TRAF6 Silencing Attenuates Multiple Myeloma Cell Adhesion to Bone Marrow Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Retroviral Transfection
2.3. Stromal Cell Co-Culture
2.4. Adhesion Assay
2.5. Real-Time PCR
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
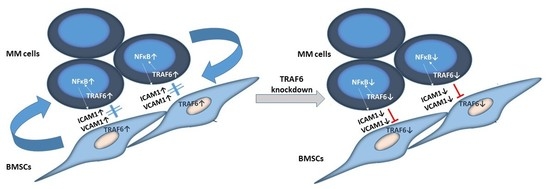
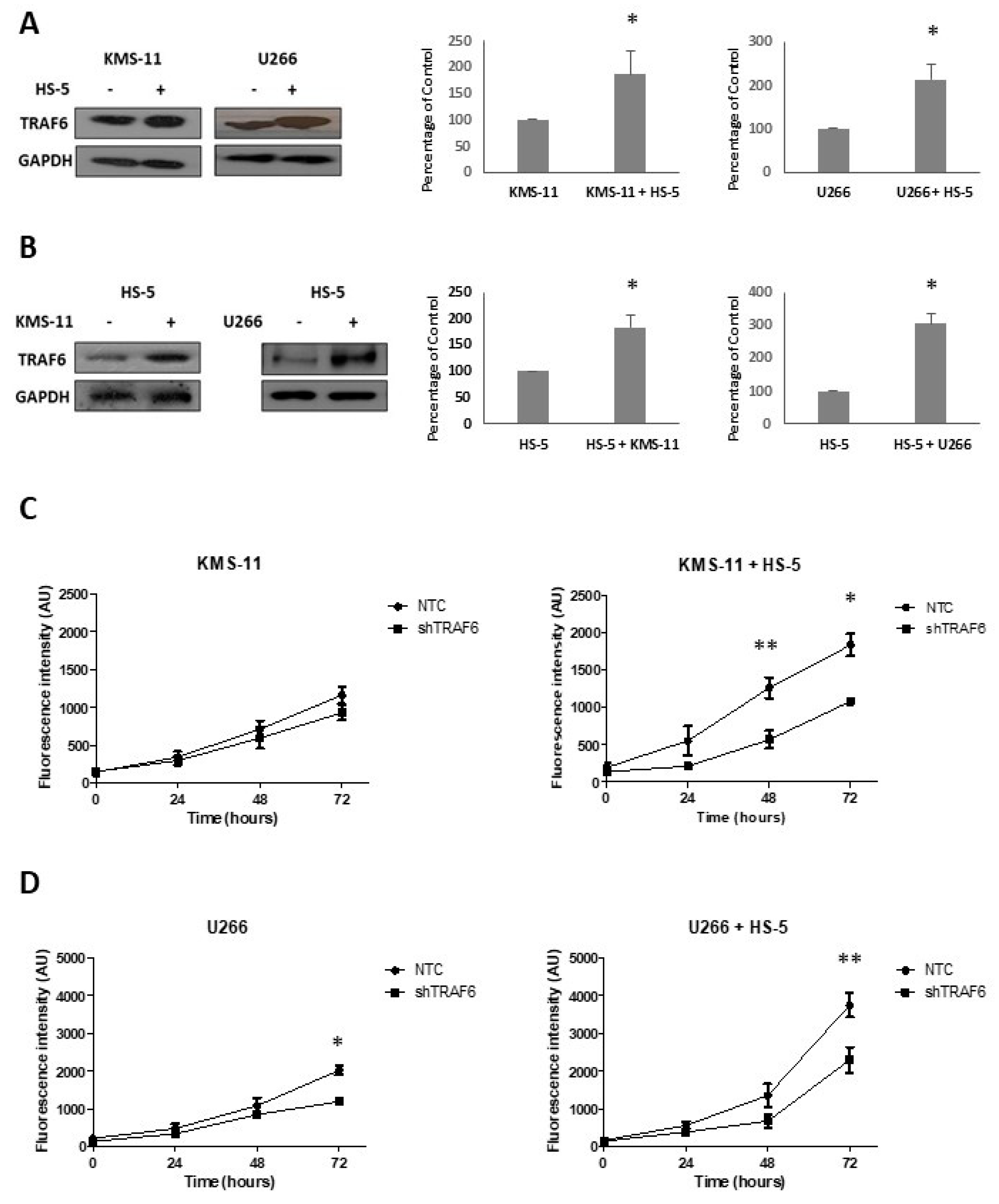
3.1. TRAF6 Expression Is Enhanced Upon Adherence of MM Cells to BMSCs
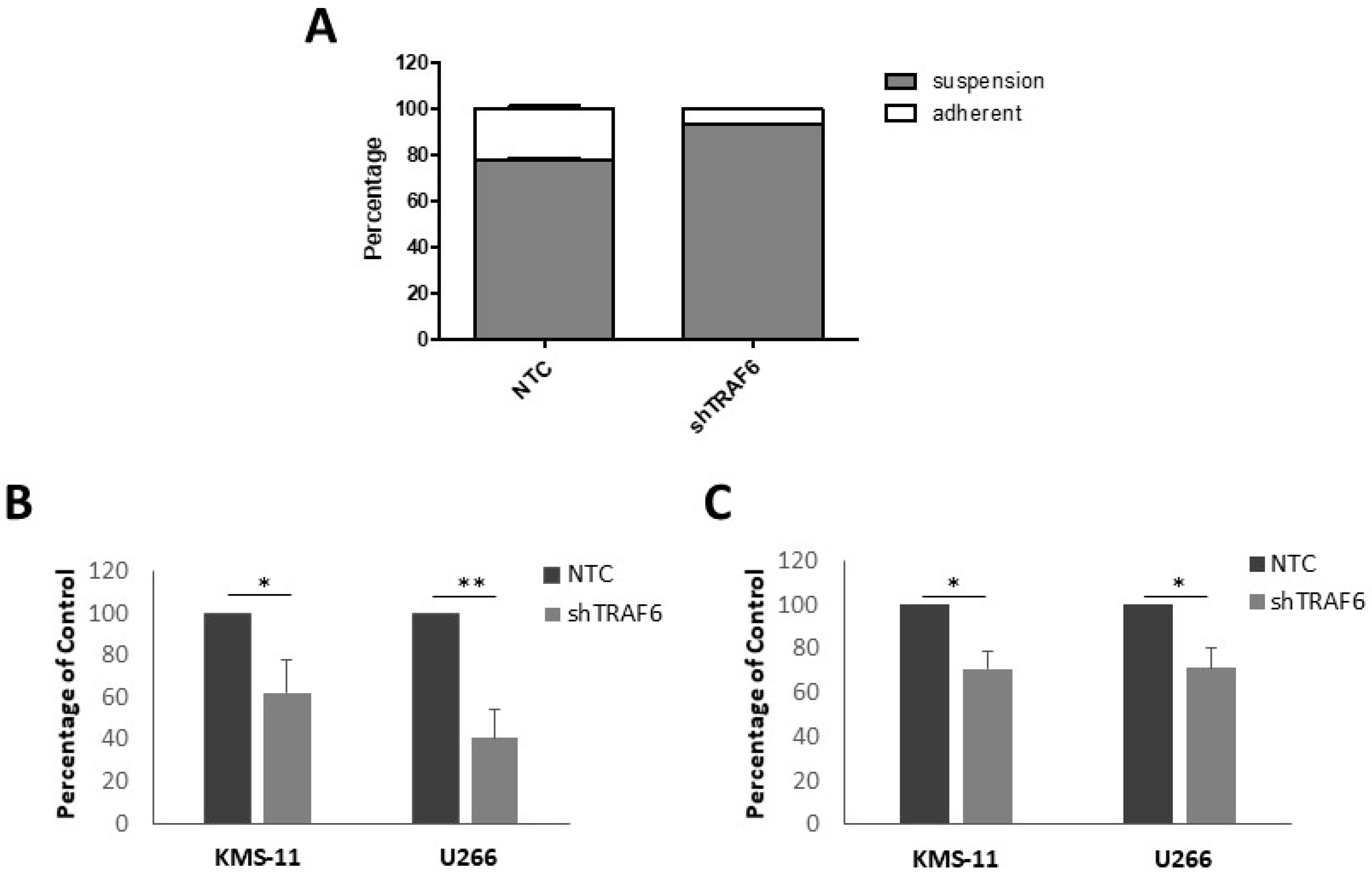
3.2. TRAF6 Knockdown Impairs Adhesion to BMSCs
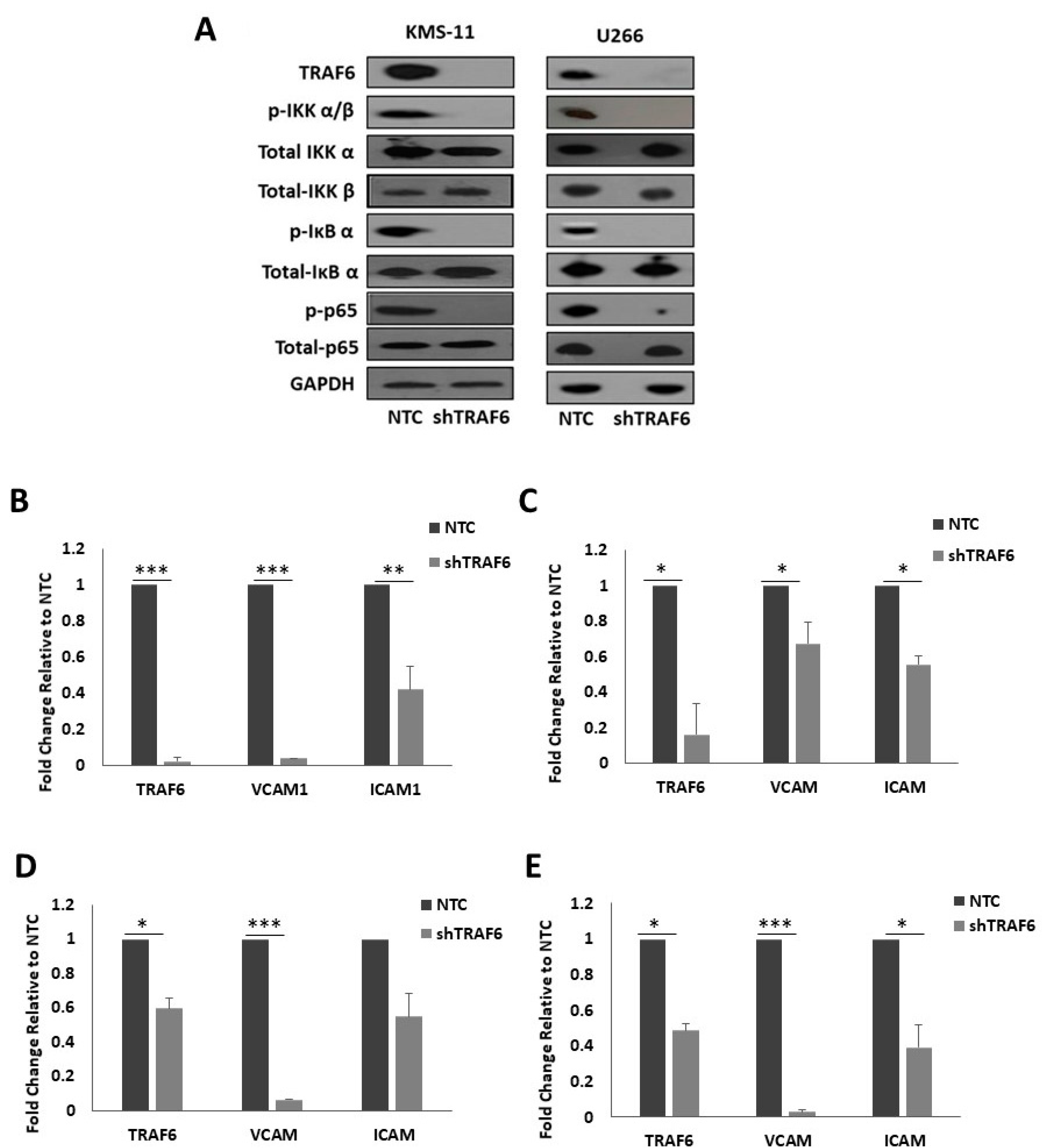
3.3. Knockdown of TRAF6 Inhibits NFκB Signalling
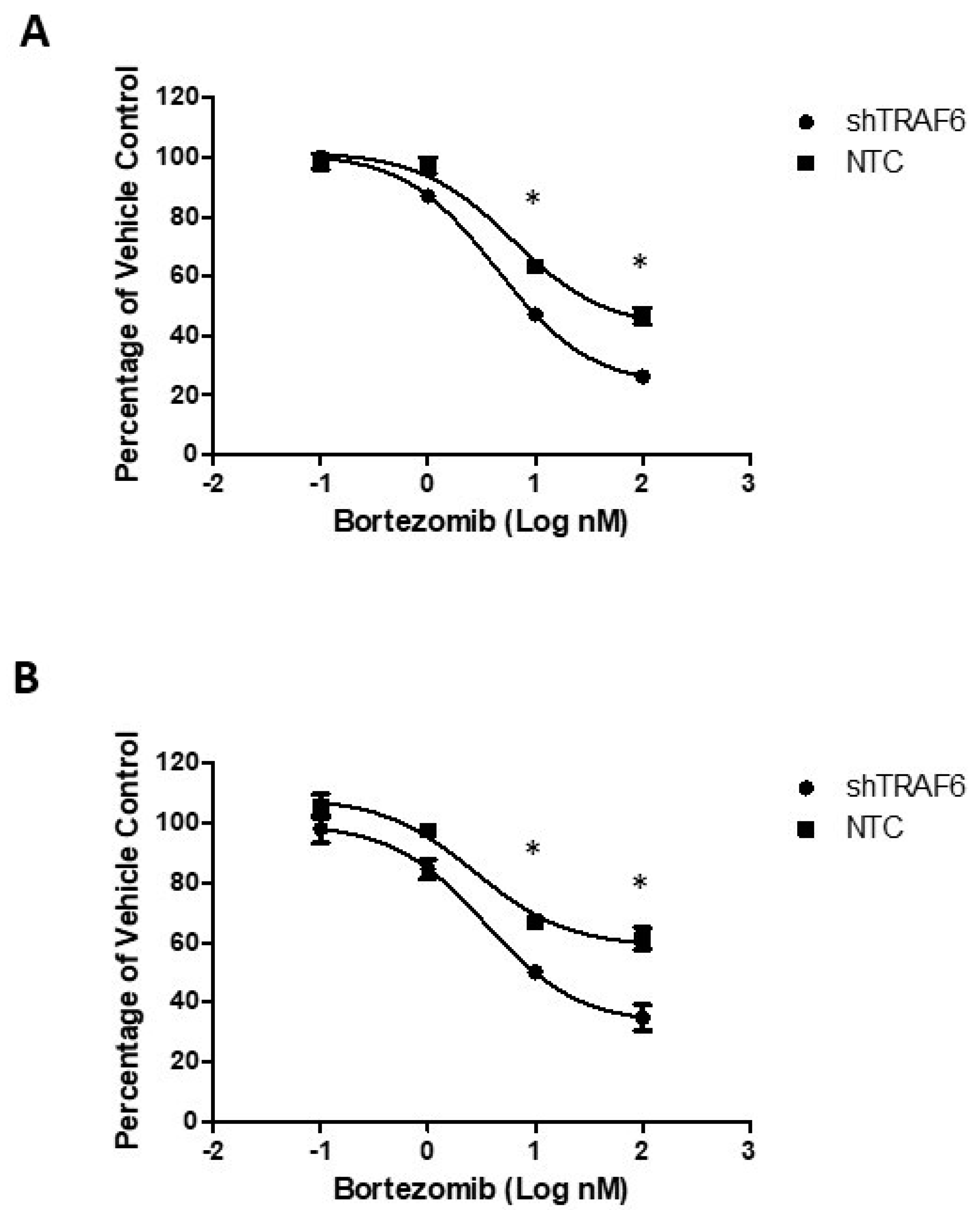
3.4. TRAF6 Knockdown Augments the Effect of Bortezomib in Stromal Co-Cultures
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BM | Bone marrow |
| BMSC | Bone marrow stromal cell |
| ICAM1 | Intracellular adhesion molecule 1 |
| MM | Multiple Myeloma |
| NFκB | Nuclear factor kappa B |
| TRAF6 | Tumour necrosis factor receptor-associated factor 6 |
| VCAM1 | Vascular adhesion molecule 1 |
References
- Guerrero-Garcia, T.A.; Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Mitsiades, C.; Anderson, K.C.; Richardson, P.G. The Power of Proteasome Inhibition in Multiple Myeloma. Expert Rev. Proteom. 2018, 15, 1033–1052. