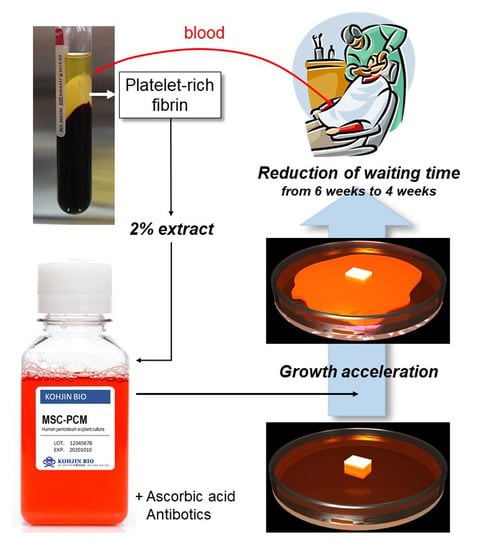
Platelet-Rich Fibrin Extract: A Promising Fetal Bovine Serum Alternative in Explant Cultures of Human Periosteal Sheets for Regenerative Therapy
Abstract
1. Introduction
2. Results
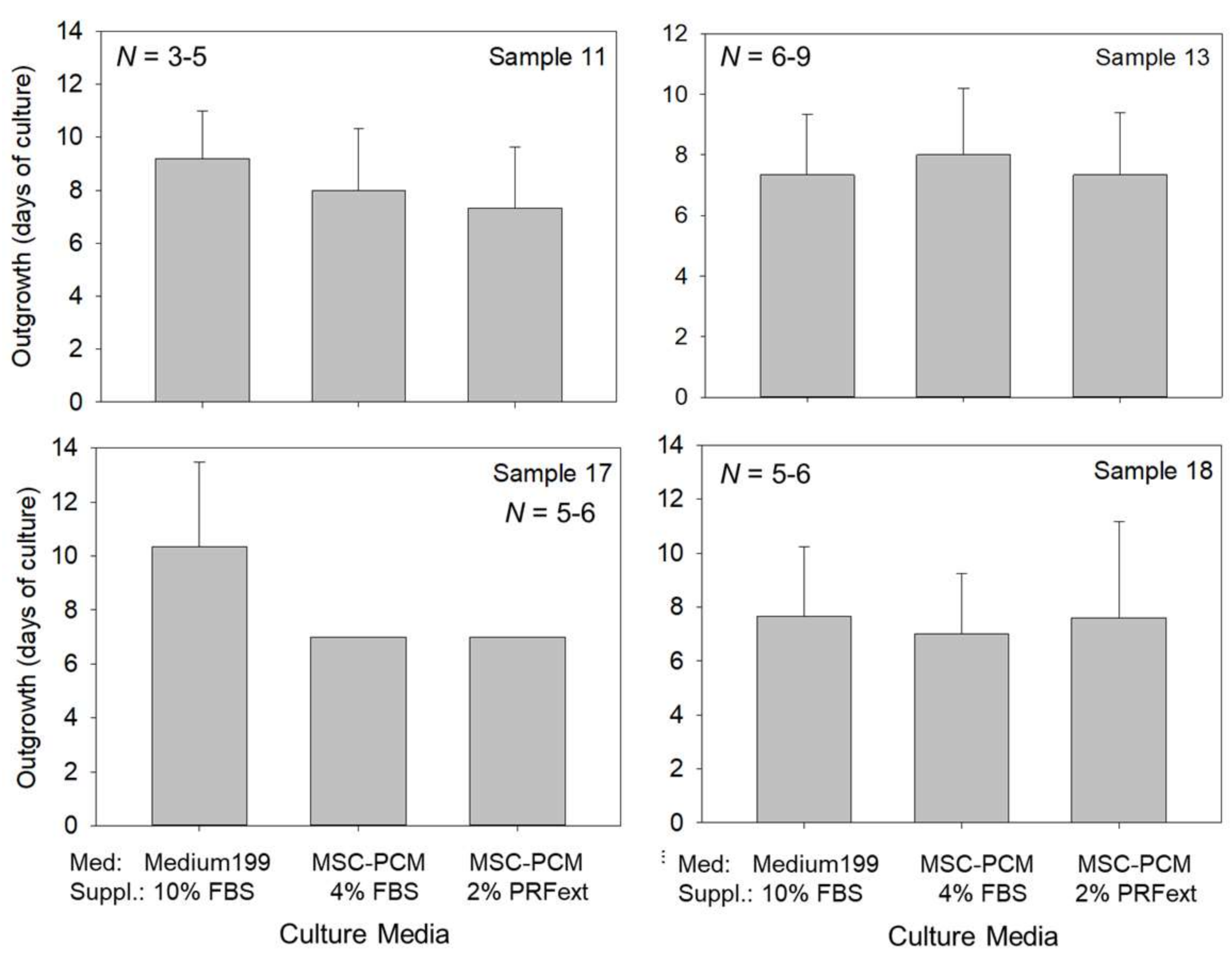
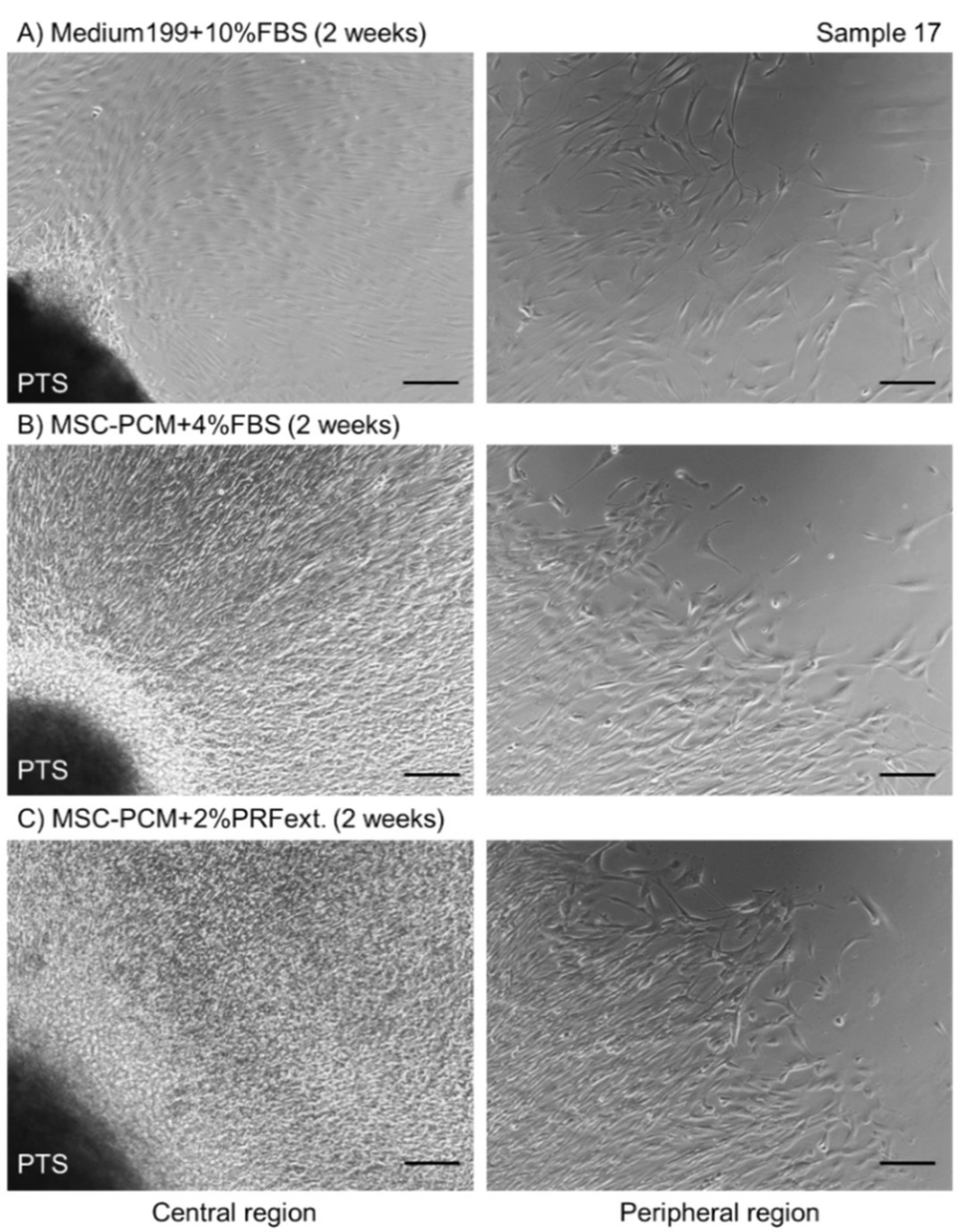
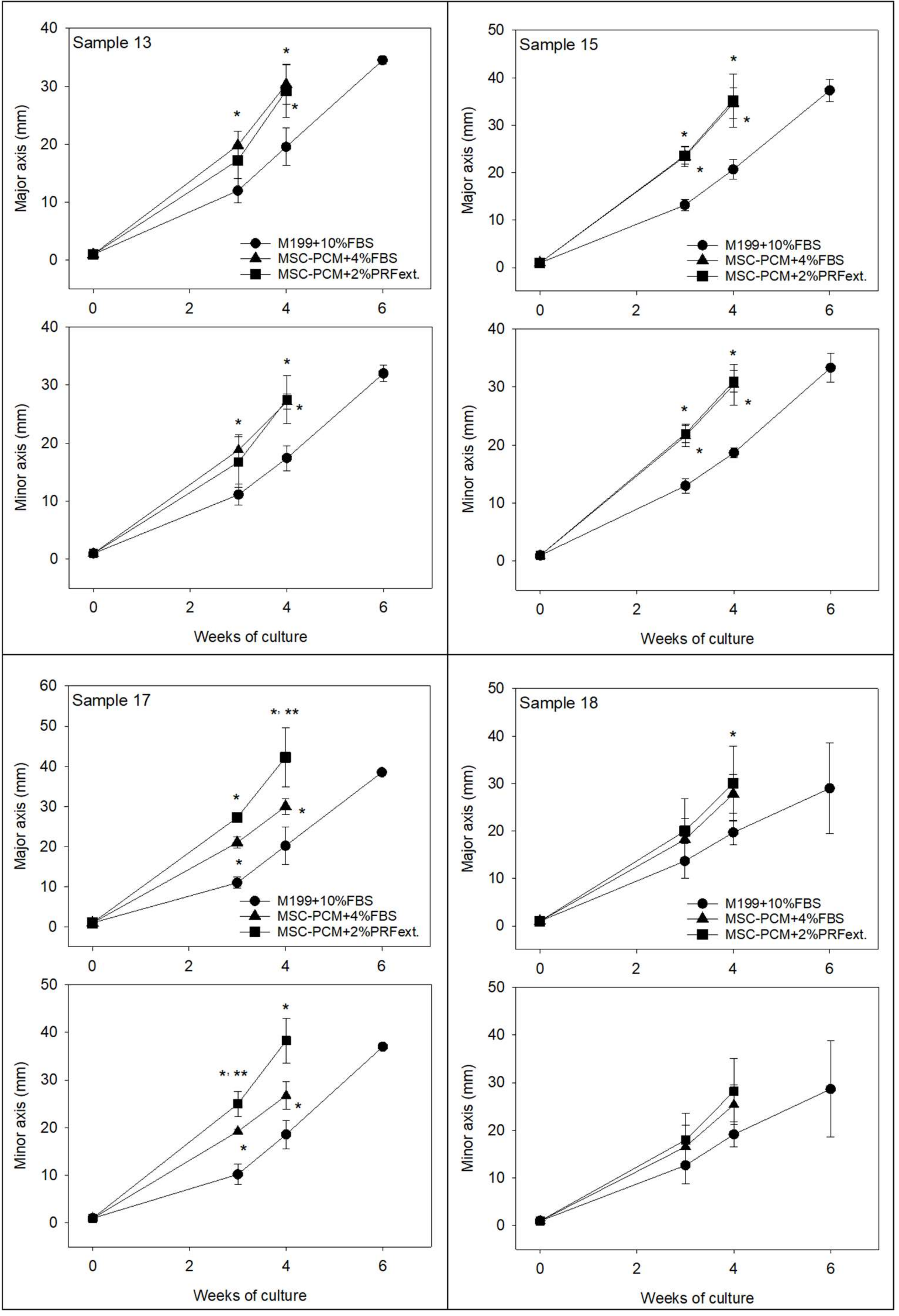
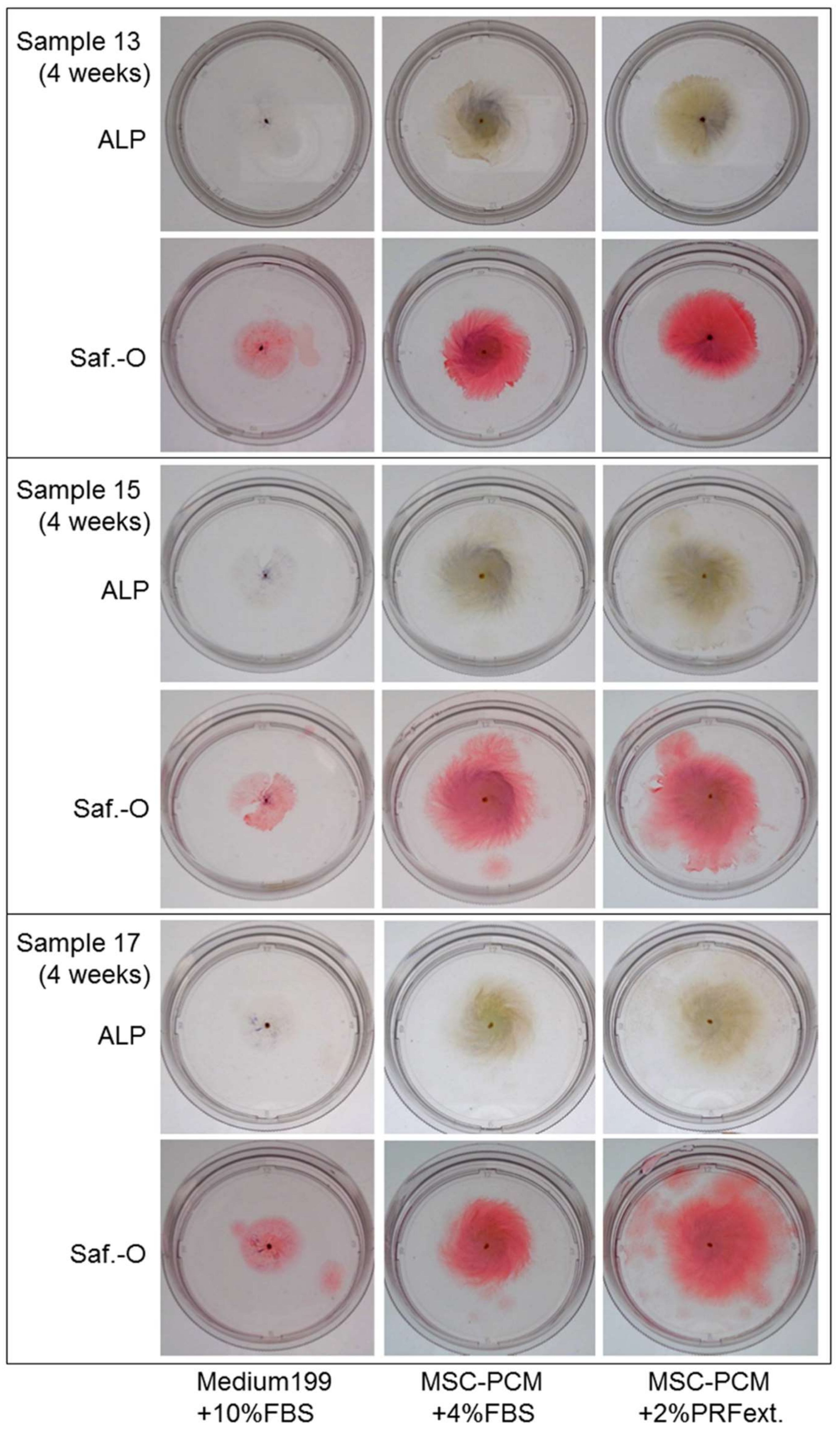
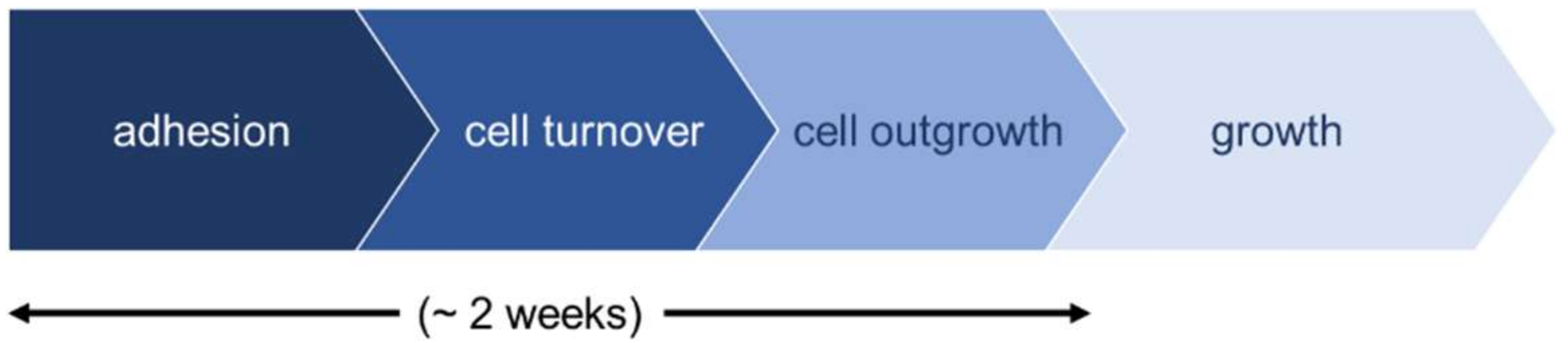
2.1. Growth of Periosteal Sheets
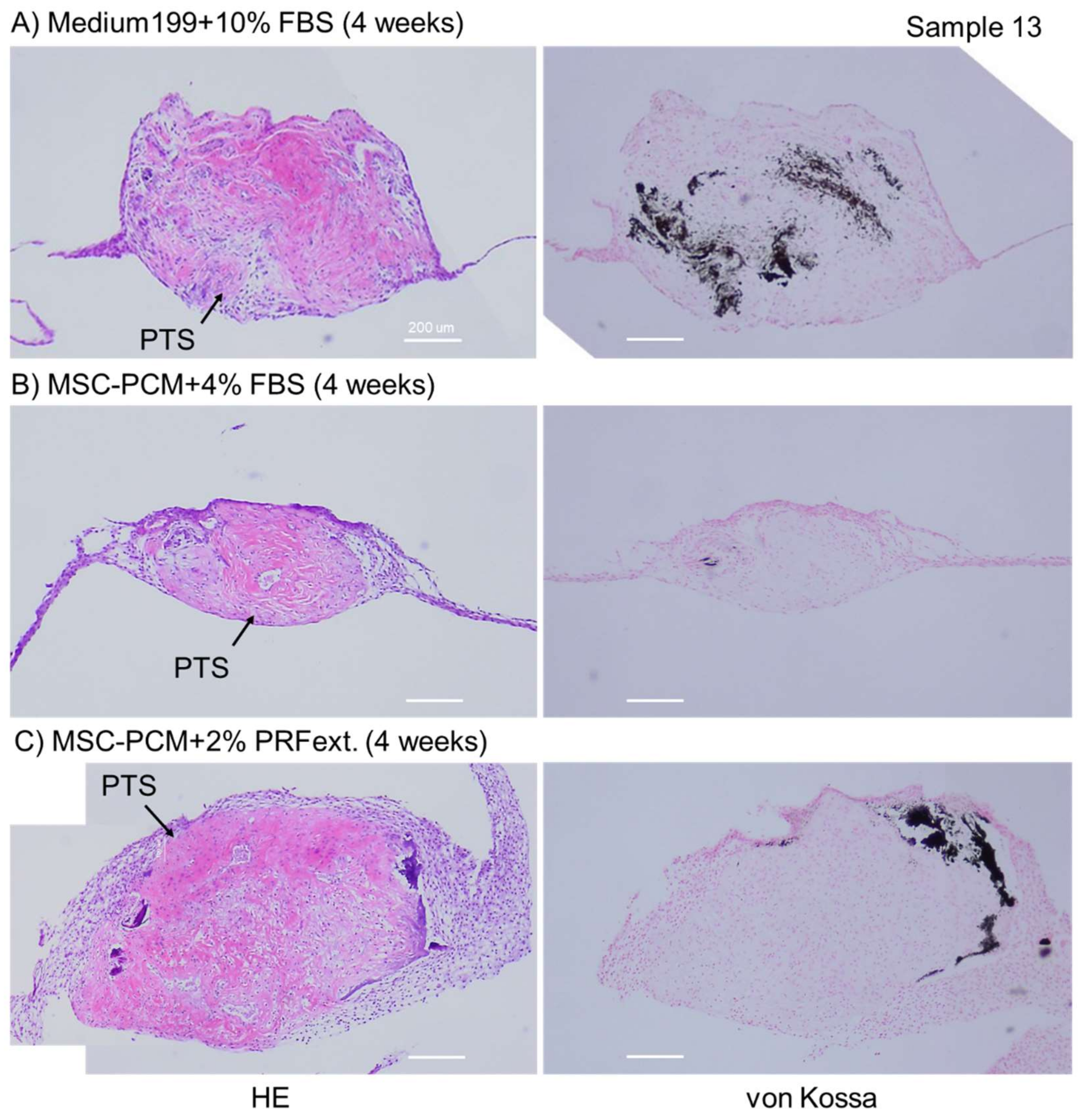
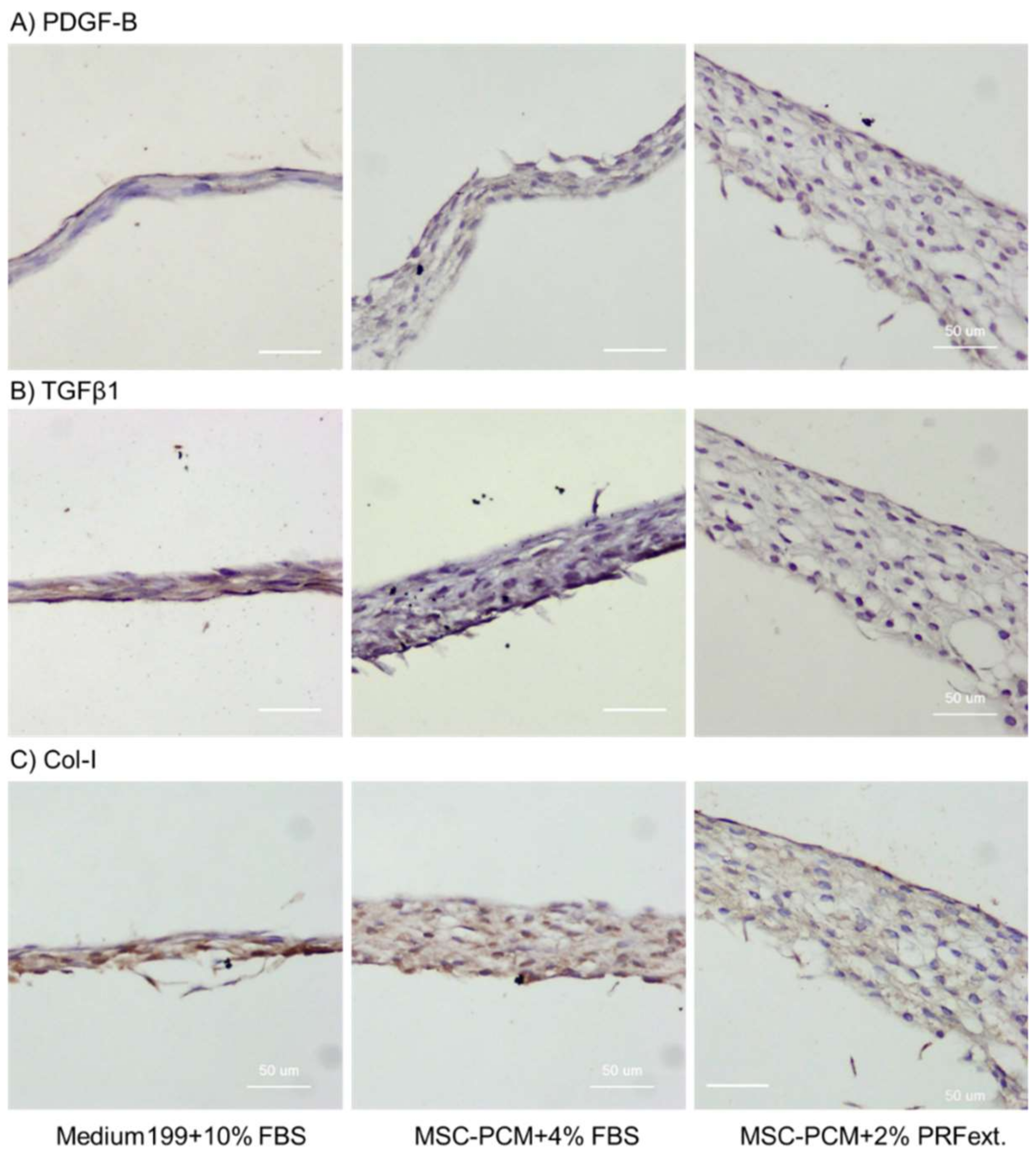
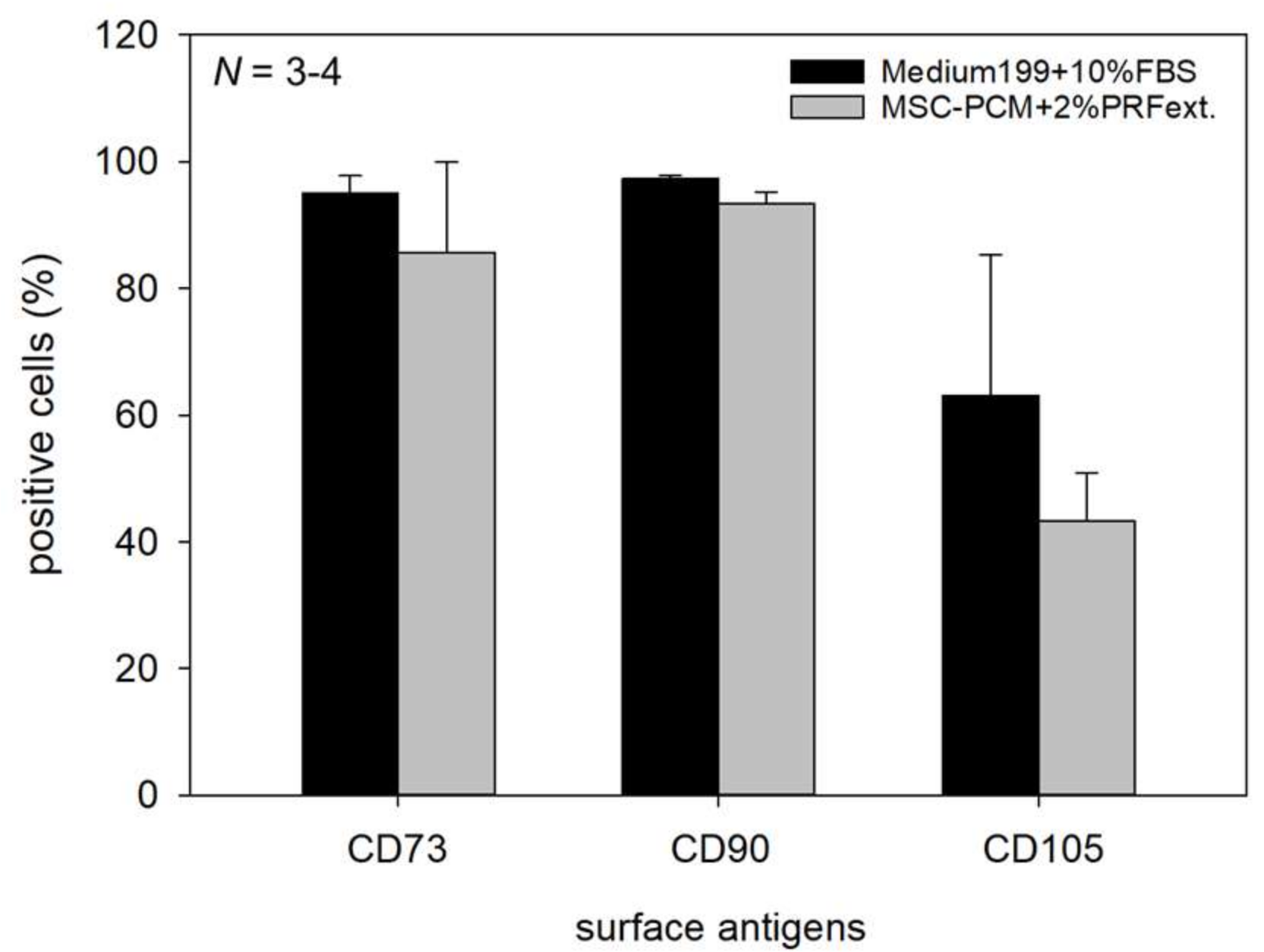
2.2. Phenotype of Periosteal Sheets
3. Discussion
4. Materials and Methods
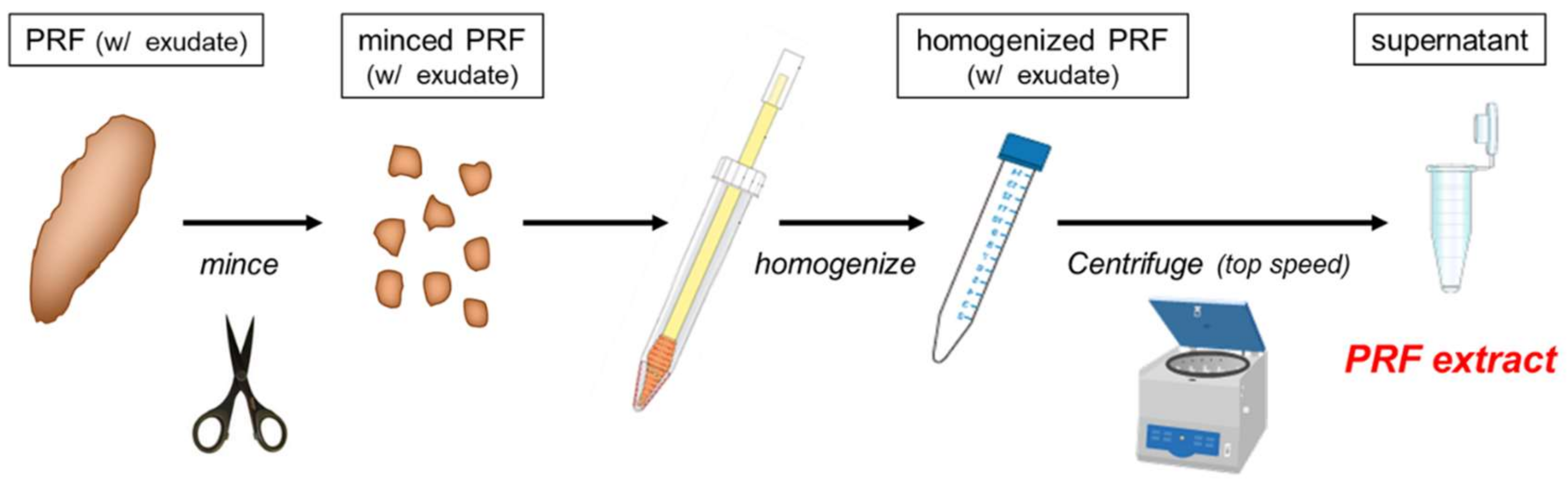
4.1. Preparation of PRFext
4.2. Explant Culture of Periosteum Tissue Segments to Form Periosteal Sheets
4.3. Evaluation of Cell Outgrowth and Growth Rate
4.4. Histological Determination of ALP Activity
