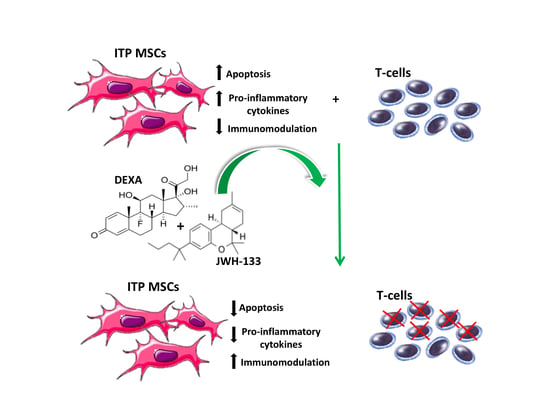
CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia
Abstract
1. Introduction
2. Results
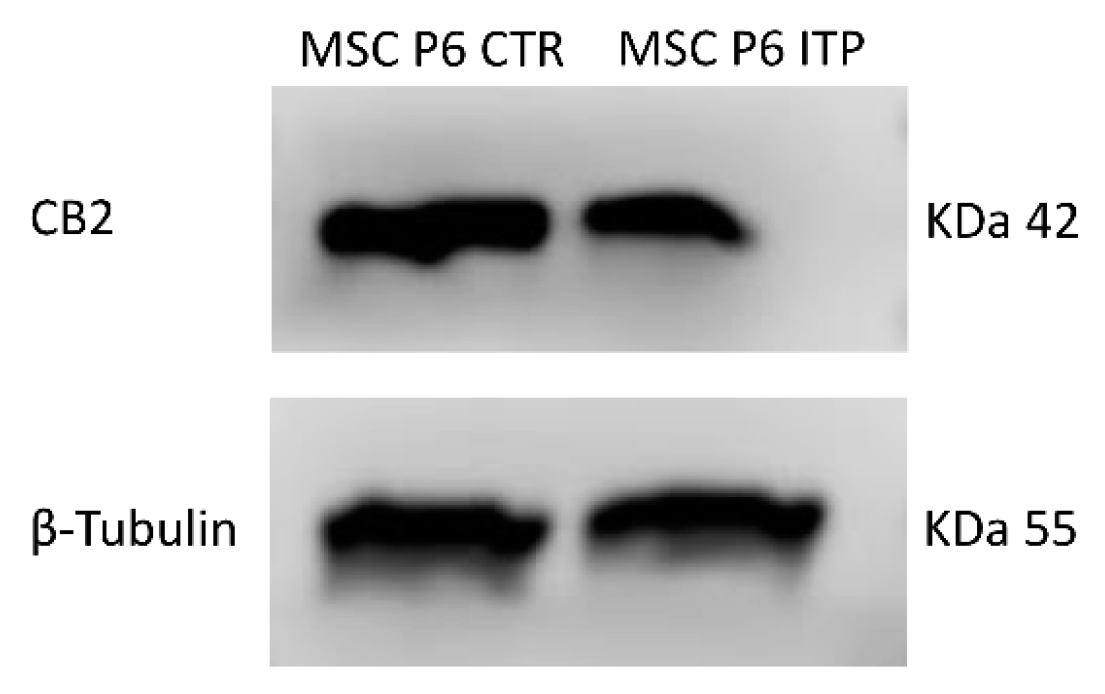
2.1. Expression of CB2 Receptor in MSCs Derived from ITP Patients
2.2. Effects of JWH-133 and Dexa on Cytokine Release
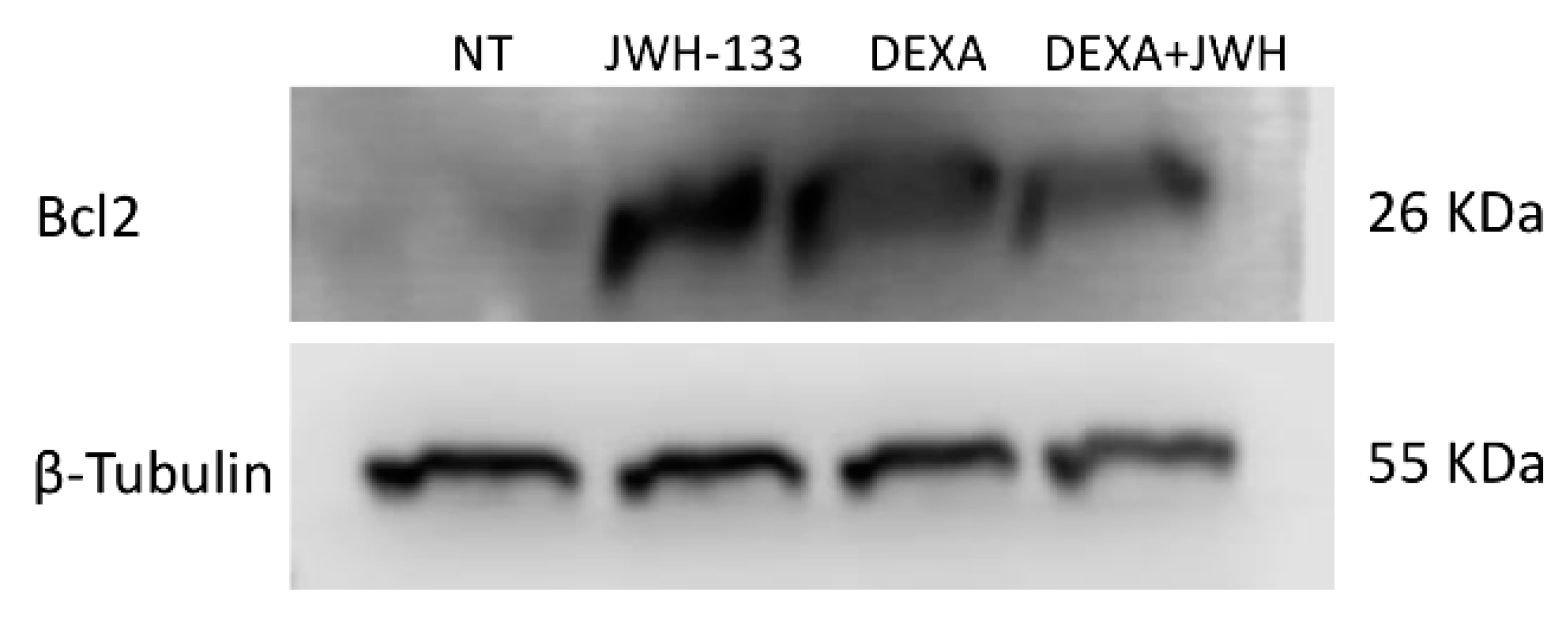
2.3. Effects of JWH-133 and Dexa on ITP-MSCs Apoptosis
2.4. Effects of JWH-133 and Dexa on ITP-MSCs’ Immunosuppression Capacity
3. Discussion
4. Materials and Methods
4.1. Source of MSCs
4.2. Isolation, Expansion, and Characterization of MSCs
4.3. MSCs and T Cells Co-Culture
4.4. Total RNA Extraction and Reverse Transcription Quantitative Polymerase Chain Reaction (RTqPCR)
4.5. Protein Isolation; Western Blot
4.6. ELISA
4.7. Annexin V & Dead Cell Assay Kit
4.8. Count and Viability Assay Kit
4.9. Drugs and Treatments
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Provan, D.; Newland, A.C. Current Management of Primary Immune Thrombocytopenia. Adv. Ther. 2015, 32, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Neunert, C.; Lim, W.; Crowther, M.; Cohen, A.; Solberg, L., Jr.; Crowther, M.A. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011, 117, 4190–4207. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Mydra, H.; Nevarez, A. Clinical Practice Updates in the Management of Immune Thrombocytopenia. Pharm. Ther. 2017, 42, 756–763. [Google Scholar]
- Audia, S.; Mahevas, M.; Samson, M.; Godeau, B.; Bonnotte, B. Pathogenesis of immune thrombocytopenia. Autoimmun. Rev. 2017, 16, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, Y.; Ruan, M.; Shi, J. The Levels of T Lymphocyte Subsets in Immune Thrombocytopenia Associated with Anti-GPIIb/IIIa- and/or Anti-GPIbalpha-Mediated Responses Are Differentially Sensitive to Dexamethasone. Acta Haematol. 2018, 140, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, K. Imbalanced immune homeostasis in immune thrombocytopenia. Semin. Hematol. 2016, 53 (Suppl. 1), S16–S19. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, H.; Poon, M.C.; Han, Z.; Gu, D.; Xu, M.; Jia, H.; Yang, R.; Han, Z.C. Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur. J. Haematol. 2007, 78, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Starc, N.; Ingo, D.; Conforti, A.; Rossella, V.; Tomao, L.; Pitisci, A.; De Mattia, F.; Brigida, I.; Algeri, M.; Montanari, M.; et al. Biological and functional characterization of bone marrow-derived mesenchymal stromal cells from patients affected by primary immunodeficiency. Sci. Rep. 2017, 7, 8153. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M.; et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Feng, F.E.; Wang, Q.M.; Zhu, X.L.; Fu, H.X.; Xu, L.P.; Liu, K.Y.; Huang, X.J.; Zhang, X.H. Platelet-Derived Growth Factor-BB Protects Mesenchymal Stem Cells (MSCs) Derived From Immune Thrombocytopenia Patients Against Apoptosis and Senescence and Maintains MSC-Mediated Immunosuppression. Stem Cells Transl. Med. 2016, 5, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Zhu, X.L.; Xue, J.; Liu, X.; Long Zheng, X.; Chang, Y.J.; Liu, K.Y.; Huang, X.J.; Zhang, X.H. Integrated mRNA and miRNA profiling revealed deregulation of cellular stress response in bone marrow mesenchymal stem cells derived from patients with immune thrombocytopenia. Funct. Integr. Genom. 2018, 18, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Perez-Simon, J.A.; Tabera, S.; Sarasquete, M.E.; Diez-Campelo, M.; Canchado, J.; Sanchez-Abarca, L.I.; Blanco, B.; Alberca, I.; Herrero-Sanchez, C.; Canizo, C.; et al. Mesenchymal stem cells are functionally abnormal in patients with immune thrombocytopenic purpura. Cytotherapy 2009, 11, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Mithoowani, S.; Gregory-Miller, K.; Goy, J.; Miller, M.C.; Wang, G.; Noroozi, N.; Kelton, J.G.; Arnold, D.M. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: A systematic review and meta-analysis. Lancet Haematol. 2016, 3, e489–e496. [Google Scholar] [CrossRef]
