Adiponectin as Link Factor between Adipose Tissue and Cancer
Abstract
1. Introduction
2. Adiponectin Structure and Function
3. Adiponectin Signaling Pathways
4. Adipose Tissue, Adiponectin and Low Chronic Inflammation
5. Adiponectin in Cancer
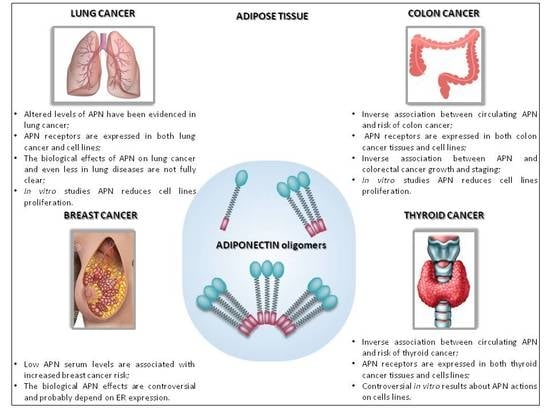
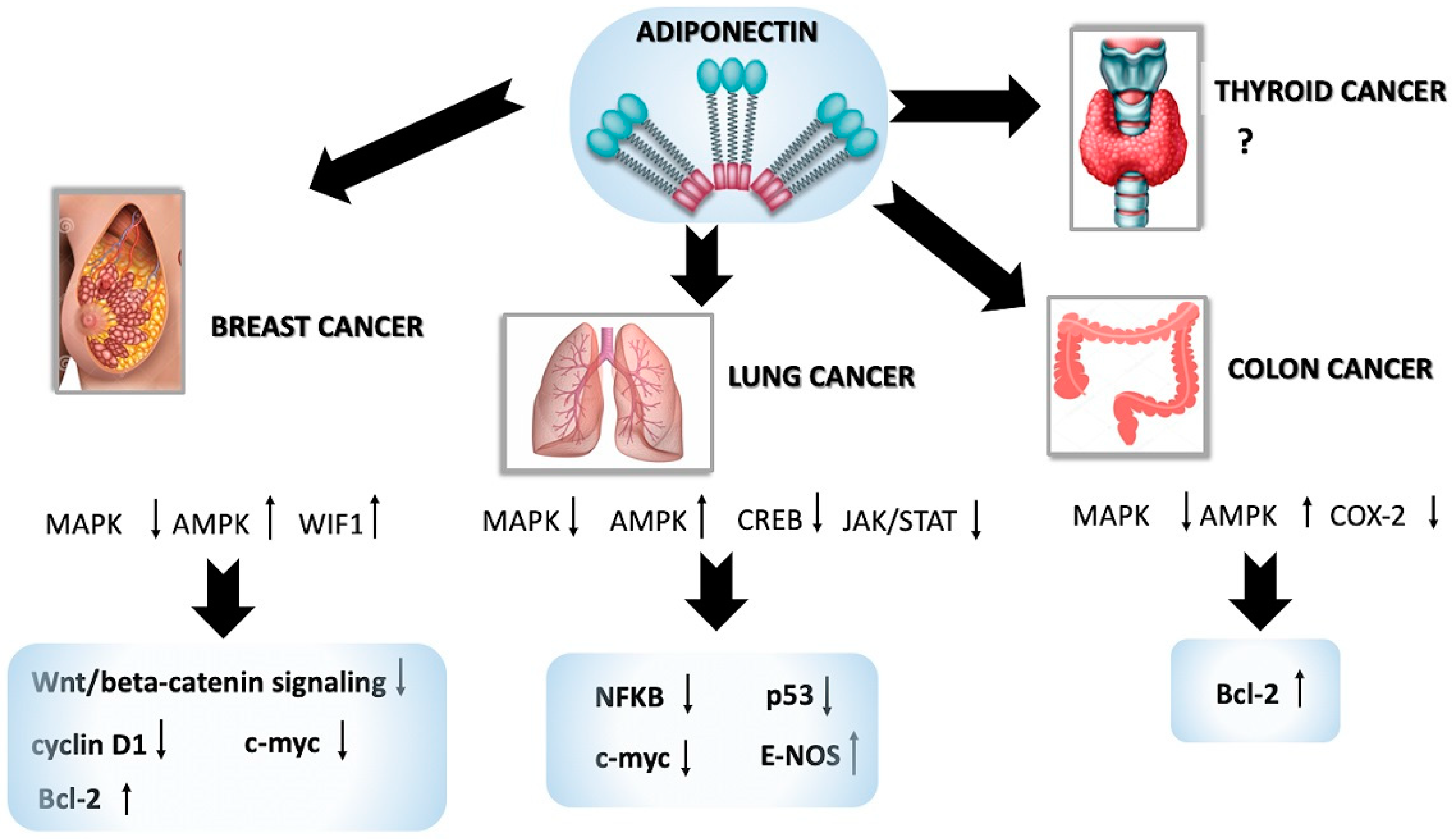
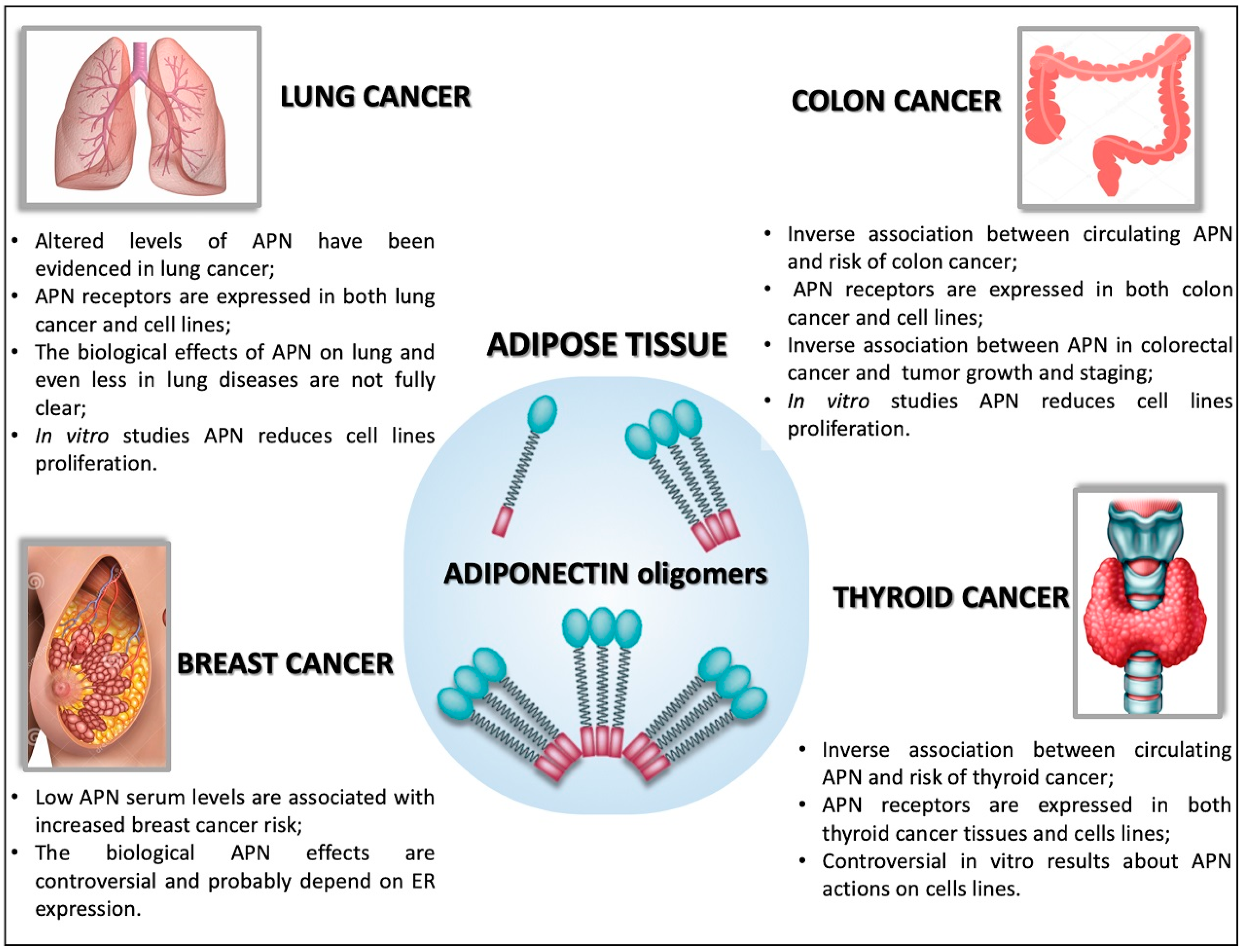
5.1. Adiponectin in Breast Cancer
5.2. Adiponectin in Lung Cancer
5.3. Adiponectin in Colon Cancer
5.4. Adiponectin in Thyroid Cancer
5.5. Adiponectin in Other Cancers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AdipoR | Adiponectin receptor |
| AMPK | 5’-adenosine monophosphate-activated protein kinase |
| APN | Adiponectin |
| BC | Breast cancer |
| BMI | Body Mass Index |
| COX-2 | Cyclooxygenase |
| CRC | Colorectal cancer |
| cPLA2 | Cytosolic phospholipase A2 |
| CREB | cAMP response element-binding protein |
| ER | Estrogen receptor |
| ERK1/2 | Extracellular signal-regulated protein kinases 1 and 2 |
| fT4 | Free thyroxine |
| HFD | High-fat diet |
| IGF-1 | Insulin-like growth factor 1 |
| IL-10 | Interleukin-10 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| JAK2 | Janus kinase 2 |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein 1 |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor-κB |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PPAR | Peroxisome proliferator-activated receptors |
| PP2A | Protein phosphatase 2 A |
| pRb | Phosphorylated retinoblastoma protein |
| ROS | Reactive oxygen species |
| STAT3 | Signal transducer and activator of transcription 3 |
| TC | Thyroid cancer |
| TNF-α | Tumor necrosis factor-α |
References
- Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults: Executive summary. Expert Panel on the Identification, Evaluation and Treatment of Overweight in Adults. Am. J. Clin. Nutr. 1998, 68, 899–917. [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects); Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G. Metabolic mediators of the effects of body-mass index, overweight and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet 2014, 383, 970–983. [Google Scholar] [CrossRef]
- Global BMI Mortality Collaboration; Di Angelantonio, E.; Bhupathiraju, S.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.; Huxley, R.; Jackson, C.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Bifulco, M.; Ciaglia, E. Updates on “adiponcosis”: More new incoming evidence strengthening the obesity-cancer link. Eur. J. Intern. Med. 2017, 41, e19–e20. [Google Scholar] [CrossRef]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Nishida, M.; Funahashi, T.; Shimomura, I. Pathophysiological significance of adiponectin. Med. Mol. Morphol. 2007, 40, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef] [PubMed]
