Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review
Abstract
1. Introduction
2. Therapeutic Potential of DPSCs in Various Systemic Diseases
2.1. Spinal Cord Injury (SCI)
2.2. Parkinson’s Disease (PD)
2.3. Alzheimer’s Disease (AD)
2.4. Cerebral Ischemia
2.5. Myocardial Infarction
2.6. Muscular Dystrophy
2.7. Diabetes
2.8. Liver Disease
2.9. Eye Disease
2.10. Immune Disease
2.11. Oral Diseases
3. Clinical Application of DPSCs
4. Current Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakaumura, S.; Ito, K.; Umemura, E.; Hara, K.; Nagasaka, T.; Abe, A.; Baba, S.; Furuichi, Y.; Izumi, Y.; et al. Injectable Bone Tissue Engineering Using Expanded Mesenchymal Stem Cells. Stem Cells 2013, 31, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; D’Antuono, M.; Glicksman, M.; Wang, J.; Jonklaas, J. A review of clinical trials: Mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus. Am. J. Stem Cells 2018, 7, 82–93. [Google Scholar] [PubMed]
- Li, Z.; Jiang, C.M.; An, S.; Cheng, Q.; Huang, Y.F.; Wang, Y.T.; Gou, Y.C.; Xiao, L.; Yu, W.J.; Wang, J. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014, 20, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ghiroldi, A.; Piccoli, M.; Cirillo, F.; Monasky, M.M.; Ciconte, G.; Pappone, C.; Anastasia, L. Cell-Based Therapies for Cardiac Regeneration: A Comprehensive Review of Past and Ongoing Strategies. Int. J. Mol. Sci. 2018, 19, 3194. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.W.; Kinder, H.A.; West, F.D. Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 2019, e01214. [Google Scholar] [CrossRef] [PubMed]
- Conrad, S.; Weber, K.; Walliser, U.; Geburek, F.; Skutella, T. Stem Cell Therapy for Tendon Regeneration: Current Status and Future Directions. Adv. Exp. Med. Biol. 2018. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Marynka-Kalmani, K.; Treves, S.; Yafee, M.; Rachima, H.; Gafni, Y.; Cohen, M.A.; Pitaru, S. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells 2010, 28, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Yamada, Y.; Ozawa, R.; Ohya, M.; Ito, K.; Ueda, M. Expression of Odontoblastic-related Genes in Human Dental Follicle Cells, Dental Pulp Stem Cells, and Oral Mucosal Cells. Int. J. Oral-Med. Sci. 2004, 3, 41–48. [Google Scholar] [CrossRef]
- Trubiani, O.; Zalzal, S.F.; Paganelli, R.; Marchisio, M.; Giancola, R.; Pizzicannella, J.; Bühring, H.J.; Piattelli, M.; Caputi, S.; Nanci, A. Expression profile of the embryonic markers nanog, OCT-4, SSEA-1, SSEA-4, and frizzled-9 receptor in human periodontal ligament mesenchymal stem cells. J. Cell. Physiol. 2010, 225, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Rajan, T.S.; Gatta, V.; D’Aurora, M.; Merciaro, I.; Marchisio, M.; Muttini, A.; Caputi, S.; Bramanti, P.; Mazzon, E.; et al. Stemness Maintenance Properties in Human Oral Stem Cells after Long-Term Passage. Stem Cells Int. 2017, 2017, 5651287. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P., Jr.; Han, J.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 127, 1671–1679. [Google Scholar] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Fujimoto, A.; Ito, A.; Yoshimi, R.; Ueda, M. Cluster analysis and gene expression profiles: A cDNA microarray system-based comparison between human dental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials 2006, 27, 3766–3781. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J. Endod. 2009, 35, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; About, I. Stem Cells of Dental Origin: Current Research Trends and Key Milestones towards Clinical Application. Stem Cells Int. 2016, 2016, 4209891. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakamura, S.; Ito, K.; Sugito, T.; Yoshimi, R.; Nagasaka, T.; Ueda, M. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng. Part A 2010, 16, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Yen, A.H.; Sharpe, P.T. Stem cells and tooth tissue engineering. Cell Tissue Res. 2008, 331, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Asatourian, A.; Sorenson, C.M.; Sheibani, N. Role of angiogenesis in endodontics: Contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J. Endod. 2015, 41, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Lim, L.W.; Wu, W.; Zhang, C. An Overview of Protocols for the Neural Induction of Dental and Oral Stem Cells In Vitro. Tissue Eng. Part B Rev. 2016, 22, 220–250. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Armiñán, A.; Gandía, C.; Bartual, M.; García-Verdugo, J.M.; Lledó, E.; Mirabet, V.; Llop, M.; Barea, J.; Montero, J.A.; Sepúlveda, P. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009, 18, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Ishkitiev, N.; Yaegaki, K.; Calenic, B.; Nakahara, T.; Ishikawa, H.; Mitiev, V.; Haapasalo, M. Deciduous and permanent dental pulp mesenchymal cells acquire hepatic morphologic and functional features in vitro. J. Endod. 2010, 36, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Kumar, B.M.; Lee, W.J.; Jeon, R.H.; Jang, S.J.; Lee, Y.M.; Park, B.W.; Byun, J.H.; Ahn, C.S.; Kim, J.W.; Rho, G.J. Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp. Cell Res. 2014, 320, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.; Zuliani, T.; Olejnik, C.; LeRoy, H.; Obriot, H.; Kerr-Conte, J.; Formstecher, P.; Bailliez, Y.; Polakowska, R.R. Human dental pulp stem cells differentiate into neural crest-derived melanocytes and have label-retaining and sphere-forming abilities. Stem Cells Dev. 2008, 17, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Concise Review: Dental Pulp Stem Cells: A Novel Cell Therapy for Retinal and Central Nervous System Repair. Stem Cells 2017, 35, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Snyder, B.R.; Cheng, P.H.; Chan, A.W. Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells 2008, 26, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, R.; Hayashi, Y.; Iohara, K.; Sugiyama, M.; Murakami, M.; Yamamoto, T.; Fukuta, O.; Nakashima, M. Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials 2013, 34, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, Z.; Karbalaie, K.; Kiani, A.; Niapour, A.; Bahramian, H.; Nasr-Esfahani, M.H.; Baharvand, H. Transplantation of undifferentiated and induced human exfoliated deciduous teeth-derived stem cells promote functional recovery of rat spinal cord contusion injury model. Stem Cells Dev. 2012, 21, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, X.; Sun, L.; Guo, W.; Tian, W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 2017, 14, 026005. [Google Scholar] [CrossRef] [PubMed]
