Influence of Chloroplast Defects on Formation of Jasmonic Acid and Characteristic Aroma Compounds in Tea (Camellia sinensis) Leaves Exposed to Postharvest Stresses
Abstract
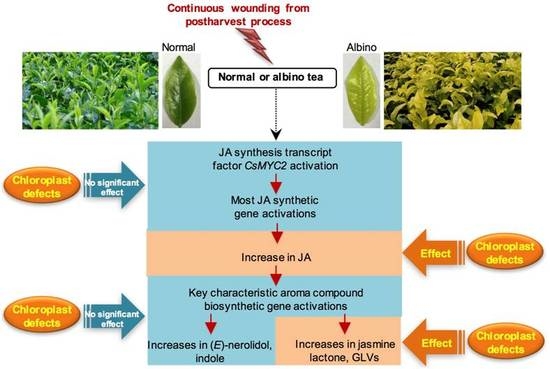
:1. Introduction
2. Results
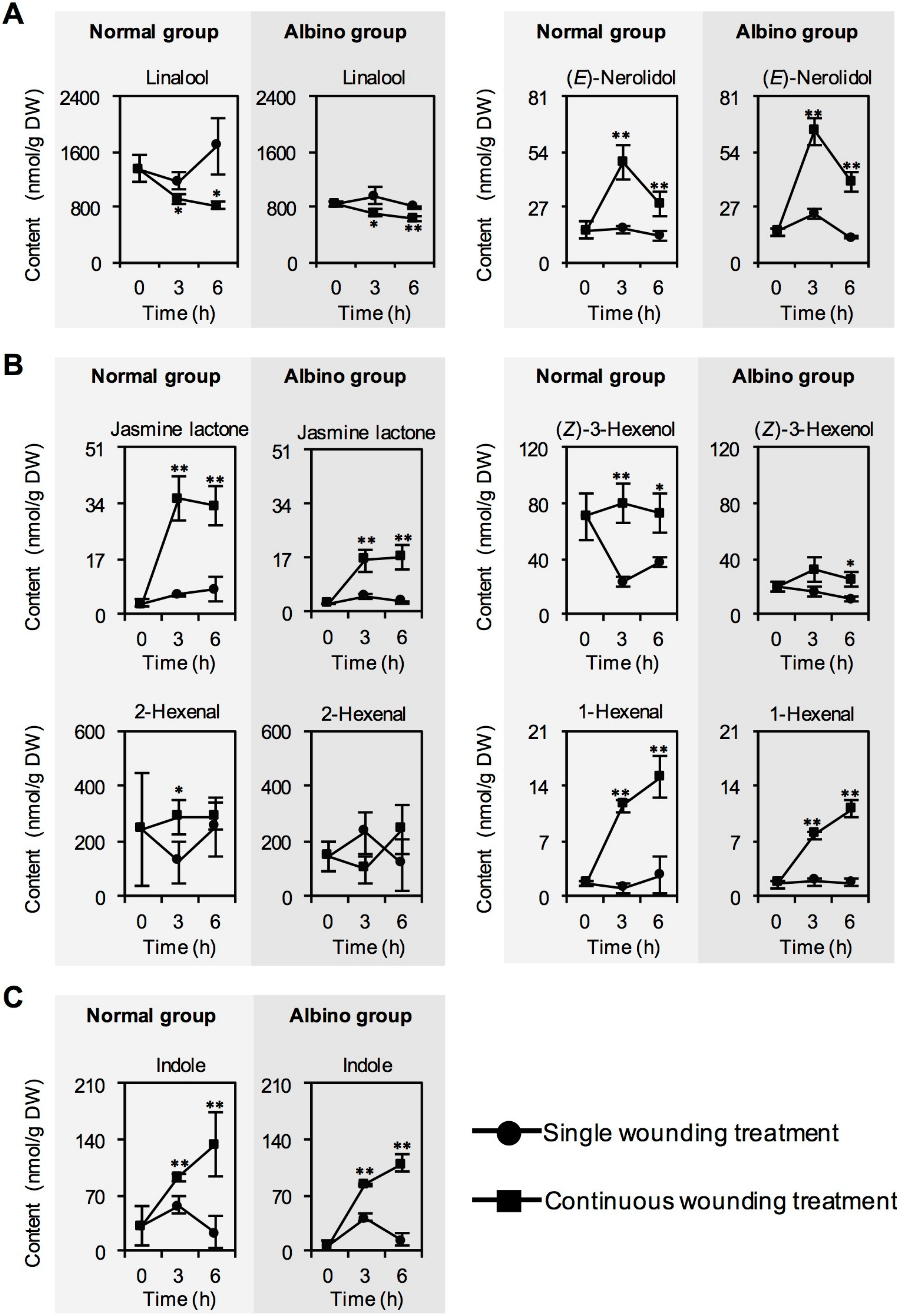
2.1. Effect of Chloroplast Defects on Characteristic Aroma Compound Contents in Response to Continuous Wounding Stress
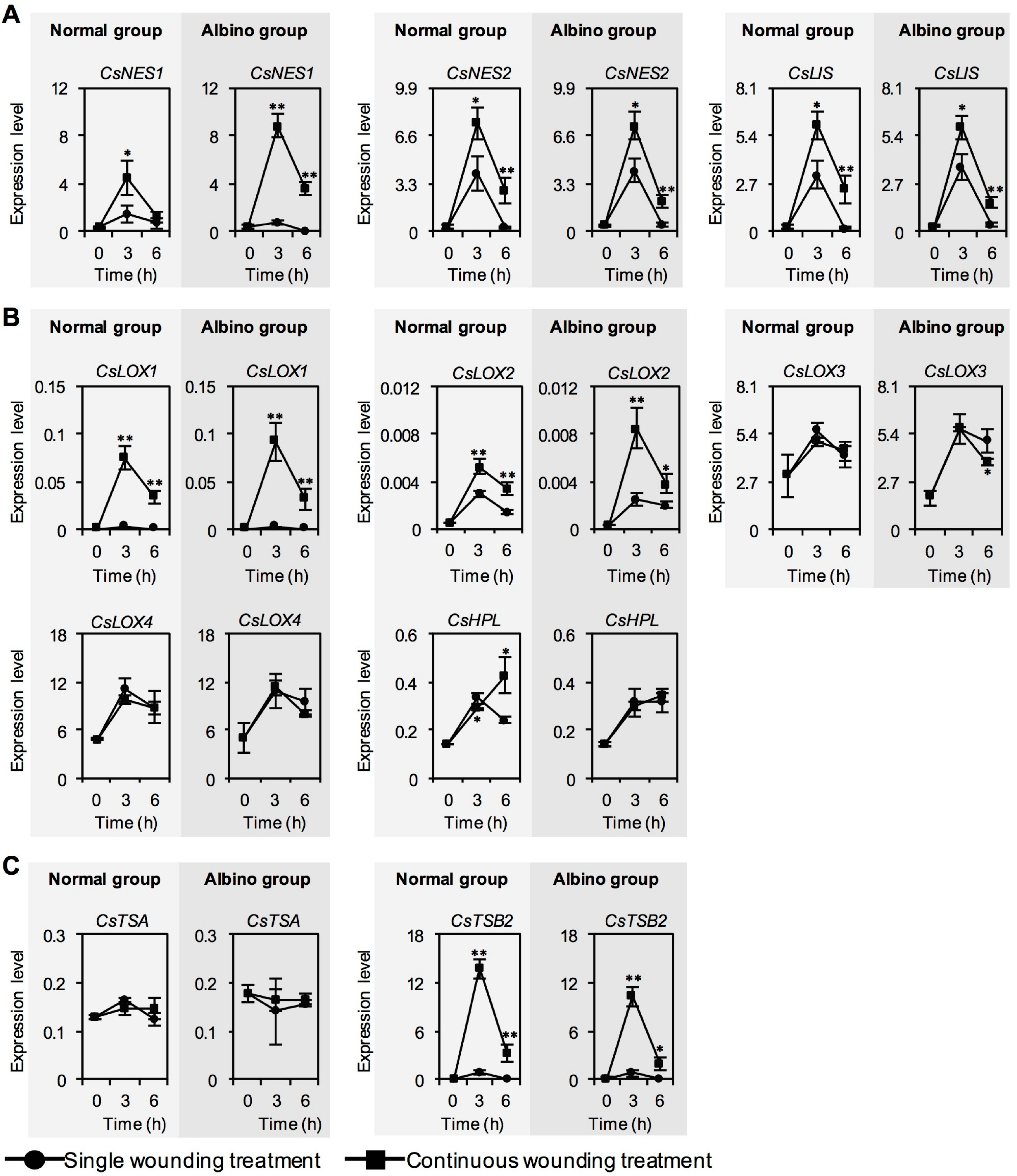
2.2. Effect of Chloroplast Defects on Expression Levels of Characteristic Genes for Aroma Compound Biosynthesis in Response to Continuous Wounding Stress
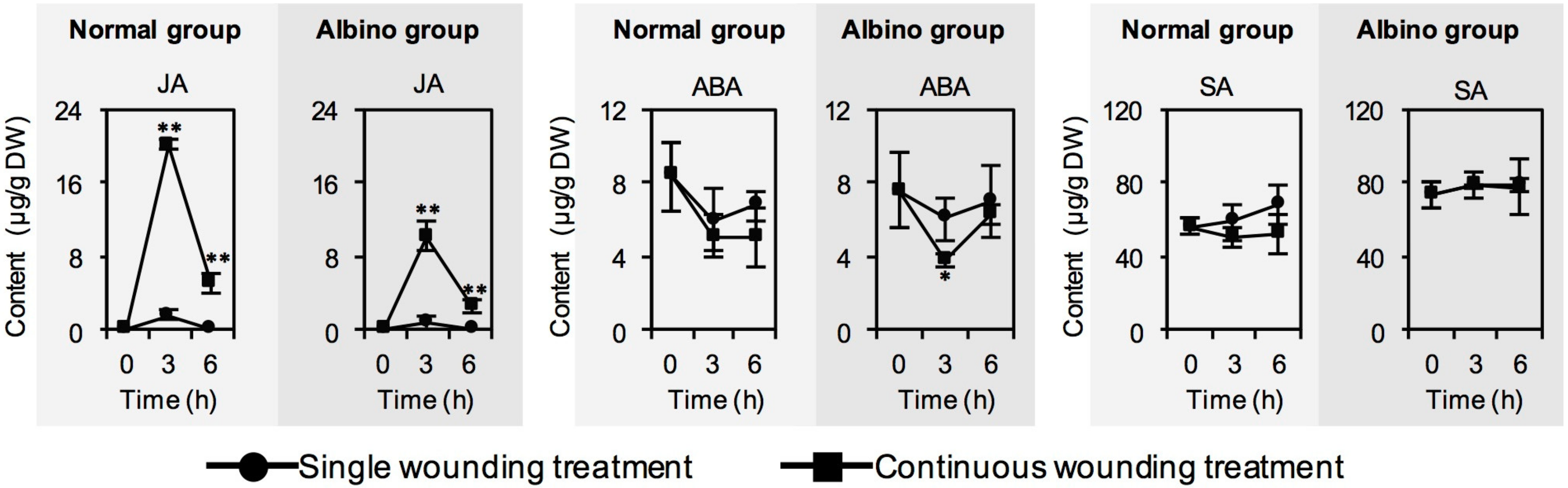
2.3. Effect of Chloroplast Defects on Phytohormone Contents in Response to Continuous Wounding Stress
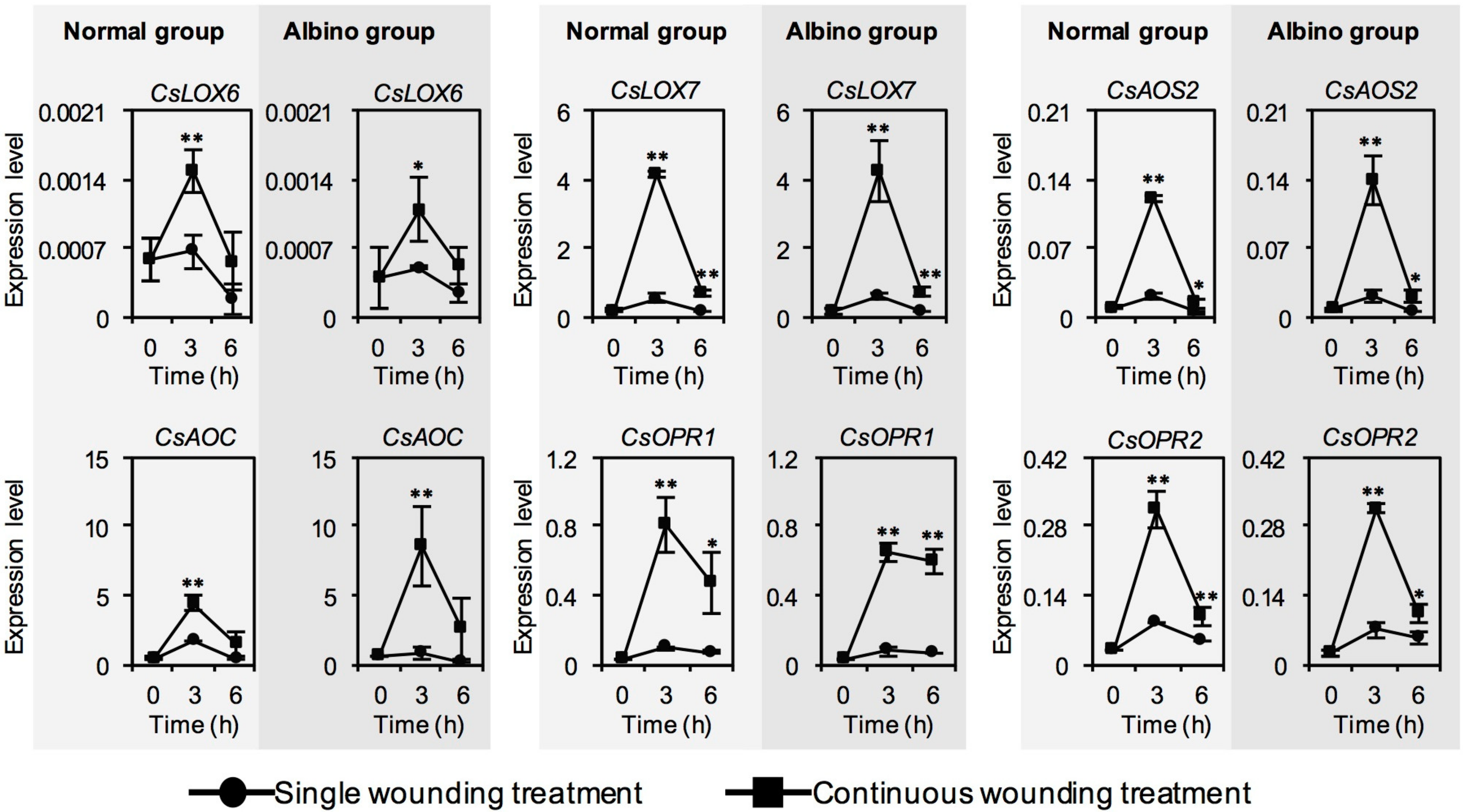
2.4. Effect of Chloroplast Defects on Expression Levels of JA Synthesis-Related Genes in Response to Continuous Wounding Stress
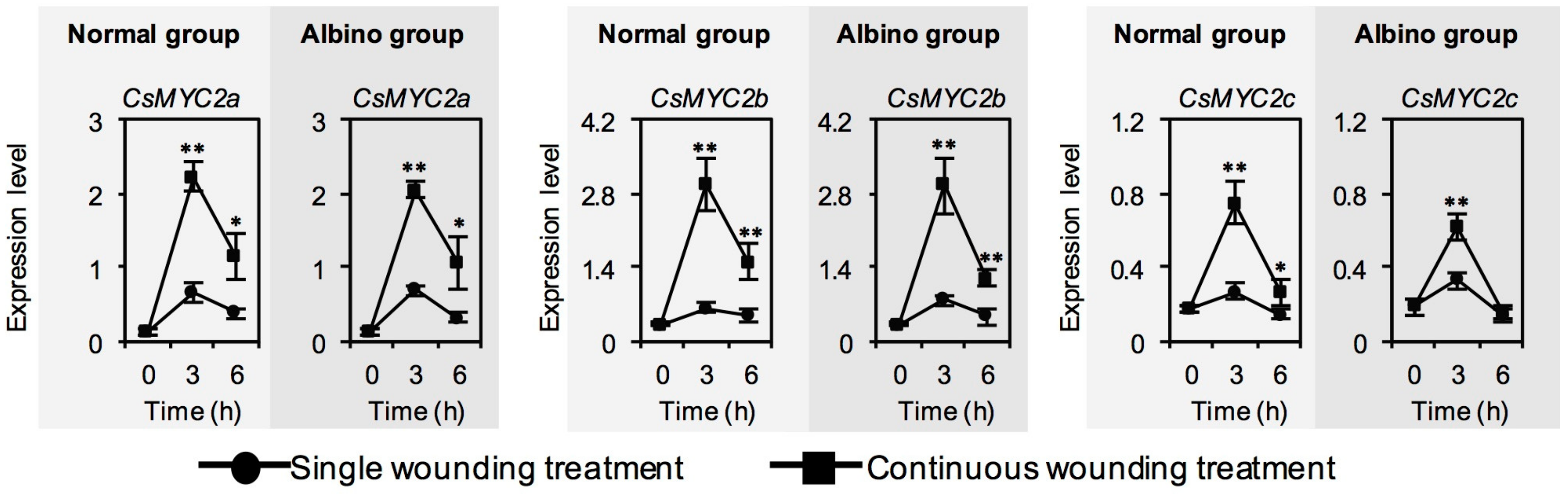
2.5. Effect of Chloroplast Defects on Expression Level of CsMYC2, a Key Transcription Factor of JA Signaling, in Response to Continuous Wounding Stress
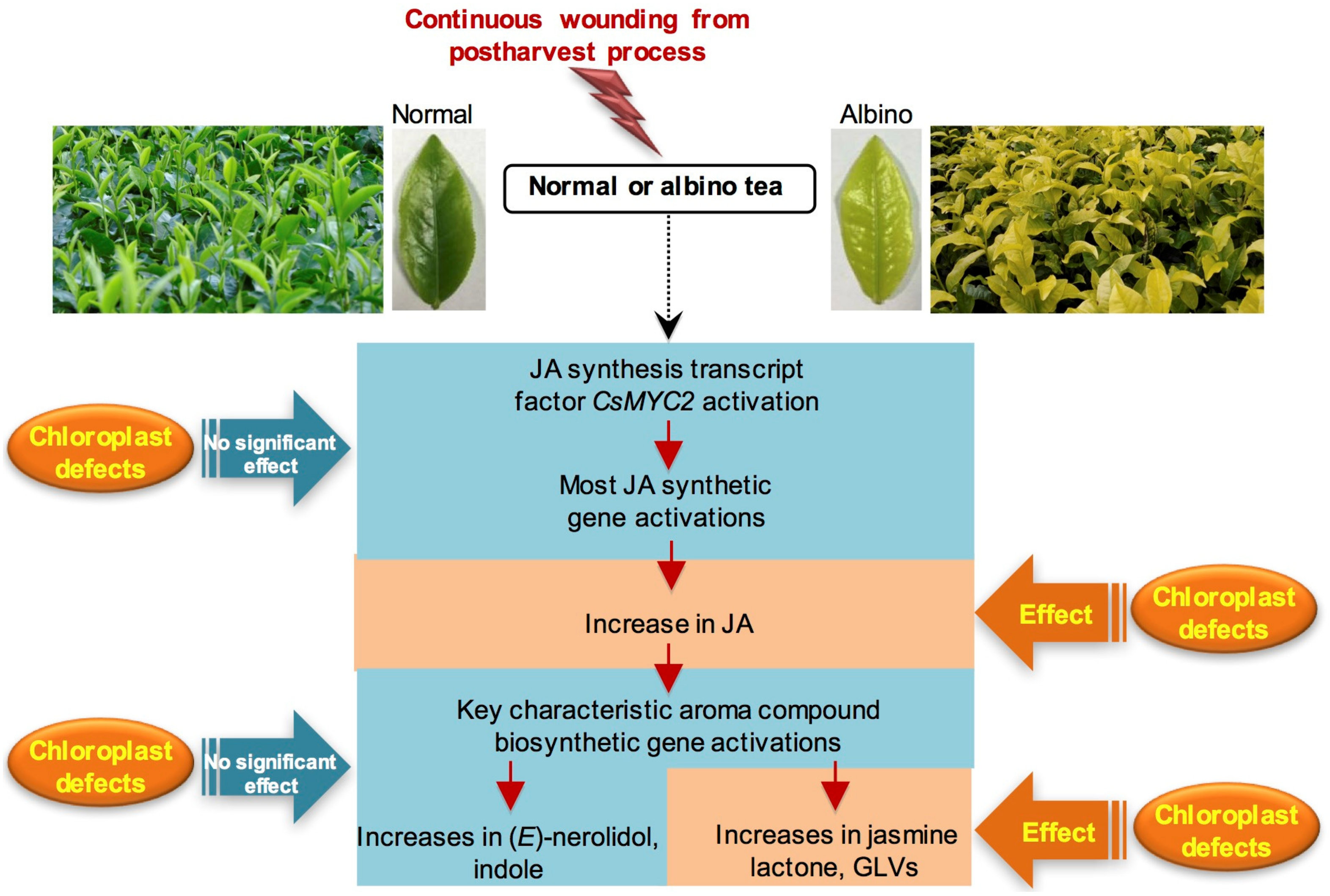
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Extraction and Analysis of Aroma Compounds in Tea Leaves
4.3. Transcript Expression Analysis of the Related Genes
4.4. Analysis of Phytohormone Contents in Tea Leaves
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| AOC | allene oxide cyclase |