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory Drugs in Multiple Myeloma: Mechanisms of Action and Clinical Experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Maglio, M.; Ghobrial, I.M.; Richardson, P.G. Current use of Monoclonal Antibodies in the Treatment of Multiple Myeloma. Br. J. Haematol. 2018, 181, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Guang, M.H.Z.; McCann, A.; Bianchi, G.; Zhang, L.; Dowling, P.; Bazou, D.; O’Gorman, P.; Anderson, K.C. Overcoming Multiple Myeloma Drug Resistance in the Era of Cancer ‘Omics’. Leuk. Lymphoma 2018, 59, 542–561. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug Resistance in Multiple Myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Chen, B.P.; Chen, S.; Pinkus, G.S.; Bronson, R.T.; Dedera, D.A.; Hoshi, Y.; Teoh, G.; Ogata, A.; Treon, S.P.; et al. The Development of a Model for the Homing of Multiple Myeloma Cells to Human Bone Marrow. Blood 1997, 90, 754–765. [Google Scholar] [PubMed]
- Chauhan, D.; Uchiyama, H.; Akbarali, Y.; Urashima, M.; Yamamoto, K.; Libermann, T.A.; Anderson, K.C. Multiple Myeloma Cell Adhesion-Induced Interleukin-6 Expression in Bone Marrow Stromal Cells Involves Activation of NF-Kappa B. Blood 1996, 87, 1104–1112. [Google Scholar]
- Michigami, T.; Shimizu, N.; Williams, P.J.; Niewolna, M.; Dallas, S.L.; Mundy, G.R.; Yoneda, T. Cell-Cell Contact between Marrow Stromal Cells and Myeloma Cells Via VCAM-1 and Alpha(4)Beta(1)-Integrin Enhances Production of Osteoclast-Stimulating Activity. Blood 2000, 96, 1953–1960. [Google Scholar]
