Halophytic Grasses, a New Source of Nutraceuticals? A Review on Their Secondary Metabolites and Biological Activities
Abstract
1. Introduction
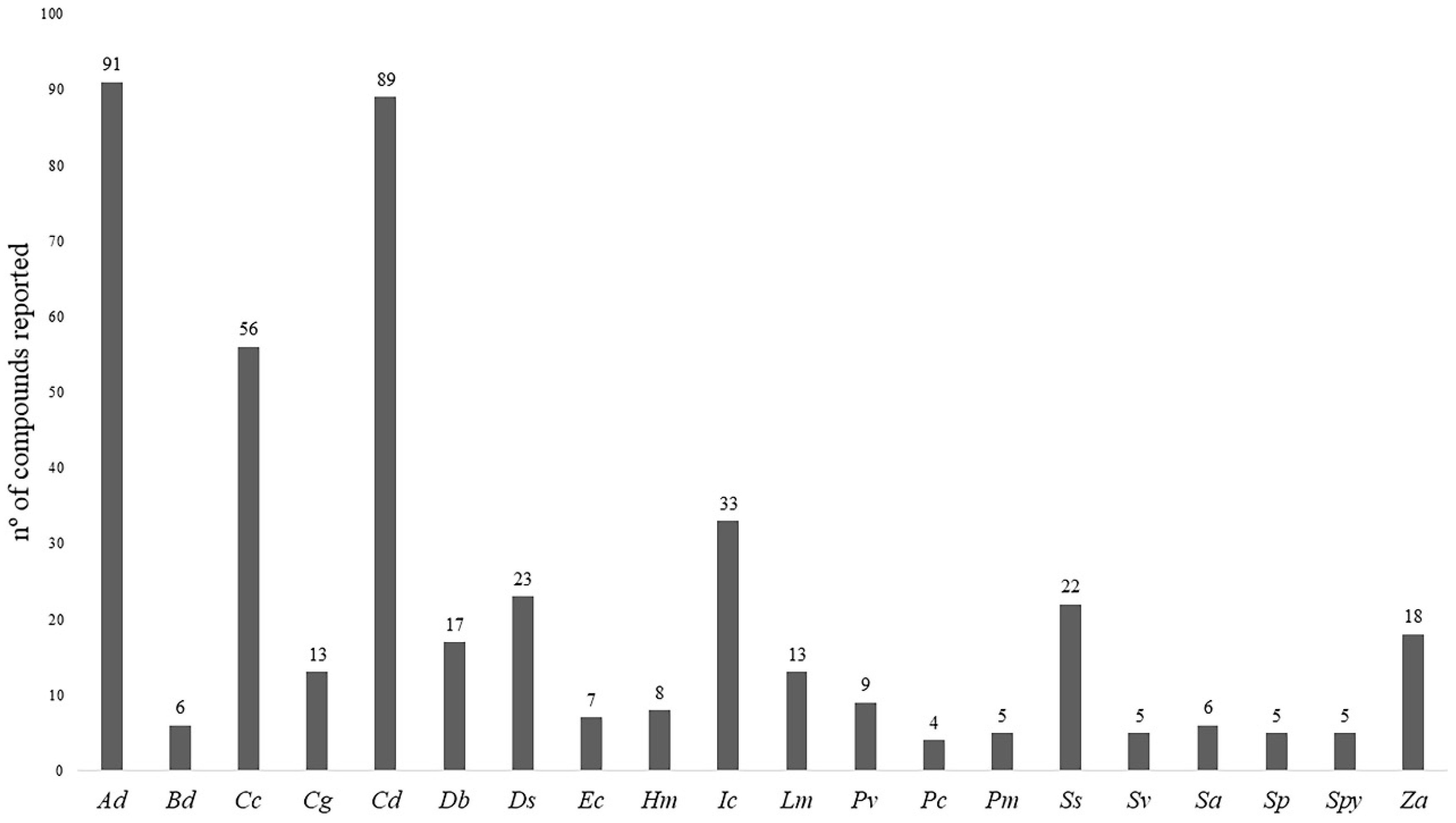
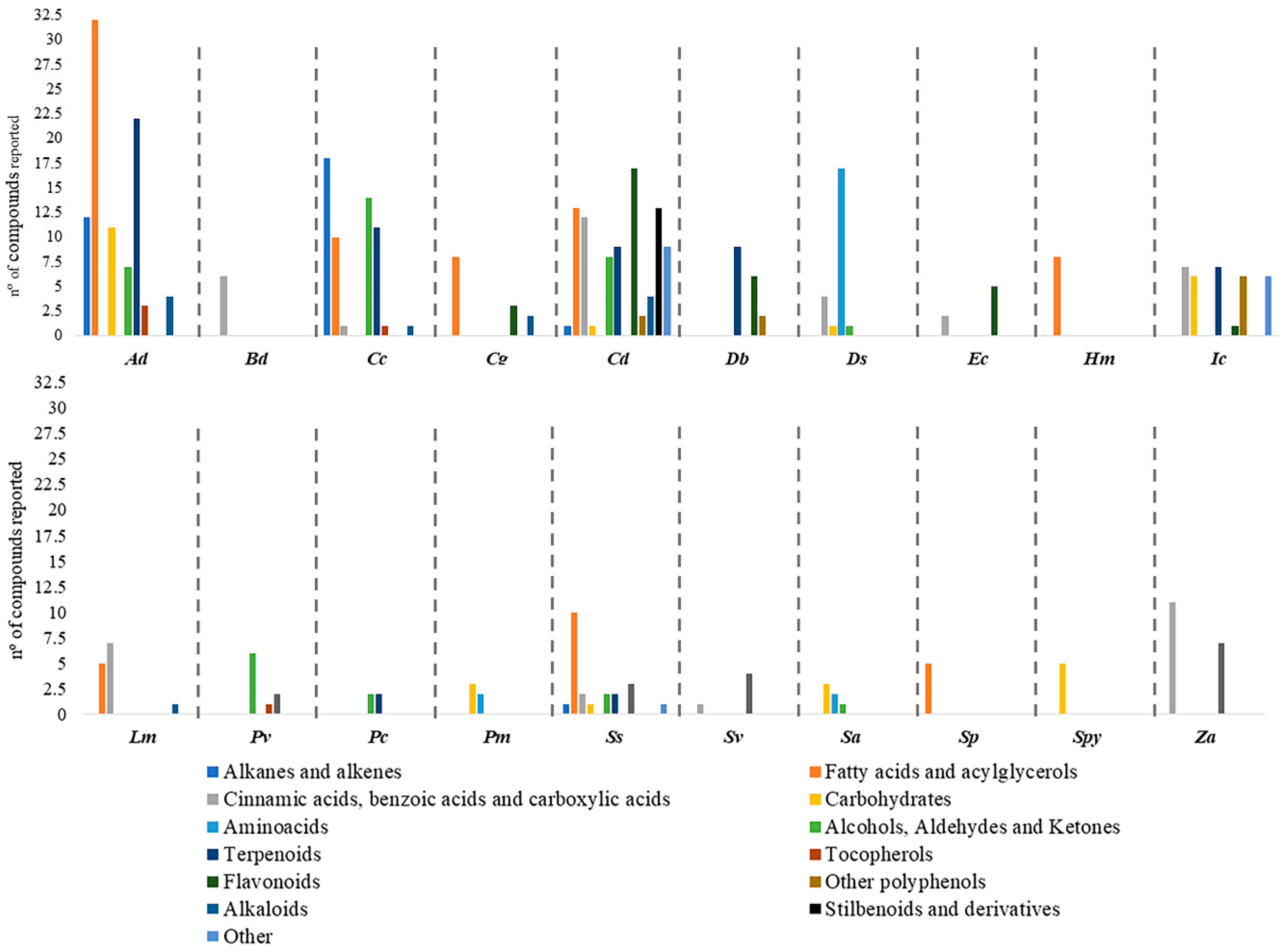
2. Phytoconstituents of Halophytic Grasses
2.1. Alkanes and Alkenes
2.2. Fatty Acids, Acylglycerols And Derivatives
2.3. Cinnamic Acids, Benzoic Acids, and Other Short Chain Carboxylic Acids
2.4. Carbohydrates and Amino Acids
2.5. Terpenoids
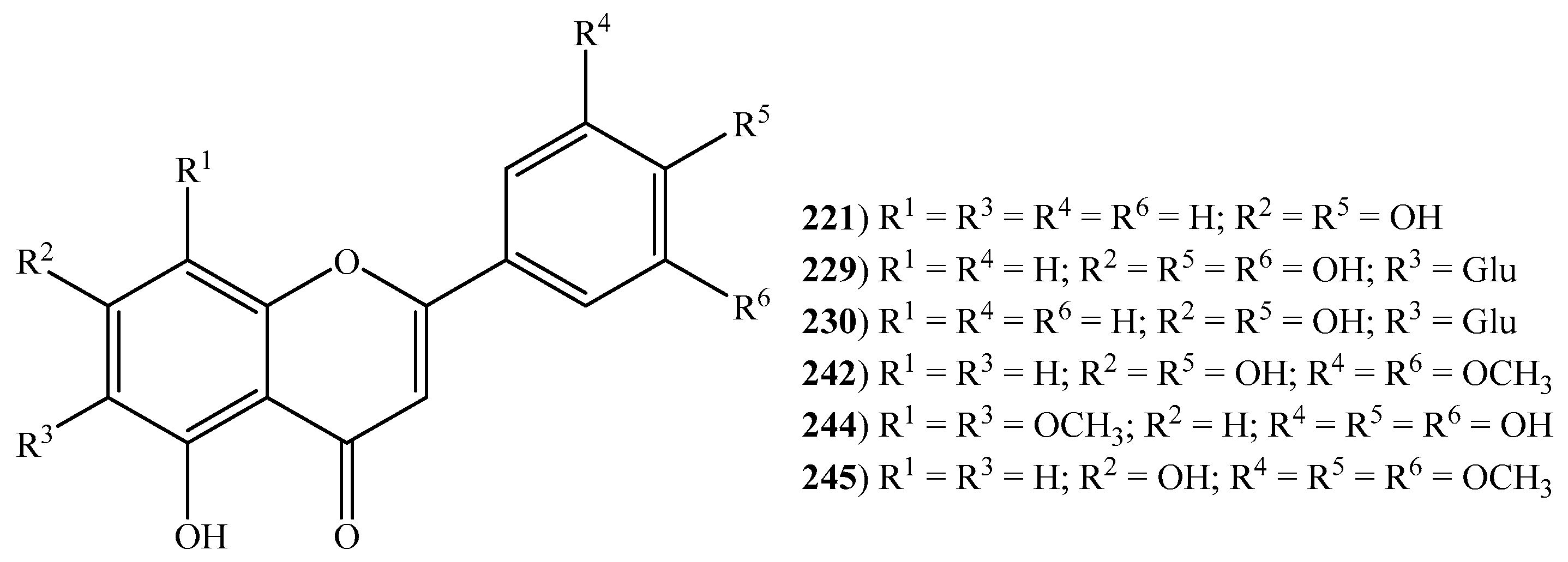
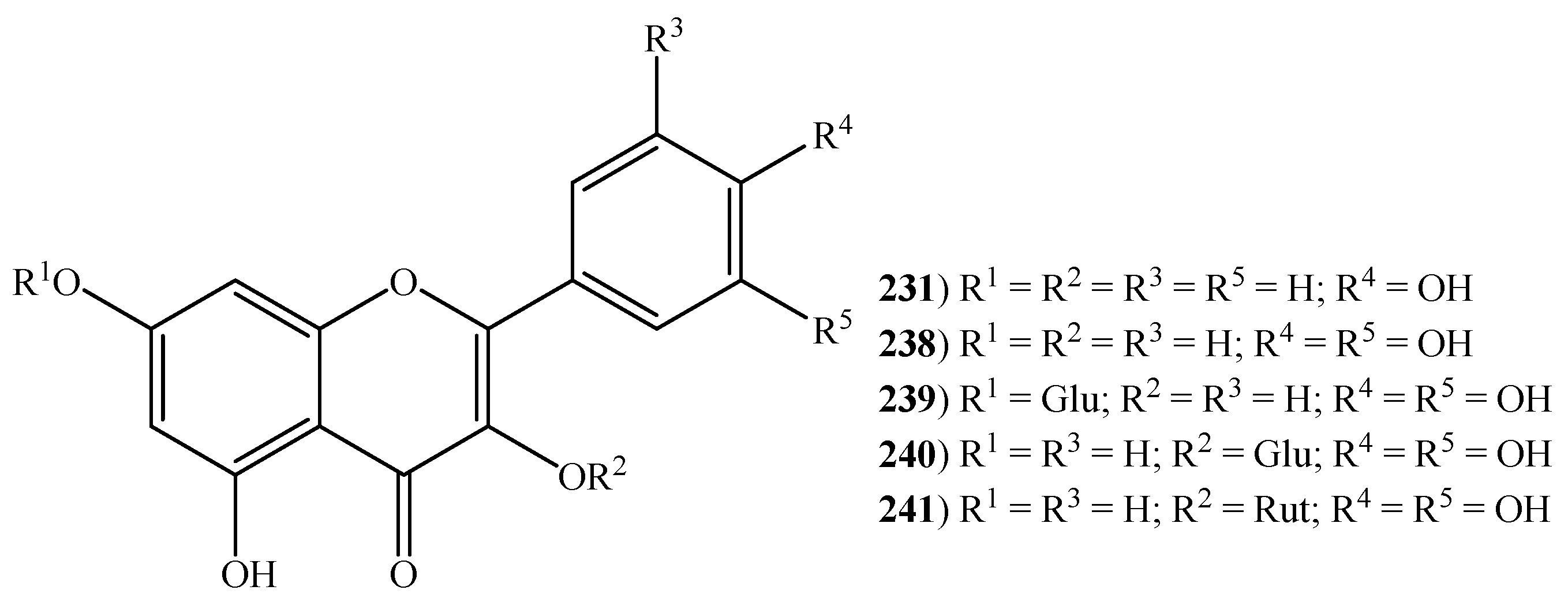
2.6. Flavonoids
2.7. Other Polyphenols
2.8. Stilbenoids and Derivatives
2.9. Miscellaneous Compounds
3. Biological Activities of Halophytic Grasses
3.1. Antibacterial, Antifungal And Antiviral Activities
3.2. Spasmolytic and Antidiarrheal Activities
3.3. Anti-Inflammatory and Antioxidant Effects
3.4. Anti-Diabetic and Anti-Obesity Activities
3.5. Anticarcinogenic Activity
3.6. Hepatoprotective Activity
3.7. Other Activities
4. Conclusion, Discussion and Future Perspectives
Supplementary Materials
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ERK | Extracellular-signal-regulated kinase |
| IC50 | Half minimum inhibitory concentration |
| LD50 | Median lethal dose |
| MIC | Minimum inhibitory concentration |
| MAPK | Mitogen-activated protein kinase |
| mRNA | Messenger ribonucleic acid |
| NF-kβ | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PPAR | Peroxisome proliferator-activated receptor |
| PUFA | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
References
- Kellogg, E.A. The Families and Genera of Vascular Plants. Flowering Plants. Monocots. Poaceae; Kubitzki, K., Ed.; Springer: Berlin, Germany, 2015; Volume 13. [Google Scholar]
- Landi, S.; Hausman, J.F.; Guerriero, G.; Esposito, S. Poaceae vs. abiotic stress: Focus on drought and salt stress, recent insights and perspectives. Front. Plant Sci. 2017, 8, 1214. [Google Scholar] [CrossRef] [PubMed]
- Dashora, K.; Gosavi, K.V.C. Grasses: An underestimated medicinal repository. J. Med. Plants Stud. 2013, 1, 151–157. [Google Scholar]
- Bennett, T.H.; Flowers, T.J.; Bromham, L. Repeated evolution of salt-tolerance in grasses. Biol. Lett. 2013, 9, 20130029. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Mangu, V.R.; Bedre, R.; Sanchez, L.; Pilcher, W.; Zandkarimi, H.; Baisakh, N. Salt adaptation mechanisms of halophytes: Improvement of salt tolerance in crop plants. In Elucidation of Abiotic Stress Signalling in Plants; Pandey, G.K., Ed.; Springer: New York, NY, USA, 2015; Volume 2, pp. 243–279. [Google Scholar]
- eHALOPH—Halophytes Database. Available online: https://www.sussex.ac.uk/affiliates/halophytes/ (accessed on 24 January 2019).
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Megdiche, W.; Ksouri, R. The halophytic genus Zygophyllum and Nitraria from North Africa: A phytochemical and pharmacological overview. In Medicinal and Aromatic Plants of the World—Africa; Medicinal and Aromatic Plants of the World; Neffati, M., Najjaa, H., Máthé, À., Eds.; Springer: Dordrecht, The Neetherlands, 2017; Volume 3, pp. 345–356. [Google Scholar]
- Alvai, N.; Parseh, I.; Ahmadi, M.; Jafarzadeh, N.; Yari, A.R.; Chehrazi, M.; Chorom, M. Phytoremediation of total petroleum hydrocarbons from highly saline and clay soil using Sorghum halepense (L.) Pers. and Aeluropus littoralis (Guna) Parl. Soil Sediment Contam. 2017, 26, 127–140. [Google Scholar] [CrossRef]
- Toderich, K.N.; Shuyskaya, E.V.; Khujanazarov, T.M.; Ismail, S.; Kawabata, Y. The structural and functional characteristics of Asiatic desert halophytes for phytostabilization of polluted sites. In Plant Adaptation and Phytoremediation; Asharaf, M., Ozturk, M., Ahmad, M.S.A., Eds.; Springer Science + Business Media B. V.: Berlin, Germany, 2010; pp. 245–274. [Google Scholar]
- Kasowska, D.; Gediga, K.; Spiak, Z. Heavy metal and nutrient uptake in plants colonizing post-flotation copper tailings. Environ. Sci. Pollut. Res. 2018, 25, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Salas-Luévano, M.A.; Mauricio-Castillo, J.A.; González-Rivera, M.L.; Vega-Carrillo, H.R.; Salas-Muñoz, S. Accumulation and phytostabilization of As, Pb and Cd in plants growing inside mine tailings reforested in Zacatecas, Mexico. Environ. Earth Sci. 2017, 76, 805–817. [Google Scholar] [CrossRef]
- Yu, S.; Sheng, L.; Zhang, C.; Deng, H. Physiological response of Arundo donax to cadmium stress by Fourier transform infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Ye, Z.H.; Wong, M.H. Lead and zinc accumulation and tolerance in populations of six wetland plants. Environ. Pollut. 2006, 141, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Caballero, M.D.R.; Alarcón-Herrera, M.T.; Valles-Aragón, M.C.; Melgoza-Castillo, A.; Ojeda-Barrios, D.L.; Leyva-Chávez, A. Germination of Bouteloua dactyloides and Cynodon dactylon in a multi-polluted soil. Sustainability 2017, 9, 81. [Google Scholar] [CrossRef]
- Abinaya, S.; Saraswathi, R.; Rajamohan, S.; Mohammed, S.A. Phyto-remediation of total dissolved solids (TDS) by Eichhornia Crassipes, Pistia Stratiotes and Chrysopogon Zizanioides from second stage RO-Brine solution. Res. J. Chem. Environ. 2018, 22, 36–41. [Google Scholar]
- Yasar, A.; Khan, M.; Tabinda, A.B.; Hayyat, M.U.; Zaheer, A. Percentage uptake of heavy metals of different macrophytes in stagnant and flowing textile effluent. J. Anim. Plant Sci. 2013, 23, 1709–1713. [Google Scholar]