- Hebbard, L.; Ranscht, B. Multifaceted roles of adiponectin in cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Katira, A.; Tan, P.H. Evolving role of adiponectin in cancer-controversies and update. Cancer Biol. Med. 2016, 13, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, V.J.; Rojas, E.; Toledo, A.; Rodríguez-Molina, D.; Vega, K.; Suárez, L.; Pacheco, M.; Canelón, R.; Arráiz, N.; Rojas, J.V.M. Single-nucleotide polymorphisms in adiponectin, AdipoR1 and AdipoR2 genes: Insulin resistance and type 2 diabetes mellitus candidate genes. Am. J. Ther. 2013, 20, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Simpson, F.; Whitehead, J.P. Adiponectin-It’s all about the modifications. Int. J. Biochem. Cell Biol. 2010, 42, 785–788. [Google Scholar] [CrossRef]
- Wang, Y.; Lam, K.S.L.; Chan, L.; Kok, W.C.; Lam, J.B.B.; Lam, M.C.; Hoo, R.C.L.; Mak, W.W.N.; Cooper, G.J.S.; Xu, A. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J. Biol. Chem. 2006, 281, 16391–16400. [Google Scholar] [CrossRef]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef]
- Chiarugi, P.; Fiaschi, T. Adiponectin in health and diseases: From metabolic syndrome to tissue regeneration. Expert Opin. Ther. Targets 2010, 14, 193–206. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Kadowaki, T. Adiponectin receptors: A review of their structure, function and how they work. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 15–23. [Google Scholar] [CrossRef]
- Hug, C.; Wang, J.; Ahmad, N.S.; Bogan, J.S.; Tsao, T.-S.; Lodish, H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. USA 2004, 101, 10308–10313. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Van Kaer, L. Contribution of lipid-reactive natural killer T cells to obesity-associated inflammation and insulin resistance. Adipocyte 2013, 2, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Frühbeck, G. Adipose tissue immunity and cancer. Front. Physiol. 2013, 4, 275. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Eckel, J. Adipose tissue inflammation: Novel insight into the role of macrophages and lymphocytes. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 366–370. [Google Scholar] [CrossRef]
- Lengyel, E.; Makowski, L.; DiGiovanni, J.; Kolonin, M.G. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer 2018, 4, 374–384. [Google Scholar] [CrossRef]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef]
- James, F.R.; Wootton, S.; Jackson, A.; Wiseman, M.; Copson, E.R.; Cutress, R.I. Obesity in breast cancer--what is the risk factor? Eur. J. Cancer 2015, 51, 705–720. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2018, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Jia, J.; Dong, S.; Zhang, C.; Yu, S.; Li, L.; Mao, C.; Wang, D.; Chen, J.; Yuan, G. Circulating adiponectin levels and the risk of breast cancer: A meta-analysis. Eur. J. Cancer Prev. 2014, 23, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.; Pellegrino, M.; De Amicis, F.; Ricchio, E.; Giordano, F.; Rizza, P.; Catalano, S.; Bonofiglio, D.; Sisci, D.; Panno, M.L.; et al. Evidences that estrogen receptor α interferes with adiponectin effects on breast cancer cell growth. Cell Cycle 2014, 13, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lam, J.B.B.; Chow, K.H.M.; Xu, A.; Lam, K.S.L.; Moon, R.T.; Wang, Y. Adiponectin stimulates Wnt inhibitory factor-1 expression through epigenetic regulations involving the transcription factor specificity protein 1. Carcinogenesis 2008, 29, 2195–2202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Otvos, L.; Knappe, D.; Hoffmann, R.; Kovalszky, I.; Olah, J.; Hewitson, T.D.; Stawikowska, R.; Stawikowski, M.; Cudic, P.; Lin, F.; et al. Development of second generation peptides modulating cellular adiponectin receptor responses. Front. Chem. 2014, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.; Naimo, G.D.; Ricchio, E.; Panno, M.L.; Andò, S. Cross-Talk between Adiponectin and IGF-IR in Breast Cancer. Front. Oncol. 2015, 5, 157. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, E.; Feola, A.; Zuchegna, C.; Romano, A.; Donini, C.F.; Bartollino, S.; Costagliola, C.; Frunzio, R.; Laccetti, P.; Di Domenico, M.; et al. The p85 regulatory subunit of PI3K mediates cAMP-PKA and insulin biological effects on MCF-7 cell growth and motility. Sci. World J. 2014, 2014, 565839. [Google Scholar] [CrossRef]
- Donini, C.F.; Coppa, A.; Di Zazzo, E.; Zuchegna, C.; Di Domenico, M.; D’Inzeo, S.; Nicolussi, A.; Avvedimento, E.V.; Coppa, A.; Porcellini, A. The p85α regulatory subunit of PI3K mediates cAMP-PKA and retinoic acid biological effects on MCF7 cell growth and migration. Int. J. Oncol. 2012, 40, 1627–1635. [Google Scholar]
- Di Zazzo, E.; Bartollino, S.; Moncharmont, B. The master regulator gene PRDM2 controls C2C12 myoblasts proliferation and Differentiation switch and PRDM4 and PRDM10 expression. Insights Biol. Med. 2017, 1, 75–91. [Google Scholar]
- Mauro, L.; Pellegrino, M.; Giordano, F.; Ricchio, E.; Rizza, P.; De Amicis, F.; Catalano, S.; Bonofiglio, D.; Panno, M.L.; Andò, S. Estrogen receptor- α drives adiponectin effects on cyclin D1 expression in breast cancer cells. FASEB J. 2015, 29, 2150–2160. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, Y.; Cui, S. Adiponectin Induces Breast Cancer Cell Migration and Growth Factor Expression. Cell Biochem. Biophys. 2014, 70, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Bong, J.G.; Park, S.H.; Choi, J.H.; Oh, H.K. Expression of leptin, leptin receptor, adiponectin and adiponectin receptor in ductal carcinoma in situ and invasive breast cancer. J. Breast Cancer 2011, 14, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Pfeiler, G.; Treeck, O.; Wenzel, G.; Goerse, R.; Hartmann, A.; Schmitz, G.; Ortmann, O. Influence of insulin resistance on adiponectin receptor expression in breast cancer. Maturitas 2009, 63, 253–256. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.; Munden, R.F. Lung cancer epidemiology, risk factors and prevention. Radiol. Clin. N. Am. 2012, 50, 863–876. [Google Scholar] [CrossRef]