- Sezer, O.; Heider, U.; Zavrski, I.; Kuhne, C.A.; Hofbauer, L.C. RANK Ligand and Osteoprotegerin in Myeloma Bone Disease. Blood 2003, 101, 2094–2098. [Google Scholar] [CrossRef]
- Inoue, J.; Gohda, J.; Akiyama, T. Characteristics and Biological Functions of TRAF6. Adv. Exp. Med. Biol. 2007, 597, 72–79. [Google Scholar]
- Deng, L.; Wang, C.; Spencer, E.; Yang, L.; Braun, A.; You, J.; Slaughter, C.; Pickart, C.; Chen, Z.J. Activation of the IkappaB Kinase Complex by TRAF6 Requires a Dimeric Ubiquitin-Conjugating Enzyme Complex and a Unique Polyubiquitin Chain. Cell 2000, 103, 351–361. [Google Scholar] [CrossRef]
- Fang, J.; Muto, T.; Kleppe, M.; Bolanos, L.C.; Hueneman, K.M.; Walker, C.S.; Sampson, L.; Wellendorf, A.M.; Chetal, K.; Choi, K.; et al. TRAF6 Mediates Basal Activation of NF-kappaB Necessary for Hematopoietic Stem Cell Homeostasis. Cell Rep. 2018, 22, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, H.; Fu, X.; Li, Y.; Wu, Z. TRAF6 Promotes Myogenic Differentiation Via the TAK1/p38 Mitogen-Activated Protein Kinase and Akt Pathways. PLoS ONE 2012, 7, e34081. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, A.; Song, J.; Thakur, N.; Itoh, S.; Marcusson, A.; Bergh, A.; Heldin, C.H.; Landstrom, M. TGF-Beta Promotes PI3K-AKT Signaling and Prostate Cancer Cell Migration through the TRAF6-Mediated Ubiquitylation of p85alpha. Sci. Signal. 2017, 10, eaal4186. [Google Scholar] [CrossRef] [PubMed]