- Takase, K.; Kada, A.; Iwasaki, H.; Yoshida, I.; Sawamura, M.; Yoshio, N.; Yoshida, S.; Iida, H.; Otsuka, M.; Takafuta, T.; et al. High-dose Dexamethasone Therapy as the Initial Treatment for Idiopathic Thrombocytopenic Purpura: Protocol for a Multicenter, Open-label, Single Arm Trial. Acta Med. Okayama 2018, 72, 197–201. [Google Scholar] [PubMed]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wu, B.; Cheng, L.; Li, F.; Zhan, Y.; Liu, C.; Ji, L.; Min, Z.; Ke, Y.; Sun, L.; et al. Distinct alterations of CD68(+)CD163(+) M2-like macrophages and myeloid-derived suppressor cells in newly diagnosed primary immune thrombocytopenia with or without CR after high-dose dexamethasone treatment. J. Transl. Med. 2018, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, M.; Zhou, S.; Ma, J.; Shi, Y.; Peng, J.; Hou, M.; Guo, C. Pulsed high-dose dexamethasone modulates Th1-/Th2-chemokine imbalance in immune thrombocytopenia. J. Transl. Med. 2016, 14, 301. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zou, S.; Hua, F.; Li, F.; Ji, L.; Wang, W.; Ye, Y.; Sun, L.; Chen, H.; Cheng, Y. High-dose dexamethasone modulates serum cytokine profile in patients with primary immune thrombocytopenia. Immunol. Lett. 2014, 160, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pang, B.; Li, Y.; Zhu, D.; Pang, T.; Liu, Y. Dexamethasone has variable effects on mesenchymal stromal cells. Cytotherapy 2012, 14, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Bernardo, M.E.; Bellini, G.; Luongo, L.; Conforti, A.; Manzo, I.; Guida, F.; Cristino, L.; Imperatore, R.; Petrosino, S.; et al. The cannabinoid receptor type 2 as mediator of mesenchymal stromal cell immunosuppressive properties. PLoS ONE 2013, 8, e80022. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Kiertscher, S.M.; Cheng, Q.; Zoumalan, R.; Tashkin, D.P.; Roth, M.D. Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J. Neuroimmunol. 2002, 133, 124–131. [Google Scholar] [CrossRef]
