Abstract
Behavioral and psychosocial factors related to development of cardiovascular disease have been gaining increased attention. Notably, sleep is considered to be one of the most important behavioral factors involved in progression of atherosclerosis and cardiovascular events, with autonomic nervous function a potential mechanism. Several studies have shown associations of sleep and autonomic dysfunction with major surrogate markers of atherosclerosis, such as carotid intima-media thickness and arterial stiffness. Endocrinological, immunological, oxidative, inflammatory, and metabolic responses, as well as endothelial dysfunction may mediate the effects of the autonomic nervous system. For this review, we examined recent findings related to sleep, autonomic nervous dysfunction, and atherosclerosis, with the aim of understanding the involved pathophysiological mechanisms.
1. Introduction
Classical cardiovascular risk factors, including obesity, hypertension, dyslipidemia, diabetes mellitus, and chronic kidney disease (CKD), are established predictors of atherosclerosis and cardiovascular disease (CVD) [1,2,3]. Recently, behavioral and psychosocial factors have been gaining increased attention in regard to development, prevention, and treatment of CVD [4], with short sleep duration and low sleep quality shown to be important behavioral factors that may be involved in its occurrence [5]. In this context, potential mechanisms associated with short sleep duration and low sleep quality include autonomic nervous function, and a previous study showed a strong association between those in a general population [6]. Additionally, autonomic nervous dysfunction has also been found to be a risk factor for CVD [7]. Potential mechanisms related to the associations among sleep, autonomic nervous function, and progression of atherosclerosis are summarized in Figure 1. It has been shown that short sleep duration, low sleep quality, and autonomic nervous dysfunction are associated with several risk factors for atherogenesis, including endocrinological, immunological, oxidative, inflammatory, and metabolic responses, as well as endothelial dysfunction.
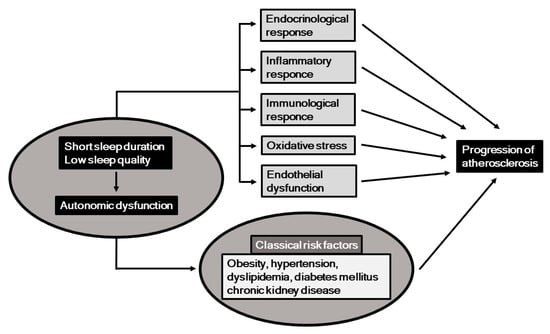
Figure 1.
Short sleep duration and low sleep quality, along with resultant autonomic nervous dysfunction may induce progression of atherosclerosis, potentially through endocrinological, immunological, inflammatory, and oxidative response, and endothelial dysfunction.
Sleep duration and quality can be measured by subjective methods, including self-reported questionnaires, the Epworth Sleepiness Scale (ESS), and Pittsburg Sleep Quality Index (PSQI), as well as by objective methods, including actigraphy [8,9], while apnea-hypopnea during sleep can be quantitatively determined using polysomnography or apnomonitor results [10,11]. Standardized autonomic nervous function tests including Valsalva maneuver, hyperventilation, standup tilt test, cold pressor, isometric handgrip, and heart rate variability (HRV) are also performed [12,13], among which HRV is a practical method to assess impaired autonomic nervous function in clinical settings [7].
Several studies have found relationships of short sleep duration, low sleep quality, and autonomic dysfunction with carotid intima-media thickness (IMT) and brachial-ankle pulse wave velocity (baPWV), which are major surrogate markers of atherosclerosis and established predictors of cardiovascular events [14,15]. We recently reported associations among sleep quality, autonomic nervous function, carotid IMT, baPWV, and nocturnal hypertension in subjects with risk factors for atherosclerosis who participated in the Hyogo Sleep Cardio-Autonomic Atherosclerosis (HSCAA) study [11,16,17,18]. The aim of this review was to summarize findings regarding the impact of sleep and autonomic nervous function on atherosclerosis, and elucidate their underlying mechanisms related to the progression of atherosclerosis.
2. Sleep and Atherosclerosis
Several epidemiological studies have suggested associations of short sleep duration, low sleep quality, and obstructive sleep apnea (OSA) with atherosclerosis. Table 1 summarizes reports of patients with atherosclerotic risk factors regarding the association between sleep and surrogate markers of atherosclerosis, carotid IMT, and baPWV, though the majority of the cohorts examined were relatively small in size. As for subclinical atherosclerosis in 86 elderly patients (73.6 ± 4.9 years old), carotid IMT in subjects with shorter sleep duration (<5 h) examined with the PSQI was found to be higher than those with the reference number of sleep h (>7 h) [19]. In a study of 201 elderly patients (79.9 ± 6.4 years old), carotid IMT was also shown to be higher in those with subjective persistent insomnia than in those with no insomnia [20]. Yoda et al. assessed using objective sleep quality and elector-encephalography findings, and reported a significant correlation between carotid IMT and rapid eye movement latency in 63 patients with type 2 diabetes mellitus [21]. Furthermore, in the HSCAA study with a relatively large number of patients (n = 330) with cardiovascular risk factors, we showed that apnea-hypopnea index (AHI) assessed by an apnomonitor and poor sleep quality determined with actigraphy were each significantly and positively associated with carotid IMT and plaque score [11]. Regarding arterial stiffness, subjective low sleep quality was reported to be correlated with higher baPWV in 724 patients with type 2 diabetes [22], while our recent study demonstrated that low sleep quality is significantly associated with impaired nocturnal blood pressure fluctuations, a risk factor for arterial stiffness [17]. Arterial stiffness was also shown to be independently associated with obstructive sleep apnea in 127 patients with ischemic stroke [10].

Table 1.
Association of subjective or objective sleep duration and quality with carotid IMT and baPWV in patients with atherosclerotic risk factors.
Until recently, no prospective study examined the association of sleep duration or quality with atherosclerotic progression in patients with atherosclerotic factors. Our report presented in 2018 of a 3-year longitudinal investigation (n = 306) in association with the HSCAA study was the first to show a relationship of low sleep quality with progression of baPWV [18]. Those findings indicated that poor sleep quality is associated with progression of arterial stiffness independent of other cardiovascular risk factors, including ambulatory blood pressure, apnea-hypopnea, and cardiac autonomic function, in patients with cardiovascular risk factors.