- Hostager, B.S. Roles of TRAF6 in CD40 Signaling. Immunol. Res. 2007, 39, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Xiong, J.; Takeuchi, M.; Kurama, T.; Goeddel, D.V. TRAF6 is a Signal Transducer for Interleukin-1. Nature 1996, 383, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Cheng, L.; Ren, Y.; Li, Z.; Li, Y.; Li, X.; Li, H.; Fu, X.Y.; Chang, Z. Interleukin-17F Signaling Requires Ubiquitination of Interleukin-17 Receptor Via TRAF6. Cell. Signal. 2007, 19, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, J.; Takaesu, G.; Akatsuka, H.; Sakurai, H.; Ninomiya-Tsuji, J.; Matsumoto, K.; Sakurai, N. Receptor Activator of NF-kappaB Ligand (RANKL) Activates TAK1 Mitogen-Activated Protein Kinase Kinase Kinase through a Signaling Complex Containing RANK, TAB2, and TRAF6. Mol. Cell. Biol. 2002, 22, 992–1000. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Sanchez, E.; Wang, C.S.; Lee, T.; Soof, C.M.; Casas, C.E.; Cao, J.; Xie, C.; Udd, K.A.; et al. Combined TRAF6 Targeting and Proteasome Blockade has Anti-Myeloma and Anti-Bone Resorptive Effects. Mol. Cancer. Res. 2017, 15, 598–609. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Campbell, R.A.; Burkhardt, K.; Zhu, D.; Li, S.G.; Lee, H.J.; Wang, C.; Zeng, Z.; Gordon, M.S.; et al. Interference with Nuclear Factor Kappa B and c-Jun NH2-Terminal Kinase Signaling by TRAF6C Small Interfering RNA Inhibits Myeloma Cell Proliferation and Enhances Apoptosis. Oncogene 2006, 25, 6520–6527. [Google Scholar] [CrossRef]
- Crawford, L.J.; Anderson, G.; Johnston, C.K.; Irvine, A.E. Identification of the APC/C Co-Factor FZR1 as a Novel Therapeutic Target for Multiple Myeloma. Oncotarget 2016, 7, 70481–70493. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sun, Z.; Wang, X.; Liu, X.; Na, W.; Xu, R.; Ding, R.; Liu, H. The Effect of Marrow Stromal Cells on TRAF6 Expression Levels in Myeloma Cells. Oncol. Lett. 2017, 14, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Treon, S.P.; Shima, Y.; Hideshima, T.; Podar, K.; Tai, Y.T.; Lin, B.; Lentzsch, S.; Davies, F.E.; Chauhan, D.; et al. Adherence of Multiple Myeloma Cells to Bone Marrow Stromal Cells Upregulates Vascular Endothelial Growth Factor Secretion: Therapeutic Applications. Leukemia 2001, 15, 1950–1961. [Google Scholar] [CrossRef] [PubMed]
- Misund, K.; Baranowska, K.A.; Holien, T.; Rampa, C.; Klein, D.C.; Borset, M.; Waage, A.; Sundan, A. A Method for Measurement of Drug Sensitivity of Myeloma Cells Co-Cultured with Bone Marrow Stromal Cells. J. Biomol. Screen. 