- Pouladi, S.F.; Anderson, B.C.; Wootton, B.; Rozema, L. Evaluation of phytodesalination potential of vegetated bioreactors treating greenhouse effluent. Water 2016, 8, 233. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, W.; Wu, L.; Bai, L. The uptake, accumulation, and toxic effects of cadmium in barnyardgrass (Echinochloa crus-galli). Pol. J. Environ. Stud. 2017, 26, 779–784. [Google Scholar] [CrossRef]
- Matsodoum, N.; Djumyom, W.; Djocgoue, P.F.; Kengne, N.; Wanko, N. Potentialities of six plant species on phytoremediation attempts of fuel oil-contaminated soils. Water. Air. Soil Pollut. 2018, 229, 88–106. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Zhao, Q.; Zhao, X.-R.; Wang, W.-W.; Wang, K.-L.; Zeng, D.-H. Assessment of phytoremediation for magnesium-rich dust contaminated soil in a magnesite mining area. Chin. J. Ecol. 2014, 33, 2782–2788. [Google Scholar]
- Boisson, S.; Le, S.; Collignon, J.; Séleck, M.; Malaisse, F.; Ngoy, S.; Faucon, M.-P.; Mahy, G. Potential of copper-tolerant grasses to implement phytostabilisation strategies on polluted soils in South D. R. Congo: Poaceae candidates for phytostabilisation. Environ. Sci. Pollut. Res. 2016, 23, 13693–13705. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.; Poschenrieder, C.; Marcenò, C.; Gallego, J.R.; Jiménez-Gámez, D.; Bueno, A.; Afif, E. Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, north of Spain. J. Geochem. Explor. 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Mahdavian, K.; Ghaderian, S.M.; Torkzadeh-Mahani, M. Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead–zinc mining area, Iran. J. Soils Sediments 2017, 17, 1310–1320. [Google Scholar] [CrossRef]
- Ng, C.C.; Law, S.H.; Amru, N.B.; Motior, M.R.; Radzi, B.M. Phyto-assessment of soil heavy metal accumulation in tropical grasses. J. Anim. Plant Sci. 2016, 26, 686–696. [Google Scholar]
- Ahsan, M.T.; Najam-ul-haq, M.; Saeed, A.; Mustafa, T.; Afzal, M. Augmentation with potential endophytes enhances phytostabilization of Cr in contaminated soil. Environ. Sci. Pollut. Res. 2018, 25, 7021–7032. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, L.; Wang, Q.; Zhang, X.; Hu, X. Effect of tea saponin on phytoremediation of Cd and pyrene in contaminated soils by Lolium multiflorum. Environ. Sci. Pollut. Res. 2017, 24, 18946–18952. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Lygeum spartum L.: A review of a candidate for West Mediterranean arid rangeland rehabilitation. Rangel. J. 2016, 38, 493–499. [Google Scholar] [CrossRef]
- McIntosh, P.; Schulthess, C.P.; Kuzovkina, Y.A.; Guillard, K. Bioremediation and phytoremediation of total petroleum hydrocarbons (TPH) under various conditions. Int. J. Phytoremediation 2017, 19, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Okem, A.; Kulkarni, M.G.; Van, S. Enhancing phytoremediation potential of Pennisetum clandestinum Hochst in cadmium-contaminated soil using smoke-water and smoke-isolated karrikinolide. Int. J. Phytoremediation 2015, 17, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, S.; Yang, H.; Huang, Y.; Zheng, L.; Yuan, J.; Zhou, S. Comparative study on effects of four energy plants growth on chemical fractions of heavy metals and activity of soil enzymes in copper mine tailings. Int. J. Phytoremediation 2018, 20, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Sepehr, M.F.; Nourozi, F. Physiological responses of Polypogon monspeliensis L. in petroleum-contaminated soils. Iran. J. Plant Physiol. 2018, 8, 2391–2401. [Google Scholar]
- Ghasemi, F.; Ebrahimi, M.; Pozesh, S. Lead phytoremediation capacity of Puccinellia distans (Jacq.) Parl. using EDTA and DTPA and associated potential leaching risk. Glob. Nest J. 2017, 19, 359–366. [Google Scholar]
- Aihemaiti, A.; Jiang, J.; Li, D.; Li, T.; Zhang, W.; Ding, X. Toxic metal tolerance in native plant species grown in a vanadium mining area. Environ. Sci. Pollut. Res. 2017, 24, 26839–26850. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.J.; Lyu, S.S.; Chen, X.G.; Jin, A.M.; Loh, P.S.; Li, F.; Jiang, Y.; Yang, X.H.; Yan, K.K.; Lou, Z.H. Heavy metal distribution and accumulation in the Spartina alterniflora from the Andong tidal flat, Hangzhou Bay, China. Environ. Earth Sci. 2017, 76, 627–641. [Google Scholar] [CrossRef]
- Agarry, S.E.; Aremu, M.O.; Aworanti, O.A. Biostimulation and phytoremediation treatment strategies of gasoline-nickel Co-contaminated soil. Soil Sediment Contam. 2014, 23, 227–244. [Google Scholar] [CrossRef]
- Eisa, S.S.; Eid, M.A. Assessment of the phytoextraction potential of some fast growing halophytes and maize plants. Aust. J. Basic Appl. Sci. 2011, 5, 88–95. [Google Scholar]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jia, S. Flora of China Forage Plants; China Agriculture Press: Beijing, China, 1987. [Google Scholar]
- Al-Snafi, A.E. The constituents and biological effects of Arundo donax—A review. Int. J. Pharm. Res. 2015, 6, 34–40. [Google Scholar]
- Golla, U.; Gajam, P.K.; Bhimathati, S.S. Evaluation of diuretic and laxative activity of hydro-alcoholic extract of Desmostachya bipinnata (L.) Stapf in rats. J. Integr. Med. 2014, 12, 372–378. [Google Scholar] [CrossRef]
- Aleem, A.; Janbaz, K.H. Ethnopharmacological evaluation of Cenchrus ciliaris for multiple gastrointestinal disorders. Bangladesh J. Pharmacol. 2017, 12, 125–132. [Google Scholar] [CrossRef]
- Kafi, M.; Khan, M.A. Crop and Forage Production Using Saline Waters; Centre for Science & Thecnology of Non-Aligned and other developing countries (NAM S&T Centre) Daya Publishing House: New Delhi, India, 2008; Volume 1. [Google Scholar]