Some largescale studies examined associations of subjective and objective sleep duration, and quality with carotid IMT and baPWV in healthy general populations (Table 2). In an investigation of 617 middle-aged healthy subjects (37–52 years old), Sands et al. showed that objective shorter sleep duration was associated with greater carotid IMT [23]. However, it is important to note that the association of subjective sleep duration with carotid IMT was U-shaped in that healthy population. Wolff et al. reported that both longer (>11 h) and shorter (<5 h) sleep duration was associated with increased risk of atherosclerosis as compared to the reference sleep duration (7–8 h) in a general population (n = 2383) [24]. Abe et al. also queried 2214 general population subjects and showed that a longer sleep duration (>7 h) was significantly correlated with the incidence of carotid artery atherosclerosis as compared with a duration of 6 h [25]. Additionally, several studies revealed that longer sleep duration has an association with the incidence of stroke and cardiovascular mortality [26,27], while several largescale studies found associations of sleep duration and quality with baPWV. Importantly, the association between sleep duration and baPWV also had a U-shape in a manner similar to the association of sleep duration with carotid IMT. In a large general population study (n = 18,106), Kim et al. reported that both longer (>8 h) and shorter (<5 h) sleep durations were associated with higher baPWV as compared with recommended sleep time (7 h) [28]. Yoshioka et al. also showed that daily sleep duration (>9 h) was associated with elevated baPWV in 4268 employees [29]. Also, in 3508 males in the general population, Tsai et al. found an association between long sleep duration and increased baPWV [30]. More recently, low sleep quality was shown to be associated with subclinical coronary atherosclerosis, as assessed by cardiac computed tomography [31].

Table 2.
Associations of subjective or objective sleep duration, and quality with carotid IMT and baPWV in healthy populations.
3. Potential Mechanisms underlying Association of Sleep with Atherosclerosis Progression
Short sleep duration and low sleep quality can have strong effects on several aspects of endocrinological, immunological, and metabolic responses. Both are known to disturb the daily rhythm of the hypothalamic-pituitary-adrenal axis. Following activation of that axis, resultant higher cortisol levels are associated with insulin resistance, cardiovascular risk factors, and coronary heart disease [32]. Also, Lee et al. showed that testosterone, a cortisol-linked stress hormone, is associated with coronary artery calcium (CAC) and carotid IMT [33]. In our study, we reported a relationship of low sleep quality with higher serum macro TSH (Table 3), which was found to be regulated in a manner distinct from free TSH, potentially due to an altered glycosylation structure [34]. In our subjects, serum macro TSH levels and sleep physical activity index values (higher value indicating poor sleep quality) were higher, while total sleep time was lower in patients with diabetes, as compared to those without (Figure 2). On the other hand, no such significant differences were found when the patients were categorized by the presence or absence of hypertension or dyslipidemia. Serum macro TSH levels were also shown to be significantly associated with fasting glucose, HbA1c, and homeostasis model assessment for insulin resistance (HOMA-R). Together, these results suggest that sleep quality is deeply related to altered endocrinological responses, which may be involved in progression of atherosclerosis through modulation of metabolic status.

Table 3.
Multiple linear regression analyses of macro TSH and sleep parameters.
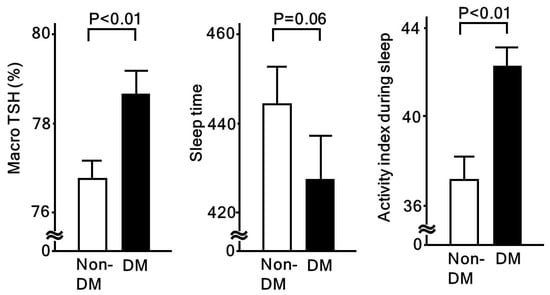
Figure 2.
Macro-TSH and sleep quality assessed by actinography in patients with and without diabetes. Macro-TSH is significantly associated with low sleep quality [34]. When compared between non-diabetic (non-DM) and diabetic (DM) patients, all macro-TSH and activity index during sleep (high values represent poor sleep quality) were significantly higher in DM patients. Values shown in each column represent the mean ± standard error. Student’s t-test was used for analyses.
It has also been reported that oxidative stress, subclinical systemic inflammation, and endothelial dysfunction may mediate the effects of short sleep duration and low sleep quality [35,36,37]. Short sleep duration and low sleep quality have been shown to induce a proinflammatory state, characterized by increased levels of several cytokines, including IL1β, TNFα, IL6, and IL17 [38,39,40]. Another study of rodents noted that chronic sleep fragmentation may induce morphological vessel changes, which are characterized by disruption and disorganization of elastic fibers, and increased recruitment of inflammatory cells [41]. An epidemiologic study found that short sleep duration (4 h) contributes to endothelial dysfunction, as measured by flow-mediated brachial artery vasodilatation (FMD) [42]. Other reports have shown that oxidative stress, such as total antioxidant capacity (TAC) and 8-hydroxy-2-deoxyguanosine (8-OHdG), is elevated in patients with OSA. Oxidative stress may be involved in dysregulation of collagen and elastin fibers of the vascular wall, leading to increased arterial stiffness [36,43]. Furthermore, study findings have indicated that OSA can lead to endothelial dysfunction and arterial disease [37], in which intermittent hypoxia and intrathoracic pressure changes may be involved.
