Accessibility of Cations to the Selectivity Filter of KcsA in the Inactivated State: An Equilibrium Binding Study
Abstract
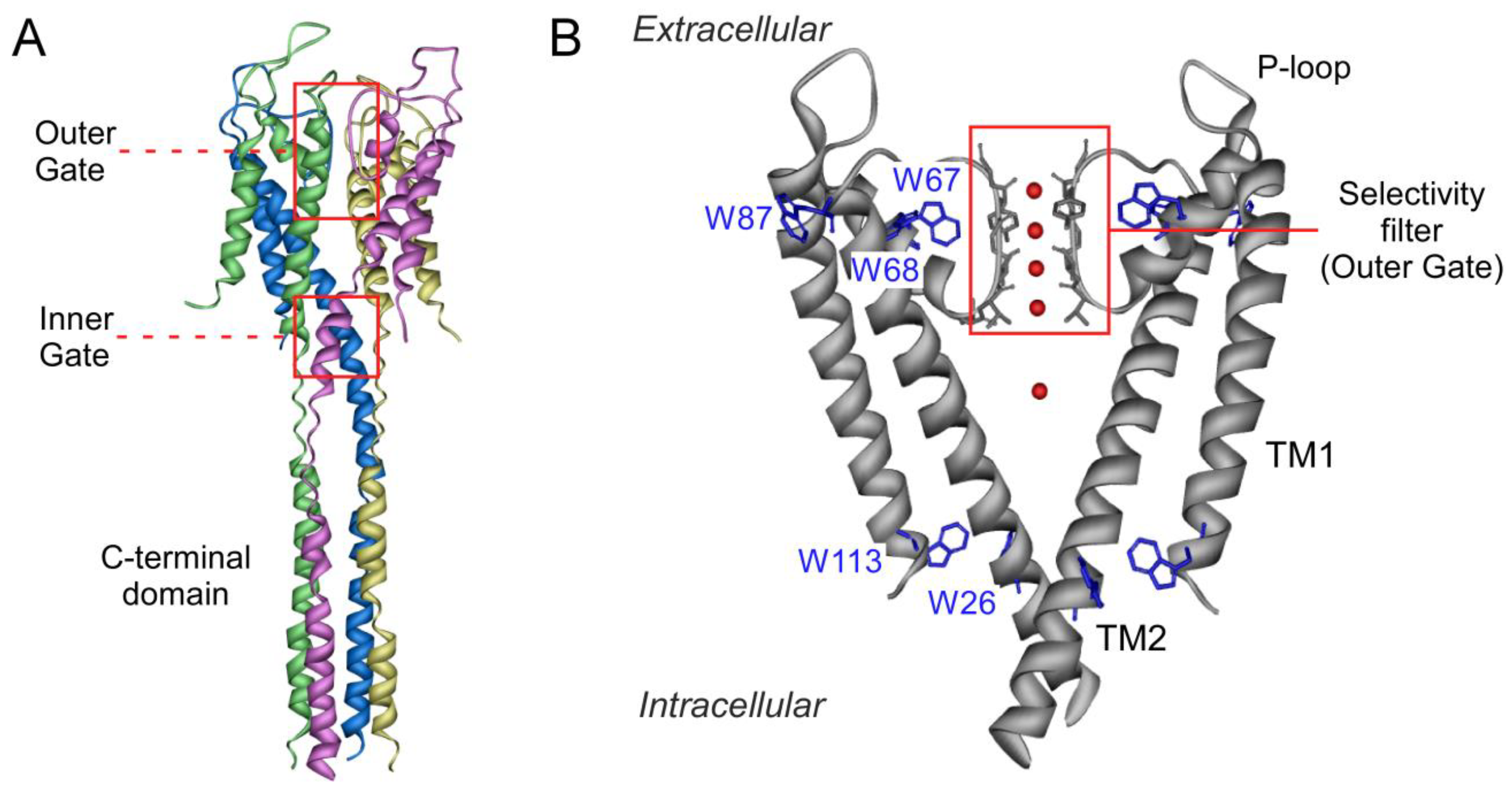
1. Introduction
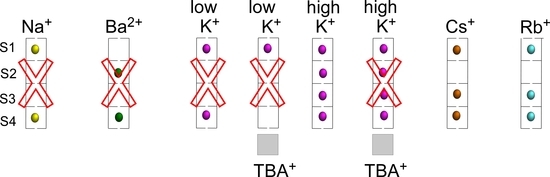
