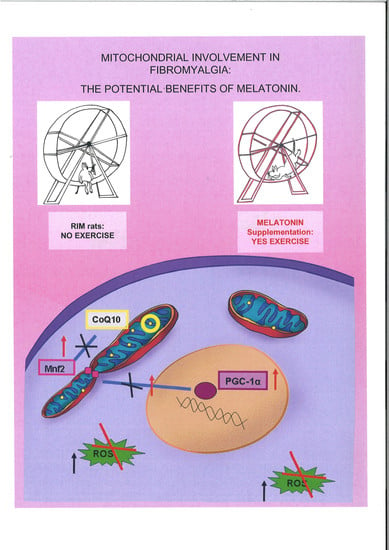
Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin
Abstract
1. Introduction
2. Results
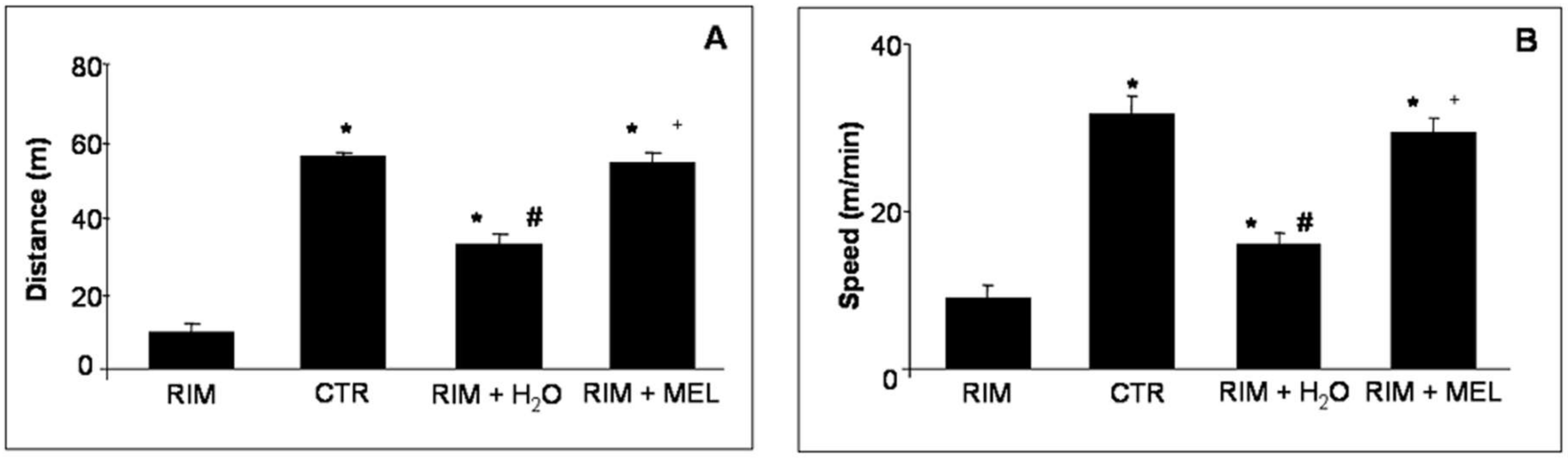
2.1. Spontaneous Locomotor Activity Monitoring