In addition to progression of atherosclerosis, several have reported close associations of sleep condition with obesity, including epidemiologic examinations that found an association between short sleep duration and weight gain [44,45,46]. Potential mechanisms underlying this relationship are feeding behavioral changes and dysregulation of the neuroendocrine system, including the leptin-ghrelin system. Fang et al. conducted a human study that showed that sleep deprivation leads to increased fat intake through brain connectivity from the dorsal anterior cingulate cortex to the putamen and anterior insula [47]. Taheri et al. also presented results showing that short sleep duration may reduce leptin and induce ghrelin [48]. Moreover, in basic research findings, chronic sleep fragmentation was indicated to induce hypothalamic endoplasmic reticulum stress, which is associated with leptin resistance, alters eating behavior, and leads to weight gain [49]. A recent study found that acute sleep loss is attributable to epigenetic changes in adipose tissue and skeletal muscle, which are the result of an alteration of metabolic fuel utilization [50]. In that study of individuals with sleep loss, down-regulated proteins in skeletal muscles were shown to include genes involved in glycolysis, such as phosphoglycerate kinase 1 (PGK1), whereas the protein is up-regulated in adipose tissue. It was also demonstrated that alterations in circadian rhythm may be involved, since protein levels of the core clock component BMAL1 were significantly higher in skeletal muscle. Indeed, significant roles of circadian rhythm and BMAL1 in metabolic utilization have been shown in animal studies [51,52].
A longitudinal study reported that low sleep quality due to insomnia increases the risk for hypertension [53]. The association between OSA and hypertension has been well established by findings of several pathophysiological and epidemiological studies, in which autonomic dysfunction, including sympathetic and parasympathetic imbalance, appears to be involved. Furthermore, cross-sectional studies have also shown a significant association between OSA and dyslipidemia [54,55]. A recent review by Barros [56] elegantly summarizes potential mechanisms related to sleep and dyslipidemia. Intermittent hypoxia due to OSA up-regulates hypoxia-induced factor-1 (HIF-1), which might be involved in lipolysis in adipose tissue lipolysis, lipid synthesis in the liver favoring secretion of very low-density lipoprotein (VLDL), and delayed clearance of triglyceride-rich lipoprotein. Excessively produced reactive oxygen species (ROS) may be involved in generation of an oxidized form of LDL cholesterol, which is known to have a more atherogenic form. Indeed, higher levels of oxidized LDL cholesterol have been found in patients with OSA [57]. Dysfunction of high-density lipoprotein (HDL) has also been suggested in OSA patients [58], which may be mediated through increased noradrenaline and cortisol secretion [59,60]. In our 1-year prospective study, we also found that low sleep quality is associated with worsening of dyslipidemia (Figure 3). Objectively measured poor sleep quality and short sleep duration are each associated with a decrease in HDL cholesterol and increase in triglyceride level, independent of sleep apnea-hypopnea index. Short sleep duration has also been shown to be associated with increased prevalence of diabetes and impaired glucose tolerance [61,62]. A 15-year longitudinal prospective study showed that difficulty with falling asleep or regular use of hypnotics is associated with diabetes incidence in middle-aged men [46]. Moreover, several epidemiological studies have found that OSA is an independent risk factor for development of diabetes [63], in which autonomic dysfunction, oxidative stress induced by intermittent hypoxia, and inflammation may be involved. The associations between OSA and atherosclerotic risk factors may not be universal among ethnic groups. Recent papers based on Multi-Ethnic Study of Atherosclerosis (MESA) results showed that low sleep quality is associated with hypertension or peripheral artery disease (PAD) in blacks, but not in whites or Hispanics. Potential mechanisms of this heterogeneity include differences in salt sensitivity and diet quality among ethnicities [64].
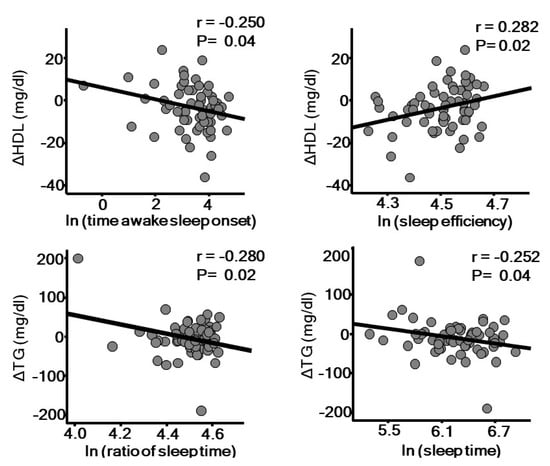
Figure 3.
Sleep quality assessed by actigraphy is significantly associated with annual changes of high density lipoprotein (HDL) cholesterol and triglyceride level. The parameters of sleep quality were natural logarithm-transformed (ln) to achieve a normal distribution. r: Pearson’s correlation coefficient.
4. Autonomic Nervous Function and Atherosclerosis
HRV provides a practical means to assess impaired autonomic nervous function in clinical settings by use of continuous electrocardiographic records, as those reflect sympatho-vagal balance and parasympathetic nervous activity.
It has been shown that reduced HRV predicts all-cause mortality and cardiovascular events [7], while that has also been recognized in patients with myocardial infarction [65], diabetes mellitus [66], and short sleep duration or low sleep quality [6,11]. Table 4 summarizes the limited numbers of studies presented thus far that have examined the relationships of HRV with carotid IMT and baPWV in specific patient groups [11,67,68,69,70,71,72]. In a longitudinal study, Gottsater et al. showed an association of low HRV with progression of carotid IMT in 61 type 2 diabetic patients over a period of 3–4 years [67]. Furthermore, Melillo et al. reported that low HRV was significantly associated with carotid IMT in 200 hypertensive patients [68]. In the HSCAA study, we found that HRV was associated with carotid IMT in patients with cardiovascular risks, independent of sleep quality and apnea-hypopnea [11]. Pizzi et al. showed an inverse association of carotid IMT or inflammatory markers (CRP, IL-6) with HRV in 391 depressed subjects with coronary risk factors [69], while Ulleryd et al. reported mutual relationships among HRV, inflammatory markers (CRP, white blood cell count), and carotid atherosclerosis in 124 men over 40 years old [70]. Together, these results suggest that inflammation plays an important role in the association of autonomic dysfunction with atherosclerosis. Recently, Pereira et al. reported that HRV noted during a deep breathing test was significantly and negatively correlated with carotid IMT in 101 patients with atherosclerotic risk factors [71]. As for arterial stiffness, Jaiswal et al. found that HRV was significantly and negatively associated with baPWV in 344 patients with type 1 diabetes [72], though no prospective studies that investigated this association have been presented.

Table 4.
Associations of HRV with carotid IMT and baPWV.