- Nicola, F.D.C.; Marques, M.R.; Odorcyk, F.; Arcego, D.M.; Petenuzzo, L.; Aristimunha, D.; Vizuete, A.; Sanches, E.F.; Pereira, D.P.; Maurmann, N.; et al. Neuroprotector effect of stem cells from human exfoliated deciduous teeth transplanted after traumatic spinal cord injury involves inhibition of early neuronal apoptosis. Brain Res. 2017, 1663, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Sun, Z.; Wang, X.; Yang, H.; Shi, S.; Wang, S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010, 19, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Matsubara, K.; Sakai, K.; Ito, M.; Ohno, K.; Ueda, M.; Yamamoto, A. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Res. 2015, 1613, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Nesti, C.; Pardini, C.; Barachini, S.; D’Alessandro, D.; Siciliano, G.; Murri, L.; Petrini, M.; Vaglini, F. Human dental pulp stem cells protect mouse dopaminergic neurons against MPP+ or rotenone. Brain Res. 2011, 1367, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Apel, C.; Forlenza, O.V.; de Paula, V.J.; Talib, L.L.; Denecke, B.; Eduardo, C.P.; Gattaz, W.F. The neuroprotective effect of dental pulp cells in models of Alzheimer’s and Parkinson’s disease. J. Neural Transm. (Vienna) 2009, 116, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jia, Y.; Liu, J.; Zhai, J.; Cao, N.; Yue, W.; He, H.; Pei, X. Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. Cell Biol. Int. 2017, 41, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Iohara, K.; Wakita, H.; Hattori, H.; Ueda, M.; Matsushita, K.; Nakashima, M. Dental pulp-derived CD31−/CD146− side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. Tissue Eng. Part A 2011, 17, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.K.; Henshall, T.L.; Arthur, A.; Kremer, K.L.; Lewis, M.D.; Helps, S.C.; Field, J.; Hamilton-Bruce, M.A.; Warming, S.; Manavis, J.; et al. Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl. Med. 2012, 1, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lee, J.H.; Bae, J.; Bu, Y.; Kim, E.C. Human Dental Pulp Stem Cells Are More Effective Than Human Bone Marrow-Derived Mesenchymal Stem Cells in Cerebral Ischemic Injury. Cell Transplant. 2017, 26, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, Y.; Li, H.; Wang, R.; Yang, D.; Li, B.; Cao, X.; Fu, J. Transplanted Dental Pulp Stem Cells Migrate to Injured Area and Express Neural Markers in a Rat Model of Cerebral Ischemia. Cell. Physiol. Biochem. 2018, 45, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Gandia, C.; Arminan, A.; Garcıa-Verdugo, J.M.; Lledó, E.; Ruiz, A.; Miñana, M.D.; Sanchez-Torrijos, J.; Payá, R.; Mirabet, V.; Carbonell-Uberos, F.; et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 2008, 26, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Ambrosio, C.E.; Kerkis, A.; Martins, D.S.; Zucconi, E.; Fonseca, S.A.; Cabral, R.M.; Maranduba, C.M.; Gaiad, T.P.; Morini, A.C.; et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic? J. Transl. Med. 2008, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Lu, A.; De Biasi, S.; Gibellini, L.; La Sala, G.B.; Bruzzesi, G.; Ferrari, A.; Huard, J.; et al. Stem cells isolated from human dental pulp and amniotic fluid improve skeletal muscle histopathology in mdx/SCID mice. Stem Cell Res. Ther. 2015, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sarrà, E.; Montori, S.; Gil-Recio, C.; Núñez-Toldrà, R.; Costamagna, D.; Rotini, A.; Atari, M.; Luttun, A.; Sampaolesi, M. Human dental pulp pluripotent-like stem cells promote wound healing and muscle regeneration. Stem Cell Res. Ther. 2017, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Kanafi, M.M.; Rajeshwari, Y.B.; Gupta, S.; Dadheech, N.; Nair, P.D.; Gupta, P.K.; Bhonde, R.R. Transplantation of islet-like cell clusters derived from human dental pulp stem cells restores normoglycemia in diabetic mice. Cytotherapy 2013, 15, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, E.T.; Cruz Gda, S.; Almeida, T.F.; Souza, B.S.; Kaneto, C.M.; Vasconcelos, J.F.; Santos, W.L.; Santos, R.R.; Villarreal, C.F.; Soares, M.B. Transplantation of stem cells obtained from murine dental pulp improves pancreatic damage, renal function, and painful diabetic neuropathy in diabetic type 1 mouse model. Cell Transplant. 