- Jia, J.; Cui, X.; Wu, J.; Wang, J.; Wang, G. Physiological and biochemical responses of halophyte Kalidium foliatum to salt stress. Afr. J. Biotechnol. 2011, 10, 11468–11476. [Google Scholar]
- Hameed, M.; Ashraf, M.; Ahmad, M.S.A.; Naz, N. Structural and functional adaptations in plants for salinity tolerance. In Plant Adaptation and Phytoremediation; Asharaf, M., Ozturk, M., Ahmad, M.S.A., Eds.; Springer: Berlin, Germany, 2010; pp. 151–173. [Google Scholar]
- Zhang, Y.; Xu, H.; Chen, H.; Wang, F.; Huai, H. Diversity of wetland plants used traditionally in China: A literature review. J. Ethnobiol. Ethnomed. 2014, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Ndathi, A.J.N.; Nyangito, M.M.; Musimba, N.K.R.; Mitaru, B.N. Farmers’ preference and nutritive value of selected indigenous plant feed materials for cattle in drylands of South-eastern Kenya. Livest. Res. Rural Dev. 2012, 24, 28. [Google Scholar]
- Dangol, D.R. Traditional uses of plants of commonland habitats in Western Chitwan, Nepal. J. Inst. Agric. Anim. Sci. 2008, 29, 71–78. [Google Scholar] [PubMed]
- Dedrilkumar, S.; Binu, M. Wild edible plants used by Meitei community of Eastern Himalayas, India. Int. J. Agric. Sci. 2016, 8, 2699–2702. [Google Scholar]
- Subramaniam, S.; Sivasubramanian, A. Tradition to therapeutics: Sacrificial medicinal grasses Desmostachya bipinnata and Imperata cylindrica of India. Boletin Latinoam. Caribe Plantas Med. Aromat. 2015, 14, 156–170. [Google Scholar]
- Svanberg, I.; Ægisson, S. Edible wild plant use in the Faroe Islands and Iceland. Acta Soc. Bot. Pol. 2012, 81, 233–238. [Google Scholar] [CrossRef]
- Bunzel, M.; Allerdings, E.; Sinwell, V.; Ralph, J.; Steinhart, H. Cell wall hydroxycinnamates in wild rice (Zizania aquatica L.) insoluble dietary fibre. Eur. Food Res. Technol. 2002, 214, 482–488. [Google Scholar] [CrossRef]
- Arora, S.; Kumar, G.; Meena, S. Gas chromatography-mass spectroscopy analysis of root of an economically important plant, Cenchrus ciliaris L. from Thar desert, Rajasthan (India). Asian J. Pharm. Clin. Res. 2017, 10, 64–69. [Google Scholar] [CrossRef]
- Al-Mazroa, S.A.; Al-Wahaibi, L.H.; Mousa, A.A.; Al-Khathlan, H.Z. Essential oil of some seasonal flowering plants grown in Saudi Arabia. Arab. J. Chem. 2015, 8, 212–217. [Google Scholar] [CrossRef]
- Asthana, A.; Kumar, A.; Dora, J.; Gangwar, S. Pharmacological perspectives of Cynodon dactylon. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 1135–1147. [Google Scholar]
- Murugasan, T.; Rangan, P.; Alagumuthu, T. Extraction and characterization of wax from Saccharum spontaneum L. Pharm. Lett. 2016, 8, 387–392. [Google Scholar]
- Bourdenx, B.; Bernard, A.; Domergue, F.; Pascal, S.; Léger, A.; Roby, D.; Pervent, M.; Vile, D.; Haslam, R.P.; Napier, J.A.; et al. Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol. 2011, 156, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Xiong, L.; Li, W.; Zhu, J.-K.; Zhu, J. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 2011, 23, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Y.M.; Yang, S.; Song, B.A.; Xu, G.F.; Bhadury, P.S.; Jin, L.H.; Hu, D.Y.; Liu, F.; Xue, W.; et al. Studies on the chemical constituents and anticancer activity of Saxifraga stolonifera (L.) Meeb. Bioorg. Med. Chem. 2008, 16, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, V.; Gupta, S.; Sharma, N.; Kumar, A.; Lone, N.A.; Khullar, M.; Dutt, P.; Sharma, P.R.; Bhagat, A.; Ahmed, Z. Anti-inflammatory potential of hentriacontane in LPS stimulated RAW 264.7 cells and mice model. Biomed. Pharmacother. 2017, 92, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Gayathri, K.; Venkatalakshmi, R.; Sasikala, C. Chemical constituents of hydro alcoholic extract and phenolic fraction of Cynodon dactylon. Int. J. ChemTech Res. 2010, 2, 149–154. [Google Scholar]
- Mozafari, A.A.; Vafaee, Y.; Shahyad, M. Phytochemical composition and in vitro antioxidant potential of Cynodon dactylon leaf and rhizome extracts as affected by drying methods and temperatures. J. Food Sci. Technol. 2018, 55, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Jananie, R.K.; Priya, V.; Vijayalakshmi, K. In vitro assessment of free radical scavenging activity of Cynodon dactylon. J. Chem. Pharm. Res. 2011, 3, 647–654. [Google Scholar]
- Weber, D.J.; Ansari, R.; Gul, B.; Ajmal, K. Potential of halophytes as source of edible oil. J. Arid Environ. 2007, 68, 315–321. [Google Scholar] [CrossRef]
- Nnabugwu, A.E.; Uchenna, A.P. Nutrient and antioxidant properties of oils from bagasses, agricultural residues, medicinal plants, and fodders. J. Am. Coll. Nutr. 2018, 38, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Matos, A.R.; Marques, J.C.; Caçador, I. Leaf fatty acid remodelling in the salt-excreting halophytic grass Spartina patens along a salinity gradient. Plant Physiol. Biochem. 2018, 124, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Fatty Acid Metabolism. In Bichemistry; W.H. Freeman: New York, NY, USA, 2002; Volume 1. [Google Scholar]
- Agostoni, C.; Moreno, L.; Shamir, R. Palmitic acid and health: Introduction. Crit. Rev. Food Sci. Nutr. 2016, 56, 1941–1942. [Google Scholar] [CrossRef] [PubMed]
- Sui, N.; Li, M.; Li, K.; Song, J.; Wang, B.S. Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica 2010, 48, 623–629. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- National Research Council Lipids; National Academies Press: Washington, DC, USA, 1989.