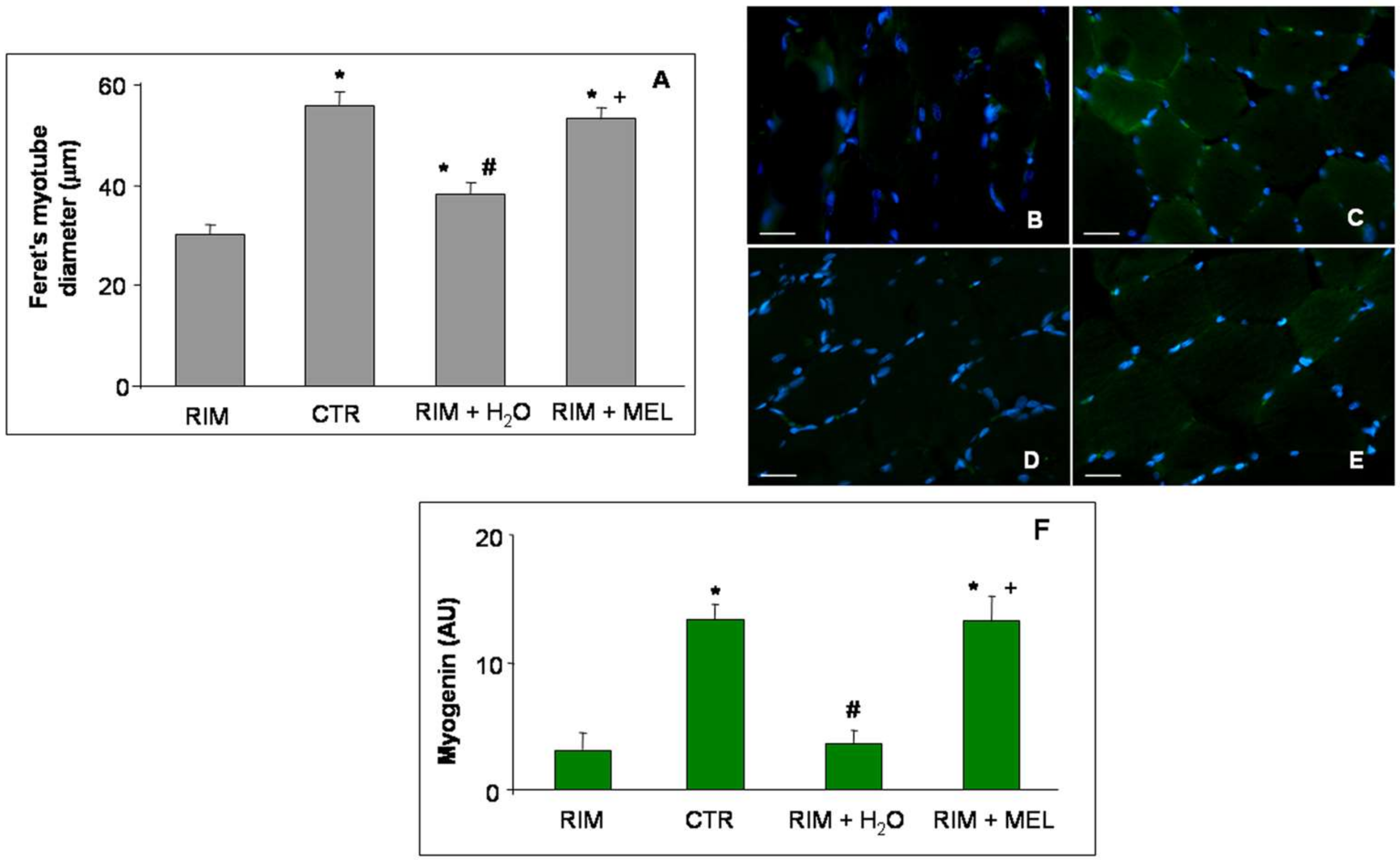
2.2. Morphological Evaluations of Gastrocnemius Muscle
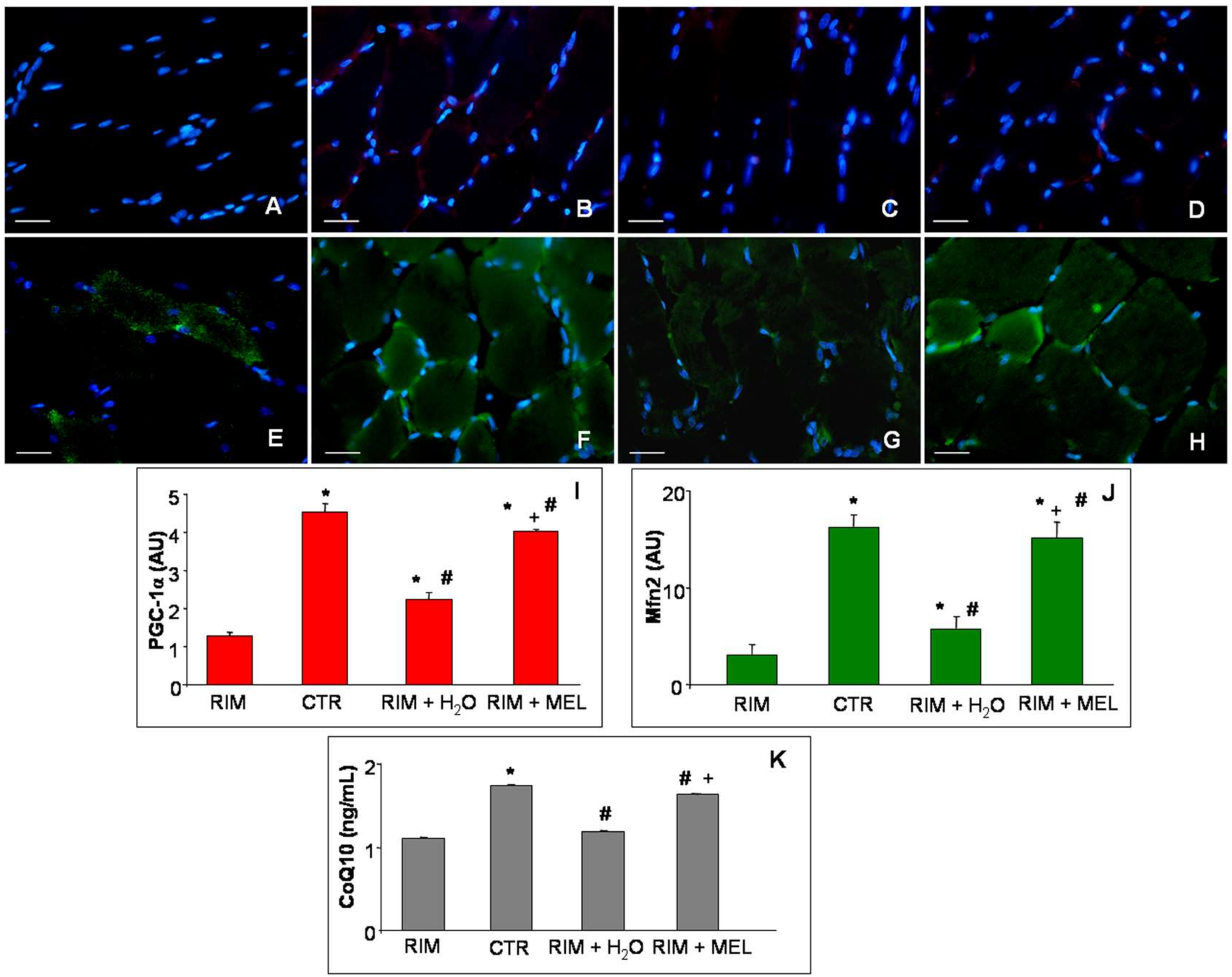
2.3. Mitochondrial Markers Evaluation
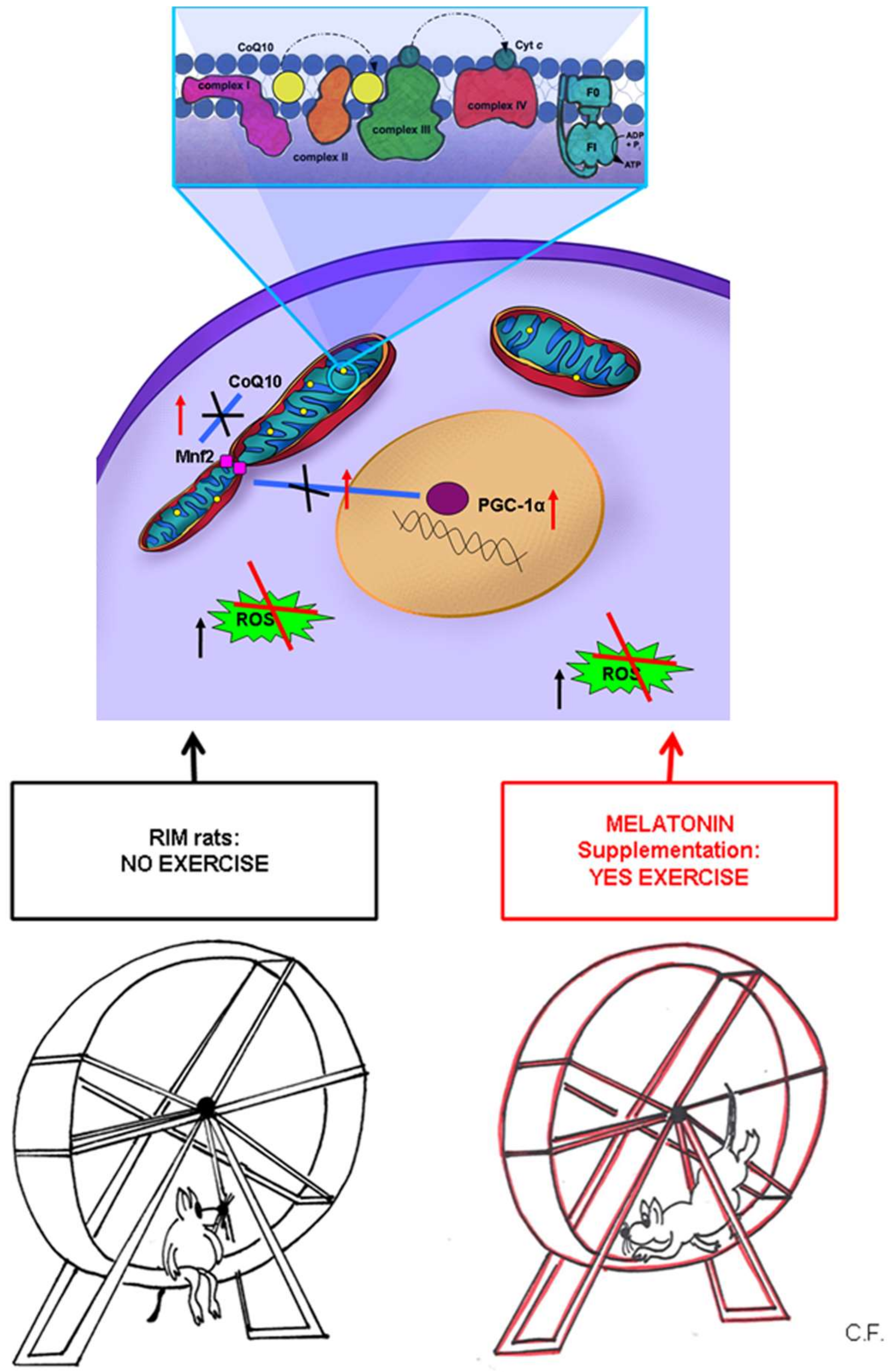
3. Discussion
4. Materials and Methods
4.1. Animal Treatment
- -
- control rats kept untreated;
- -
- control rats treated with melatonin for two months;
- -
- control rats treated with the vehicle of melatonin;
- -
- control rats treated with vehicle of reserpine;
- -
- reserpine-induced myalgic rats (RIM);
- -
- RIM rats plus tap water for two months (RIM + H2O);
- -
- RIM rats treated with melatonin for two months (RIM + MEL).