- Klein, T.W.; Lane, B.; Newton, C.A.; Friedman, H. The cannabinoid system and cytokine network. Proc. Soc. Exp. Biol. Med. 2000, 225, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: Novel role for endogenous and exogenous cannabinoids in immunoregulation. J. Immunol. 2004, 173, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Mancusi, S.; Bellini, G.; Roberti, D.; Punzo, F.; Vetrella, S.; Matarese, S.M.; Nobili, B.; Maione, S.; Perrotta, S. CNR2 functional variant (Q63R) influences childhood immune thrombocytopenic purpura. Haematologica 2011, 96, 1883–1885. [Google Scholar] [CrossRef] [PubMed]
- Kared, H.; Camous, X.; Larbi, A. T cells and their cytokines in persistent stimulation of the immune system. Curr. Opin. Immunol. 2014, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, A.; Kapur, R.; Semple, J.W. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J. Clin. Med. 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Oppenheim, J.J. Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 2011, 585, 3611–3618. [Google Scholar] [CrossRef] [PubMed]
- Culic, S.; Salamunic, I.; Konjevoda, P.; Dajak, S.; Pavelic, J. Immune thrombocytopenia: Serum cytokine levels in children and adults. Med. Sci. Monit. 2013, 19, 797–801. [Google Scholar] [PubMed]
- Buron, F.; Perrin, H.; Malcus, C.; Hequet, O.; Thaunat, O.; Kholopp-Sarda, M.N.; Moulin, F.T.; Morelon, E. Human mesenchymal stem cells and immunosuppressive drug interactions in allogeneic responses: An in vitro study using human cells. Transp. Proc. 2009, 41, 3347–3352. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, M.G.; Fettucciari, K.; Montuschi, P.; Ronchetti, S.; Cari, L.; Migliorati, G.; Mazzon, E.; Bereshchenko, O.; Bruscoli, S.; Nocentini, G.; et al. Transcriptional regulation of kinases downstream of the T cell receptor: Another immunomodulatory mechanism of glucocorticoids. BMC Pharmacol. Toxicol. 2014, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gan, Y.; Li, W.; Su, J.; Zhang, Y.; Huang, Y.; Roberts, A.I.; Han, Y.; Li, J.; Wang, Y.; et al. The interaction between mesenchymal stem cells and steroids during inflammation. Cell Death Dis. 2014, 5, e1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Shen, H.; Jordan, C.J.; Liu, Q.R.; Gardner, E.L.; Bonci, A.; Xi, Z.X. CB2 receptor antibody signal specificity: Correlations with the use of partial CB2-knockout mice and anti-rat CB2 receptor antibodies. Acta Pharmacol. Sin. 2018. [Google Scholar] [CrossRef] [PubMed]