- Morise, A.; Sérougne, C.; Gripois, D.; Blouquit, M.F.; Lutton, C.; Hermier, D. Effects of dietary alpha linolenic acid on cholesterol metabolism in male and female hamsters of the LPN strain. J. Nutr. Biochem. 2004, 15, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P. Effects of dietary fatty acids on breast and prostate cancers: Evidence from in vitro experiments and animal studies. Am. J. Clin. Nutr. 1997, 66, 1513S–1522S. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H. Evaluation of anti-tubercular activity of linolenic acid and conjugated-linoleic acid as effective inhibitors against Mycobacterium tuberculosis. Asian Pac. J. Trop. Med. 2016, 9, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Toomey, S.; Harhen, B.; Roche, H.M.; Fitzgerald, D.; Belton, O. Profound resolution of early atherosclerosis with conjugated linoleic acid. Atherosclerosis 2006, 187, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Cagliari, A.; Margis, R.; dos Santos Maraschin, F.; Turchetto Zolet, A.C.; Loss, G.; Margis-Pinheiro, M. Biosynthesis of triacylglycerols (TAGs) in plants and alga. Int. J. Plant Biol. 2011, 2, 40–52. [Google Scholar] [CrossRef]
- Li, Y.; Beisson, F.; Ohlrogge, J.; Pollard, M. Monoacylglycerols are components of root waxes and can be produced in the aerial cuticle by ectopic expression of a suberin-associated acyltransferase. Plant Physiol. 2007, 144, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, M.G. The essentials of essential fatty acids. J. Diet. Suppl. 2009, 6, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.K.; Pandit, S.; Chakrabarti, S.; Banerjee, S.; Poyra, N.; Seal, T. Evaluation of Cynodon dactylon for wound healing activity. J. Ethnopharmacol. 2017, 197, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, R.; Devadasu, C.; Srinivasa, B. Isolation, characterization, and RP-HPLC estimation of p-coumaric acid from methanolic extract of durva grass (Cynodon dactylon Linn.) (Pers.). Int. J. Anal. Chem. 2015, 2015, 201386. [Google Scholar] [CrossRef] [PubMed]
- Hartley, R.D.; Buchan, H. High-performance liquid chromatography of phenolic acids and aldehydes derived from plants or from the decomposition of organic matter in soil. J. Chromatogr. A 1979, 180, 139–143. [Google Scholar] [CrossRef]
- O’Donovan, D.G.; Horan, H. The biosynthesis of annuloline, a unique oxazole alkaloid. J. Chem. Soc. C Org. Chem. 1971, 1971, 331–334. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.-F.; Yang, L.; Chou, G.-X.; Wang, Z.-T. Four new compounds from Imperata cylindrica. J. Nat. Med. 2014, 68, 295–301. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Nugroho, A.; Song, B.M.; Park, H.J. Isoeugenin, a novel nitric oxide synthase inhibitor isolated from the rhizomes of Imperata cylindrica. Molecules 2015, 20, 21336–21345. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, B.; Chou, G.; Yang, L.; Wang, Z. Chemical constituents from Imperata cylindrica. Zhongguo Zhongyao Zazhi 2012, 37, 2296–2300. [Google Scholar] [PubMed]
- Wu, L.; Guo, X.; Harivandi, M.A. Allelopathic effects of phenolic acids detected in buffalograss (Buchloe dactyloides) clippings on growth of annual bluegrass (Poa annua) and buffalograss seedlings. Environ. Exp. Bot. 1998, 39, 159–167. [Google Scholar] [CrossRef]
- El, M.; Motaal, A.A.; El, H.; El, F. Cytotoxic activity of phenolic constituents from Echinochloa crus-galli against four human cancer cell lines. Braz. J. Pharmacogn. 2016, 26, 62–67. [Google Scholar]
- Yong, S.K.; Eun, Y.K.; Won, J.K.; Woo, K.K.; Chang, M.K. Antioxidant constituents from Setaria viridis. Arch. Pharm. Res. 2002, 25, 300–305. [Google Scholar]
- Sumczynski, D.; Kotásková, E.; Orsavová, J.; Valášek, P. Contribution of individual phenolics to antioxidant activity and in vitro digestibility of wild rices (Zizania aquatica L.). Food Chem. 2017, 218, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Yoon, B.H.; Jung, J.W.; Lee, J.-J.; Cho, Y.W.; Jang, C.G.; Jin, C.; Oh, T.H.; Ryu, J.H. Anxiolytic-like effects of sinapic acid in mice. Life Sci. 2007, 81, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Drãgan, M.; Stan, C.D.; Pânzariu, A.; Profire, L. Evaluation of anti-inflammatory potential of some new ferulic acid derivatives. Farmacia 2016, 64, 194–197. [Google Scholar]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Serafim, T.; Carvalho, S.F.; Marques, M.P.M.; Cruz, R.C.; Silva, T.B.; Garrido, J.M.P.J.; Milhaze, N.; Borges, F.; Roleira, F.M.F.; Silva, E.T.; et al. Lipophilic caffeic and ferulic acid derivatives presenting cytotoxicity against human breast cancer cells. Chem. Res. Toxicol. 2011, 24, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Kallepalli, S.; Alok, A.; Chaudhury, N.k. Protective effect of ferulic acid on ionizing radiation induced damage in bovine serum albumin. Int. J. Radiat. Res. 2014, 12, 113–121. [Google Scholar]
- Su, P.; Shi, Y.; Wang, J.; Shen, X.; Zhang, J. Anticancer agents derived from natural cinnamic acids. Anticancer Agents Med. Chem. 2015, 15, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Lin, C.; Zheng, Y.; Hou, J.; Li, D. The cardioprotective effects of citric acid and L-malic acid on myocardial ischemia/reperfusion injury. J. Evid. Based Complement. Altern. Med. 2013, 2013, 820695. [Google Scholar] [CrossRef] [PubMed]
- Leleka, M.; Zalis’ka, O.; Kozyr, G. Screening research of pharmaceutical compositions based on succinic acid, ascorbic acid and rutin. J. Pharm. Pharmacol. 2016, 4, 486–491. [Google Scholar]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Moscatello, S.; Fagnano, M.; Fiorentino, N.; Impagliazzo, A.; Battistelli, A. Chemical composition and yield of rhizome biomass of Arundo donax L. grown for biorefinery in the Mediterranean environment. Biomass Bioenergy 2017, 107, 191–197. [Google Scholar] [CrossRef]
- Pinilla, V.; Luu, B. Isolation and partial characterization of immunostimulating polysaccharides from Imperata cylindrica. Planta Med. 1999, 65, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, H.R.; Gaff, D.F.; Williams, R.P.W.; Gianello, R.D. Contents of sugars in leaves of drying desiccation tolerant flowering plants, particularly grasses. Plant Growth Regul. 1998, 24, 185–191. [Google Scholar] [CrossRef]
- Gorham, J.; Hughes, L.; Wyn, J. Chemical composition of salt-marsh plants from Ynys Môn (Anglesey): The concept of physiotypes. Plant Cell Environ. 1980, 3, 309–318. [Google Scholar] [CrossRef]