5. Potential Mechanisms underlying Association of Autonomic Nervous Dysfunction and Progression of Atherosclerosis
Autonomic nervous dysfunction can cause alterations in immunological response and endothelial dysfunction, which eventually could lead to progression of atherosclerosis, while an increased level of inflammatory cytokines may mediate the effects of autonomic nervous dysfunction on atherosclerosis progression [69,70]. Similar to the association between low sleep quality and atherosclerosis, augmented recruitment of inflammatory cells into vessel walls induces morphological changes in vessel cells. Another recent study suggested that autonomic dysfunction dysregulates neurotransmitters, such as norepinephrine (NE), adenosine triphosphate (ATP), neuropeptide Y (NPY), and acetylcholine (Ach), which are released from autonomic nerve terminal varicosities, and become diffusely distributed to smooth muscle cells and endothelial cells in the vessel [73]. The effects of autonomic function and these neuropeptides on vascular functions including arterial tone may be mediated by nitric oxide (NO) and endothelin, vasoactive factors produced by endothelial cells [74]. Additionally, platelet aggregation might serve to mediate the effects of autonomic nervous dysfunction on progression of atherosclerosis [75].
Furthermore, autonomic nervous dysfunction disturbs metabolic factors and may be attributable to classical atherosclerotic risk factors, such as hypertension, dyslipidemia, and diabetes mellitus. Yamada and Katagiri showed that the autonomic nervous system plays an important role in communicating organ-to-organ metabolic information [76]. Using these systems, the brain obtains information regarding peripheral metabolic status and processes the signals to regulate peripheral metabolism. Indeed, a relationship between autonomic nervous function and metabolic syndrome was shown in a large prospective cohort study conducted over a 2-year period of follow-up examinations [77]. Their findings indicated that an index of autonomic dysfunction was associated with increased numbers of metabolic syndrome components, such as high blood pressure and low HDL cholesterol.
The effects of autonomic dysfunction on atherosclerosis may be mediated by endocrinological alterations, including circulating epinephrine [78], as well as insulin resistance and adipocytokines, including leptin. Recent findings in animal models clearly showed that leptin, which reduces food intake, is significantly associated with autonomic nervous function [79]. The leptin receptor is expressed in the central nervous system. In our study conducted with the HSCAA cohort and performed under clinical conditions, we showed an association between plasma leptin and autonomic nervous function in patients with type 2 diabetes, and that association was independent of other clinical factors [80]. Additionally, brain-derived neurotrophic factor (BDNF) may be another key factor related to the effects of autonomic nervous function [81], as it has critical roles in survival, growth, maintenance, and death of central and peripheral neurons, and is also involved in regulation of the autonomic nervous system. In the HSCAA cohort study, we found a positive and significant association between plasma BDNF and autonomic nervous function in patients with cardiovascular risk factors [16].
6. Fatigue, Atherosclerosis and Cardiovascular Diseases
Fatigue is a common symptom in patients with a variety of conditions. Although the pathophysiological details have not been well characterized, it is known to be associated with continuous short sleep deprivation and low sleep quality. Recent epidemiological studies have found that fatigue is more common in patients with cardiovascular risk factors, such as diabetes [82], obesity [83], and sleep apnea [84]. We showed that higher fatigue score, determined using a recently established fatigue questionnaire, is a predictor of cardiovascular events in patients undergoing hemodialysis, with an impact independent of the presence of diabetes or past history of cardiovascular diseases [85]. Although the underlying mechanisms of those findings are not clear at present, potential candidates include short sleep duration, low sleep quality, autonomic nervous dysfunction such as elevated sympathetic nervous function, and altered endocrine and immune functions. We reported that loading of psychological fatigue in rats was associated with disturbance of neuroendocrinological functions [86]. Furthermore, in the HSCAA cohort study, fatigue score was found to be closely associated with plasma leptin level in patients with cardiovascular risk factors [87]. The leptin receptor is expressed in the central nervous system and may be involved in cardiac autonomic nerve function [80]. We have also shown that low plasma BDNF level, another potential biomarker indicating fatigue, is independently associated with a reverse-dipper pattern of nocturnal blood pressure [16] and development of chronic kidney disease (CKD) [88], in which autonomic nervous dysfunction may be involved, at least in part. Additional studies are needed to explore the mechanisms related to the contributions of fatigue to progression of atherosclerosis and cardiovascular diseases via autonomic dysfunction.
7. Conclusions and Perspectives
Behavioral and psychosocial factors, especially sleep, have been gaining increased attention in regard to their relationship with development of cardiovascular disease. In this review, we aimed to focus on the pathogenic impact of sleep problems, autonomic nervous function, and fatigue in relation to development of atherosclerosis and cardiovascular diseases (Figure 4). It is possible that inflammatory and oxidative response, endothelial dysfunction, endocrinological and immunological factors, and metabolic responses, as well as yet unveiled mechanisms underlie the effects of sleep problems, autonomic dysfunction, and fatigue on atherosclerosis progression. As shown in Figure 4, these psycho-behavior factors might accelerate the degree of progression, which is induced by classical risk factors such as diabetes, hypertension, obesity, and others. As highlighted in this report, the association between sleep problems and atherosclerosis has mainly been demonstrated in healthy populations, and results of a prospective large cohort study of patients with atherosclerotic risks are definitely needed. Moreover, no randomized controlled trial to examine the impact of improvement of these psycho-behavior problems on progression of atherosclerosis and occurrence of cardiovascular diseases has been reported. Additionally, basic studies are mandatory to identify feasible biomarkers for assessment of sleep problems and autonomic dysfunction in clinical settings. Fortunately, greater attention and additional investigations have brought focus to this important research field, and we believe that behavior factors will be recognized in the near future as promising clinical targets for prevention of atherosclerotic diseases.
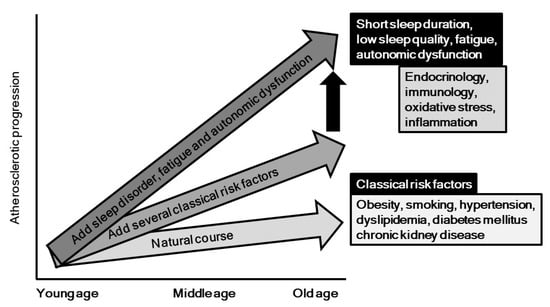
Figure 4.