| AOS | allene oxide synthase |
| EF1 | encoding elongation factor 1 |
| GC–MS | gas chromatography–mass spectrometry |
| HPL | hydroperoxide lyase |
| JA | jasmonic acid |
| LIS | linalool synthase |
| LOX | lipoxygenase |
| NES | (E)-nerolidol synthase |
| OPR | 12-oxo-phytodienoic acid reductase |
| SA | salicylic acid |
| TSA | tryptophan synthase α-subunit |
| TSB | tryptophan synthase β-subunit |
| UPLC–QTOF-MS | ultra performance liquid chromatography/quadrupole time-of-flight mass spectrometry |
References
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Dong, F.; Fu, X.M.; Watanabe, N.; Su, X.G.; Yang, Z.Y. Recent advances in the emission and functions of plant vegetative volatiles. Molecules 2016, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.T.; Watanabe, N.; Yang, Z.Y. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Baldermann, S.; Watanabe, N. Recent studies of the aroma compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Cho, J.Y.; Mizutani, M.; Shimizu, B.; Kinoshita, T.; Ogura, M.; Tokoro, K.; Lin, M.L.; Sakata, K. Chemical profiling and gene expression profiling during the manufacturing process of Taiwan oolong tea “Oriental Beauty”. Biosci. Biotechnol. Biochem. 2007, 71, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Liu, X.Y.; Zhou, Y.; Wang, X.Q.; Zeng, L.T.; Fu, X.M.; Li, J.L.; Tang, J.C.; Dong, F.; Yang, Z.Y. Formation and emission of linalool in tea (Camellia sinensis) leaves