The Involvement of Ethylene in Calcium-Induced Adventitious Root Formation in Cucumber under Salt Stress
Abstract
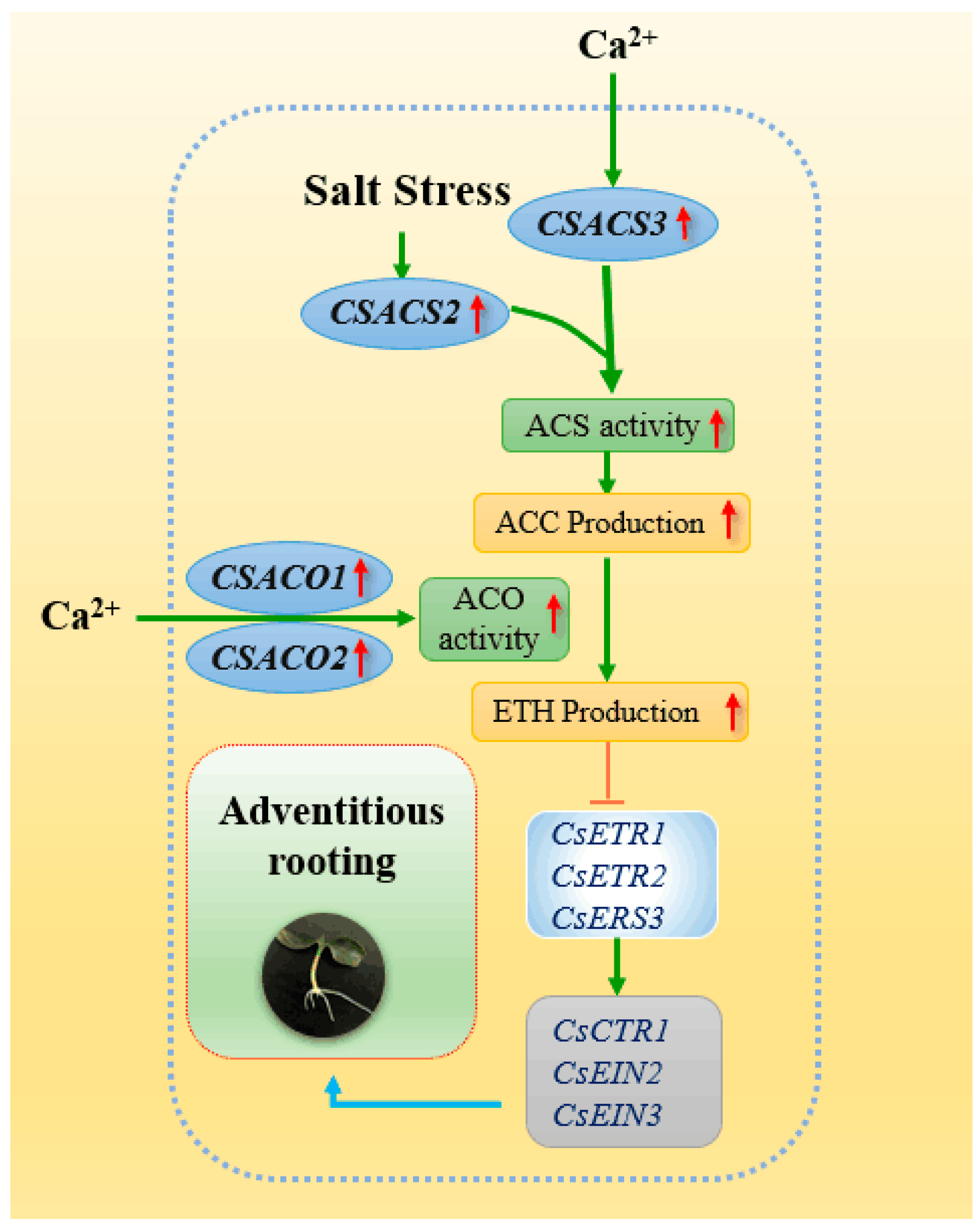
1. Introduction
2. Results
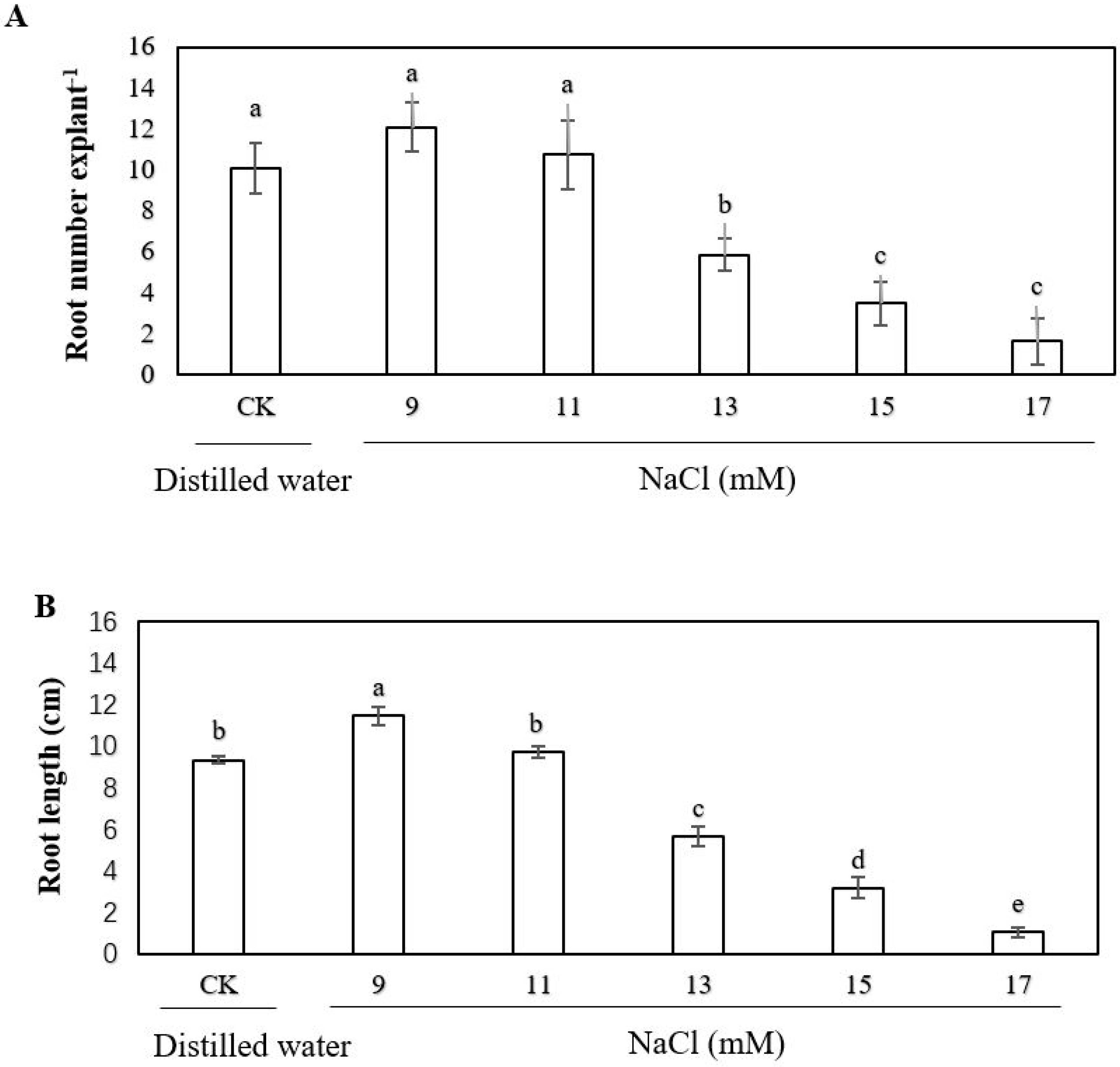
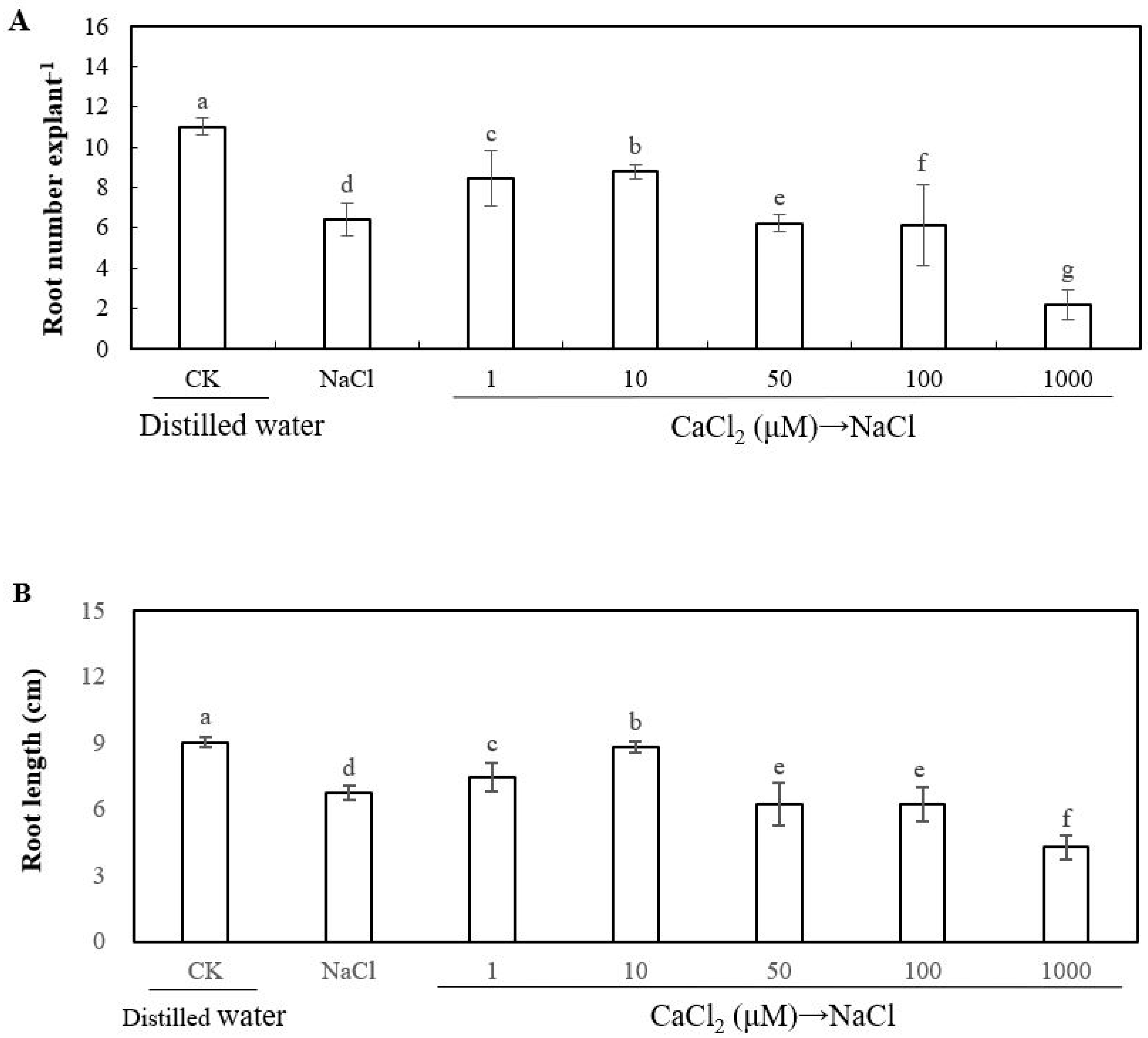
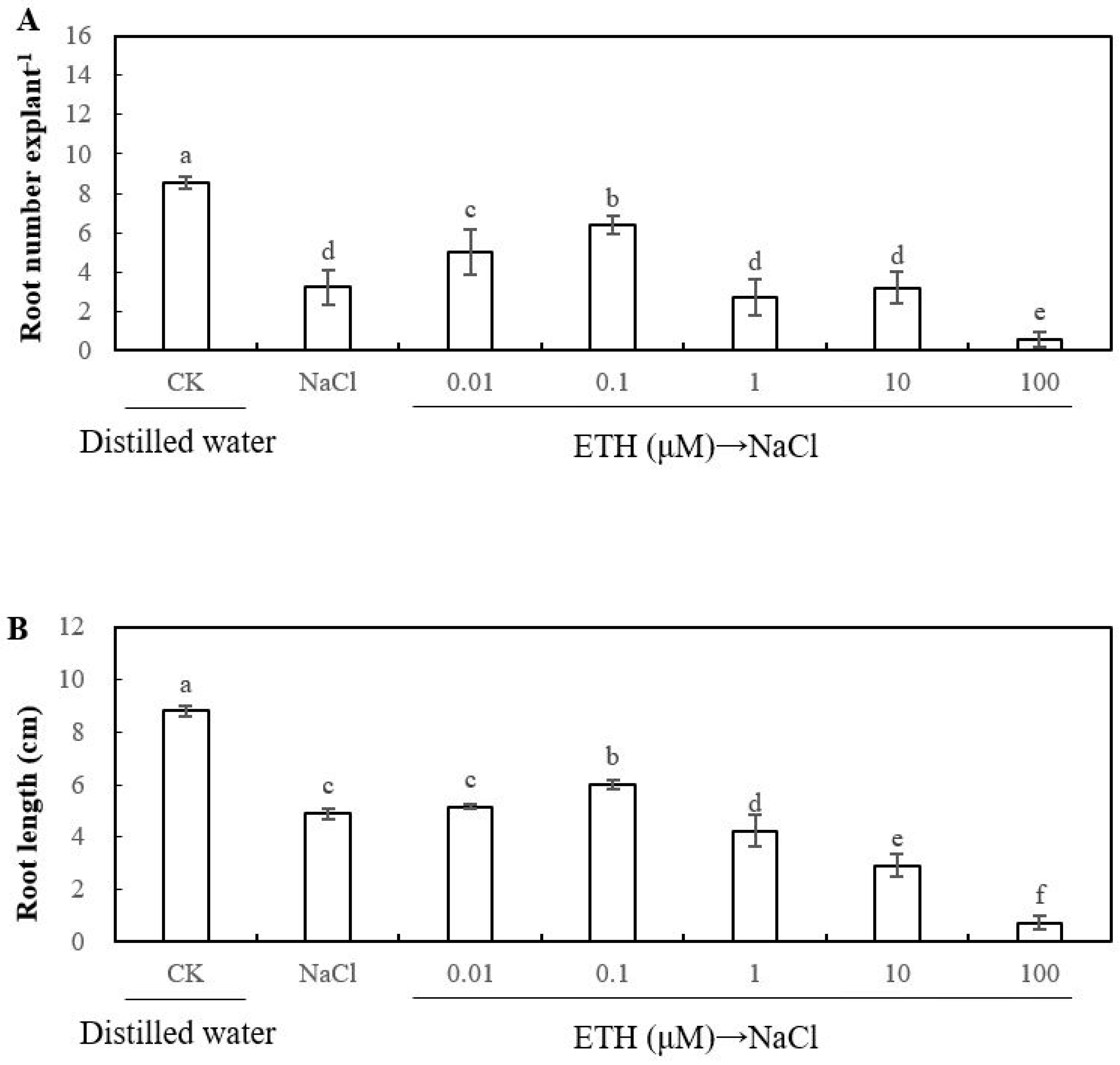
2.1. Effect of Exogenous Sodium Chloride (NaCl), Calcium and Ethylene on the Adventitious Rooting of Cucumber Explant under Salt Stress
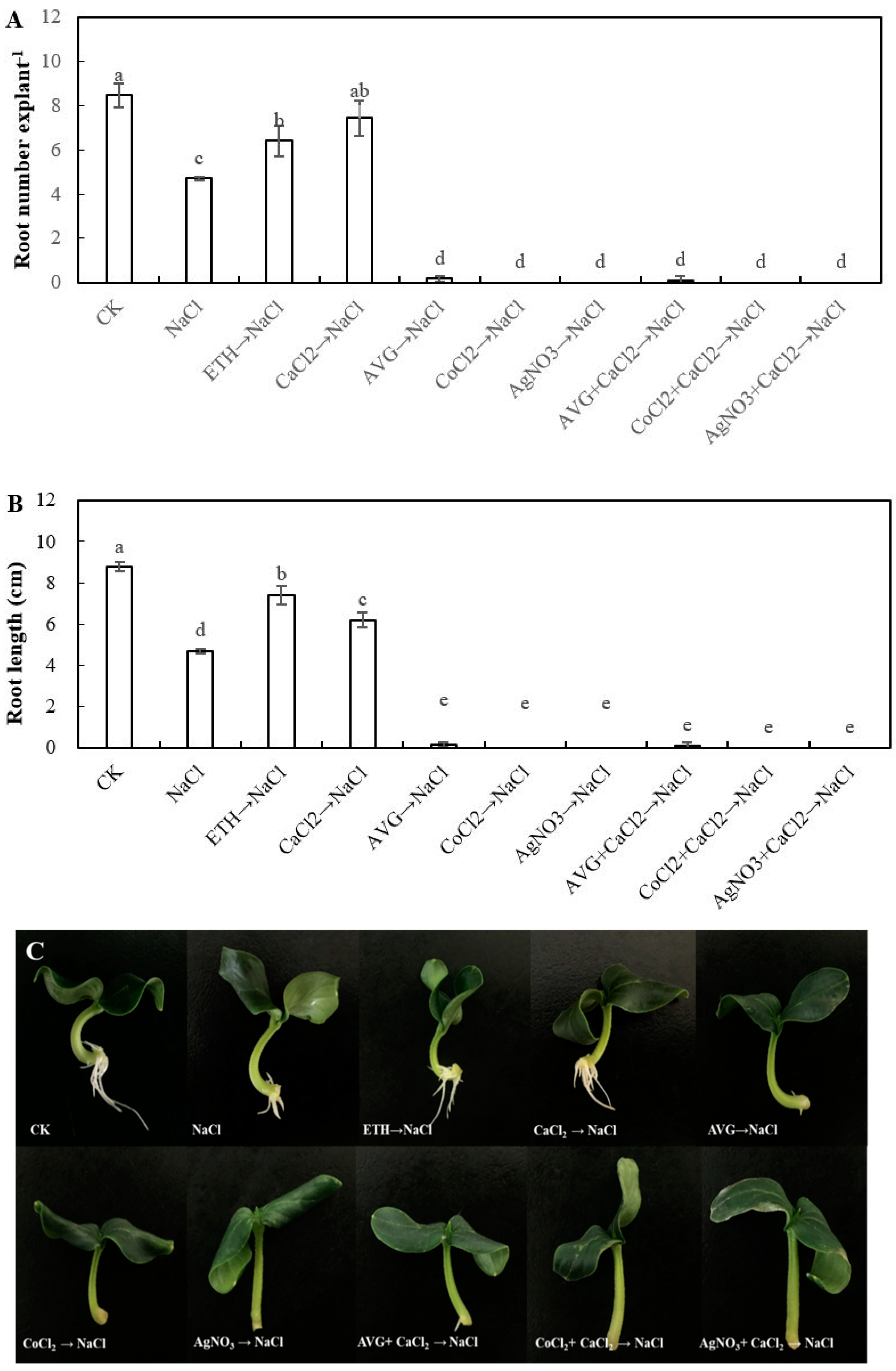
2.2. Effect of Ethylene Aminoethoxyvinyl Glycine (AVG), Cobalt Chloride (CoCl2) and Sliver Nitrate (AgNO3) on the Adventitious Rooting of Cucumber Explant under Salt Stress
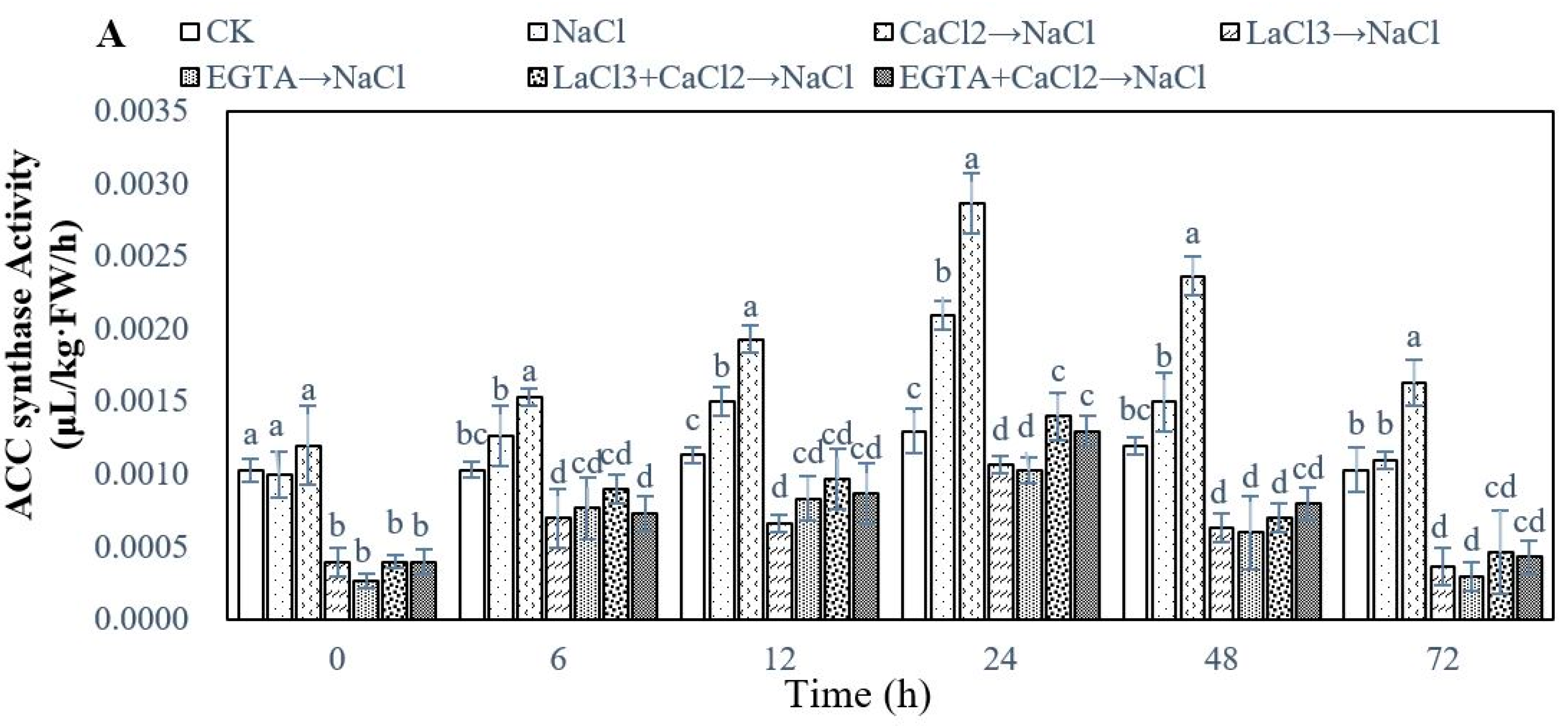
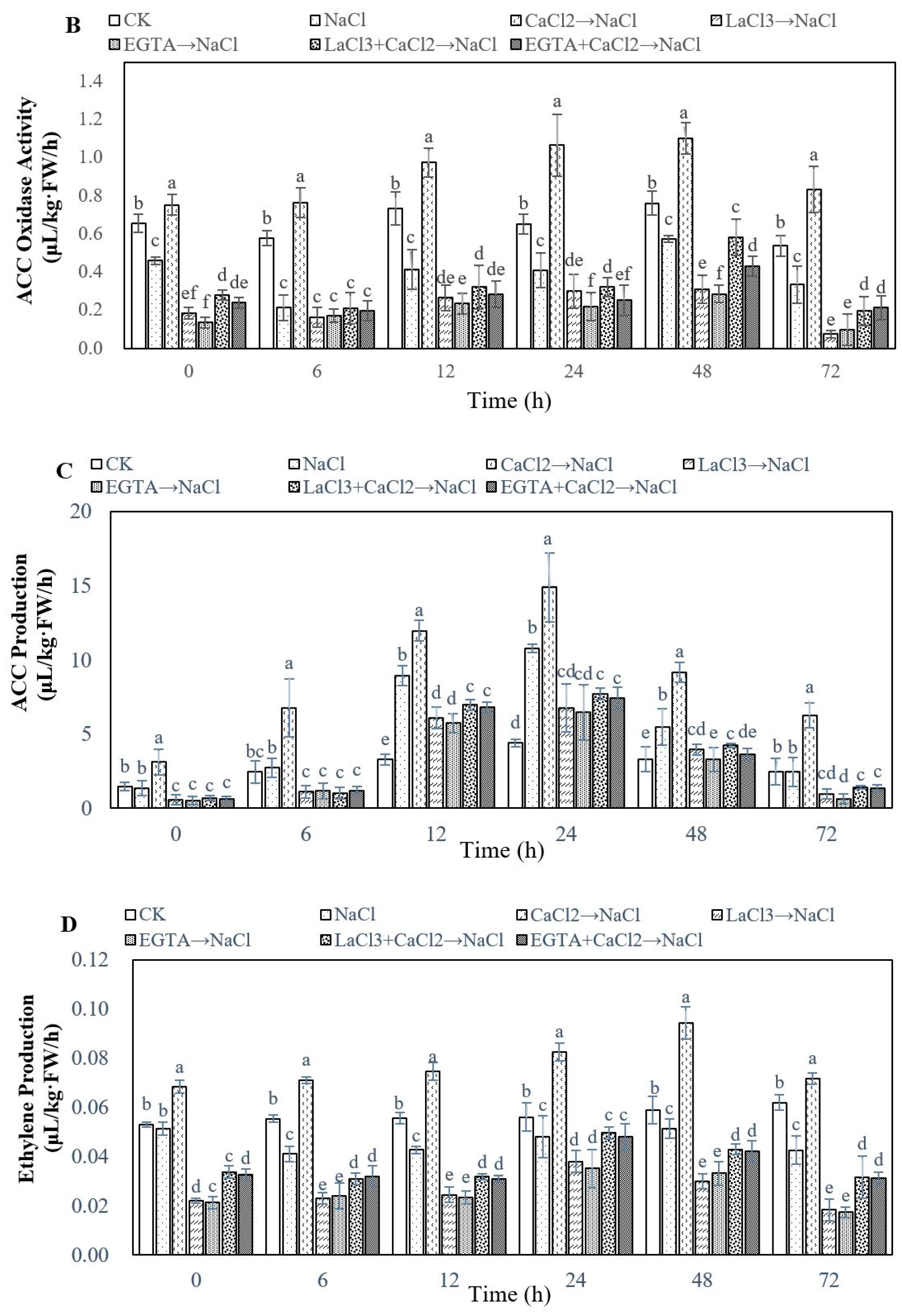
2.3. Effect of Ca2+ on Ethylene Biosynthesis During Adventitious Rooting in Cucumber under Salt Stress
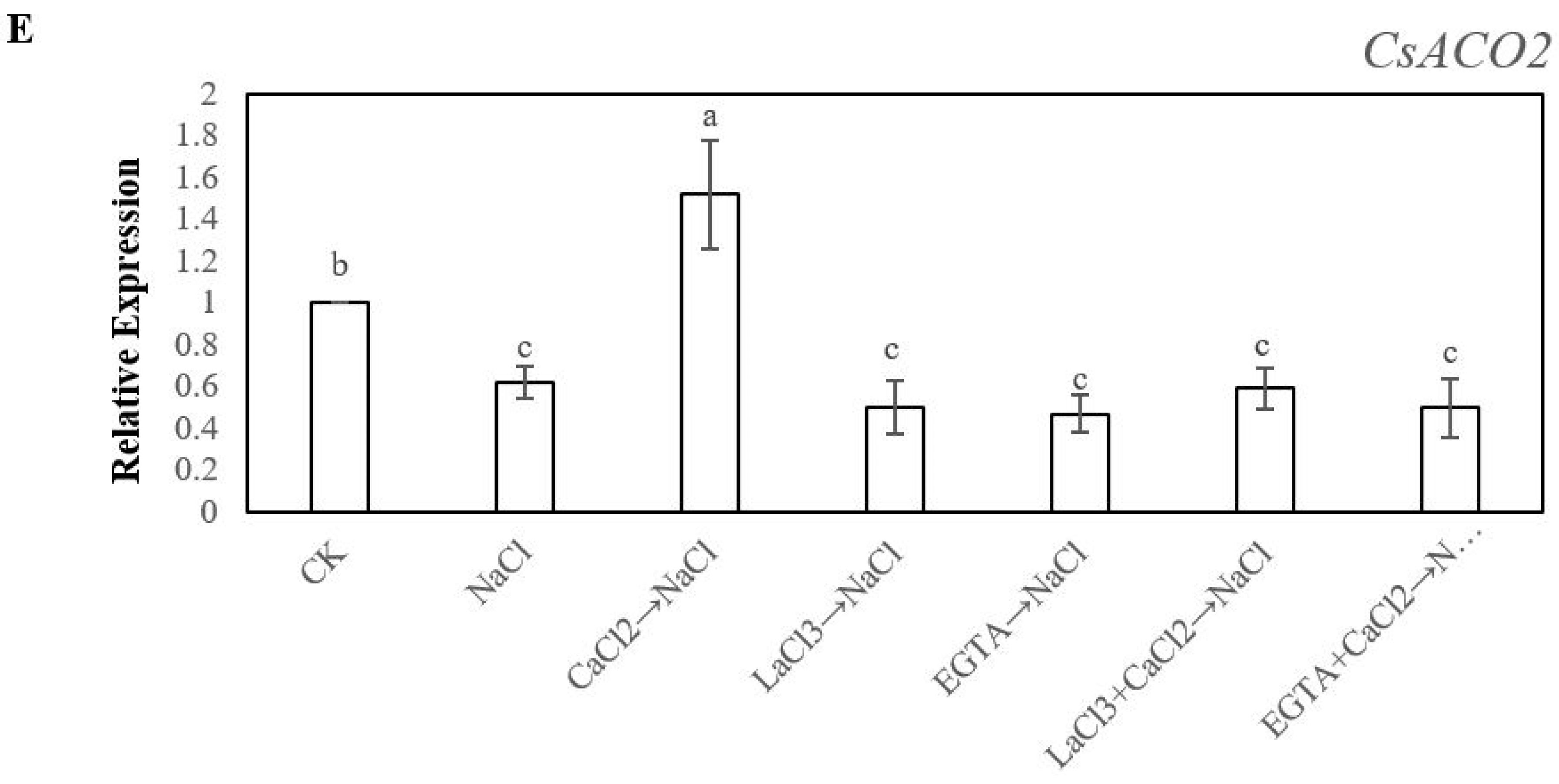
2.4. Effects of Ca2+ on the Expression Levels of CsACS1, CsACS2, CsACS3, CsACO1and CsACO2 under Salt Stress During Adventitious Rooting in Cucumber
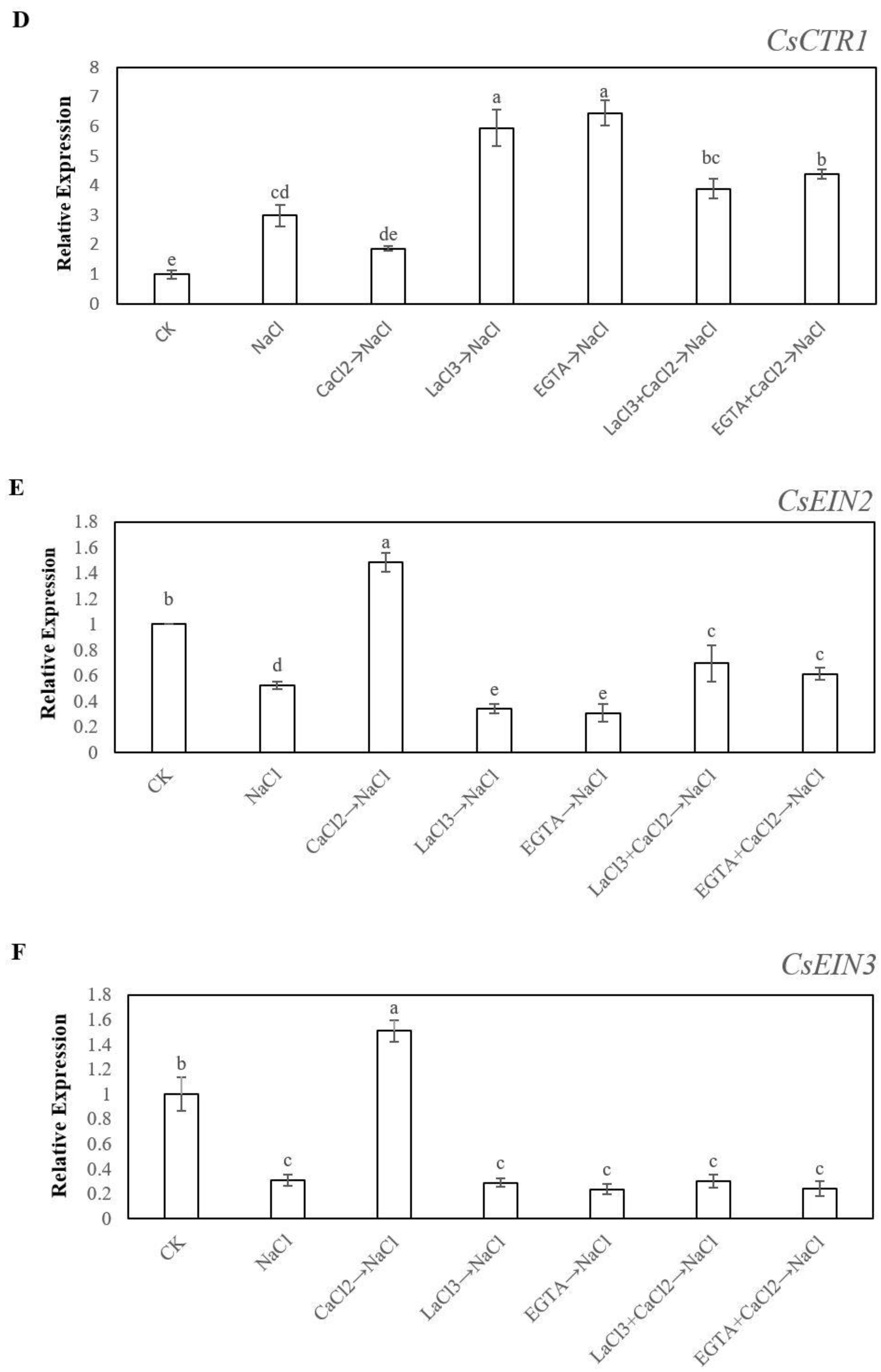
2.5. Effects of Ca2+ on the Expression Levels of CsETR1, CsETR2, CsERS, CsCTR1, CsEIN2 and CsEIN3 under Salt Stress During Adventitious Rooting in Cucumber
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Treatments of Explants
4.3. Determination of ACC Production
4.4. Ethylene Measurement
4.5. Determination of ACO Activity
4.6. Determination of ACS Activity
4.7. Gene Expression Analyses by RT-qPCR
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Kong, D.; Ju, C.; Parihar, A.; Kim, S.; Cho, D.; Kwak, J.M. Arabidopsis Glutamate Receptor Homolog AtGLR3.5 Modulates Cytosolic Ca2+ Level to Counteract Effect of Abscisic Acid in Seed Germination. Plant Physiol. 2015, 167, 1630–1642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pan, Y.; Tian, W.; Dong, M.; Zhu, H.; Luan, S.; Li, L. Arabidopsis CNGC14 mediates calcium influx required for tip growth in root hairs. Mol. Plant 2017, 10, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Himschoot, E.; Beeckman, T.; Friml, J.; Vanneste, S. Calcium is an organizer of cell polarity in plants. Biochim. Biophys. Acta 2015, 1853, 2168–2172. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.H.; Shun, D.S.; Xiao, H.; Tian, H.Q. Calcium distribution during pollen development in Bauhinia blakeana. Trees 2018, 32, 465–472. [Google Scholar] [CrossRef]