4.2. Morphometrical Analyses
4.3. Immunofluorescence Assay
4.4. Coenzyme Q10 ELISA Evaluation
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cordero, M.D.; Alcocer-Gómez, E.; Culic, O.; Carrión, A.M.; de Miguel, M.; Díaz-Parrado, E.; Pérez-Villegas, E.M.; Bullón, P.; Battino, M.; Sánchez-Alcazar, J.A. NLRP3 inflammasome is activated in fibromyalgia: The effect of coenzyme Q10. Antioxid. Redox Signal. 2014, 20, 1169–80. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; D’Arcy, Y.; Gebke, K.; Semel, D.; Pauer, L.; Jones, K.D. Normalizing fibromyalgia as a chronic illness. Postgrad. Med. 2018, 130, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Bennett, R.M.; Crofford, L.J.; Dean, L.E.; Clauw, D.J.; Goldenberg, D.L.; Fitzcharles, M.A.; Paiva, E.S.; Staud, R.; Sarzi-Puttini, P.; et al. AAPT diagnostic criteria for fibromyalgia. J. Pain 2018, 1–18. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; Culic, O.; Navarro-Pando, J.M.; Sánchez-Alcázar, J.A.; Bullón, P. Effect of coenzyme Q(10) on psychopathological symptoms in fibromyalgia patients. CNS Neurosci. Ther. 2017, 23, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Basso, V.; Marchesan, E.; Peggion, C.; Chakraborty, J.; von Stockum, S.; Giacomello, M.; Ottolini, D.; Debattisti, V.; Caicci, F.; Tasca, E.; et al. Regulation of ER-mitochondria contacts by Parkin via Mfn2. Pharmacol. Res. 2018, 138, 43–56. [Google Scholar] [CrossRef]
- Mourier, A.; Motori, E.; Brandt, T.; Lagouge, M.; Atanassov, I.; Galinier, A.; Rappl, G.; Brodesser, S.; Hultenby, K.; Dieterich, C.; et al. Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J. Cell Biol. 2015, 208, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; Sánchez-Alcázar, J.A.; Cordero, M.D. Coenzyme q10 regulates serotonin levels and depressive symptoms in fibromyalgia patients: Results of a small clinical trial. J. Clin. Psychopharmacol. 2014, 34, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Procaccio, V.; Bris, C.; Chao de la Barca, J.M.; Oca, F.; Chevrollier, A.; Amati-Bonneau, P.; Bonneau, D.; Reynier, P. Perspectives of drug-based neuroprotection targeting mitochondria. Rev. Neurol. (Paris) 2014, 170, 390–400. [Google Scholar] [CrossRef]
- Nagakura, Y.; Oe, T.; Aoki, T.; Matsuoka, N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 2009, 146, 26–33. [Google Scholar] [CrossRef]
- Atzeni, F.; Gerardi, M.C.; Masala, I.F.; Alciati, A.; Batticciotto, A.; Sarzi-Puttini, P. An update on emerging drugs for fibromyalgia treatment. Expert Opin. Emerg. Drugs. 2017, 22, 357–367. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; Dincer, F.; Leino-Arjas, P.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Higgs, J.B. Fibromyalgia in Primary Care. Prim. Care 2018, 45, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.A.; Fatima, G.; Das, S.K.; Verma, N.S. Abnormality of circadian rhythm of serum melatonin and other biochemical parameters in fibromyalgia syndrome. Indian J. Biochem. Biophys. 2011, 48, 82–87. [Google Scholar] [PubMed]
- Citera, G.; Arias, M.A.; Maldonado-Cocco, J.A.; Lázaro, M.A.; Rosemffet, M.G.; Brusco, L.I.; Scheines, E.J.; Cardinalli, D.P. The effect of melatonin in patients with fibromyalgia: A pilot study. Clin Rheumatol. 2000, 19, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Reiter, R.J. Melatonin therapy in fibromyalgia. J Pineal Res. 2006, 40, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barceló, E.J.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Clinical uses of melatonin: Evaluation of human trials. Curr Med Chem. 2010, 17, 2070–2095. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef]
- Rohr, U.D.; Herold, J. Melatonin deficiencies in women. Maturitas 2002, 41, 85–104. [Google Scholar] [CrossRef]
- Reiter, R.J.; Acuna-Castroviejo, D.; Tan, D.X. Melatonin therapy in fibromyalgia. Curr. Pain Headache Rep. 2007, 11, 339–342. [Google Scholar] [CrossRef]