2013, 22, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Hata, M.; Nakamura, N.; Miyabe, M.; Kobayashi, Y.; Kamiya, H.; Nakamura, J.; Ozawa, S.; Tanaka, Y.; Takebe, J.; et al. Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy. J. Diabetes Investig. 2016, 7, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Datta, I.; Bhadri, N.; Shahani, P.; Majumdar, D.; Sowmithra, S.; Razdan, R.; Bhonde, R. Functional recovery upon human dental pulp stem cell transplantation in a diabetic neuropathy rat model. Cytotherapy 2017, 19, 1208–1224. [Google Scholar] [CrossRef] [PubMed]
- Yamaza, T.; Alatas, F.S.; Yuniartha, R.; Yamaza, H.; Fujiyoshi, J.K.; Yanagi, Y.; Yoshimaru, K.; Hayashida, M.; Matsuura, T.; Aijima, R.; et al. In vivo hepatogenic capacity and therapeutic potential of stem cells from human exfoliated deciduous teeth in liver fibrosis in mice. Stem Cell Res. Ther. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Noh, K.; Jue, S.S.; Lee, S.Y.; Kim, E.C. Melatonin promotes hepatic differentiation of human dental pulp stem cells: Clinical implications for the prevention of liver fibrosis. J. Pineal Res. 2015, 58, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Cho, Y.A.; Lee, Y.M.; Lee, S.Y.; Bae, W.J.; Kim, E.C. PIN1 Suppresses the Hepatic Differentiation of Pulp Stem Cells via Wnt3a. J. Dent. Res. 2016, 95, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Syed-Picard, F.N.; Du, Y.; Lathrop, K.L.; Mann, M.M.; Funderburgh, M.L.; Funderburgh, J.L. Dental pulp stem cells: A new cellular resource for corneal stromal regeneration. Stem Cells Transl. Med. 2015, 4, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.; Monteiro, B.G.; Melo, G.B.; Smith, R.L.; da Silva, M.C.P.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Kushnerev, E.; Shawcross, S.G.; Sothirachagan, S.; Carley, F.; Brahma, A.; Yates, J.M.; Hillarby, M.C. Regeneration of Corneal Epithelium with Dental Pulp Stem Cells Using a Contact Lens Delivery System. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5192–5199. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Hill, L.J.; Blanch, R.J.; Ward, K.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016, 18, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Yamaza, T.; Kentaro, A.; Chen, C.; Liu, Y.; Shi, Y.; Gronthos, S.; Wang, S.; Shi, S. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res. Ther. 2010, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng. Part A 2011, 17, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Zhang, Z.; Grande, R.H.; Nör, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, A.; Eslaminejad, M.B.; Arabsolghar, M.; Paknejad, M.; Ghaedi, B.; Rokn, A.R.; Moslemi, N.; Nazarian, H.; Jahangir, S. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J. Oral Implantol. 2013, 39, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Nosrat, I.V.; Widenfalk, J.; Olson, L.; Nosrat, C.A. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev. Biol. 2001, 238, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D.; Kanafi, M.; Bhonde, R.; Gupta, P.; Datta, I. Differential Neuronal Plasticity of Dental Pulp Stem Cells from Exfoliated Deciduous and Permanent Teeth Towards Dopaminergic Neurons. J. Cell. Physiol. 