- Lanteri, M.L.; Pagnussat, G.C.; Lamattina, L. Calcium and calcium-dependent protein kinases are involved in nitric oxide-and auxin-induced adventitious root formation in cucumber. J. Exp. Bot. 2006, 57, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.R.; Moustafa, A.N.; Bassuony, F.M.; Haroun, S.A. Kinetin and/or calcium affect growth of Phaseolus vulgaris L. plant grown under heavy metals stress. J. Environ. Sci. 2017, 46, 103–120. [Google Scholar]
- Huang, D.; Gong, X.; Liu, Y.; Zeng, G.; Lai, C.; Bashir, H.; Zhou, L.; Wang, D.; Xu, P.; Cheng, M.; et al. Effects of calcium at toxic concentrations of cadmium in plants. Planta 2017, 245, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Anwar, Y.; Hasan, M.M.; Iqbal, A.; Ali, M.; Alharby, H.F.; Hakeem, K.R.; Hasanuzzaman, M. Attenuation of drought stress in brassica seedlings with exogenous application of Ca2+ and H2O2. Plants 2017, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Cui, H.; Lv, X.; Yang, Y.; Wang, Y.; Lu, X. Exogenous CaCl2 reduces salt stress in sour jujube by reducing Na+ and increasing K+, Ca2+, and Mg2+ in different plant organs. J. Hortic. Sci. Biotech. 2017, 92, 98–106. [Google Scholar] [CrossRef]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.B.; Zhang, M.L.; Huang, G.B.; Yu, J.H. Ca2+ and CaM are involved in NO- and H2O2-induced adventitious root development in marigold. J. Plant Growth Regul. 2012, 31, 253–264. [Google Scholar] [CrossRef]
- Niu, L.; Yu, J.; Liao, W.; Yu, J.; Zhang, M.; Dawuda, M.M. Calcium and calmodulin are involved in Nitric Oxide-induced adventitious rooting of cucumber under simulated osmotic stress. Front. Plant Sci. 2017, 8, 1684. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Pang, X.; Li, H.; Feng, X.; Xu, H. Exogenous application of poly-γ-glutamic acid enhances stress defense in Brassica napus L. seedlings by inducing cross-talks between Ca2+, H2O2, brassinolide, and jasmonic acid in leaves. Plant Physiol. Biochem. 2017, 118, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Qi, F.; Zhang, Y.; Cao, H.; Zhang, J.; Wang, R.; Shen, W. Methane-rich water induces cucumber adventitious rooting through heme oxygenase1/carbon monoxide and Ca2+ pathways. Plant Cell Rep. 2015, 34, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.O.; Yang, S.F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA 1979, 76, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Ann. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Ribeiro, R.P.; Costa, L.C.; Medina, E.F.; Araújo, W.L.; Zsögön, A.; Ribeiro, D.M. Ethylene coordinates seed germination behavior in response to low soil pH in Stylosanthes humilis. Plant Soil 2018, 425, 87. [Google Scholar] [CrossRef]
- Xu, X.T.; Jin, X.; Liao, W.B.; Dawuda, M.M.; Li, X.P.; Wang, M.; Niu, L.J.; Ren, P.J.; Zhu, Y.C. Nitric oxide is involved in ethylene-induced adventitious root development in cucumber (Cucumis sativus L.) explants. Sci. Hortic. 2017, 215, 65–71. [Google Scholar] [CrossRef]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.G.; Gubrium, E.K.; Barrett, J.E.; Nell, T.A.; Klee, H.J. Root formation in ethylene-insensitive plants. Plant Physiol. 1999, 121, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 1642, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, H.; Liu, W.; Zhang, X.; Lu, Y.T. Ethylene inhibits root elongation during alkaline stress through AUX1 and associated changes in auxin accumulation. Plant Physiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Kim, H.; Bakshi, A.; Binder, B. The Ethylene Receptors, ETHYLENE RESPSONE 1 and 2, Have Contrasting Roles in Seed Germination of Arabidopsis thaliana During Salt Stress. Plant Physiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.J.; Chen, H.W.; Ma, B.; Zhang, W.K.; Chen, S.Y.; Zhang, J.S. The role of ethylene in plants under salinity stress. Front. Plant Sci. 2015, 6, 1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Vandenbussche, F.; Van Der Straeten, D. Regulation of seedling growth by ethylene and the ethylene–auxin crosstalk. Planta 2017, 245, 467–489. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Ortiz, S.; Garnica-Vergara, A.; Esparza-Reynoso, S.; García-Cárdenas, E.; Raya-González, J.; Ruiz-Herrera, L.F.; López-Bucio, J. Jasmonic Acid-Ethylene Crosstalk via ETHYLENE INSENSITIVE 2 Reprograms Arabidopsis Root System Architecture Through Nitric Oxide Accumulation. J. Plant Growth Regul. 2018, 37, 438–451. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.X.; He, X.L.; Liu, L.J.; Wu, H.; Tang, C.; Zhang, Y.S.; Jin, C.W. Ethylene and nitric oxide interact to regulate the magnesium deficiency-induced root hair development in Arabidopsis. New Phytol. 2017, 213, 1242–1256. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.R.; Monteiro, C.C.; Carvalho, R.F.; Ribeiro, P.C.; Tezotto, T.; Azevedo, R.A.; Gratão, P.L. Cadmium stress related to root-to-shoot communication depends on ethylene and auxin in tomato plants. Environ. Exp. Bot. 2017, 134, 102–115. [Google Scholar] [CrossRef]
- Kissoudis, C.; Seifi, A.; Yan, Z.; Islam, A.T.M.; van der Schoot, H.; van de Wiel, C.; Visser, R.G.F.; van der Linden, C.G.; Bai, Y. Ethylene and abscisic acid signaling pathways differentially influence tomato resistance to combined powdery mildew and salt stress. Front. Plant Sci. 2017, 7, 2009. [Google Scholar] [CrossRef] [PubMed]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Zhu, C.Q.; Wang, C.; Dong, X.Y.; Shen, R.F. Nitric oxide acts upstream of ethylene in cell wall phosphorus reutilization in phosphorus-deficient rice. J. Exp. Bot. 2017, 68, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, I.B. Calcium stimulation of ethylene production induced by 1-aminocyclopropane-1-carboxylic acid and indole-3-acetic acid. J. Plant Growth Regul. 1983, 2, 205–214. [Google Scholar] [CrossRef]
- Hasenstein, K.H.; Evans, M.L. Calcium ion dependency of ethylene production in segments of primary roots of Zea mays. Physiol. Plant. 1986, 67, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Lee, J.H.; Cho, M.H.; Kim, W.T. Induction of 1-aminocyclopropane-1-carboxylate oxidase mRNA by ethylene in mung bean roots: Possible involvement of Ca2+ and phosphoinositides in ethylene signalling. Plant Cell Environ. 2000, 23, 205–213. [Google Scholar] [CrossRef]
- Zielińska, M.; Michniewicz, M. The effect of calcium on the production of ethylene and abscisic acid by fungus Fusarium culmorum and by wheat seedlings infected with that pathogen. Acta Physiol. Plant. 2001, 23, 79–85. [Google Scholar] [CrossRef]