- Hussain, S.A.; Al-Khalifa, I.I.; Jasim, N.A.; Gorial, F.I. Adjuvant use of melatonin for treatment of fibromyalgia. J. Pineal Res. 2011, 50, 267–271. [Google Scholar] [CrossRef]
- Sánchez-Domínguez, B.; Bullón, P.; Román-Malo, L.; Marín-Aguilar, F.; Alcocer-Gómez, E.; Carrión, A.M.; Sánchez-Alcazar, J.A.; Cordero, M.D. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with Fibromyalgia. Mitochondrion 2015, 21, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Danilov, A.; Kurganova, J. Melatonin in chronic pain syndromes. Pain Ther. 2016, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Trapletti, V.; Bonomini, F.; Stacchiotti, A.; Lavazza, A.; Rodella, L.F.; Rezzani, R. Oral supplementation of melatonin protects against fibromyalgia-related skeletal muscle alterations in reserpine-induced myalgia rats. Int. J. Mol. Sci. 2017, 18, 1389. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.G.; Acuña, M.J.; Cabrera, D.; Goldschmeding, R.; Brandan, E. The pro-fibrotic connective tissue growth factor (CTGF/CCN2) correlates with the number of necrotic-regenerative foci in dystrophic muscle. J. Cell. Commun. Signal. 2018, 12, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gómez, E.; Marín-Aguilar, F.; Rybkina, T.; Cotán, D.; Pérez-Pulido, A.; Alvarez-Suarez, J.M.; Battino, M.; Sánchez-Alcazar, J.A.; Carrión, A.M.; et al. Mutation in cytochrome b gene of mitochondrial DNA in a family with fibromyalgia is associated with NLRP3-inflammasome activation. J. Med. Genet. 2016, 53, 113–122. [Google Scholar] [CrossRef]
- Blasco-Serra, A.; Escrihuela-Vidal, F.; González-Soler, E.M.; Martínez-Expósito, F.; Blasco-Ausina, M.C.; Martínez-Bellver, S.; Cervera-Ferri, A.; Teruel-Martí, V.; Valverde-Navarro, A.A. Depressive-like symptoms in a reserpine-induced model of fibromyalgia in rats. Physiol. Behav. 2015, 151, 456–462. [Google Scholar] [PubMed]
- Maeda, T.; Kudo, Y.; Horiuchi, T.; Makino, N. Clinical and anti-aging effect of mud-bathing therapy for patients with fibromyalgia. Mol. Cell. Biochem. 2018, 444, 87–92. [Google Scholar] [CrossRef]
- Segura-Jiménez, V.; Borges-Cosic, M.; Soriano-Maldonado, A.; Estévez-López, F.; Álvarez-Gallardo, I.C.; Herrador-Colmenero, M.; Delgado-Fernández, M.; Ruiz, J.R. Association of sedentary time and physical activity with pain, fatigue, and impact of fibromyalgia: The al-Ándalus study. Scand. J. Med. Sci. Sports 2017, 27, 83–92. [Google Scholar] [CrossRef]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. (Maywood) 2018, 243, 118–128. [Google Scholar] [CrossRef]
- Siu, P.M.; Donley, D.A.; Bryner, R.W.; Always, S.E. Myogenin and oxidative enzyme gene expression levels are elevated in rat soleus muscles after endurance training. J. Appl. Physiol. (1985) 2004, 97, 277–285. [Google Scholar] [CrossRef]
- Bazgir, B.; Fathi, R.; Rezazadeh Valojerdi, M.; Mozdziak, P.; Asgari, A. Satellite cells contribution to exercise mediated muscle hypertrophy and repair. Cell. J. 2017, 18, 473–484. [Google Scholar] [PubMed]
- Chung, C.P.; Titova, D.; Oeser, A.; Randels, M.; Avalos, I.; Milne, G.L.; Morrow, J.D.; Stein, C.M. Oxidative stress in fibromyalgia and its relationship to symptoms. Clin. Rheumatol. 2009, 28, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Cannavino, J.; Brocca, L.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. PGC1-α over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J. Physiol. 2014, 592, 4575–4589. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Robinson, M.M.; Nair, K.S. Skeletal muscle aging and the mitochondrion. Trends Endocrinol. Metab. 2013, 24, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Tryon, L.D.; Carter, H.N.; Kim, Y.; Chen, C.C. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem. J. 2016, 473, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Hancock, C.R.; Terada, S.; Higashida, K.; Holloszy, J.O.; Han, D.H. PPARβ is essential for maintaining normal levels of PGC-1α and mitochondria and for the increase in muscle mitochondria induced by exercise. Cell. Metab. 2017, 25, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Triolo, M.; Hood, D.A. Mitochondrial breakdown in skeletal muscle and the emerging role of the lysosomes. Arch. Biochem. Biophys. 2018, 661, 66–73. [Google Scholar] [CrossRef]