4.5. Histological and Immunohistochemical Examination for Calcium Deposition, Growth Factor Expression, and Collagen Accumulation
4.6. Flow Cytometric Evaluation of CD73-, CD90-, and CD105-Positive Periosteal Cells
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgement
Conflicts of Interest
Abbreviations
| PRF | platelet-rich fibrin |
| PRFext | platelet-rich fibrin extract |
| PRP | platelet-rich plasma |
| bFGF | basic fibroblast growth factor |
| ALP | alkaline phosphatase |
| Saf.-O | Safranin-O |
| IgG | immunoglobulin |
| HRP | horseradish peroxidase |
| DAB | 3,3′-diaminobenzidine tetrahydrochloride |
| PDGF-B | Platelet-derived growth factor-B |
| TGFβ1 | Transforming growth factor beta 1 |
| EDTA | Ethylenediaminetetraacetic acid |
References
- Karnieli, O.; Friedner, O.M.; Allickson, J.G.; Zhang, N.; Jung, S.; Fiorentini, D.; Abraham, E.; Eaker, S.S.; Yong, T.K.; Chan, A.; et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy 2017, 19, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontics 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Okuda, K.; Kogami, H.; Nakayama, H.; Nagata, M.; Nakata, K.; Yoshie, H. Characterization of human cultured periosteal sheets expressing bone-forming potential: In vitro and in vivo animal studies. J. Tissue Engin. Regener. Med. 2009, 3, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Yamamiya, K.; Okuda, K.; Kawase, T.; Hata, K.; Wolff, L.F.; Yoshie, H. Tissue-engineered cultured periosteum used with platelet-rich plasma and hydroxyapatite in treating human osseous defects. J. Periodontol. 2008, 79, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Hoshina, H.; Li, M.; Arasawa, M.; Uematsu, K.; Ogawa, S.; Yamada, K.; Kawase, T.; Suzuki, K.; Ogose, A.; et al. A clinical study of alveolar bone tissue engineering with cultured autogenous periosteal cells: Coordinated activation of bone formation and resorption. Bone 2012, 50, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Hoshina, H.; Nakata, K.; Yamada, K.; Uematsu, K.; Kawase, T.; Takagi, R.; Nagata, M. High-Resolution Three-Dimensional Computed Tomography Analysis of the Clinical Efficacy of Cultured Autogenous Periosteal Cells in Sinus Lift Bone Grafting. Clin. Implant Dent. Relat. Res. 2016, 18, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, K.; Kawase, T.; Nagata, M.; Suzuki, K.; Okuda, K.; Yoshie, H.; Burns, D.M.; Takagi, R. Tissue culture of human alveolar periosteal sheets using a stem-cell culture medium (MesenPRO-RS): In vitro expansion of CD146-positive cells and concomitant upregulation of osteogenic potential in vivo. Stem cell Res. 2013, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, S.; Berreur, M.; Trichet, V.; Pilet, P.; Louarn, G.; Layrolle, P. Adhesion and osteogenic differentiation of human mesenchymal stem cells on titanium nanopores. Eur. Cells Mater. 2011, 22, 84–96. [Google Scholar] [CrossRef]
- Lavenus, S.; Pilet, P.; Guicheux, J.; Weiss, P.; Louarn, G.; Layrolle, P. Behaviour of mesenchymal stem cells, fibroblasts and osteoblasts on smooth surfaces. Acta Biomater. 2011, 7, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarrete, R.; Hyzy, S.L.; Hutton, D.L.; Erdman, C.P.; Wieland, M.; Boyan, B.D.; Schwartz, Z. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials 2010, 31, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Okuda, K. Method for culturing human periosteum. U.S. Patent 8,420,392B2, 16 April 2013. [Google Scholar]
- Kawase, T.; Kogami, H.; Nagata, M.; Uematsu, K.; Okuda, K.; Burns, D.M.; Yoshie, H. Manual cryopreservation of human alveolar periosteal tissue segments: Effects of pre-culture on recovery rate. Cryobiology 2011, 62, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Catalina, P.; Cobo, F.; Cortes, J.L.; Nieto, A.I.; Cabrera, C.; Montes, R.; Concha, A.; Menendez, P. Conventional and molecular cytogenetic