- Li, S.; Han, X.; Yang, L.; Deng, X.; Wu, H.; Zhang, M.; Liu, Y.; Zhang, S.; Xu, J. Mitogen-activated protein kinases and calcium-dependent protein kinases are involved in wounding-induced ethylene biosynthesis in Arabidopsis thaliana. Plant. Cell Environ. 2018, 41, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.G.; Tian, Q.Y.; Zhang, W.H. Ethylene activates a plasma membrane Ca2+-permeable channel in tobacco suspension cells. New Phytol. 2007, 174, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Cheng, M.C.; Liao, P.M.; Kuo, W.W.; Lin, T.P. The Arabidopsis ETHYLENE-RESPONSE-FACTOR1 regulates abiotic-stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- West, G.; Inzé, D.; Beemster, G.T. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, K.; Li, X. Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana. J. Plant Physiol. 2009, 166, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Dietrich, D.; Ng, C.H.; Chan, P.M.Y.; Bhalerao, R.; Bennett, M.J.; Dinneny, J.R. Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 2013. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Fang, L.; Ren, X.; Wang, W.; Jiang, J. MPK6 controls H2O2-induced root elongation by mediating Ca2+ influx across the plasma membrane of root cells in Arabidopsis seedlings. New Phytol. 2015, 205, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.J.; Li, X.D.; Ratnasekera, D.; Wang, C.; Liu, W.X.; Song, L.F.; Zhang, W.Z.; Wu, W.H. Arabidopsis Calcium-Dependent Protein Kinse8 and Catalase3 function in Abscisic Acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 2015, 27, 1445–1460. [Google Scholar] [CrossRef] [PubMed]
- Abd_Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Wirth, S.; Egamberdieva, D. Calcium application enhances growth and alleviates the damaging effects induced by Cd stress in sesame (Sesamum indicum L.). J. Plant Interact. 2017, 12, 237–243. [Google Scholar] [CrossRef]
- Manishankar, P.; Wang, N.; Köster, P.; Alatar, A.A.; Kudla, J. Calcium Signaling during Salt Stress and in the Regulation of Ion Homeostasis. J. Exp. Bot. 2018, 69, 4215–4226. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Xue, L. The interaction between H2O2 and NO, Ca2+, cGMP, and MAPKs during adventitious rooting in mung bean seedlings. In Vitro Cell. Dev.-Biol. 2010, 46, 142–148. [Google Scholar] [CrossRef]
