes endometrial stromal tumors from uterine smooth muscle tumors. Am. J. Surg. Pathol. 2001, 25, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rush, D.S.; Tan, J.; Baergen, R.N.; Soslow, R.A. H-caldesmon, a novel smooth muscle-specific antibody, distinguishes between cellular leiomyoma and endometrial stromal sarcoma. Am. J. Surg. Pathol. 2001, 25, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.G.; Arber, D.A.; Weiss, L.M.; Chang, K.L. Utility of cd10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: An immunohistochemical comparison of 34 cases. Mod. Pathol. 2001, 14, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Shi, Y.F.; Cheng, X.D.; Zhao, C.L.; Wu, Y.Z. Immunohistochemical markers in differential diagnosis of endometrial stromal sarcoma and cellular leiomyoma. Gynecol. Oncol. 2004, 92, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Baek, M.H.; Park, Y.; Kim, Y.T.; Nam, J.H. Investigation of hormone receptor expression and its prognostic value in endometrial stromal sarcoma. Virchows Arch. 2018, 473, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, W.P.; Olszewski, W.T. The role of pathologist in cancer patients selection for EGFR-targeted therapy. Onkol. Prak. Klin. 2010, 6, 228–235. [Google Scholar]
- Hewedi, I.H.; Radwan, N.A.; Shash, L.S. Diagnostic value of progesterone receptor and p53 expression in uterine smooth muscle tumors. Diagn. Pathol. 2012, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M.; Soslow, R.A.; Nonaka, D.; Olshen, A.B.; Aghajanian, C.; Sabbatini, P.; Dupont, J.; Hensley, M.; Sonoda, Y.; Barakat, R.R.; et al. Tissue microarray immunohistochemical expression of estrogen, progesterone, and androgen receptors in uterine leiomyomata and leiomyosarcoma. Cancer 2004, 101, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.L.; Kobayashi, Y.; Mori, A.; Orii, A.; Nikaido, T.; Konishi, I.; Fujii, S. Expression of steroid receptors, Ki-67, and p53 in uterine leiomyosarcomas. Int. J. Gynecol. Pathol. 1999, 18, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.H.; Park, J.Y.; Park, Y.; Kim, K.R.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Androgen receptor as a prognostic biomarker and therapeutic target in uterine leiomyosarcoma. J. Gynecol. Oncol. 2018, 29, e30. [Google Scholar] [CrossRef] [PubMed]
- Borahay, M.A.; Asoglu, M.R.; Mas, A.; Adam, S.; Kilic, G.S.; Al-Hendy, A. Estrogen receptors and signaling in fibroids: Role in pathobiology and therapeutic implications. Reprod. Sci. 2017, 24, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wu, T.; Chen, X.Y.; Xie, L.; Yang, J. Selective estrogen receptor modulators (SERMs) for uterine leiomyomas. Cochrane Database Syst. Rev. 2012, 10, CD005287. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Tomaszewski, J.; Vazquez, F.; Bouchard, P.; Lemieszczuk, B.; Baro, F.; Nouri, K.; Selvaggi, L.; Sodowski, K.; Bestel, E.; et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N. Engl. J. Med. 2012, 366, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Chaudhry, Z.T.; Al-Hendy, A. Successes and failures of uterine leiomyoma drug discovery. Expert Opin. Drug Discov. 2018, 13, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.; Dutta, D. Esmya((r)) and the pearl studies: A review. Womens Health (Lond.) 2016, 12, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Murji, A.; Whitaker, L.; Chow, T.L.; Sobel, M.L. Selective progesterone receptor modulators (sprms) for uterine fibroids. Cochrane Database Syst. Rev. 2017, 4, CD010770. [Google Scholar] [CrossRef] [PubMed]
- Flyckt, R.; Coyne, K.; Falcone, T. Minimally invasive myomectomy. Clin. Obstet. Gynecol. 2017, 60, 252–272. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, C.E.; Jallad, K.; Paraiso, M.F.R. Minimally invasive hysterectomy for benign indications: An update. Minerva Ginecol. 2017, 69, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.R.; Wright, J.D. Risk of occult uterine sarcoma in presumed uterine fibroids. Clin. Obstet. Gynecol. 2016, 59, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Sagae, S.; Yamashita, K.; Ishioka, S.; Nishioka, Y.; Terasawa, K.; Mori, M.; Yamashiro, K.; Kanemoto, T.; Kudo, R. Preoperative diagnosis and treatment results in 106 patients with uterine sarcoma in hokkaido, japan. Oncology 2004, 67, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Takai, Y.; Akahori, T.; Ishida, H.; Hanaoka, T.; Uotani, T.; Sato, S.; Matsunaga, S.; Baba, K.; Seki, H. Novel uterine sarcoma preoperative diagnosis score predicts the need for surgery in patients presenting with a uterine mass. Springerplus 2014, 3, 678. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. Available online: https://wayback.archive-it.org/7993/20170406071822/https:/www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm (accessed on 8 October 2018).