- Hidayat, K.; Du, X.; Chen, G.; Shi, M.; Shi, B. Abdominal Obesity and Lung Cancer Risk: Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2016, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dong, J.; Sun, K.; Zhao, L.; Zhao, F.; Wang, L.; Jiao, Y. Obesity and incidence of lung cancer: A meta-analysis. Int. J. Cancer 2013, 132, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.; De Rosa, A.; Nigro, E.; Scudiero, O.; Capasso, M.; Masullo, M.; de Laurentiis, G.; Oriani, G.; Sofia, M.; Bianco, A. Adiponectin oligomerization state and adiponectin receptors airway expression in chronic obstructive pulmonary disease. Int. J. Biochem. Cell Biol. 2012, 44, 563–569. [Google Scholar] [CrossRef]
- Petridou, E.T.; Mitsiades, N.; Gialamas, S.; Angelopoulos, M.; Skalkidou, A.; Dessypris, N.; Hsi, A.; Lazaris, N.; Polyzos, A.; Syrigos, C.; et al. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: Two case-control studies. Oncology 2007, 73, 261–269. [Google Scholar] [CrossRef]
- Gulen, S.T.; Karadag, F.; Karul, A.B.; Kilicarslan, N.; Ceylan, E.; Kuman, N.K.; Cildag, O. Adipokines and Systemic Inflammation in Weight-Losing Lung Cancer Patients. Lung 2012, 190, 327–332. [Google Scholar] [CrossRef]
- Karapanagiotou, E.M.; Tsochatzis, E.A.; Dilana, K.D.; Tourkantonis, I.; Gratsias, I.; Syrigos, K.N. The significance of leptin, adiponectin and resistin serum levels in non-small cell lung cancer (NSCLC). Lung Cancer 2008, 61, 391–397. [Google Scholar] [CrossRef]
- Denzel, M.S.; Scimia, M.-C.; Zumstein, P.M.; Walsh, K.; Ruiz-Lozano, P.; Ranscht, B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Investig. 2010, 120, 4342–4352. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Deng, A.; Wang, X.; Wang, B.; Mao, W.; Feng, X.; Hua, F. The role of adiponectin (ADIPOQ) gene polymorphisms in the susceptibility and prognosis of non-small cell lung cancer. Biochem. Cell Biol. 2011, 89, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Sarnataro, D.; Mazzarella, G.; Sofia, M.; Bianco, A.; Daniele, A. Adiponectin affects lung epithelial A549 cell viability counteracting TNFα and IL-1ß toxicity through AdipoR1. Int. J. Biochem. Cell Biol. 2013, 45, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Illiano, M.; Nigro, E.; Sapio, L.; Caiafa, I.; Spina, A.; Scudiero, O.; Bianco, A.; Esposito, S.; Mazzeo, F.; Pedone, P.V.; et al. Adiponectin down-regulates CREB and inhibits proliferation of A549 lung cancer cells. Pulm. Pharmacol. Ther. 2017, 45, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Yang, C.-M.; Chang, J.-F.; Wu, C.-S.; Sia, K.-C.; Lin, W.-N. AdipoR-increased intracellular ROS promotes cPLA2 and COX-2 expressions via activation of PKC and p300 in adiponectin-stimulated human alveolar type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L255–L269. [Google Scholar] [PubMed]