diagnostic methods in stem cell research: A concise review. Cell Biol. Int. 2007, 31, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Mohn, F.; Schubeler, D. Genetics and epigenetics: Stability and plasticity during cellular differentiation. Trends Genet. 2009, 25, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Kamiya, M.; Hayama, K.; Nagata, M.; Okuda, K.; Yoshie, H.; Burns, D.M.; Tsuchimochi, M.; Nakata, K. X-ray and ultraviolet C irradiation-induced gamma-H2AX and p53 formation in normal human periosteal cells in vitro: Markers for quality control in cell therapy. Cytotherapy 2015, 17, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.C.; Frolik, C.A. Physiological and pharmacological regulation of biological calcification. Int. Rev. Cytol. 1991, 126, 195–292. [Google Scholar] [PubMed]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant Dent. 2016, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kawase, T.; Horimizu, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals 2012, 40, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Tsukioka, T.; Hiratsuka, T.; Nakamura, M.; Watanabe, T.; Kitamura, Y.; Isobe, K.; Okudera, T.; Okudera, H.; Azuma, A.; Uematsu, K.; et al. An on-site preparable, novel bone-grafting complex consisting of human platelet-rich fibrin and porous particles made of a recombinant collagen-like protein. J. Biomed. Mater. Res. B Appl. Biomater. 2018. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawase, T.; Nagata, M.; Okuda, K.; Ushiki, T.; Fujimoto, Y.; Watanabe, M.; Ito, A.; Nakata, K. Platelet-Rich Fibrin Extract: A Promising Fetal Bovine Serum Alternative in Explant Cultures of Human Periosteal Sheets for Regenerative Therapy. Int. J. Mol. Sci. 2019, 20, 1053. https://doi.org/10.3390/ijms20051053