infested by tea green leafhopper (Empoasca (Matsumurasca) onukii Matsuda. Food Chem. 2017, 237, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.M.; Chen, Y.Y.; Mei, X.; Katsuno, T.; Kobayashi, E.; Dong, F.; Watanabe, N.; Yang, Z.Y. Regulation of formation of aroma compounds of tea (Camellia sinensis) leaves by single light wavelength. Sci. Rep. 2015, 5, 16858. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Kobayashi, E.; Katsuno, T.; Asanuma, T.; Fujimori, T.; Ishikawa, T.; Tomomura, M.; Mochizuki, K.; Watase, T.; Nakamura, Y.; et al. Characterisation of volatile and non-volatile metabolites in etiolated leaves of tea (Camellia sinensis) plants in the dark. Food Chem. 2012, 135, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.D.; Fu, X.M.; Zhou, Y.; Katsuno, T.; Mei, X.; Deng, R.F.; Xu, X.L.; Zhang, L.Y.; Dong, F.; Watanabe, N.; et al. Does enzymatic hydrolysis of glycosidically bound aroma compounds really contribute to the formation of aroma compounds during the oolong tea manufacturing process? J. Agric. Food Chem. 2015, 63, 6905–6914. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.T.; Zhou, Y.; Gui, J.D.; Fu, X.M.; Mei, X.; Zhen, Y.P.; Ye, T.X.; Du, B.; Dong, F.; Watanabe, N.; et al. Formation of volatile tea constituent indole during the oolong tea manufacturing process. J. Agric. Food Chem. 2016, 64, 5011–5019. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, L.T.; Liu, X.Y.; Gui, J.D.; Mei, X.; Fu, X.M.; Dong, F.; Tang, J.C.; Zhang, L.Y.; Yang, Z.Y. Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chem. 2017, 231, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.T.; Zhou, Y.; Fu, X.M.; Mei, X.; Cheng, S.H.; Gui, J.D.; Dong, F.; Tang, J.C.; Ma, S.Z.; Yang, Z.Y. Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? Contribution of the stem to oolong tea aroma. Food Chem. 2017, 237, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.T.; Zhou, Y.; Fu, X.M.; Liao, Y.Y.; Yuan, Y.F.; Jia, Y.X.; Dong, F.; Yang, Z.Y. Biosynthesis of jasmine lactone in tea (Camellia sinensis) leaves and its formation in response to multiple stresses. J. Agric. Food Chem. 2018, 66, 3899–3909. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Yang, Z.Y.; Baldermann, S.; Sato, Y.; Asai, T.; Watanabe, N. Herbivore-induced volatiles from tea (Camellia sinensis) plants and their involvement in intraplant communication and changes in endogenous nonvolatile metabolites. J. Agric. Food Chem. 2011, 59, 13131–13135. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.T.; Liao, Y.Y.; Li, J.L.; Zhou, Y.; Tang, J.C.; Dong, F.; Yang, Z.Y. α-Farnesene and ocimene induce metabolite changes by volatile signaling in neighboring tea (Camellia sinensis) plants. Plant Sci. 2017, 264, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Song, W.C.; Brash, A.R. Purification of an allene oxide synthase and identification of the enzyme as a