- Hyatt, H.; Deminice, R.; Yoshihara, T.; Powers, S.K. Mitochondrial dysfunction induces muscle atrophy during prolonged T inactivity: A review of the causes and effects. Arch. Biochem. Biophys. 2019, 662, 49–60. [Google Scholar] [CrossRef]
- Filadi, R.; Pendin, D.; Pizzo, P. Mitofusin 2: From functions to disease. Cell. Death. Dis. 2018, 9, 330. [Google Scholar] [CrossRef]
- Zorzano, A. Regulation of mitofusin-2 expression in skeletal muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 433–439. [Google Scholar] [CrossRef]
- Marino Gammazza, A.; Macaluso, F.; Di Felice, V.; Cappello, F.; Barone, R. Hsp60 in skeletal muscle fiber biogenesis and homeostasis: From physical exercise to skeletal muscle pathology. Cells 2018, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gómez, E.; de Miguel, M.; Cano-García, F.J.; Luque, C.M.; Fernández-Riejo, P.; Fernández, A.M.; Sánchez-Alcazar, J.A. Coenzyme Q(10): A novel therapeutic approach for fibromyalgia? case series with 5 patients. Mitochondrion 2011, 11, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Cotán, D.; del-Pozo-Martín, Y.; Carrión, A.M.; de Miguel, M.; Bullón, P.; Sánchez-Alcazar, J.A. Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient. Nutrition 2012, 28, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, T.; Seki, M.; Naga, T.; Uchino, S.; Asazuma, H.; Yoshida, T.; Iizuka, Y.; Kikuchi, M.; Imagawa, T.; Natsumeda, Y.; et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: Amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep. 2013, 18, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Aaseth, J. Fibromyalgia and nutrition: Therapeutic possibilities? Biomed. Pharmacother. 2018, 103, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and its metabolites ameliorate UVR-induced mitochondrial oxidative stress in human MNT-1 melanoma cells. Int. J. Mol. Sci. 2018, 19, 3786. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. PNAS 2017, 114, 7997–8006. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; El-Sawi, M.R. Melatonin reduces oxidant damage and promotes mitochondrial respiration: Implications for aging. Ann. N. Y. Acad. Sci. 2002, 959, 238–250. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, S.; Pei, Z.; Drozda, M.; Stavrovskaya, I.G.; Del Signore, S.J.; Cormier, K.; Shimony, E.M.; Wang, H.; Ferrante, R.J.; et al. Inhibitors of cytochrome c release with therapeutic potential for Huntington’s disease. J. Neurosci. 2008, 28, 9473–9485. [Google Scholar] [CrossRef]
- Wang, X.; Sirianni, A.; Pei, Z.; Cormier, K.; Smith, K.; Jiang, J.; Zhou, S.; Wang, H.; Zhao, R.; Yano, H.; et al. The melatonin MT1 receptor axis modulates mutant Huntingtin-mediated toxicity. J. Neurosci. 2011, 31, 14496–14507. [Google Scholar] [CrossRef]
- Zhang, Y.; Cook, A.; Kim, J.; Baranov, S.V.; Jiang, J.; Smith, K.; Cormier, K.; Bennett, E.; Browser, R.P.; Day, A.L.; et al. Melatonin inhibits the caspase 1/cytochrome c/caspase-3 cell death pathway, inhibits MT1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2013, 55, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Calpena, A.C.; Clares, B. Evaluating the oxidative stress in inflammation: Role of melatonin. Int. J. Mol. Sci. 2015, 16, 16981–17004. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective Effects of Melatonin and Mitochondria-targeted Antioxidants Against Oxidative Stress: A Review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef] [PubMed]