Potential impacts of psycho-behavior factors on progression of atherosclerosis. As shown in vertical black arrow, psycho-behavior factors might accelerate the degree of atherosclerotic progression, which is induced by classical risk factors such as diabetes, hypertension, obesity, and others.
Funding
This work was supported by grants from JSPS KAKENHI (JP16K19562 to M.K., JP18K08531 to H.K.).
Acknowledgments
We are grateful for the work of all our colleagues, even those whose names we could not cite other than indirectly through other publications, due to space limitations. We thank all of the investigators and staff members, as well as participants in the Hyogo Sleep Cardio-Autonomic Atherosclerosis (HSCAA) study, for their valuable contributions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fujiyoshi, A.; Ohkubo, T.; Miura, K.; Murakami, Y.; Nagasawa, S.Y.; Okamura, T.; Ueshima, H. Observational Cohorts in Japan Research group. Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens. Res. 2012, 35, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Doi, Y.; Arima, H.; Yonemoto, K.; Hata, J.; Kubo, M.; Tanizaki, Y.; Ibayashi, S.; Iida, M.; Kiyohara, Y. LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: The Hisayama study. Stroke 2009, 40, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, S.; Okamura, T.; Hozawa, A.; Kadowaki, T.; Kadota, A.; Murakami, Y.; Nakamura, K.; Saitoh, S.; Nakamura, Y.; Hayakawa, T.; et al. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia 2008, 51, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Krantz, D.S.; Sheps, D.S.; Carney, R.M.; Natelson, B.H. Effects of mental stress in patients with coronary artery disease: Evidence and clinical implications. JAMA 2000, 283, 1800–1802. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Castro-Diehl, C.; Diez Roux, A.V.; Redline, S.; Seeman, T.; McKinley, P.; Sloan, R.; Shea, S. Sleep Duration and Quality in Relation to Autonomic Nervous System Measures: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2016, 39, 1927–1940. [Google Scholar] [CrossRef]
- Tsuji, H.; Larson, M.G.; Venditti, F.J., Jr.; Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996, 94, 2850–2855. [Google Scholar] [CrossRef]
- Buysse, D.J.; Hall, M.L.; Strollo, P.J.; Kamarck, T.W.; Owens, J.; Lee, L.; Reis, S.E.; Matthews, K.A. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J. Clin. Sleep Med. 2008, 4, 563–5671. [Google Scholar]
- Practice parameters for the use of actigraphy in the clinical assessment of sleep disorders. American Sleep Disorders Association. Sleep 1995, 18, 285–287. [CrossRef]
- Chen, C.Y.; Chen, C.L.; Yu, C.C. Obstructive sleep apnea is independently associated with arterial stiffness in ischemic stroke patients. J. Neurol. 2015, 262, 1247–1254. [Google Scholar] [CrossRef]
- Kadoya, M.; Koyama, H.; Kurajoh, M.; Kanzaki, A.; Kakutani-Hatayama, M.; Okazaki, H.; Shoji, T.; Moriwaki, Y.; Yamamoto, T.; Emoto, M.; et al. Sleep, cardiac autonomic function, and carotid atherosclerosis in patients with cardiovascular risks: HSCAA study. Atherosclerosis 2015, 238, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Low, P.A. Testing the autonomic nervous system. Semin. Neurol. 2003, 23, 407–421. [Google Scholar] [PubMed]
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [CrossRef]
- Polak, J.F.; Pencina, M.J.; Pencina, K.M.; O’Donnell, C.J.; Wolf, P.A.; D’Agostino, R.B., Sr. Carotid-wall intima-media thickness and cardiovascular events. N. Engl. J. Med. 2011, 365, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, M.; Koyama, H.; Kanzaki, A.; Kurajoh, M.; Hatayama, M.; Shiraishi, J.; Okazaki, H.; Shoji, T.; Moriwaki, Y.; Yamamoto, T.; et al. Plasma brain-derived neurotrophic factor and reverse dipping pattern of nocturnal blood pressure in patients with cardiovascular risk factors. PLoS ONE 2014, 9, e105977. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, M.; Koyama, H.; Kurajoh, M.; Naka, M.; Miyoshi, A.; Kanzaki, A.; Kakutani, M.; Shoji, T.; Moriwaki, Y.; Yamamoto, T.; et al. Associations of Sleep Quality and Awake Physical Activity with Fluctuations in Nocturnal Blood Pressure in Patients with Cardiovascular Risk Factors. PLoS ONE 2016, 11, e0155116. [Google Scholar] [CrossRef]
- Kadoya, M.; Kurajoh, M.; Kakutani-Hatayama, M.; Morimoto, A.; Miyoshi, A.; Kosaka-Hamamoto, K.; Shoji, T.; Moriwaki, Y.; Inaba, M.; Koyama, H. Low sleep quality is associated with progression of arterial stiffness in patients with cardiovascular risk factors: HSCAA study. Atherosclerosis 2018, 270, 95–101. [Google Scholar] [CrossRef]
- Nakazaki, C.; Noda, A.; Koike, Y.; Yamada, S.; Murohara, T.; Ozaki, N. Association of insomnia and short sleep duration with atherosclerosis risk in the elderly. Am. J. Hypertens. 2012, 25, 1149–1155. [Google Scholar]
- Nagai, M.; Hoshide, S.; Nishikawa, M.; Shimada, K.; Kario, K. Sleep duration and insomnia in the elderly: Associations with blood pressure variability and carotid artery remodeling. Am. J. Hypertens. 2013, 26, 981–989. [Google Scholar]
- Yoda, K.; Inaba, M.; Hamamoto, K.; Yoda, M.; Tsuda, A.; Mori, K.; Imanishi, Y.; Emoto, M.; Yamada, S. Association between poor glycemic control, impaired sleep quality, and increased arterial thickening in type 2 diabetic patients. PLoS ONE 2015, 10, e0122521. [Google Scholar] [CrossRef] [PubMed]