2016, 231, 2048–2063. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.; Valenzuela, M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther. 2017, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Zheng, L.; Wake, H.; Ito, M.; Nabekura, J.; Wakita, H.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. A novel stem cell source for vasculogenesis in ischemia: Subfraction of side population cells from dental pulp. Stem Cells 2008, 26, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Shibata, R.; Yamamoto, N.; Nishikawa, M.; Hibi, H.; Tanigawa, T.; Ueda, M.; Murohara, T.; Yamamoto, A. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci. Rep. 2015, 5, 16295. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, V.; Ronald, V.S.; Abdullah, A.N.; Ganesan Nathan, K.R.; Ab. Aziz, Z.A.C.; Abdullah, M.; Musa, S.; Anu Kasim, N.H.; Bhonde, R.R. Differentiation of dental pulp stem cells into islet-like aggregates. J. Dent. Res. 2011, 90, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.G.; Serafim, R.C.; Melo, G.B.; Silva, M.C.; Lizier, N.F.; Maranduba, C.M.; Smith, R.L.; Kerkis, A.; Cerruti, H.; Gomes, J.A.; et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009, 42, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Berry, M.; Logan, A.; Scott, R.A.; Leadbeater, W.; Scheven, B.A. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015, 14, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Roozafzoon, R.; Lashay, A.; Vasei, M.; Ai, J.; Khoshzaban, A.; Keshel, S.H.; Barabadi, Z.; Bahrami, H. Dental pulp stem cells differentiation into retinal ganglion-like cells in a three-dimensional network. Biochem. Biophys. Res. Commun. 2015, 457, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Kwack, K.H.; Lee, J.M.; Park, S.H.; Lee, H.W. Human Dental Pulp Stem Cells Suppress Alloantigen-induced Immunity by Stimulating T Cells to Release Transforming Growth Factor Beta. J. Endod. 2017, 43, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K. Recent Progress in Translation from Bench to a Pilot Clinical Study on Total Pulp Regeneration. J. Endod. 2017, 43, S82–S86. [Google Scholar] [CrossRef] [PubMed]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ito, K.; Nakamura, S.; Ueda, M.; Nagasaka, T. Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transplant. 2011, 20, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Yamada, Y.; Nakamura, S.; Ueda, M. Osteogenic potential of effective bone engineering using dental pulp stem cells, bone marrow stem cells, and periosteal cells for osseointegration of dental implants. Int. J. Oral Maxillofac. Implants 2011, 26, 947–954. [Google Scholar] [PubMed]
- Hara, K.; Yamada, Y.; Nakamura, S.; Umemura, E.; Ito, K.; Ueda, M. Potential characteristics of stem cells from human exfoliated deciduous teeth compared with bone marrow-derived mesenchymal stem cells for mineralized tissue-forming cell biology. J. Endod. 2011, 37, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakamura, S.; Kazaoka, Y. Dental Pulp Stem Cell Therapy in Bone Regeneration for Dental Implants. In Proceedings of the 31th Anniversary Meeting of Academy of Osseointegration, San Diego, CA, USA, 17–20 February 2016. [Google Scholar]