- US Food and Drug Administration. FDA Updated Assessment of the Use of Laparoscopic Power Morcellators to Treat Uterine Fibroids. Available online: https://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/SurgeryandLifeSupport/UCM584539 (accessed on 8 October 2018).
- Shikeeva, A.A.; Kekeeva, T.V.; Zavalishina, L.E.; Andreeva, I.; Frank, G.A. [The loss of heterozygosity and microsatellite instability analysis in differential diagnostics of leiomyosarcoma and proliferative leiomyoma of the uterus]. Arkh. Patol. 2011, 73, 47–50. [Google Scholar] [PubMed]
- Amada, S.; Nakano, H.; Tsuneyoshi, M. Leiomyosarcoma versus bizarre and cellular leiomyomas of the uterus: A comparative study based on the MIB-1 and proliferating cell nuclear antigen indices, p53 expression, DNA flow cytometry, and muscle specific actins. Int. J. Gynecol. Pathol. 1995, 14, 134–142. [Google Scholar] [CrossRef] [PubMed]
| Myometrial Neoplasms | |
| Benign Lesions | |
| leiomyoma | leiomyoma with Unusual Presentation |
| cellular leiomyoma | diffuse leiomyomatosis |
| epithelioid leiomyoma | intravenous leiomyomatosis |
| myxoid leiomyoma | benign metastasizing leiomyoma |
| atypical leiomyoma | |
| lipoleiomyoma | |
| Uncertain Potential | |
| Smooth Muscle Tumor of Uncertain Malignant Potential (STUMP) | |
| Malignant | |
| leiomyosarcoma | |
| epithelioid variant | |
| myxoid variant | |
| Benign | Malignant |
|---|---|
|
|
| Leiomyoma (requires all below) | Smooth Muscle Tumor of Uncertain Malignant Potential (used for any of below) | Leiomyosarcoma (requires any one of below) |
|---|---|---|
| Cytologically bland | Bland but 1-4 mitotic figures/50 High-Power Field | Cytologic pleomorphism or atypia |
| <1 mitotic figure/50 High-Power Field | Multiple recurrences but lacking other atypical features | >4 mitotic figures/50 High-Power Field |
| No tumor cell necrosis | Coagulative tumor cell necrosis |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubisz, P.; Ciebiera, M.; Hirnle, L.; Zgliczyńska, M.; Łoziński, T.; Dzięgiel, P.; Kobierzycki, C. The Usefulness of Immunohistochemistry in the Differential Diagnosis of Lesions Originating from the Myometrium. Int. J. Mol. Sci. 2019, 20, 1136. https://doi.org/10.3390/ijms20051136
Rubisz P, Ciebiera M, Hirnle L, Zgliczyńska M, Łoziński T, Dzięgiel P, Kobierzycki C. The Usefulness of Immunohistochemistry in the Differential Diagnosis of Lesions Originating from the Myometrium. International Journal of Molecular Sciences. 2019; 20(5):1136. https://doi.org/10.3390/ijms20051136
Chicago/Turabian StyleRubisz, Piotr, Michał Ciebiera, Lidia Hirnle, Magdalena Zgliczyńska, Tomasz Łoziński, Piotr Dzięgiel, and Christopher Kobierzycki. 2019. "The Usefulness of Immunohistochemistry in the Differential Diagnosis of Lesions Originating from the Myometrium" International Journal of Molecular Sciences 20, no. 5: 1136. https://doi.org/10.3390/ijms20051136
APA StyleRubisz, P., Ciebiera, M., Hirnle, L., Zgliczyńska, M., Łoziński, T., Dzięgiel, P., & Kobierzycki, C. (2019). The Usefulness of Immunohistochemistry in the Differential Diagnosis of Lesions Originating from the Myometrium. International Journal of Molecular Sciences, 20(5), 1136. https://doi.org/10.3390/ijms20051136