- Osonoi, Y.; Mita, T.; Osonoi, T.; Saito, M.; Tamasawa, A.; Nakayama, S.; Someya, Y.; Ishida, H.; Kanazawa, A.; Gosho, M.; et al. Poor sleep quality is associated with increased arterial stiffness in Japanese patients with type 2 diabetes mellitus. BMC Endocr. Disord. 2015, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Sands, M.R.; Lauderdale, D.S.; Liu, K.; Knutson, K.L.; Matthews, K.A.; Eaton, C.B.; Linkletter, C.D.; Loucks, E.B. Short sleep duration is associated with carotid intima-media thickness among men in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Stroke 2012, 43, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.; Volzke, H.; Schwahn, C.; Robinson, D.; Kessler, C.; John, U. Relation of self-reported sleep duration with carotid intima-media thickness in a general population sample. Atherosclerosis 2008, 196, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Aoki, T.; Yata, S.; Okada, M. Sleep duration is significantly associated with carotid artery atherosclerosis incidence in a Japanese population. Atherosclerosis 2011, 217, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Giles, W.H.; Croft, J.B.; Bliwise, D.L. Habitual sleep patterns and risk for stroke and coronary heart disease: A 10-year follow-up from NHANES I. Neurology 1997, 48, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Burazeri, G.; Gofin, J.; Kark, J.D. Over 8 h of sleep-marker of increased mortality in Mediterranean population: Follow-up population study. Croat. Med. J. 2003, 44, 193–198. [Google Scholar]
- Kim, C.W.; Chang, Y.; Zhao, D.; Cainzos-Achirica, M.; Ryu, S.; Jung, H.S.; Yun, K.E.; Choi, Y.; Ahn, J.; Zhang, Y.; et al. Sleep Duration, Sleep Quality, and Markers of Subclinical Arterial Disease in Healthy Men and Women. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2238–2245. [Google Scholar] [CrossRef]
- Yoshioka, E.; Saijo, Y.; Kita, T.; Okada, E.; Satoh, H.; Kawaharada, M.; Kishi, R. Relation between self-reported sleep duration and arterial stiffness: A cross-sectional study of middle-aged Japanese civil servants. Sleep 2011, 34, 1681–1686. [Google Scholar] [CrossRef]
- Tsai, T.C.; Wu, J.S.; Yang, Y.C.; Huang, Y.H.; Lu, F.H.; Chang, C.J. Long sleep duration associated with a higher risk of increased arterial stiffness in males. Sleep 2014, 37, 1315–1320. [Google Scholar] [CrossRef]
- Dominguez, F.; Fuster, V.; Fernandez-Alvira, J.M.; Fernandez-Friera, L.; Lopez-Melgar, B.; Blanco-Rojo, R.; Fernandez-Ortiz, A.; Garcia-Pavia, P.; Sanz, J.; Mendiguren, J.M.; et al. Association of Sleep Duration and Quality with Subclinical Atherosclerosis. J. Am. Coll. Cardiol. 2019, 73, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Ben-Shlomo, Y.; Beswick, A.; Yarnell, J.; Lightman, S.; Elwood, P. Cortisol, testosterone, and coronary heart disease: Prospective evidence from the Caerphilly study. Circulation 2005, 112, 332–340. [Google Scholar] [CrossRef]
- Lee, J.M.; Colangelo, L.A.; Schwartz, J.E.; Yano, Y.; Siscovick, D.S.; Seeman, T.; Schreiner, P.J.; Liu, K.J.; Lloyd-Jones, D.M.; Greenland, P. Associations of cortisol/testosterone and cortisol/sex hormone-binding globulin ratios with atherosclerosis in middle-age women. Atherosclerosis 2016, 248, 203–209. [Google Scholar] [CrossRef]
- Kadoya, M.; Koyama, S.; Morimoto, A.; Miyoshi, A.; Kakutani, M.; Hamamoto, K.; Kurajoh, M.; Shoji, T.; Moriwaki, Y.; Koshiba, M.; et al. Serum Macro TSH Level is Associated with Sleep Quality in Patients with Cardiovascular Risks—HSCAA Study. Sci. Rep. 2017, 7, 44387. [Google Scholar] [CrossRef] [PubMed]
- Cereda, C.W.; Tamisier, R.; Manconi, M.; Andreotti, J.; Frangi, J.; Pifferini, V.; Bassetti, C.L. Endothelial dysfunction and arterial stiffness in ischemic stroke: The role of sleep-disordered breathing. Stroke 2013, 44, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.L.; Yu, C.C.; Chen, T.T.; Tseng, S.T.; Ho, C.H. Association of inflammation and oxidative stress with obstructive sleep apnea in ischemic stroke patients. Sleep Med. 2015, 16, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.; Stradling, J.R. Mechanisms of vascular damage in obstructive sleep apnea. Nat. Rev. Cardiol. 2010, 7, 677–685. [Google Scholar] [CrossRef]
- Everson, C.A. Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1054–R1063. [Google Scholar] [CrossRef]
- Irwin, M.R.; Wang, M.; Campomayor, C.O.; Collado-Hidalgo, A.; Cole, S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern. Med. 2006, 166, 1756–1762. [Google Scholar] [CrossRef]
- Yehuda, S.; Sredni, B.; Carasso, R.L.; Kenigsbuch-Sredni, D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J. Interferon Cytokine Res. 2009, 29, 393–398. [Google Scholar] [CrossRef]
- Carreras, A.; Zhang, S.X.; Peris, E.; Qiao, Z.; Gileles-Hillel, A.; Li, R.C.; Wang, Y.; Gozal, D. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep 2014, 37, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Calvin, A.D.; Covassin, N.; Kremers, W.K.; Adachi, T.; Macedo, P.; Albuquerque, F.N.; Bukartyk, J.; Davison, D.E.; Levine, J.A.; Singh, P.; et al. Experimental sleep restriction causes endothelial dysfunction in healthy humans. J. Am. Heart Assoc. 2014, 3, e001143. [Google Scholar] [CrossRef] [PubMed]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Mueller, W.H.; Chan, W.; Meininger, J.C. Is obesity associated with poor sleep quality in adolescents? Am. J. Hum. Biol. 2002, 14, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; Buysse, D.J.; Klaghofer, R.; Gamma, A.; Ajdacic, V.; Eich, D.; Rossler, W.; Angst, J. The association between short sleep duration and obesity in young adults: A 13-year prospective study. Sleep 2004, 27, 661–666. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Roost, M.; Engstrom, G.; Hedblad, B.; Berglund, G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care 2004, 27, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Spaeth, A.M.; Ma, N.; Zhu, S.; Hu, S.; Goel, N.; Detre, J.A.; Dinges, D.F.; Rao, H. Altered salience network connectivity predicts macronutrient intake after sleep deprivation. Sci. Rep. 2015, 5, 8215. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- Hakim, F.; Wang, Y.; Carreras, A.; Hirotsu, C.; Zhang, J.; Peris, E.; Gozal, D. Chronic sleep fragmentation during the sleep period induces hypothalamic endoplasmic reticulum stress and PTP1b-mediated leptin resistance in male mice. Sleep 2015, 38, 31–40. [Google Scholar] [CrossRef]
- Cedernaes, J.; Schonke, M.; Westholm, J.O.; Mi, J.; Chibalin, A.; Voisin, S.; Osler, M.; Vogel, H.; Hornaeus, K.; Dickson, S.L.; et al. Acute sleep loss results in tissue-specific alterations in genome-wide DNA methylation state and metabolic fuel utilization in humans. Sci. Adv. 2018, 4, eaar8590. [Google Scholar] [CrossRef]
- Thurley, K.; Herbst, C.; Wesener, F.; Koller, B.; Wallach, T.; Maier, B.; Kramer, A.; Westermark, P.O. Principles for circadian orchestration of metabolic pathways. Proc. Natl. Acad. Sci. USA 2017, 114, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Peek, C.B.; Levine, D.C.; Cedernaes, J.; Taguchi, A.; Kobayashi, Y.; Tsai, S.J.; Bonar, N.A.; McNulty, M.R.; Ramsey, K.M.; Bass, J. Circadian Clock Interaction with HIF1alpha Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2017, 25, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Liao, D.; Bixler, E.O.; Chrousos, G.P.; Vela-Bueno, A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009, 32, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Trzepizur, W.; Le Vaillant, M.; Meslier, N.; Pigeanne, T.; Masson, P.; Humeau, M.P.; Bizieux-Thaminy, A.; Goupil, F.; Chollet, S.; Ducluzeau, P.H.; et al. Independent association between nocturnal intermittent hypoxemia and metabolic dyslipidemia. Chest 2013, 143, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yi, H.; Zou, J.; Meng, L.; Tang, X.; Zhu, H.; Yu, D.; Zhou, H.; Su, K.; Guan, J.; et al. Independent Association between Sleep Fragmentation and Dyslipidemia in Patients with Obstructive Sleep Apnea. Sci. Rep. 2016, 6, 26089. [Google Scholar] [CrossRef] [PubMed]
- Barros, D.; Garcia-Rio, F. Obstructive sleep apnea and dyslipidemia. From animal models to clinical evidence. Sleep 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Savransky, V.; Nanayakkara, A.; Smith, P.L.; O’Donnell, C.P.; Polotsky, V.Y. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J. Appl. Physiol. (1985) 2007, 102, 557–563. [Google Scholar] [CrossRef]
- Tan, K.C.; Chow, W.S.; Lam, J.C.; Lam, B.; Wong, W.K.; Tam, S.; Ip, M.S. HDL dysfunction in obstructive sleep apnea. Atherosclerosis 2006, 184, 377–382. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Somers, V.K. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand. 2003, 177, 385–390. [Google Scholar] [CrossRef]
- Ottosson, M.; Vikman-Adolfsson, K.; Enerback, S.; Olivecrona, G.; Bjorntorp, P. The effects of cortisol on the regulation of lipoprotein lipase activity in human adipose tissue. J. Clin. Endocrinol. Metab. 1994, 79, 820–825. [Google Scholar]
- Gottlieb, D.J.; Punjabi, N.M.; Newman, A.B.; Resnick, H.E.; Redline, S.; Baldwin, C.M.; Nieto, F.J. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch. Intern. Med. 2005, 165, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Rafalson, L.; Donahue, R.P.; Stranges, S.; Lamonte, M.J.; Dmochowski, J.; Dorn, J.; Trevisan, M. Short sleep duration is associated with the development of impaired fasting glucose: The Western New York Health Study. Ann. Epidemiol. 2010, 20, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.D.; Grote, L.; Ryan, S.; Pepin, J.L.; Bonsignore, M.R.; Tkacova, R.; Saaresranta, T.; Verbraecken, J.; Levy, P.; Hedner, J.; et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: The European Sleep Apnea Cohort (ESADA) study. Chest 2014, 146, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, M.; Lutsey, P.L.; Benkeser, D.; Wassel, C.L.; Folsom, A.R.; Shahar, E.; Iso, H.; Allison, M.A.; Criqui, M.H.; Redline, S. Association of sleep apnea and sleep duration with peripheral artery disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2016, 251, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Liberty, I.F.; Porath, A.; Ovsyshcher, I.; Prystowsky, E.N. A simple bedside test of 1-minute heart rate variability during deep breathing as a prognostic index after myocardial infarction. Am. Heart J. 1999, 138, 32–38. [Google Scholar] [CrossRef]
- Valensi, P.; Sachs, R.N.; Harfouche, B.; Lormeau, B.; Paries, J.; Cosson, E.; Paycha, F.; Leutenegger, M.; Attali, J.R. Predictive value of cardiac autonomic neuropathy in diabetic patients with or without silent myocardial ischemia. Diabetes Care 2001, 24, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Gottsater, A.; Ahlgren, A.R.; Taimour, S.; Sundkvist, G. Decreased heart rate variability may predict the progression of carotid atherosclerosis in type 2 diabetes. Clin. Auton. Res. 2006, 16, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Melillo, P.; Izzo, R.; De Luca, N.; Pecchia, L. Heart rate variability and target organ damage in hypertensive patients. BMC Cardiovasc. Disord. 2012, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, C.; Manzoli, L.; Mancini, S.; Bedetti, G.; Fontana, F.; Costa, G.M. Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors. Atherosclerosis 2010, 212, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Ulleryd, M.A.; Prahl, U.; Borsbo, J.; Schmidt, C.; Nilsson, S.; Bergstrom, G.; Johansson, M.E. The association between autonomic dysfunction, inflammation and atherosclerosis in men under investigation for carotid plaques. PLoS ONE 2017, 12, e0174974. [Google Scholar] [CrossRef]