cytochrome P-450. Science 1991, 253, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Song, W.C.; Funk, C.D.; Brash, A.R. Molecular cloning of an allene oxide synthase: A cytochrome P-450 specialized for metabolism of fatty acid hydroperoxides. Proc. Natl. Acad. Sci. USA 1993, 90, 8519–8523. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; Ellis, C.; Devoto, A. The jasmonate signal pathway. Plant Cell 2002, 14, S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.Y.; Zhou, Y.; Liao, Y.Y.; Zeng, L.T.; Xu, X.L.; Jia, Y.X.; Dong, F.; Li, J.L.; Tang, J.C.; Yang, Z.Y. Functional characterization of an allene oxide synthase involved in biosynthesis of jasmonic acid and its influence on metabolite profiles and ethylene formation in tea (Camellia sinensis) flowers. Int. J. Mol. Sci. 2018, 19, 2440. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zeng, L.T.; Yu, Z.M.; Li, J.L.; Tang, J.C.; Su, X.G.; Yang, Z.Y. Differential accumulation of aroma compounds in normal green and albino-induced yellow tea (Camellia sinensis) leaves. Molecules 2018, 23, 2677. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.R.; Li, M.; Zhang, L.J.; Liang, Y.R. Studies on classification of albino tea resources. J. Tea 2015, 41, 126–129. (In Chinese) [Google Scholar]
- Wang, W.; Guo, Y.L. Development and application of albino tea varieties. J. Food Safety Quality 2017, 8, 3104–3110. (In Chinese) [Google Scholar]