2013, 18, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Sarkar, U.A.; Basak, S. The NF-kappaB Activating Pathways in Multiple Myeloma. Biomedicines 2018, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Podar, K.; Richardson, P.G.; Hideshima, T.; Chauhan, D.; Anderson, K.C. The Malignant Clone and the Bone-Marrow Environment. Best Pract. Res. Clin. Haematol. 2007, 20, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-kappaB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Migkou, M.; Christoulas, D.; Gavriatopoulou, M.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Iakovaki, M.; Panagiotidis, I.; Ziogas, D.C.; Fotiou, D.; et al. Increased Circulating VCAM-1 Correlates with Advanced Disease and Poor Survival in Patients with Multiple Myeloma: Reduction by Post-Bortezomib and Lenalidomide Treatment. Blood Cancer. J. 2016, 6, e428. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Rhyasen, G.; Bolanos, L.; Rasch, C.; Varney, M.; Wunderlich, M.; Goyama, S.; Jansen, G.; Cloos, J.; Rigolino, C.; et al. Cytotoxic Effects of Bortezomib in Myelodysplastic syndrome/acute Myeloid Leukemia Depend on Autophagy-Mediated Lysosomal Degradation of TRAF6 and Repression of PSMA1. Blood 2012, 120, 858–867. [Google Scholar] [CrossRef]
- Murray, M.Y.; Auger, M.J.; Bowles, K.M. Overcoming Bortezomib Resistance in Multiple Myeloma. Biochem. Soc. Trans. 2014, 42, 804–808. [Google Scholar] [CrossRef]