- Fan, T.W.M.; Colmer, T.D.; Lane, A.N.; Higashi, R.M. Determination of metabolites by 1H NMR and GC: Analysis for organic osmolytes in crude tissue extracts. Anal. Biochem. 1993, 214, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Tanaka, M.; Honda, S. Trehalose extends longevity in the nematode Caenorhabditis elegans. Aging Cell 2010, 9, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.C.C.; Scio, E. The biological activities and chemical composition of Pereskia species (Cactaceae)—A review. Plant Foods Hum. Nutr. 2014, 69, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Quintão, N.L.M.; Rocha, L.W.; Silva, G.F.; Reichert, S.; Claudino, V.D.; Lucinda-Silva, R.M.; Malheiros, A.; Souza, M.M.D.; Filho, V.C.; Bellé Bresolin, T.M. Contribution of α,β-amyrenone to the anti-inflammatory and antihypersensitivity effects of Aleurites moluccana (L.) Willd. BioMed Res. Int. 2014, 2014, 636839. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, S.D.; Elmore, C.J.; Bongers, K.S.; Ebert, S.M.; Fox, D.K.; Dyle, M.C.; Bullard, S.A.; Adams, C.M. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver sisease. PLoS ONE 2012, 7, e39332. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; Perazzo, F.F.; Tavares Carvalho, J.C.; Bastos, J.K. Anti-inflammatory and analgesic activities of the ethanolic extracts from Zanthoxylum riedelianum (Rutaceae) leaves and stem bark. J. Pharm. Pharmacol. 2007, 59, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Casuga, F.P.; Castillo, A.L.; Corpuz, M.J.-A.T. GC–MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco) (Moraceae) leaves. Asian Pac. J. Trop. Biomed. 2016, 6, 957–961. [Google Scholar] [CrossRef]
- Borrione, P.; Rizzo, M.; Quaranta, F.; Ciminelli, E.; Fagnani, F.; Parisi, A.; Pigozzi, F. Consumption and biochemical impact of commercially available plant-derived nutritional supplements. An observational pilot-study on recreational athletes. J. Int. Soc. Sports Nutr. 2012, 9, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.W.; Jones, P.J. Dietary phytosterols: A review of metabolism, benefits and side effects. Life Sci. 1995, 57, 195–206. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.; Uckoo, R.; Patil, B. Inhibition of Escherichia coli O157:H7 motility and biofilm by β-Sitosterol glucoside. Biochim. Biophys. Acta 2013, 1830, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Antwi, A.O.; Obiri, D.D.; Osafo, N. Stigmasterol modulates allergic airway inflammation in guinea pig model of ovalbumin-induced asthma. Mediat. Inflamm. 2017, 2017, 2953930. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.G.; Li, G.; Barbosa-Lorenzi, V.C.; Hao, M. Stigmasterol prevents glucolipotoxicity induced defects in glucose-stimulated insulin secretion. Sci. Rep. 2017, 7, 9536. [Google Scholar] [CrossRef] [PubMed]
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a renewable source of biologically active compounds. Pure Appl. Chem. 2017, 89, 1105–1118. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-α-Pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cho, S.K. Phytol induces apoptosis and ROS-mediated protective autophagy in human gastric adenocarcinoma AGS cells. Biochem. Anal. Biochem. 2015, 4, 211–212. [Google Scholar]
- Olofsson, P.; Hultqvist, M.; Hellgren, L.I.; Holmdahl, R. Phytol: A chlorophyll component with anti-inflammatory and metabolic properties. In Recent Advances in Redox Active Plant and Microbial Products; Jacob, C., Kirsch, G., Slusarenko, A.J., Winyard, P.G., Burkholz, T., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 345–359. [Google Scholar]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids-food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar] [PubMed]
- Bonesi, M.; Loizzo, M.R.; Menichini, F.; Tundis, R. Flavonoids in treating psoriasis. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 281–294. [Google Scholar]
- Bone, K.; Mills, S. How to use the monographs. In Principles and Practice of Phytotherapy, 2nd ed.; Bone, K., Mills, S., Eds.; Churchill Livingstone: London, UK, 2013; pp. 353–961. [Google Scholar]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Ramirez-Sanchez, I.; Perkins, G.A.; Murphy, A.; Taub, P.R.; Ceballos, G.; Villarreal, F.J.; Hogan, M.C.; Malek, M.H. (−)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J. Physiol. 2011, 589, 4615–4631. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sanchez, I.; Nogueira, L.; Moreno, A.; Murphy, A.; Taub, P.; Perkins, G.; Ceballos, G.; Hogan, M.; Malek, M.; Villarreal, F. Stimulatory effects of the flavanol (−)-epicatechin on cardiac angiogenesis: Additive effects with exercise. J. Cardiovasc. Pharmacol. 2012, 60, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Tatsimo, S.J.N.; de D. Tamokou, J.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Nass, J.; Saeed, M.E.M.; Schuler, B.; Efferth, T. Kaempferol as an anti-inflammatory compound with activity towards NF-κB pathway proteins. Anticancer Res. 2015, 35, 2645–2650. [Google Scholar] [PubMed]
- Cho, H.J.; Park, J.H.Y. Kaempferol induces cell cycle arrest in HT-29 Human colon cancer cells. J. Cancer Prev. 2013, 18, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Amaro, M.; Rocha, J.; Vila-Real, H.; Eduardo-Figueira, M.; Mota-Filipe, H.; Sepodes, B.; Ribeiro, M.H. Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Res. Int. 2009, 42, 1010–1017. [Google Scholar] [CrossRef]
- Visnagri, A.; Adil, M.; Kandhare, A.D.; Bodhankar, S.L. Effect of naringin on hemodynamic changes and left ventricular function in renal artery occluded renovascular hypertension in rats. J. Pharm. Bioallied Sci. 2015, 7, 121–127. [Google Scholar] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.; Ghiasvand, R.; Feizi, A.; Ghanadian, S.M.; Karimian, J. The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J. Res. Med. Sci. 2012, 17, 637–641. [Google Scholar] [PubMed]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Hornick, A.; Lieb, A.; Vo, N.P.; Rollinger, J.M.; Stuppner, H.; Prast, H. The coumarin scopoletin potentiates acetylcholine release from synaptosomes, amplifies hippocampal long-term potentiation and ameliorates anticholinergic- and age-impaired memory. Neuroscience 2011, 197, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Muthu, R.; Selvaraj, N.; Vaiyapuri, M. Anti-inflammatory and proapoptotic effects of umbelliferone in colon carcinogenesis. Hum. Exp. Toxicol. 2016, 35, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khan, R.; Khan, H.; Pervez, S.; Saboor Pirzada, A. In vivo antinociceptive and anti-inflammatory activities of umbelliferone isolated from Potentilla evestita. Nat. Prod. Res. 2014, 28, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-J.; Liu, Y.; Gu, A.-T.; Wang, F. Chemical constituents of Cynodon dactylon. Chin. Tradit. Herb. Drugs 2017, 48, 62–66. [Google Scholar]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Gromek, S.; Niesen, D.; Seeram, N.P.; Henry, G.E. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. J. Agric. Food Chem. 2011, 59, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Slater, S.J.; Seiz, J.L.; Cook, A.C.; Stagliano, B.A.; Buzas, C.J. Inhibition of protein kinase C by resveratrol. Biochim. Biophys. Acta 2003, 1637, 59–69. [Google Scholar] [CrossRef]