- d’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cell Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Manescu, A.; Langer, M.; Rustichelli, F.; Desiderio, V.; Paino, F.; De Rosa, A.; Laino, L.; d’Aquino, R.; Tirino, V.; et al. Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: Biological and clinical implications. Stem Cells Transl. Med. 2013, 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Ferrarotti, F.; Gamba, M.N.; Giraudi, M.; Romano, F. Regenerative Treatment of Periodontal Intrabony Defects Using Autologous Dental Pulp Stem Cells: A 1-Year Follow-Up Case Series. Int. J. Periodont. Restor. Dent. 2018, 38, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, A.; Kremer, K.L.; Hamilton-Bruce, M.A.; Kaidonis, X.; Milton, A.G.; Levi, C.; Shi, S.; Carey, L.; Hillier, S.; Rose, M.; et al. TOOTH (The Open study Of dental pulp stem cell Therapy in Humans): Study protocol for evaluating safety and feasibility of autologous human adult dental pulp stem cell therapy in patients with chronic disability after stroke. Int. J. Stroke 2016, 11, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H.; et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed]
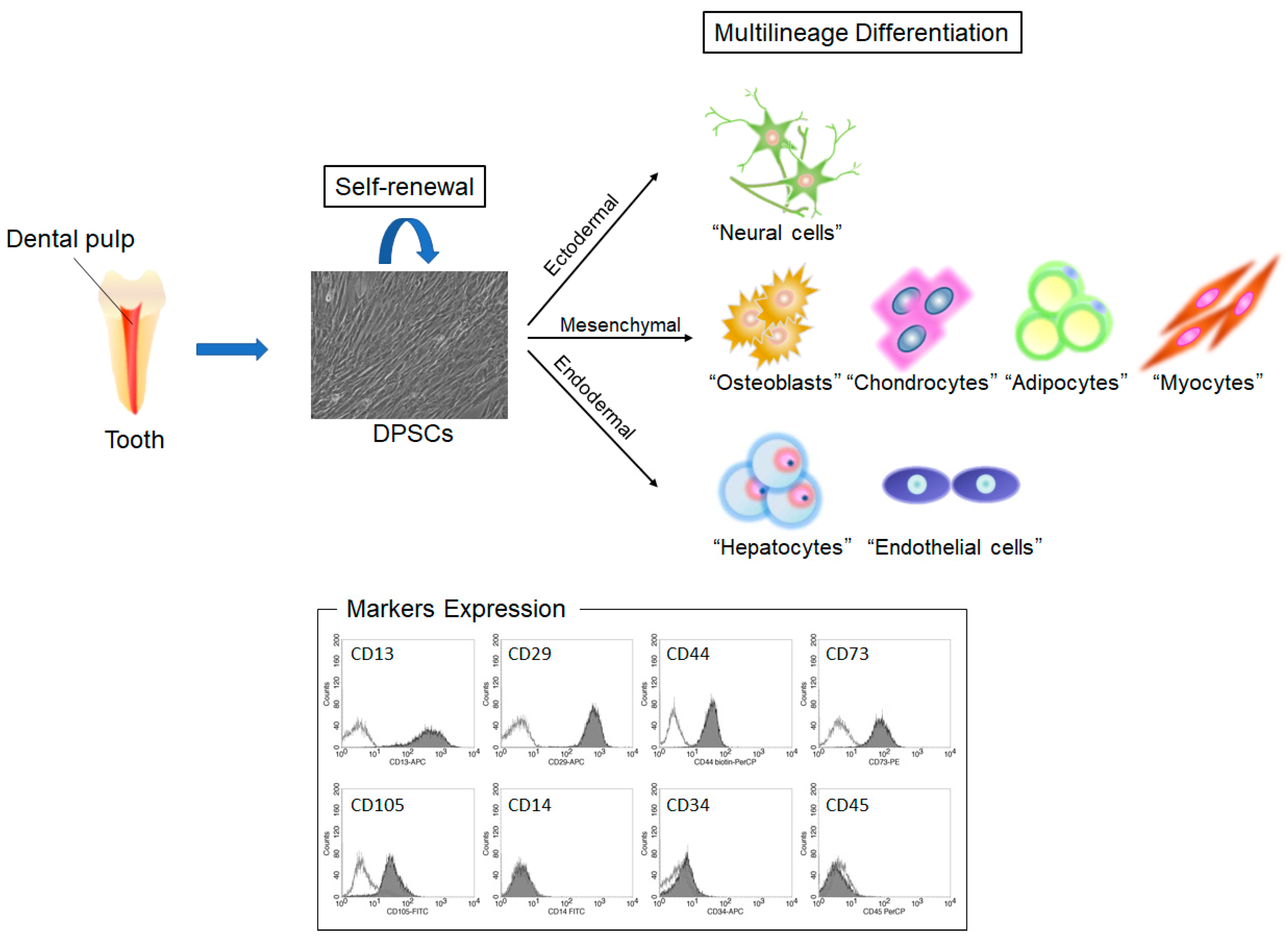
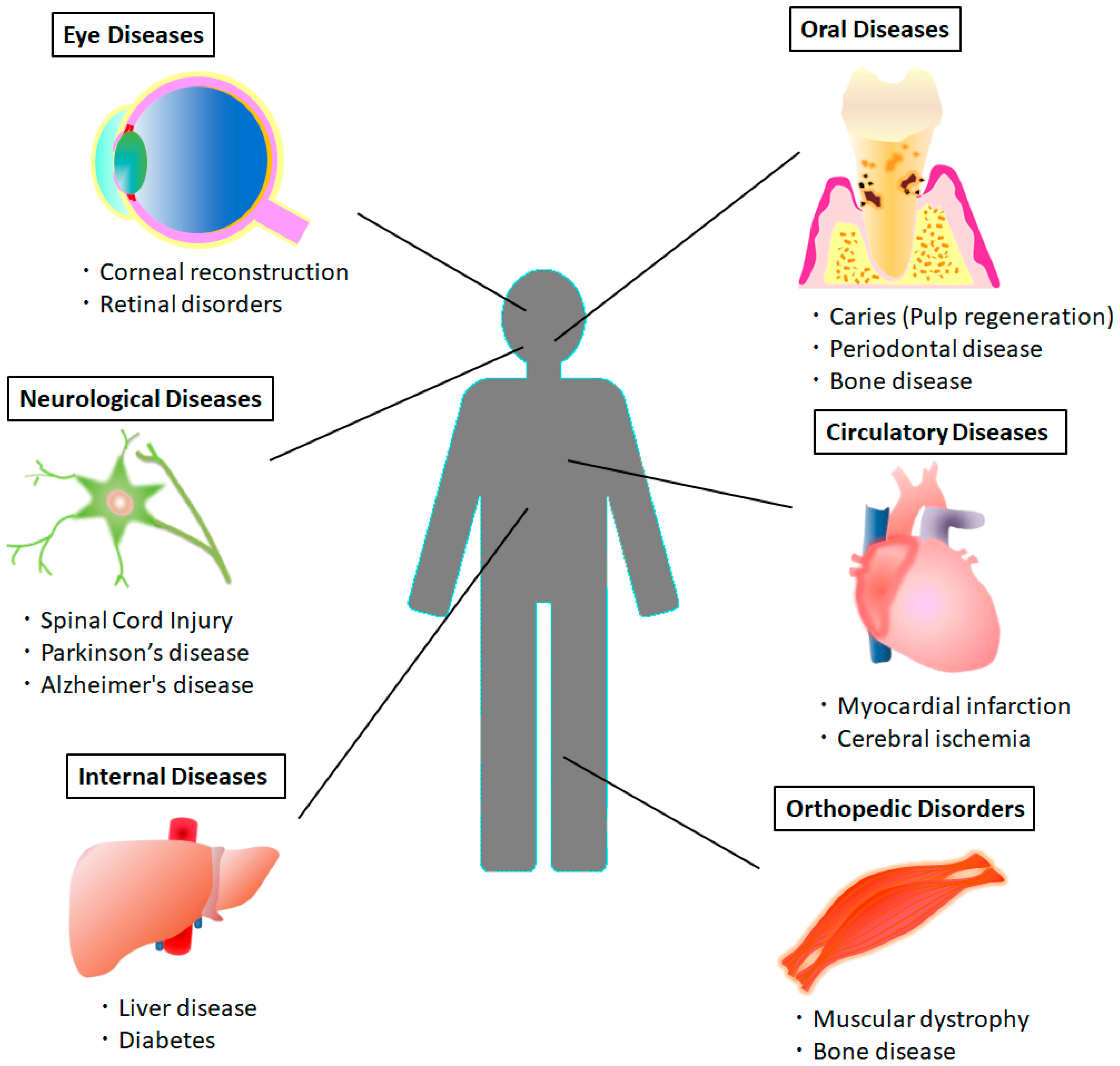
| Disease | Cells | Study Model (Application Method) | Outcome | Ref. |
|---|---|---|---|---|
| Spinal cord injury (SCI) | Human DPSCs, SHED (stem cells from human exfoliated deciduous teeth) | Transplantation into a completely transected rat SCI model | DPSCs and SHED had neuroregenerative activities including inhibition of apoptosis of neurons, astrocytes, and oligodendrocytes, promoted the regeneration of transected axons, and replaced lost cells that fulfill many requirements for functional recovery after SCI. | [31] |
| SHED, neural induced SHED (iSHED) | Injection into the spinal cord lesions of rat acute contused SCI model | Transplanted SHED and iSHED showed neuronal and glial differentiation and displayed significant locomotor functional recovery. | [34] | |
| Human DPSCs | Transplanted into completely transected rat spinal cord | DPSCs presented tissue regenerative capability after SCI through their immunomodulatory, differentiation, and protection capacity. | [35] | |
| SHED | Transplanted into completely injured rat spinal cord | The acute transplantation of SHED reduces early neuronal apoptosis, contributing to tissue and motor neuron preservation and hind limb functional recovery. | [36] | |
| Parkinson’s disease (PD) | SHED | Intrastriatal transplantation of SHED and SHED-derived spheres in Parkinsonian rats | SHED and SHED-derived spheres differentiated into dopaminergic neurons and ameliorated behavioral impairment. | [37] |
| SHED | Intrastriatal transplantation of SHED and SHED-derived dopaminergic neuron-like cells (dSHED) in Parkinsonian rats | Engrafted dSHEDs survived in the striatum of Parkinsonian rats, improved the dopamine level more efficiently than engrafted undifferentiated SHED, and promoted recovery from neurological deficits. | [38] | |
| Human DPSCs | MPP+ or rotenone-induced in vitro model of PD; indirect co-culture system with mesencephalic cell cultures | DPSCs showed a protective effect on dopamine neurons; dopamine uptake, and an increased number of spared tyrosinehydroxylase (TH)+ cells. | [39] | |
| Alzheimer’s disease (AD) | Rat dental pulp cells | In vitro model of AD | The co-culture with dental pulp cells significantly attenuated 6-hydroxydopamine and Abeta(1-42)-induced toxicity in primary cultures of hippocampal neurons. | [40] |
| Human DPSCs | In vitro model of AD established by okadaic acid (OA)-induced damage to human neuroblastoma cell line | DPSCs caused a significant increase in the viability and a decrease in apoptosis of AD model cells and phosphorylation at Ser 396 of Tau protein was significantly suppressed. | [41] | |