2. Results
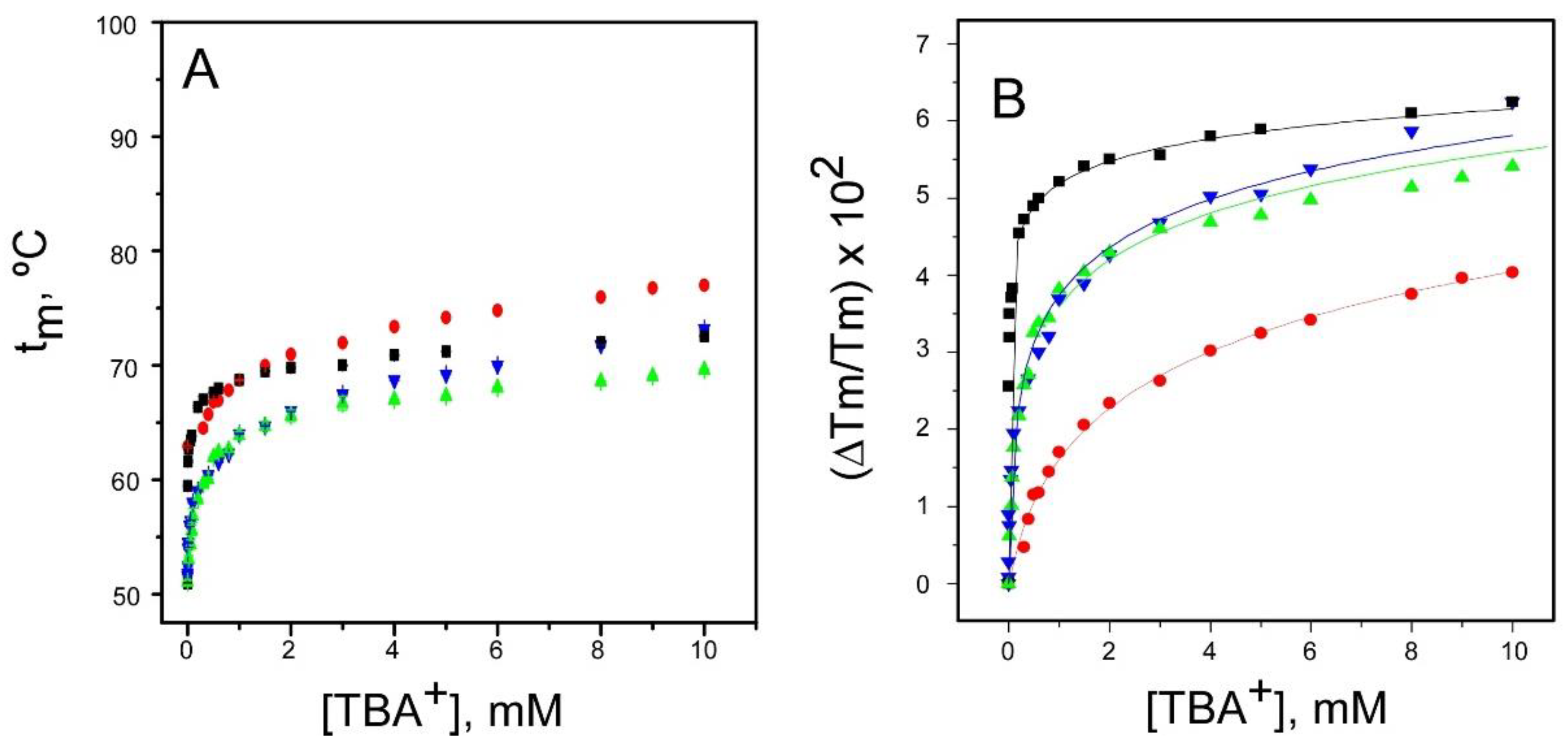
2.1. Binding of TBA+