- Kim, H.J.; Saleem, M.; Seo, S.H.; Jin, C.; Lee, Y.S. Two new antioxidant stilbene dimers, parthenostilbenins A and B from Parthenocissus tricuspidata. Planta Med. 2005, 71, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Do, B.K.; Shim, S.M.; Kwon, H.; Lee, D.H.; Nah, A.H.; Choi, Y.J.; Lee, S.Y. Determination of cyanogenic compounds in edible plants by ion chromatography. Toxicol. Res. 2013, 29, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Golla, U.; Gajam, P.K.; Solomon, S.R. The effect of Desmostachya bipinnata (Linn.) extract on physiologically altered glycemic status in non-diabetic rats. J. Med. Sci. Faisalabad 2013, 13, 221–225. [Google Scholar] [CrossRef]
- Abdur, R.; Bashir, S.; Gilani, A.H. Calcium channel blocking activity in Desmostachya bipinnata (L.) explains its use in gut and airways disorders. Phytother. Res. 2013, 27, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-L.; Xing, F.-W. Ethnobotanical study on medicinal plants used by local Hoklos people on Hainan Island, China. J. Ethnopharmacol. 2016, 194, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.A.; Sharvanee, S.; Patel, J.; Choudhary, R.K. Chemical composition and antimicrobial activity of the essential oil of Desmostachya bipinnata Linn. Int. J. Phytomedicine 2010, 2, 436–439. [Google Scholar]
- Zorofchian, M.S.; Abdul, K.H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar]
- Singariya, P.; Kumar, P.; Mourya, K.K. Isolation of some new steroids and evaluation of bio-activity of Cenchrus ciliaris. Int. J. Res. Pharm. Sci. 2013, 3, 678–684. [Google Scholar]
- Padalia, H.; Rathod, T.; Chanda, S. Evaluation of antimicrobial potential of different solvent extracts of some medicinal plants of semi-arid region. Asian J. Pharm. Clin. Res. 2017, 10, 295–299. [Google Scholar] [CrossRef]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and analysis of terpenes/terpenoids. Curr. Protoc. Plant Biol. 2016, 1, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Shakila, R.; Meeradevi Sri, P.; Arul Antony, S.; Gopakumar, K. Antimicrobial studies on Desmostachya bipinnata Rootstock. J. Pharm. Chem. Biol. Sci. 2014, 2, 197–201. [Google Scholar]
- Ibrahim, N.H.; Awaad, A.S.; Alnafisah, R.A.; Alqasoumi, S.I.; El-Meligy, R.M.; Mahmoud, A.Z. In vitro activity of Desmostachya bipinnata (L.) Stapf successive extracts against Helicobacter pylori clinical isolates. Saudi Pharm. J. 2018, 26, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.P.; Vadia, S.H. In-vitro antioxidant activity and antibacterial assay of minor millet extracts. J. Chem. Pharm. Res. 2014, 6, 2343–2350. [Google Scholar]
- Boz, H. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Soni, A.; Dahiya, P. Screening of phytochemicals and antimicrobial potential of extracts of Vetiver zizanoides and Phragmites karka against clinical isolates. Int. J. Appl. Pharm. 2015, 7, 22–24. [Google Scholar]
- Al-Zubairi, A.S.; Abdul, A.B.; Abdelwahab, S.I.; Peng, C.Y.; Mohan, S.; Elhassan, M.M. Eleucine indica possesses antioxidant, antibacterial and citotoxic properties. Evid. Based Complement. Alternat. Med. 2011, 2011, 965370. [Google Scholar] [CrossRef] [PubMed]
- Iberahim, R.; Bahtiar, A.A.; Ibrahim, N. Anti-herpes simplex virus type-1 activity of Eleusine indica methanol extract. Malays. J. Microbiol. 2016, 12, 471–474. [Google Scholar]
- Ajaib, M.; Khan, K.M.; Perveen, S.; Shah, S. Antimicrobial and antioxidant activities of Echinochloa colona (Linn.) link and Sporobolus coromandelianus (Retz.) kunth. J. Chem. Soc. Pak. 2013, 35, 960–965. [Google Scholar]
- Gilani, A.H.; Rahman, A. Trends in ethnopharmacology. J. Ethnopharmacol. 2005, 100, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Memon, R.; Gilani, A.H. Antispasmodic and antidiarrheal activities of Valeriana hardwickii Wall. rhizome are putatively mediated through calcium channel blockade. Evid.-Based Complement. Altern. Med. 2011, 2011, 304960. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Mahmood, K.; Yusoff, I.; Qureshi, A.K. Chemical constituents of Cenchrus ciliaris L. from the Cholistan desert, Pakistan. Arch. Biol. Sci. 2013, 65, 1473–1478. [Google Scholar] [CrossRef]
- Hedge, M.; Lakshman, K.; Girija, K.; Kumar, A.; Lakshmiprasanna, V. Assessment of antidiarrheal activity of Desmostachya bipinnata L. (Poaceae) root extracts. Boletin Latinoam. Caribe Plantas Med. Aromat. 2010, 9, 312–318. [Google Scholar]
- Janbaz, K.H.; Saqib, F. Pharmacological evaluation of Dactyloctenium aegyptium: An indigenous plant used to manage gastrointestinal ailments. Bangladesh J. Pharmacol. 2015, 10, 295–302. [Google Scholar] [CrossRef]
- Gerber, M.; Boutron-Ruault, M.C.; Riboli, E.; Scalbert, A.; Siess, M.H. Food and cancer: State of the art about the protective effect of fruits and vegetables. Bull. Cancer 2002, 89, 293–312. [Google Scholar] [PubMed]
- Bhatia, S.; Shukla, R.; Venkata Madhu, S.; Kaur Gambhir, J.; Madhava Prabhu, K. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin. Biochem. 2003, 36, 557–562. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Patro, J.V.; Pradhan, D.K.; Jana, G.K. Analgesic, antipyretic and anti-inflammatory effect of the whole plant extract of Desmostachya bipinnata Stapf (Poaceae) in Albino Rats. Drug Invent. Today 2009, 1, 150–153. [Google Scholar]
- Golla, U.; Bhimathati, S.S.R. Evaluation of antioxidant and DNA damage protection activity of the hydroalcoholic extract of Desmostachya bipinnata L. Stapf. Sci. World J. 2014, 12, 372–378. [Google Scholar]
- Orfali, G.C.; Duarte, A.C.; Bonadio, V.; Martinez, N.P.; de Araújo, M.E.M.B.; Priviero, F.B.M.; Carvalho, P.O.; Priolli, D.G. Review of anticancer mechanisms of isoquercitin. World J. Clin. Oncol. 2016, 7, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Kazibwe, Z.; Pandurangan, M.; Judy, G.; Kim, D.H. Probing the impact of quercetin-7-O-glucoside on influenza virus replication influence. Phytomedicine 2016, 23, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Ko, S.H.; Yoo, D.Y.; Lee, J.Y.; Kim, Y.J.; Choi, S.M.; Kang, K.K.; Yoon, H.J.; Kim, H.; Youn, J. 5,7-Dihydroxy-3,4,6-trimethoxyflavone inhibits intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 via the Akt and nuclear factor-κB-dependent pathway, leading to suppression of adhesion of monocytes and eosinophils to bronchial epithelial cells. Immunology 2012, 137, 98–113. [Google Scholar] [PubMed]