- Wang, K.K.; Li, N.N.; Du, Y.Y.; Liang, Y.R. Effect of sunlight shielding on leaf structure and amino acids concentration of light sensitive albino tea plan. Afr. J. Biotechnol. 2013, 12, 5535–5539. [Google Scholar]
- Song, L.; Ma, Q.; Zou, Z.; Sun, K.; Yao, Y.; Tao, J.; Kaleri, N.A.; Li, X. Molecular Link between Leaf coloration and gene expression of flavonoid and carotenoid biosynthesis in Camellia sinensis cultivar ‘Huangjinya’. Front. Plant Sci. 2017, 8, 803. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Han, Z.X.; Feng, L.; Gao, L.P.; Gao, M.J.; Gruber, M.Y.; Zhang, Z.L.; Xia, T.; Wan, X.C.; Wei, S. Metabolic flux redirection and transcriptomic reprogramming in the albino tea cultivar ‘Yu-Jin-Xiang’ with an emphasis on catechin production. Sci. Rep. 2017, 7, 45062. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Fu, X.M.; Liao, Y.Y.; Xu, X.L.; Zeng, L.T.; Tang, J.C.; Li, J.L.; Lai, J.H.; Yang, Z.Y. Differential accumulation of specialized metabolite L-theanine in green and albino-induced yellow tea (Camellia sinensis) leaves. Food Chem. 2019, 276, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science 2006, 331, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Liu, J.J.; He, Z.R.; Wang, F.M.; Yang, H.; Yan, Y.F.; Gao, M.J.; Gruber, M.Y.; Wan, X.C.; Wei, S. Implementation of CsLIS/NES in linalool biosynthesis involves transcript splicing regulation in Camellia sinensis. Plant Cell Environ. 