- Manni, S.; Carrino, M.; Semenzato, G.; Piazza, F. Old and Young Actors Playing Novel Roles in the Drama of Multiple Myeloma Bone Marrow Microenvironment Dependent Drug Resistance. Int. J. Mol. Sci. 2018, 19, 1512. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, L.; Desantis, V.; Solimando, A.G.; Ruggieri, S.; Annese, T.; Nico, B.; Fumarulo, R.; Vacca, A.; Frassanito, M.A. Microenvironment Drug Resistance in Multiple Myeloma: Emerging New Players. Oncotarget 2016, 7, 60698–60711. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, J.J.; McAvera, R.M.; Crawford, L.J. TRAF6 Silencing Attenuates Multiple Myeloma Cell Adhesion to Bone Marrow Stromal Cells. Int. J. Mol. Sci. 2019, 20, 702. https://doi.org/10.3390/ijms20030702
Morgan JJ, McAvera RM, Crawford LJ. TRAF6 Silencing Attenuates Multiple Myeloma Cell Adhesion to Bone Marrow Stromal Cells. International Journal of Molecular Sciences. 2019; 20(3):702. https://doi.org/10.3390/ijms20030702
Chicago/Turabian StyleMorgan, Jonathan J., Roisin M. McAvera, and Lisa J. Crawford. 2019. "TRAF6 Silencing Attenuates Multiple Myeloma Cell Adhesion to Bone Marrow Stromal Cells" International Journal of Molecular Sciences 20, no. 3: 702. https://doi.org/10.3390/ijms20030702
APA StyleMorgan, J. J., McAvera, R. M., & Crawford, L. J. (2019). TRAF6 Silencing Attenuates Multiple Myeloma Cell Adhesion to Bone Marrow Stromal Cells. International Journal of Molecular Sciences, 20(3), 702. https://doi.org/10.3390/ijms20030702