- Cramer, G.R.; Läuchli, A.; Epstein, E. Effects of NaCl and CaCl2 on ion activities in complex nutrient solutions and root growth of cotton. Plant Physiol. 1986, 81, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Veloccia, A.; Fattorini, L.; Della Rovere, F.; Sofo, A.; D’angeli, S.; Betti, C.; Falasca, G.; Altamura, M.M. Ethylene and auxin interaction in the control of adventitious rooting in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6445–6458. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Elhiti, M.; Wang, H.; Xu, A.; Brown, D.; Wang, A. Adventitious root formation of in vitro peach shoots is regulated by auxin and ethylene. Sci. Hortic. 2017, 226, 250–260. [Google Scholar] [CrossRef]
- Coleman, W.K.; Huxter, T.J.; Reid, D.M.; Thorpe, T.A. Ethylene as an endogenous inhibitor of root regeneration in tomato leaf discs cultured in vitro. Physiol. Plant. 1980, 48, 519–525. [Google Scholar] [CrossRef]
- Negi, S.; Sukumar, P.; Liu, X.; Cohen, J.D.; Muday, G.K. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J. 2010, 61, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ye, N.; Zhu, F.; Li, H.; Wang, J.; Jiang, L.; Zhang, J. Calcium-dependent protein kinase CPK 28 targets the methionine adenosyltransferases for degradation by the 26S proteasome and affects ethylene biosynthesis and lignin deposition in Arabidopsis. Plant J. 2017, 90, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.A.; Saitoh, H.; Felix, G.; Freymark, G.; Miersch, O.; Wasternack, C.; Boller, T.; Jones, J.D.G.; Romeis, T. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc. Natl. Acad. Sci. USA 2005, 102, 10736–10741. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.K.; Evensen, K.B. Ca2+ effects on ethylene, carbon dioxide and 1-aminocyclopropane-1-carboxylic acid synthase activity. Physiol. Plant. 1986, 66, 609–615. [Google Scholar] [CrossRef]
- Cheverry, J.L.; Pouliquen, J.; Le Guyader, H.; Marcellin, P. Calcium regulation of exogenous and endogenous 1-aminocylopropane-1-carboxylic acid bioconversion to ethylene. Physiol. Plant. 1988, 74, 53–57. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Z.; Gao, J.; Gong, Z. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J. 2014, 79, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.H.; Lee, S.H. The Requirements for Ca2+, Protein Phosphorylation, and Dephosphorylation for Ethylene Signal Transduction in Piswn sativum L. Plant Cell Physiol. 