- Tarasiuk, A.; Mosińska, P.; Fichna, J. The mechanisms linking obesity to colon cancer: An overview. Obes. Res. Clin. Pract. 2018, 12, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Riondino, S.; Roselli, M.; Palmirotta, R.; Della-Morte, D.; Ferroni, P.; Guadagni, F. Obesity and colorectal cancer: Role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014, 20, 5177–5190. [Google Scholar] [CrossRef]
- Moon, H.-S.; Liu, X.; Nagel, J.M.; Chamberland, J.P.; Diakopoulos, K.N.; Brinkoetter, M.T.; Hatziapostolou, M.; Wu, Y.; Robson, S.C.; Iliopoulos, D.; et al. Salutary effects of adiponectin on colon cancer: In vivo and in vitro studies in mice. Gut 2013, 62, 561–570. [Google Scholar] [CrossRef]
- Saetang, J.; Boonpipattanapong, T.; Palanusont, A.; Maneechay, W.; Sangkhathat, S. Alteration of Leptin and Adiponectin in Multistep Colorectal Tumorigenesis. Asian Pac. J. Cancer Prev. 2016, 17, 2119–2123. [Google Scholar] [CrossRef]
- Xu, X.T.; Xu, Q.; Tong, J.L.; Zhu, M.M.; Huang, M.L.; Ran, Z.H.; Xiao, S.D. Meta-analysis: Circulating adiponectin levels and risk of colorectal cancer and adenoma. J. Dig. Dis. 2011, 12, 234–244. [Google Scholar] [CrossRef]
- Tae, C.H.; Kim, S.-E.; Jung, S.-A.; Joo, Y.-H.; Shim, K.-N.; Jung, H.-K.; Kim, T.H.; Cho, M.-S.; Kim, K.H.; Kim, J.S. Involvement of adiponectin in early stage of colorectal carcinogenesis. BMC Cancer 2014, 14, 811. [Google Scholar] [CrossRef] [PubMed]
- Kumor, A.; Daniel, P.; Pietruczuk, M.; Małecka-Panas, E. Serum leptin, adiponectin and resistin concentration in colorectal adenoma and carcinoma (CC) patients. Int. J. Colorectal Dis. 2009, 24, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ayyildiz, T.; Dolar, E.; Ugras, N.; Adim, S.B.; Yerci, O. Association of adiponectin receptor (Adipo-R1/-R2) expression and colorectal cancer. Asian Pac. J. Cancer Prev. 2014, 15, 9385–9390. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-Z.; Huo, J.-R. Correlation between T-cadherin gene expression and aberrant methylation of T-cadherin promoter in human colon carcinoma cells. Med. Oncol. 2012, 29, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Huang, T.; Li, J.; Zhou, C.; Yang, P.; Ni, C.; Chen, S. Role of CDH13 promoter methylation in the carcinogenesis, progression and prognosis of colorectal cancer: A systematic meta-analysis under PRISMA guidelines. Medicine 2017, 96, e5956. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.K.; Yi, J.W.; Chai, Y.J.; Park, K.J. Upregulation of the adipokine genes ADIPOR1 and SPP1 is related to poor survival outcomes in colorectal cancer. J. Surg. Oncol. 2018, 117, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Takahashi, H.; Hosono, K.; Endo, H.; Kato, S.; Yoneda, K.; Nozaki, Y.; Fujita, K.; Yoneda, M.; Wada, K.; et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int. J. Oncol. 2009, 34, 339–344. [Google Scholar]