- Vellosa, J.C.R.; Regasini, L.O.; Khalil, N.M.; da Silva Bolzani, V.; Khalil, O.A.K.; Manente, F.A.; Pasquini Netto, H.; de Faria Oliveira, O.M.M. Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclética Quím. 2011, 36, 7–20. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.-R.; Hou, Z.-X.; Liu, P.; Wang, S.-S. Anti-inflammatory effect of Imperata cylindrica. Chin. J. Clin. Rehabil. 2006, 10, 85–87. [Google Scholar]
- Choi, K.C.; Son, Y.O.; Hwang, J.M.; Kim, B.T.; Chae, M.; Lee, J.C. Antioxidant, anti-inflammatory and anti-septic potential of phenolic acids and flavonoid fractions isolated from Lolium multiflorum. Pharm. Biol. 2017, 55, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Amutha, I.D.; Kottai, M. Evaluation of in-vitro free radical scavenging potential of whole plant of Saccharum spontaneum (Linn). Int. J. PharmTech Res. 2014, 6, 1436–1440. [Google Scholar]
- Sathya, M.; Kokilavani, R. Phytochemical screening and in vitro antioxidant activity of Saccharum spontaneum Linn. Int. J. Pharm. Sci. Rev. Res. 2013, 18, 75–79. [Google Scholar]
- Devi, J.A.I.; Muthu, A.K. Isolation and characterization of active components derived from whole plant of Saccharum spontaneum (linn.). Pharm. Lett. 2015, 7, 197–203. [Google Scholar]
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.; Kim, J.; Himmeldirk, K.; Cao, Y.; Chen, X. Antidiabetes and anti-obesity activity of Lagerstroemia speciosa. Evid.-Based Complement. Altern. Med. 2007, 4, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Nagarjuna, S.; Gopala, K.M.; Srinivasa, R. Anti-diabetic activity of different solvent extracts of Dactyloctenium aegyptium in streptozotocin induced diabetic rats. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 485–493. [Google Scholar]
- Gao, D.; Zhang, Y.; Yang, F.; Lin, Y.; Zhang, Q.; Xia, Z. In vitro screening and evaluation of 37 traditional Chinese medicines for their potential to activate peroxisome proliferator-activated receptors-γ. Pharmacogn. Mag. 2016, 12, 120–127. [Google Scholar] [PubMed]
- Ong, S.L.; Nalamolu, K.R.; Lai, H.Y. Potential lipid-lowering effects of Eleusine indica (L) Gaertn. extract on high-fat-diet-induced hyperlipidemic rats. Pharmacogn. Mag. 2017, 13, S1–S9. [Google Scholar] [PubMed]
- Ong, S.L.; Paneerchelvan, S.; Lai, H.Y.; Rao, N.K. In vitro lipase inhibitory effect of thirty two selected plants in Malaysia. Asian J. Pharm. Clin. Res. 2014, 7, 19–24. [Google Scholar]
- Okokon, J.E.; Odomena, C.S.; Effiong, I.; Obot, J.; Udobang, J.A. Antiplasmodial and antidiabetic activities of Eleusine indica. Int. J. Drug Dev. Res. 2010, 2, 493–500. [Google Scholar]
- Bayala, B.; Bassole, I.H.; Scifo, R.; Gnoula, C.; Morel, L.; Lobaccaro, J.M.A.; Simpore, J. Anticancer activity of essential oils and their chemical components—A review. Am. J. Cancer Res. 2014, 4, 591–607. [Google Scholar] [PubMed]
- Rahate, K.P.; Rajasekaran, A.; Arulkumaran, K.S.G. Potential of Desmostachya bipinnata Stapf (Poaceae) root extracts in inhibition of cell proliferation of cervical cancer cell lines. Int. J. Res. Pharm. Sci. 2012, 3, 5–11. [Google Scholar]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Alothman, E.A.; Awaas, A.S.; Al-Qurayn, N.A.; Al-Kanhal, H.F.; El-Meligy, R.M.; Zain, Y.M.; Alasmary, F.A.; Alqasoumi, S.I. Anticancer effect of Cenchrus ciliaris L. Saudi Pharm. J. 2018, 26, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Hansakul, P.; Wongnoppavich, A.; Ingkaninan, K.; Seewaboon, S.; Watcharin, P. Apoptotic induction activity of Dactyloctenium aegyptium (L.) P.B. and Eleusine indica (L.) Gaerth. extracts on human lung and cervical cancer cell lines. Songklanakarin J. Sci. Technol. 2009, 31, 273–279. [Google Scholar]
- Keshava, R.; Muniyappa, N.; Gope, R.; Ramaswamaiah, A.S. Anti-cancer effects of Imperata cylindrica leaf extract on human oral squamous carcinoma cell line SCC-9 in vitro. Asian Pac. J. Cancer Prev. 2016, 17, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Sandjo, L.P.; Wiench, B.; Efferth, T. Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J. Ethnopharmacol. 2013, 149, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.P.I.; Tsai, S.Y.; Kuo, Y.H.; Pai, M.H.; Chiu, H.L.; Rodriguez, R.L.; Tang, F.Y. Caffeic acid derivatives inhibit the growth of colon cancer: Involvement of the PI3-K/Akt and AMPK signalling pathways. PLoS ONE 2014, 9, e99631. [Google Scholar] [CrossRef] [PubMed]
- Mujeeb, M.; Alam Khan, S.; Aeri, V.; Ali, B. Hepatoprotective activity of the ethanolic extract of Ficus carica Linn. leaves in carbon tetrachloride-induced hepatotoxicity in rats. Iran. J. Pharm. Res. IJPR 2011, 10, 301–306. [Google Scholar] [PubMed]
- Rahate, K.P.; Rajasekaran, A. Hepatoprotection by active fractions from Desmostachya bipinnata stapf (L.) against tamoxifen-induced hepatotoxicity. Indian J. Pharmacol. 2015, 47, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Praneetha, P.; Durgaiah, G.; Narsimha, R.; Ravi, K. In vitro hepatoprotective effect of Echinochloa colona on ethanol-induced oxidative damage in HEPG2 cells. Asian J. Pharm. Clin. Res. 2017, 10, 259–261. [Google Scholar]
- Iqbal, M.; Gnanaraj, C. Eleusine indica L. possesses antioxidant activity and precludes carbon tetrachloride (CCl 4)-mediated oxidative hepatic damage in rats. Environ. Health Prev. Med. 2012, 17, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Liaquat, M.; Asghar, R. Evaluation of methanolic extract of Phragmites karka on carbon tetrachloride-induced liver fibrosis in rat. Bangladesh J. Pharmacol. 2017, 12, 276–281. [Google Scholar] [CrossRef]
- Sreedhar Naik, B.; Dangi, N.B.; Sapkota, H.P.; Wagle, N.; Nagarjuna, S.; Sankaranand, R.; Anantha kumari, B. Phytochemical screening and evaluation of anti-fertility activity of Dactyloctenium aegyptium in male albino rats. Asian Pac. J. Reprod. 2016, 5, 51–57. [Google Scholar] [CrossRef]
- Naga, K.; Mangilal, T.; Anjaneyulu, N.; Abhinayani, G.; Sravya, N. Investigation of anti-urolithiatic activity of Brassica oleracea gongylodes and Desmostachya bipinnata in experimentally induced urolithiasis in animal models. Int. J. Pharm. Pharm. Sci. 2014, 6, 602–604. [Google Scholar]
- Ojha, S.N.; Nagore, D.H.; Ganu, G.P. In vitro and in vivo anticoagulant activity of Imperata cylindrica a novel anticoagulant lead from natural origin. Pharmacogn. J. 2010, 2, 38–43. [Google Scholar]
- Sultan, R.A.; Kabir, M.S.H.; Uddin, M.M.N.; Uddin, M.; Mahmud, Z.A.; Raihan, S.Z.; Qais, N. Ethnopharmacological investigation of the aerial part of Phragmites karka (Poaceae). J. Basic Clin. Physiol. Pharmacol. 2017, 28, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.A.S.; Varadharajan, R.; Muthumani, P.; Meera, R.; Devi, P.; Kameswari, B. Psychopharmacological studies on the stem of Saccharum spontaneum. Int. J. PharmTech Res. 2010, 2, 319–321. [Google Scholar]