2018, 41, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Parris, K.D.; Ahmed, S.A.; Miles, E.W.; Davies, D.R. Exchange of K+ or Cs+ for Na+ induces local and long-range changes in the three-dimensional structure of the tryptophan synthase α2β2 complex. Biochemistry 1996, 35, 4211–4221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Wang, X.W.; Guo, L.X.; Xu, Q.S.; Zhao, S.Q.; Li, F.D.; Yan, X.M.; Liu, S.R.; Wei, C.L. Characterization and alternative splicing profiles of lipoxygenase gene family in tea plant (Camellia sinensis). Plant Cell Physiol. 2018, 59, 1765–1781. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.W.; Wu, Y.L.; Li, Y.Y.; Tan, Z.; Wei, C.L. Molecular cloning and characterization of hydroperoxide lyase gene in the leaves of tea plant (Camellia sinensis). J. Agric. Food Chem. 2016, 64, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Negre-Zakharov, F.; Long, M.C.; Dudareva, N. Floral scents and fruit aromas inspired by nature. In Plant-Derived Natural Products, 1st ed.; Osbourn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 405–431. [Google Scholar]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M.; et al. MYC2 differentially modulates diverse jasmonate-dependent function in Arabidopsis. Plant Cell 2007, 19, 2225–2245. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Horvath, D.P.; Chao, W.S.; Yang, Y.; Wang, X.; Xiao, B. Identification and evaluation of reliable reference genes for quantitative real-time PCR analysis in tea plant (Camellia sinensis (L.) O. Kuntze). Int. J. Mol. Sci. 2014, 15, 22155–22172. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zeng, L.; Liao, Y.; Gu, D.; Tang, J.; Yang, Z. Influence of Chloroplast Defects on Formation of Jasmonic Acid and Characteristic Aroma Compounds in Tea (Camellia sinensis) Leaves Exposed to Postharvest Stresses. Int. J. Mol. Sci. 2019, 20, 1044. https://doi.org/10.3390/ijms20051044
Li J, Zeng L, Liao Y, Gu D, Tang J, Yang Z. Influence of Chloroplast Defects on Formation of Jasmonic Acid and Characteristic Aroma Compounds in Tea (Camellia sinensis) Leaves Exposed to Postharvest Stresses. International Journal of Molecular Sciences. 2019; 20(5):1044. https://doi.org/10.3390/ijms20051044
Chicago/Turabian StyleLi, Jianlong, Lanting Zeng, Yinyin Liao, Dachuan Gu, Jinchi Tang, and Ziyin Yang. 2019. "Influence of Chloroplast Defects on Formation of Jasmonic Acid and Characteristic Aroma Compounds in Tea (Camellia sinensis) Leaves Exposed to Postharvest Stresses" International Journal of Molecular Sciences 20, no. 5: 1044. https://doi.org/10.3390/ijms20051044
APA StyleLi, J., Zeng, L., Liao, Y., Gu, D., Tang, J., & Yang, Z. (2019). Influence of Chloroplast Defects on Formation of Jasmonic Acid and Characteristic Aroma Compounds in Tea (Camellia sinensis) Leaves Exposed to Postharvest Stresses. International Journal of Molecular Sciences, 20(5), 1044. https://doi.org/10.3390/ijms20051044