- Kiso, T.; Moriyama, A.; Furutani, M.; Matsuda, R.; Funatsu, Y. Effects of pregabalin and duloxetine on neurotransmitters in the dorsal horn of the spinal cord in a rat model of fibromyalgia. Eur. J. Pharmacol. 2018, 827, 117–124. [Google Scholar] [CrossRef]
- Ogino, S.; Nagakura, Y.; Tsukamoto, M.; Watabiki, T.; Ozawa, T.; Oe, T.; Shimizu, Y.; Ito, H. Systemic administration of 5-HT(2C) receptor agonists attenuates muscular hyperalgesia in reserpine-induced myalgia model. Pharmacol. Biochem. Behav. 2013, 108, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Stacchiotti, A.; Castrezzati, S.; Bonomini, F.; Albanese, M.; Rezzani, R.; Rodella, L.F. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr. Res. 2015, 35, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Favero, G.; Stacchiotti, A.; Rodella, L.F. Endothelial and vascular smooth muscle cell dysfunction mediated by cyclophylin A and the atheroprotective effects of melatonin. Life Sci. 2013, 92, 875–882. [Google Scholar] [CrossRef]
- Agabiti-Rosei, C.; De Ciuceis, C.; Rossini, C.; Porteri, E.; Rodella, L.F.; Withers, S.B.; Heagerty, A.M.; Favero, G.; Agabiti-Rosei, E.; Rizzoni, D.; et al. Anticontractile activity of perivascular fat in obese mice and the effect of long-term treatment with melatonin. J. Hypertens. 2014, 32, 1264–1274. [Google Scholar] [CrossRef]
- Agabiti-Rosei, C.; Favero, G.; De Ciuceis, C.; Rossini, C.; Porteri, E.; Rodella, L.F.; Franceschetti, L.; Maria Sarkar, A.; Agabiti-Rosei, E.; Rizzoni, D.; et al. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens. Res. 2017, 40, 41–50. [Google Scholar] [CrossRef]
- Rodella, L.F.; Rossini, C.; Favero, G.; Foglio, E.; Loreto, C.; Rezzani, R. Nicotine-induced morphological changes in rat aorta: The protective role of melatonin. Cells Tissues Organs 2012, 195, 252–259. [Google Scholar] [CrossRef]
- Favero, G.; Paini, A.; De Ciuceis, C.; Rodella, L.F.; Moretti, E.; Porteri, E.; Rossini, C.; Ministrini, S.; Solaini, L.; Stefano, C.; et al. Changes in extracellular matrix in subcutaneous small resistance arteries of patients with essential hypertension. Blood Press. 2018, 27, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.A.; Oliveira, C.S.; Ineu, R.P.; Moraes-Silva, L.; de Siqueira, L.F.; Pereira, M.E. Lactating and non-lactating rats differ in sensitivity to HgCl(2): Protective effect of ZnCl(2). J. Trace Elem. Med. Biol. 2014, 28, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Favero, G.; Rodella, L.F.; Moghadasian, M.H.; Rezzani, R. Melatonin modulation of sirtuin-1 attenuates liver injury in a hypercholesterolemic mouse model. BioMed Res. Int. 2018, 2018, 7968452. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Borgonovo, A.; Rezzani, R.; Santoro, F. TGF-beta1 and VEGF after fresh frozen bone allograft insertion in oral-maxillo-facial surgery. Histol. Histopathol. 2010, 25, 463–471. [Google Scholar] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favero, G.; Bonomini, F.; Franco, C.; Rezzani, R. Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin. Int. J. Mol. Sci. 2019, 20, 765. https://doi.org/10.3390/ijms20030765
Favero G, Bonomini F, Franco C, Rezzani R. Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin. International Journal of Molecular Sciences. 2019; 20(3):765. https://doi.org/10.3390/ijms20030765
Chicago/Turabian StyleFavero, Gaia, Francesca Bonomini, Caterina Franco, and Rita Rezzani. 2019. "Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin" International Journal of Molecular Sciences 20, no. 3: 765. https://doi.org/10.3390/ijms20030765
APA StyleFavero, G., Bonomini, F., Franco, C., & Rezzani, R. (2019). Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin. International Journal of Molecular Sciences, 20(3), 765. https://doi.org/10.3390/ijms20030765