- Parvathy, N.G.; Padma, R.; Renjith, V.; Rahate, K.P.; Saranya, T.S. Phytochemical screening and anthelmintic activity of methanolic extract of Imperata cylindrica. Int. J. Pharm. Pharm. Sci. 2012, 4, 232–234. [Google Scholar]
- Manibusan, M.K.; Odin, M.; Eastmond, D.A. Postulated carbon tetrachloride mode of action: A review. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2007, 25, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. 2010, 67, 3–12. [Google Scholar] [PubMed]
- Chemat, F.; Vian, M.A. Alternative Solvents for Natural Products Extraction, 1st ed.; Springer: Berlin, Germany, 2014. [Google Scholar]
- United Nations. Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or Not Approved by Governments: Chemicals; United Nations Publications: New York, NY, USA, 2009. [Google Scholar]
- Dutta, D.; Puzari, K.C.; Gogoi, R.; Dutta, P.; Dutta, D.; Puzari, K.C.; Gogoi, R.; Dutta, P. Endophytes: Exploitation as a tool in plant protection. Braz. Arch. Biol. Technol. 2014, 57, 621–629. [Google Scholar] [CrossRef]
- Lee, C.; Kim, S.; Li, W.; Bang, S.; Lee, H.; Lee, H.J.; Noh, E.Y.; Park, J.E.; Bang, W.Y.; Shim, S.H. Bioactive secondary metabolites produced by an endophytic fungus Gaeumannomyces sp. JS0464 from a maritime halophyte Phragmites communis. J. Antibiot. 2017, 70, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L. Epichloë festucae and related mutualistic symbionts of grasses. Fungal Genet. Biol. 2001, 33, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Gundel, P.E.; Helander, M. Chemical ecology mediated by fungal endophytes in grasses. J. Chem. Ecol. 2013, 39, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.G.B.; Kouznetsov, V.V. Antimycobacterial susceptibility testing methods for natural products research. Braz. J. Microbiol. 2010, 41, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Luber, P.; Bartelt, E.; Genschow, E.; Wagner, J.; Hahn, H. Comparison of broth microdilution, E test, and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 2003, 41, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Lehoczky, E.; Nelima, M.O.; Szabó, R.; Szalai, A.; Nagy, P. Allelopathic effect of Bromus spp. and Lolium spp. shoot extracts on some crops. Commun. Agric. Appl. Biol. Sci. 2011, 76, 537–544. [Google Scholar] [PubMed]
- Liu, Q.R.; Li, J.; Zhao, X.F.; Xu, B.; Peng, W.D.; Li, S.X. Studies on constituents of rhizome of Arundo donax L. Planta Med. 2016, 82, PC41. [Google Scholar] [CrossRef]
- Verma, S.C.; Jain, C.L.; Padhi, M.M. Microwave-assisted extraction of Cynodon dactylon Linn. whole plant and simultaneous analysis of four phenolics by diode array detection with RP-HPLC. Asian J. Chem. 2011, 23, 3663–3666. [Google Scholar]
- Franscisco, L.; Juan Carlos, G.; Antonio, P.; Javier, F.M.; Minerva, A.M.Z.; Gil, G. Chemical and energetic characterization of species with a high-biomass production: Fractionation of their components. Environ. Prog. Sustain. Energy 2010, 29, 499–509. [Google Scholar] [CrossRef]
- Perera, R.M.M.; Marriott, P.J.; Galbally, I.E. Headspace solid-phase microextraction—Comprehensive two-dimensional gas chromatography of wound induced plant volatile organic compound emissions. Analyst 2002, 127, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Karthik, D.; Ravikumar, S. Proteome and phytochemical analysis of Cynodon dactylon leaves extract and its biological activity in diabetic rats. Biomed. Prev. Nutr. 2011, 1, 49–56. [Google Scholar] [CrossRef]
- Ravindranath, S.V.; Uppugundla, N.; Lay, J.O.; Clausen, E.C.; Wilkins, M.; Ingraham, R.G.; West, C.; Wu, Y.; Carrier, D.J. Policosanol, r-tocopherol, and moisture content as a function of timing of harvest of switchgrass (Panicum Virgatum L.). J. Agric. Food Chem. 2009, 57, 3500–3505. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Abdel-Lateff, A.; Fouad, M.A.; Ibrahim, S.R.M.; Elkhayat, E.S.; Okino, T. Chemical composition and hepato-protective activity of Imperata cylindrica Beauv. Pharmacogn. Mag. 2009, 4, 28–36. [Google Scholar]
- Matsunaga, K.; Shibuya, M.; Ohizumi, Y. Cylindrene, a novel sesquiterpenoid from Imperata cylindrica with inhibitory activity on contractions of vascular smooth muscle. J. Nat. Prod. 1994, 57, 1183–1184. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.D.; Kaliyaperumal, A.; Subramaniyan, A. Identification and determination of flavonoids, carotenoids and chlorophyll concentration in Cynodon dactylon (L.) by HPLC analysis. Nat. Prod. Res. 2015, 29, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Annapurna, H.V.; Apoorva, B.; Ravichandran, N.; Arun, K.P.; Brindha, P.; Swaminathan, S.; Vijayalakshmi, M.; Nagarajan, A. Isolation and in silico evaluation of antidiabetic molecules of Cynodon dactylon (L.). J. Mol. Graph. Model. 2013, 39, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Uppugundla, N.; Engelberth, A.; Ravindranath, S.V.; Clausen, E.C.; Lay, J.O.; Giddens, J.; Carrier, D.J.; Martin, R.E. Switchgrass water extracts: Extraction, separation and biological activity of rutin and quercitrin. J. Agric. Food Chem. 2009, 57, 7763–7770. [Google Scholar] [CrossRef] [PubMed]
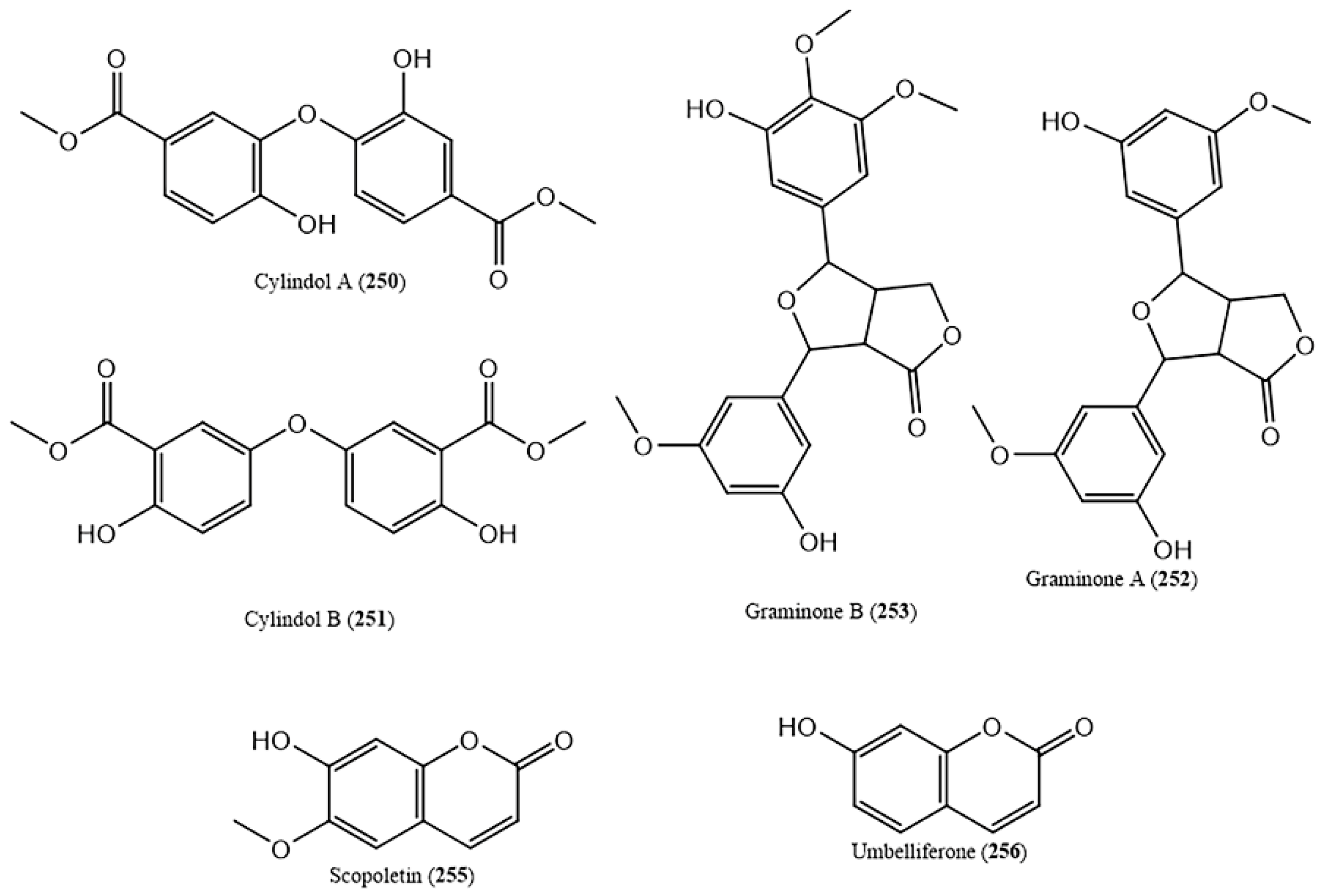
- Matsunaga, K.; Ikeda, M.; Shibuya, M.; Ohizumi, Y. Cylindol A, a novel biphenyl ether with 5-lipoxygenase inhibitory activity, and a related compound from Imperata cylindrica. J. Nat. Prod. 1994, 57, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Shibuya, M.; Ohizumi, Y. Graminone B, a novel lignan with vasodilative activity from Imperata cylindrica. J. Nat. Prod. 1994, 57, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
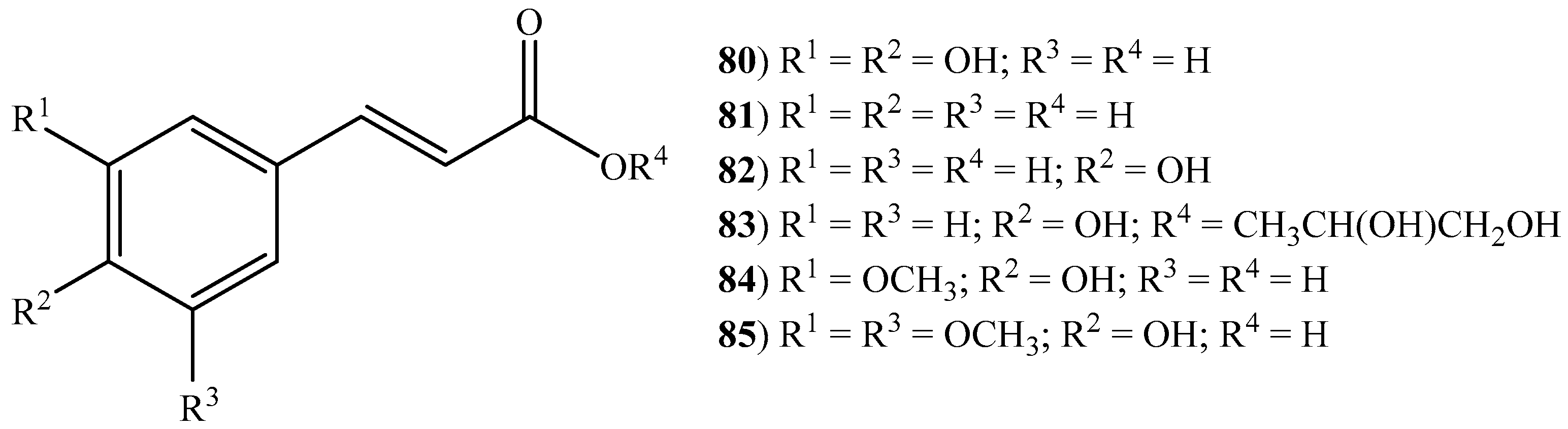
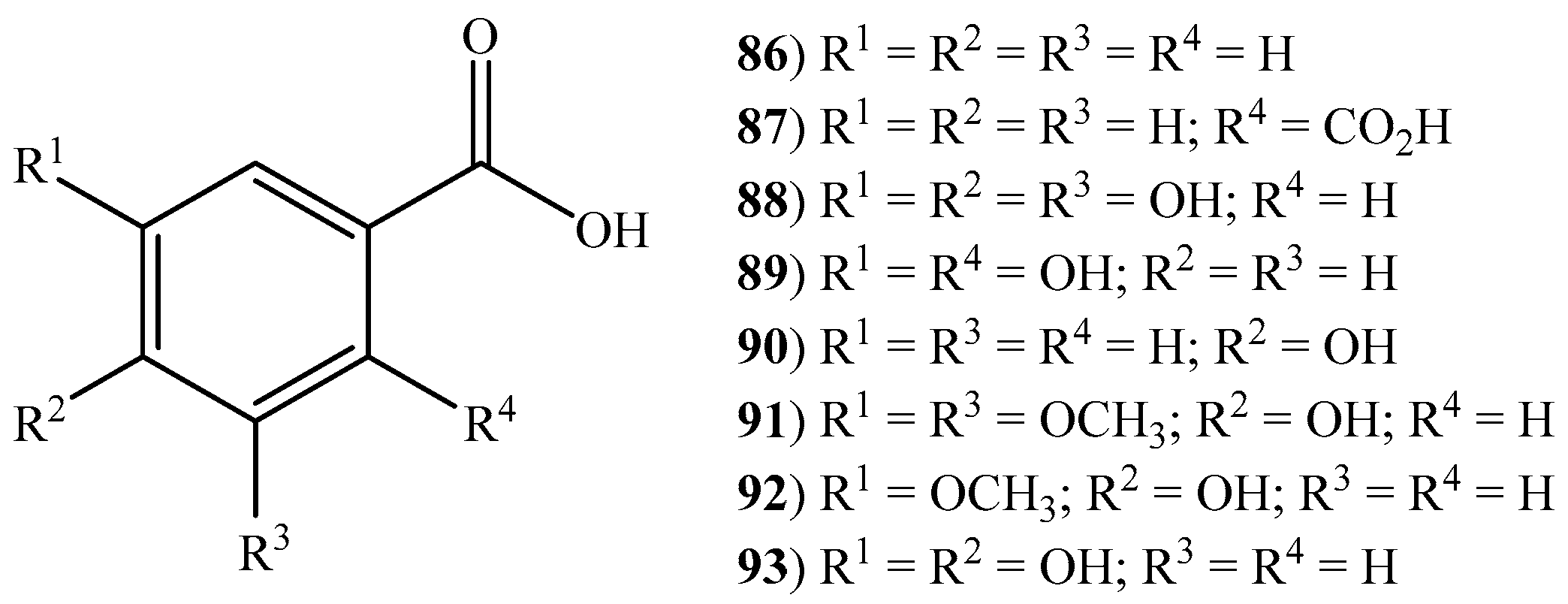

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faustino, M.V.; Faustino, M.A.F.; Pinto, D.C.G.A. Halophytic Grasses, a New Source of Nutraceuticals? A Review on Their Secondary Metabolites and Biological Activities. Int. J. Mol. Sci. 2019, 20, 1067. https://doi.org/10.3390/ijms20051067
Faustino MV, Faustino MAF, Pinto DCGA. Halophytic Grasses, a New Source of Nutraceuticals? A Review on Their Secondary Metabolites and Biological Activities. International Journal of Molecular Sciences. 2019; 20(5):1067. https://doi.org/10.3390/ijms20051067
Chicago/Turabian StyleFaustino, Maria V., Maria A. F. Faustino, and Diana C. G. A. Pinto. 2019. "Halophytic Grasses, a New Source of Nutraceuticals? A Review on Their Secondary Metabolites and Biological Activities" International Journal of Molecular Sciences 20, no. 5: 1067. https://doi.org/10.3390/ijms20051067
APA StyleFaustino, M. V., Faustino, M. A. F., & Pinto, D. C. G. A. (2019). Halophytic Grasses, a New Source of Nutraceuticals? A Review on Their Secondary Metabolites and Biological Activities. International Journal of Molecular Sciences, 20(5), 1067. https://doi.org/10.3390/ijms20051067