1997, 38, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, J.; Su, X.; Li, L.; Pang, X.; Zhang, Z. MaCDPK7, a calcium-dependent protein kinase gene from banana is involved in fruit ripening and temperature stress responses. J. Hortic. Sci. Biotech. 2017, 92, 240–250. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Xia, X.; Zhang, W.H. Ameliorative effect of brassinosteroid and ethylene on germination of cucumber seeds in the presence of sodium chloride. Plant Growth Regul. 2011, 65, 407. [Google Scholar] [CrossRef]
- Shi, J.; Drummond, B.; Wang, H.; Archibald, R.L.; Habben, J.E. Maize and Arabidopsis ARGOS proteins interact with ethylene receptor signaling complex, supporting a regulatory role for ARGOS in ethylene signal transduction. Plant Physiol. 2016, 171, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.R.; Chen, S.Y.; Zhang, J.S. Ethylene signaling regulates salt stress response: An overview. Plant Signal. Behav. 2008, 3, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Thongkum, M.; McAtee, P.M.; Schaffer, R.J.; Allan, A.C.; Ketsa, S. Characterization and differential expression of ethylene receptor genes during fruit development and dehiscence of durian (Durio zibethinus). Sci. Hortic. 2018, 240, 623–630. [Google Scholar] [CrossRef]
- Duckett, C.M.; Grierson, C.; Linstead, P.; Schneider, K.; Lawson, E.; Dean, C.; Poethig, C.; Roberts, K. Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development 1994, 120, 2465–2474. [Google Scholar]
- Pitts, R.J.; Cernac, A.; Estelle, M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 1998, 16, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, M.; Lizada, C.; Yang, S.F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal. Biochem. 1979, 100, 140–145. [Google Scholar] [CrossRef]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk complexities between auxin, cytokinin and ethylene in Arabidopsis root development: From experiments to systems modelling, and back again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, W.; Xu, Y.P.; Cao, J.Y.; Braam, J.; Cai, X.Z. Genome-wide identification and functional analyses of calmodulin genes in Solanaceous species. BMC Plant Biol. 2013, 13, 70. [Google Scholar] [CrossRef] [PubMed]



| Gene Symbol | Accession Number | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Primer Efficiency |
|---|---|---|---|---|
| Actin | AB010922.1 | 5′-TTGAATCCCAAGGCGAATAG-3′ | 5′-TGCGACCACTGGCATAAAG-3′ | 1.98 |