- Pereira, V.L., Jr.; Dobre, M.; Dos Santos, S.G.; Fuzatti, J.S.; Oliveira, C.R.; Campos, L.A.; Brateanu, A.; Baltatu, O.C. Association between Carotid Intima Media Thickness and Heart Rate Variability in Adults at Increased Cardiovascular Risk. Front Physiol. 2017, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Urbina, E.M.; Wadwa, R.P.; Talton, J.W.; D’Agostino, R.B., Jr.; Hamman, R.F.; Fingerlin, T.E.; Daniels, S.R.; Marcovina, S.M.; Dolan, L.M.; et al. Reduced heart rate variability is associated with increased arterial stiffness in youth with type 1 diabetes: The SEARCH CVD study. Diabetes Care 2013, 36, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zhu, L. The crosstalk between autonomic nervous system and blood vessels. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 17–28. [Google Scholar] [PubMed]
- Gamboa, A.; Figueroa, R.; Paranjape, S.Y.; Farley, G.; Diedrich, A.; Biaggioni, I. Autonomic Blockade Reverses Endothelial Dysfunction in Obesity-Associated Hypertension. Hypertension 2016, 68, 1004–1010. [Google Scholar] [PubMed]
- Badimon, L.; Martinez-Gonzalez, J.; Royo, T.; Lassila, R.; Badimon, J.J. A sudden increase in plasma epinephrine levels transiently enhances platelet deposition on severely damaged arterial wall—Studies in a porcine model. Thromb. Haemost. 1999, 82, 1736–1742. [Google Scholar] [PubMed]
- Yamada, T.; Katagiri, H. Avenues of communication between the brain and tissues/organs involved in energy homeostasis. Endocr. J. 2007, 54, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Licht, C.M.; de Geus, E.J.; Penninx, B.W. Dysregulation of the autonomic nervous system predicts the development of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.S.; Critchley, J.A.; Tomlinson, B.; Young, R.P.; Thomas, G.N.; Cockram, C.S.; Chan, T.Y.; Chan, J.C. Urinary epinephrine and norepinephrine interrelations with obesity, insulin, and the metabolic syndrome in Hong Kong Chinese. Metabolism 2001, 50, 135–143. [Google Scholar] [CrossRef]
- Simonds, S.E.; Pryor, J.T.; Ravussin, E.; Greenway, F.L.; Dileone, R.; Allen, A.M.; Bassi, J.; Elmquist, J.K.; Keogh, J.M.; Henning, E.; et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 2014, 159, 1404–1416. [Google Scholar] [CrossRef]
- Kurajoh, M.; Koyama, H.; Kadoya, M.; Naka, M.; Miyoshi, A.; Kanzaki, A.; Kakutani-Hatayama, M.; Okazaki, H.; Shoji, T.; Moriwaki, Y.; et al. Plasma leptin level is associated with cardiac autonomic dysfunction in patients with type 2 diabetes: HSCAA study. Cardiovasc. Diabetol. 2015, 14, 117. [Google Scholar]
- Nakahashi, T.; Fujimura, H.; Altar, C.A.; Li, J.; Kambayashi, J.; Tandon, N.N.; Sun, B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000, 470, 113–117. [Google Scholar] [CrossRef]
- Drivsholm, T.; de Fine Olivarius, N.; Nielsen, A.B.; Siersma, V. Symptoms, signs and complications in newly diagnosed type 2 diabetic patients, and their relationship to glycaemia, blood pressure and weight. Diabetologia 2005, 48, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Hong, S.; Nelesen, R.; Dimsdale, J.E. The association of obesity, cytokine levels, and depressive symptoms with diverse measures of fatigue in healthy subjects. Arch Intern. Med. 2005, 165, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Bardwell, W.A.; Moore, P.; Ancoli-Israel, S.; Dimsdale, J.E. Fatigue in obstructive sleep apnea: Driven by depressive symptoms instead of apnea severity? Am. J. Psychiatry 2003, 160, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Fukuda, S.; Shoji, T.; Inaba, M.; Tsujimoto, Y.; Tabata, T.; Okuno, S.; Yamakawa, T.; Okada, S.; Okamura, M.; et al. Fatigue is a predictor for cardiovascular outcomes in patients undergoing hemodialysis. Clin. J. Am. Soc. Nephrol. 2010, 5, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, A.; Okauchi, T.; Hu, D.; Shingaki, T.; Katayama, Y.; Koyama, H.; Watanabe, Y.; Cui, Y. Extension of recovery time from fatigue by repeated rest with short-term sleep during continuous fatigue load: Development of chronic fatigue model. J. Neurosci. Res. 2016, 94, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Kurajoh, M.; Kadoya, M.; Morimoto, A.; Naka, M.; Miyoshi, A.; Kanzaki, A.; Kakutani-Hatayama, M.; Hamamoto, K.; Shoji, T.; Moriwaki, Y.; et al. Plasma leptin concentration is associated with fatigue severity in patients with cardiovascular risk factors—HSCAA study. Psychoneuroendocrinology 2016, 74, 7–12. [Google Scholar] [CrossRef]
- Kurajoh, M.; Kadoya, M.; Morimoto, A.; Miyoshi, A.; Kanzaki, A.; Kakutani-Hatayama, M.; Hamamoto, K.; Shoji, T.; Moriwaki, Y.; Yamamoto, T.; et al. Plasma brain-derived neurotrophic factor concentration is a predictor of chronic kidney disease in patients with cardiovascular risk factors—Hyogo Sleep Cardio-Autonomic Atherosclerosis study. PLoS ONE 2017, 12, e0178686. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).