Kawase T, Nagata M, Okuda K, Ushiki T, Fujimoto Y, Watanabe M, Ito A, Nakata K. Platelet-Rich Fibrin Extract: A Promising Fetal Bovine Serum Alternative in Explant Cultures of Human Periosteal Sheets for Regenerative Therapy. International Journal of Molecular Sciences. 2019; 20(5):1053. https://doi.org/10.3390/ijms20051053
Chicago/Turabian StyleKawase, Tomoyuki, Masaki Nagata, Kazuhiro Okuda, Takashi Ushiki, Yoko Fujimoto, Mari Watanabe, Akira Ito, and Koh Nakata. 2019. "Platelet-Rich Fibrin Extract: A Promising Fetal Bovine Serum Alternative in Explant Cultures of Human Periosteal Sheets for Regenerative Therapy" International Journal of Molecular Sciences 20, no. 5: 1053. https://doi.org/10.3390/ijms20051053
APA StyleKawase, T., Nagata, M., Okuda, K., Ushiki, T., Fujimoto, Y., Watanabe, M., Ito, A., & Nakata, K. (2019). Platelet-Rich Fibrin Extract: A Promising Fetal Bovine Serum Alternative in Explant Cultures of Human Periosteal Sheets for Regenerative Therapy. International Journal of Molecular Sciences, 20(5), 1053. https://doi.org/10.3390/ijms20051053