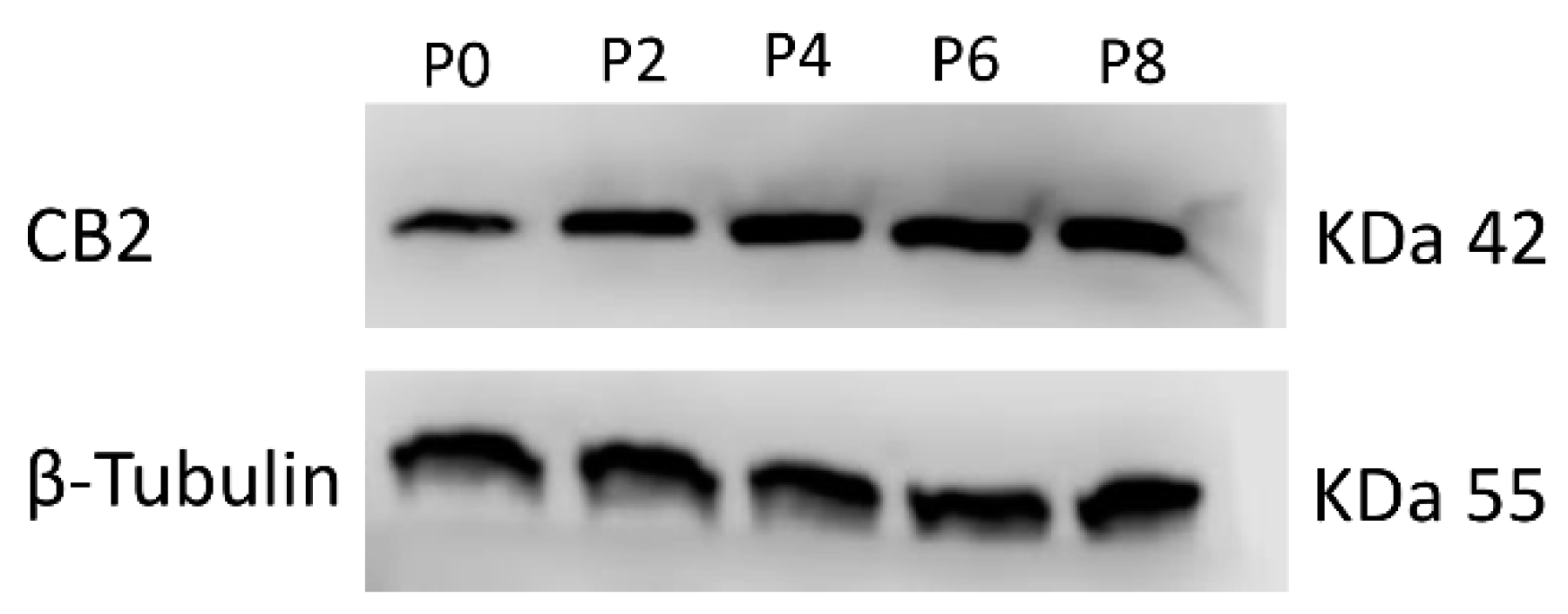
| (A) | CB2 Relative Quantification (2−∆∆Ct) | ||||
| Passages | |||||
| Samples | P0 | P2 | P4 | P6 | P8 |
| MSC ITP-1 | 1 | 2.3 * | 2.5 * | 4.8 * | 6.0 * |
| MSC ITP-2 | 1 | 3.0 * | 1.9 * | 4.6 * | 5.4 * |
| (B) | CB2 Protein signal density | ||||
| Passages | |||||
| Samples | P0 | P2 | P4 | P6 | P8 |
| MSC ITP-1 | 1 | 1.72 * | 2.10 * | 2.26 * | 2.90 * |
| MSC ITP-2 | 1 | 1.80 * | 2.11 * | 2.29 * | 2.44 * |
| (A) | |
| Samples | CB2 Relative Quantification (2−∆∆Ct) |
| MSC P6 CTR-1 | 1 |
| MSC P6 CTR-2 | 1 |
| MSC P6 ITP-1 | 0.54 * |
| MSC P6 ITP-2 | 0.62 * |
| (B) | |
| Samples | CB2 Protein Signal Density |
| MSC P6 CTR-1 | 1 |
| MSC P6 CTR-2 | 1 |
| MSC P6 ITP-1 | 0.53 * |
| MSC P6 ITP-2 | 0.49 * |
| (A) IL-6 | |||||||
| Samples | CTR-1 | ITP-1 NT | ITP-1 JWH-133 | ITP-1 DEXA | ITP-1 D + J | ITP-1 AM630 | ITP-1 A + J |
| pg/mL | 7.04 | 12.87 * | 9.02 ^ | 7.70 ^ | 11.01 ^ | 17.05 ^ | 15.41 ^ |
| Samples | CTR-2 | ITP-2 NT | ITP-2 JWH-133 | ITP-2 DEXA | ITP-2 D + J | ITP-2 AM630 | ITP-2 A + J |
| pg/mL | 7.18 | 16.02 * | 9.83 ^ | 8.63 ^ | 9.44 ^ | 17.38 ^ | 17.52 ^ |
| (B) IL-4 | |||||||
| Samples | CTR-1 | ITP-1 NT | ITP-1 JWH-133 | ITP-1 DEXA | ITP-1 D + J | ITP-1 AM630 | ITP-1 A + J |
| pg/mL | 44.61 | 31.53 * | 43.11 ^ | 45.26 ^ | 39.38 ^ | 28.89 ^ | 27.48 ^ |
| Samples | CTR-2 | ITP-2 NT | ITP-2 JWH-133 | ITP-2 DEXA | ITP-2 D + J | ITP-2 AM630 | ITP-2 A + J |
| pg/mL | 45,82 | 32.68 * | 42.91 ^ | 45.03 ^ | 40.67 ^ | 29.41 ^ | 30.05 ^ |
| (A) | Percentage of Total Apoptotic ITP-MSCs | |||
| Treatments | ||||
| Samples | NT | JWH-133 | DEXA | J + D |
| MSC ITP-1 | 41.56 | 34.24 * | 30.53 * | 27.56 * |
| MSC ITP-2 | 44.94 | 31.26 * | 35.27 * | 31.08 * |
| (B) | Bcl2 Protein Signal Density | |||
| Treatments | ||||
| Samples | NT | JWH-133 | DEXA | J + D |
| MSC ITP-1 | 1 | 7.94 * | 10.25 * | 7.85 * |
| MSC ITP-2 | 1 | 8.48 * | 8.15 * | 6.67 * |