| Cerebral ischemia | CD31−/CD146− side population (SP) cells from porcine dental pulp | Transplanted into brain of middle cerebral artery occlusion rat | The cells promoted migration and differentiation of the endogenous neuronal progenitor cells, induced vasculogenesis, and ameliorated ischemic brain injury. | [42] |
| Human DPSCs | Intracerebral transplantation of human DPSCs 24 h following focal cerebral ischemia in rat model | Human DPSC treatment enhanced functional recovery of post-stroke sensorimotor deficits. | [43] | |
| Human DPSCs | Intravenous transplantation of human DPSCs in a rat stroke model | DPSCs had a better effect on infarct size and significantly decreased reactive gliosis compared with bone-marrow-derived MSCs. | [44] | |
| Rat DPSCs | Intravenous administration in rat model of focal cerebral ischemia | DPSCs migrated into the boundary of ischemic areas and expressed neural specific markers, reducing infarct volume and cerebral edema. | [45] | |
| Myocardial Infarction | Human DPSCs | Intramyocardial injection into nude rats with acute myocardial infarction | Cell-treated animals showed an improvement in cardiac function, observed by changes in anterior wall thickening, and a reduction in infarct size; angiogenesis was increased. | [46] |
| Muscular dystrophy | SHED | Systemic or intramuscular transplantation of SHED in golden retriever muscular dystrophy dogs | Donor SHED can engraft, differentiate, and persist in the host muscle. Systemic multiple deliveries seemed more effective than local injections. | [47] |
| Human DPSCs | Intramuscular injection of pre-differentiated DPSCs into mdx/SCID mice, an immune-compromised animal model of Duchenne muscular dystrophy | DPSCs engrafted within the host muscle, promoted angiogenesis, and reduced fibrosis. | [48] | |
| Human dental pulp pluripotent-like stem cells (DPPSC) | DPPSC injection in dystrophic mice | DPPSC differentiated into both endothelial cells and smooth muscle cells and contributed to myogenic regeneration. | [49] | |
| Diabetes | Human DPSCs, SHED | Transplantation of islet-like cell clusters (ICCs) derived from SHED into streptozotocin-induced diabetic mice | Mice transplanted with macro-capsules containing ICCs were restored to normoglycemia. | [50] |
| Mouse DPSCs | Endovenous transplantation of DPSCs into streptozotocin-induced diabetes type 1 model | Transplanted DPSCs were confirmed in or surrounding pancreatic islets and DPSC transplantation improved pancreatic damage, renal function, and painful neuropathy. | [51] | |
| Rat DPSCs | Transplantation into the unilateral hind limb skeletal muscles of diabetic rat | DPSC transplantation improved sciatic nerve conduction velocity and sciatic nerve blood flow, ameliorated sural nerve axonal circularity, and decreased the macrophages in diabetic sciatic nerves. | [52] | |
| Human DPSCs | Intravenous or intramuscular transplantation of DPSCs into streptozotocin -induced neuropathic rats | DPSC transplantation through both routes was beneficial for the retrieval of neuropathic parameters of diabetic neuropathy. | [53] | |
| Liver disease | SHED | Intrasplenic transplantation into the liver dysfunction of carbon tetrachloride-treated mice | Transplanted SHED directly transformed into hepatocytes, improved hepatic dysfunction, and led to anti-fibrotic and anti-inflammatory effects in the recipient livers. | [54] |