| CsACS1 | AB006803.1 | 5′-ACGGTCACGGCGAGGATTCAC-3′ | 5′-AGTTCAGGAGACCTTCGTCGGTAC-3′ | 1.96 |
| CsACS2 | AB006804.1 | 5′-ATGTCACCACACTCACCGTTGC-3′ | 5′-ATGACTGATTGGCGGTCGTCTTG-3′ | 1.99 |
| CsACS3 | AB006805.1 | 5′-CCTTGCAGAGGCTGGCGATG-3′ | 5′-GGTGACTTGGAAGCCGTTGGAG-3′ | 1.99 |
| CsACO1 | AB006806.1 | 5′-AGGTAGGTGGCCTGCAACTCC-3′ | 5′-CTCCGAGGTTGACGACAATGGC-3′ | 1.99 |
| CsACO2 | AB006807.2 | 5′-CAGTCTCCAACATCGCGGATCTC-3′ | 5′-GCAGGAGTTCGGCGAGTACTTG-3′ | 1.92 |
| CSETR1 | AB026498.1 | 5′-AATGAGGAGCGTGTTGTCGGAAC-3′ | 5′-TCTCAAGATCACCACCACAATGCC-3′ | 1.96 |
| ‘CSETR2 | NM_001308840.1 | 5′-GCCATGCCTGAACCTGGAGAATC-3′ | 5′-GCTGGTGCCATGACTGTGAGAC-3′ | 1.97 |
| CSERS | AB026499.1 | 5′-AGAAGTTGTTGCAGTGCGAGTCC-3′ | 5′-GCTACCTGGTCTGCGACAACATC-3′ | 1.95 |
| CsCTR1 | NM_001305781.1 | 5′-TGTTGACTCCAGCATCGCTTCATC-3′ | 5′-CAAGTGATTGCATACCAGCTTCGC-3′ | 1.97 |
| CsEIN2 | NM_KF245636.1 | 5′-ATTATCAGCCTGCCACAGTCCATG-3′ | 5′-CAAGCCTGCACCACCACCAC-3′ | 1.99 |
| CsEIN3 | XM_004144061.2 | 5′-GCACCTGCTCGGCTGATGAAC-3′ | 5′-GGCAGTTGTTGCTGTTGGTTGTG-3′ | 1.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Niu, L.; Yu, J.; Liao, W.; Xie, J.; Lv, J.; Feng, Z.; Hu, L.; Dawuda, M.M. The Involvement of Ethylene in Calcium-Induced Adventitious Root Formation in Cucumber under Salt Stress. Int. J. Mol. Sci. 2019, 20, 1047. https://doi.org/10.3390/ijms20051047
Yu J, Niu L, Yu J, Liao W, Xie J, Lv J, Feng Z, Hu L, Dawuda MM. The Involvement of Ethylene in Calcium-Induced Adventitious Root Formation in Cucumber under Salt Stress. International Journal of Molecular Sciences. 2019; 20(5):1047. https://doi.org/10.3390/ijms20051047
Chicago/Turabian StyleYu, Jian, Lijuan Niu, Jihua Yu, Weibiao Liao, Jianming Xie, Jian Lv, Zhi Feng, Linli Hu, and Mohammed Mujitaba Dawuda. 2019. "The Involvement of Ethylene in Calcium-Induced Adventitious Root Formation in Cucumber under Salt Stress" International Journal of Molecular Sciences 20, no. 5: 1047. https://doi.org/10.3390/ijms20051047
APA StyleYu, J., Niu, L., Yu, J., Liao, W., Xie, J., Lv, J., Feng, Z., Hu, L., & Dawuda, M. M. (2019). The Involvement of Ethylene in Calcium-Induced Adventitious Root Formation in Cucumber under Salt Stress. International Journal of Molecular Sciences, 20(5), 1047. https://doi.org/10.3390/ijms20051047