- Kim, A.Y.; Lee, Y.S.; Kim, K.H.; Lee, J.H.; Lee, H.K.; Jang, S.-H.; Kim, S.-E.; Lee, G.Y.; Lee, J.-W.; Jung, S.-A.; et al. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol. Endocrinol. 2010, 24, 1441–1452. [Google Scholar] [CrossRef]
- Davis, J.E.; Gabler, N.K.; Walker-Daniels, J.; Spurlock, M.E. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity 2008, 16, 1248–1255. [Google Scholar] [CrossRef]
- Priego, T.; Sánchez, J.; Picó, C.; Palou, A. Sex-differential expression of metabolism-related genes in response to a high-fat diet. Obesity 2008, 16, 819–826. [Google Scholar] [CrossRef]
- Boddicker, R.L.; Whitley, E.M.; Davis, J.E.; Birt, D.F.; Spurlock, M.E. Low-dose dietary resveratrol has differential effects on colorectal tumorigenesis in adiponectin knockout and wild-type mice. Nutr. Cancer 2011, 63, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Ha, J.; Park, I.-J.; Lee, S.-K.; Baik, H.W.; Kim, Y.M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Schettino, P.; Polito, R.; Scudiero, O.; Monaco, M.L.; De Palma, G.D.; Daniele, A. Adiponectin and colon cancer: Evidence for inhibitory effects on viability and migration of human colorectal cell lines. Mol. Cell. Biochem. 2018, 448, 125–135. [Google Scholar] [CrossRef]
- Saxena, A.; Chumanevich, A.; Fletcher, E.; Larsen, B.; Lattwein, K.; Kaur, K.; Fayad, R. Adiponectin deficiency: Role in chronic inflammation induced colon cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Ogunwobi, O.O.; Beales, I.L.P. Adiponectin stimulates proliferation and cytokine secretion in colonic epithelial cells. Regul. Pept. 2006, 134, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, B.S.; Kitayama, J.; Nagawa, H. Adiponectin supports cell survival in glucose deprivation through enhancement of autophagic response in colorectal cancer cells. Cancer Sci. 2011, 102, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Franceschi, S.; Biessy, C.; Navionis, A.-S.; Travis, R.C.; Weiderpass, E.; Scalbert, A.; Romieu, I.; Tjønneland, A.; Olsen, A.; et al. Adipokines and inflammation markers and risk of differentiated thyroid carcinoma: The EPIC study. Int. J. Cancer 2018, 142, 1332–1342. [Google Scholar] [CrossRef]
- Abooshahab, R.; Yaghmaei, P.; Ghadaksaz, H.G.; Hedayati, M. Lack of Association between Serum Adiponectin/Leptin Levels and Medullary Thyroid Cancer. Asian Pac. J. Cancer Prev. 2016, 17, 3861–3864. [Google Scholar]
- Cheng, S.-P.; Liu, C.-L.; Hsu, Y.-C.; Chang, Y.-C.; Huang, S.-Y.; Lee, J.-J. Expression and biologic significance of adiponectin receptors in papillary thyroid carcinoma. Cell Biochem. Biophys. 2013, 65, 203–210. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Huang, S.-C.; Sheu, W.H.-H. Circulating adiponectin concentrations were related to free thyroxine levels in thyroid cancer patients after thyroid hormone withdrawal. Metabolism 2010, 59, 195–199. [Google Scholar] [CrossRef]