| Human DPSCs | Transplantation of hepatically differentiated DPSCs into carbon tetrachloride-treated mice | The combination of melatonin and hDPSC significantly suppressed liver fibrosis and restored alanine transaminase, aspartate transaminase, and ammonia levels. | [55] | |
| Human DPSCs | Transplantation of hepatically differentiated DPSCs into carbon tetrachloride-treated mice | The combination of hDPSCs and PIN1 inhibitor juglone into CCl4-injured mice significantly suppressed liver fibrosis and restored serum levels of alanine transaminase, aspartate transaminase, and ammonia. | [56] | |
| Eye disease | Human DPSCs | Keratocyte differentiated DPSCs were injected into mouse corneal stroma | Human DPSCs produced corneal stromal extracellular matrix and did not affect corneal transparency or induce immunological rejection. | [57] |
| SHED sheet | Transplantation onto the corneal bed in rabbit model of limbal stem cell deficiency | Transplantation of a tissue-engineered human undifferentiated immature DPSC sheet was successful for the reconstruction of corneal epithelium. | [58] | |
| Human DPSCs | Contact lenses pre-seeded with DPSCs were transferred onto corneas | DPSCs transdifferentiated into corneal epithelial progenitors and established a barrier to the invasion of the cornea. | [59] | |
| Human DPSCs | Intravitreal transplantation in rodent model of glaucoma | DPSC provided significant protection from retinal ganglion cell (RGC) loss and preserved visual function. | [60] | |
| Immune disease | SHED | Systemic transplantation to SLE-like murine model | SHED transplantation was capable of effectively reversing systemic lupus erythematosus (SLE)-associated disorders in SLE-like mice. | [61] |
| Oral disease | Canine CD105+ DPSCs | Transplantation into an adult canine model of pulpectomy | Complete pulp regeneration with neurogenesis and vasculogenesis occurred after transplantation of CD105+ DPSCs in pulpectomized root canals in canines. | [62] |
| SHED | Injection into full-length human root canals model of pulpectomy | SHED survive and differentiate into odontoblasts when transplanted into full-length human root canals with injectable scaffolds. | [63] | |
| Canine DPSCs | Implantation into canine periodontitis model | Regeneration of cementum, bone, and periodontal ligament was observed. | [64] | |
| Canine DPSCs, deciduous tooth stem cells | Transplantation into canine alveolar bone atrophy model | Regeneration of well-formed mature bone was observed and dental implants were successfully installed in the regenerated bone. | [21] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, Y.; Nakamura-Yamada, S.; Kusano, K.; Baba, S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int. J. Mol. Sci. 2019, 20, 1132. https://doi.org/10.3390/ijms20051132
Yamada Y, Nakamura-Yamada S, Kusano K, Baba S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. International Journal of Molecular Sciences. 2019; 20(5):1132. https://doi.org/10.3390/ijms20051132
Chicago/Turabian StyleYamada, Yoichi, Sayaka Nakamura-Yamada, Kaoru Kusano, and Shunsuke Baba. 2019. "Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review" International Journal of Molecular Sciences 20, no. 5: 1132. https://doi.org/10.3390/ijms20051132
APA StyleYamada, Y., Nakamura-Yamada, S., Kusano, K., & Baba, S. (2019). Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. International Journal of Molecular Sciences, 20(5), 1132. https://doi.org/10.3390/ijms20051132