| T Cell Viability | |||||
| Sample 1 | T cells | T cells + MSC | T cells + MSC JWH-133 | T cells + MSC DEXA | T cells + MSC J + D |
| (Number of cells × 106) | 6.68 | 7.01 | 5.56 * | 6.44 * | 6.23 * |
| Sample 2 | T cells | T cells + MSC | T cells + MSC JWH-133 | T cells + MSC DEXA | T cells + MSC J + D |
| (Number of cells × 106) | 7.20 | 7.12 | 5.75 * | 6.37 * | 6.11 * |
| TNF-α | |||||||
| Sample 1 | T cells | T cells + MSC | T cells + LPS | T cells + MSC LPS | T cells + MSC L + JWH-133 | T cells + MSC L + DEXA | T cells + MSC L + J + D |
| pg/mL | 44.61 | 31.53 * | 43.11 ^ | 45.26 ^ | 39.38 ° | 28.89 ° | 27.48 ° |
| Sample 2 | T cells | T cells + MSC | T cells + LPS | T cells + MSC LPS | T cells + MSC L + JWH-133 | T cells + MSC L + DEXA | T cells + MSC L + J + D |
| pg/mL | 45.82 | 32.68 * | 42.91 ^ | 45.03 ^ | 40.67 ° | 29.41 ° | 30.05 ° |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, F.; Tortora, C.; Palumbo, G.; Punzo, F.; Argenziano, M.; Casale, M.; Di Paola, A.; Locatelli, F.; Perrotta, S. CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia. Int. J. Mol. Sci. 2019, 20, 1049. https://doi.org/10.3390/ijms20051049
Rossi F, Tortora C, Palumbo G, Punzo F, Argenziano M, Casale M, Di Paola A, Locatelli F, Perrotta S. CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia. International Journal of Molecular Sciences. 2019; 20(5):1049. https://doi.org/10.3390/ijms20051049
Chicago/Turabian StyleRossi, Francesca, Chiara Tortora, Giuseppe Palumbo, Francesca Punzo, Maura Argenziano, Maddalena Casale, Alessandra Di Paola, Franco Locatelli, and Silverio Perrotta. 2019. "CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia" International Journal of Molecular Sciences 20, no. 5: 1049. https://doi.org/10.3390/ijms20051049
APA StyleRossi, F., Tortora, C., Palumbo, G., Punzo, F., Argenziano, M., Casale, M., Di Paola, A., Locatelli, F., & Perrotta, S. (2019). CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia. International Journal of Molecular Sciences, 20(5), 1049. https://doi.org/10.3390/ijms20051049