- Mitsiades, N.; Pazaitou-Panayiotou, K.; Aronis, K.N.; Moon, H.-S.; Chamberland, J.P.; Liu, X.; Diakopoulos, K.N.; Kyttaris, V.; Panagiotou, V.; Mylvaganam, G.; et al. Circulating adiponectin is inversely associated with risk of thyroid cancer: In vivo and in vitro studies. J. Clin. Endocrinol. Metab. 2011, 96, E2023–E2028. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.G.; Park, J.W.; Willingham, M.C.; Cheng, S. Diet-induced obesity increases tumor growth and promotes anaplastic change in thyroid cancer in a mouse model. Endocrinology 2013, 154, 2936–2947. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Yu, C.; Wen, J.; Jia, W.; Li, G.; Ke, Y.; Zhao, S.; Campell, W. Adiponectin induces interleukin-6 production and activates STAT3 in adult mouse cardiac fibroblasts. Biol. Cell 2009, 101, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Disney-Hogg, L.; Sud, A.; Law, P.J.; Cornish, A.J.; Kinnersley, B.; Ostrom, Q.T.; Labreche, K.; Eckel-Passow, J.E.; Armstrong, G.N.; Claus, E.B.; et al. Influence of obesity-related risk factors in the aetiology of glioma. Br. J. Cancer 2018, 118, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Porcile, C.; Di Zazzo, E.; Monaco, M.L.; D’Angelo, G.; Passarella, D.; Russo, C.; Di Costanzo, A.; Pattarozzi, A.; Gatti, M.; Bajetto, A.; et al. Adiponectin as novel regulator of cell proliferation in human glioblastoma. J. Cell. Physiol. 2014, 229, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Castoria, G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front. Oncol. 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Zazzo, E.; Polito, R.; Bartollino, S.; Nigro, E.; Porcile, C.; Bianco, A.; Daniele, A.; Moncharmont, B. Adiponectin as Link Factor between Adipose Tissue and Cancer. Int. J. Mol. Sci. 2019, 20, 839. https://doi.org/10.3390/ijms20040839
Di Zazzo E, Polito R, Bartollino S, Nigro E, Porcile C, Bianco A, Daniele A, Moncharmont B. Adiponectin as Link Factor between Adipose Tissue and Cancer. International Journal of Molecular Sciences. 2019; 20(4):839. https://doi.org/10.3390/ijms20040839
Chicago/Turabian StyleDi Zazzo, Erika, Rita Polito, Silvia Bartollino, Ersilia Nigro, Carola Porcile, Andrea Bianco, Aurora Daniele, and Bruno Moncharmont. 2019. "Adiponectin as Link Factor between Adipose Tissue and Cancer" International Journal of Molecular Sciences 20, no. 4: 839. https://doi.org/10.3390/ijms20040839
APA StyleDi Zazzo, E., Polito, R., Bartollino, S., Nigro, E., Porcile, C., Bianco, A., Daniele, A., & Moncharmont, B. (2019). Adiponectin as Link Factor between Adipose Tissue and Cancer. International Journal of Molecular Sciences, 20(4), 839. https://doi.org/10.3390/ijms20040839