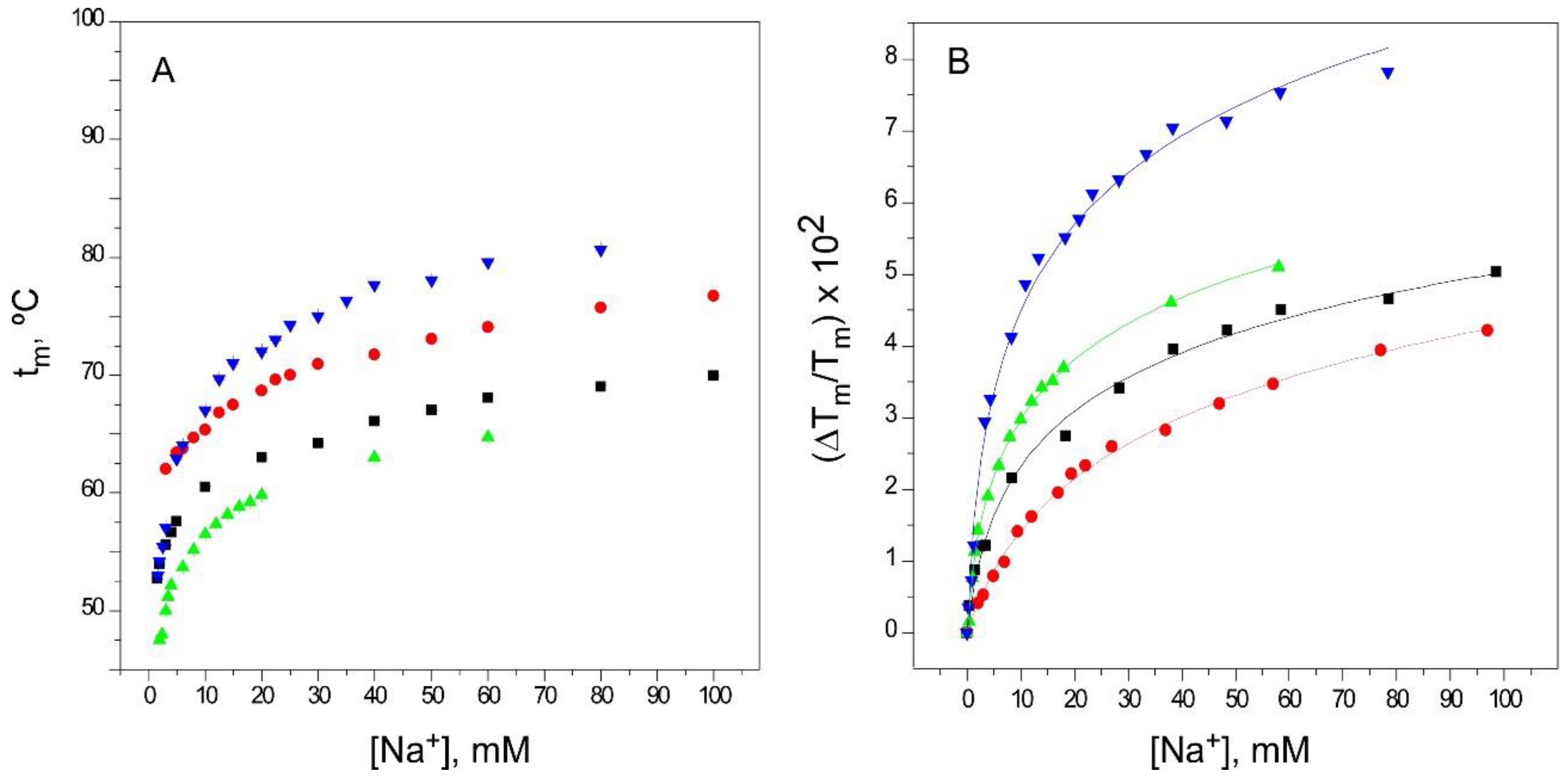
2.2. Binding of Na+
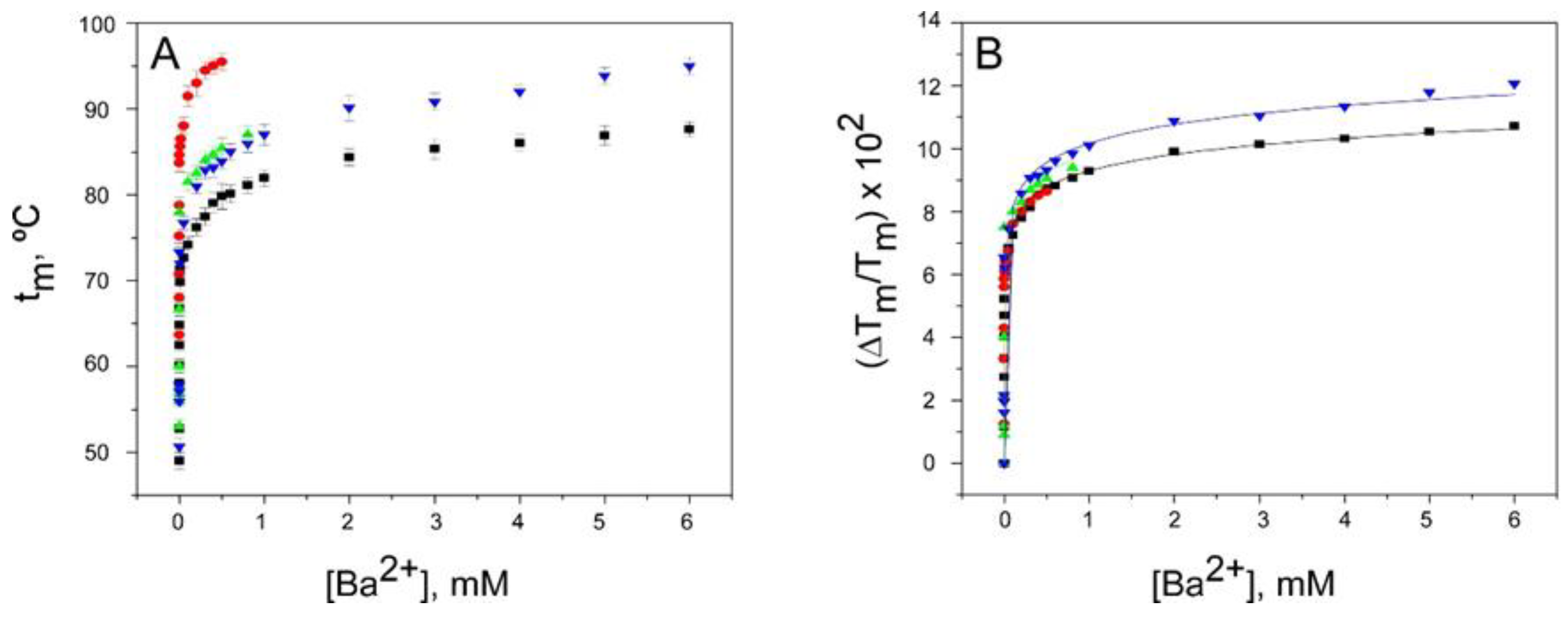
2.3. Binding of Ba2+
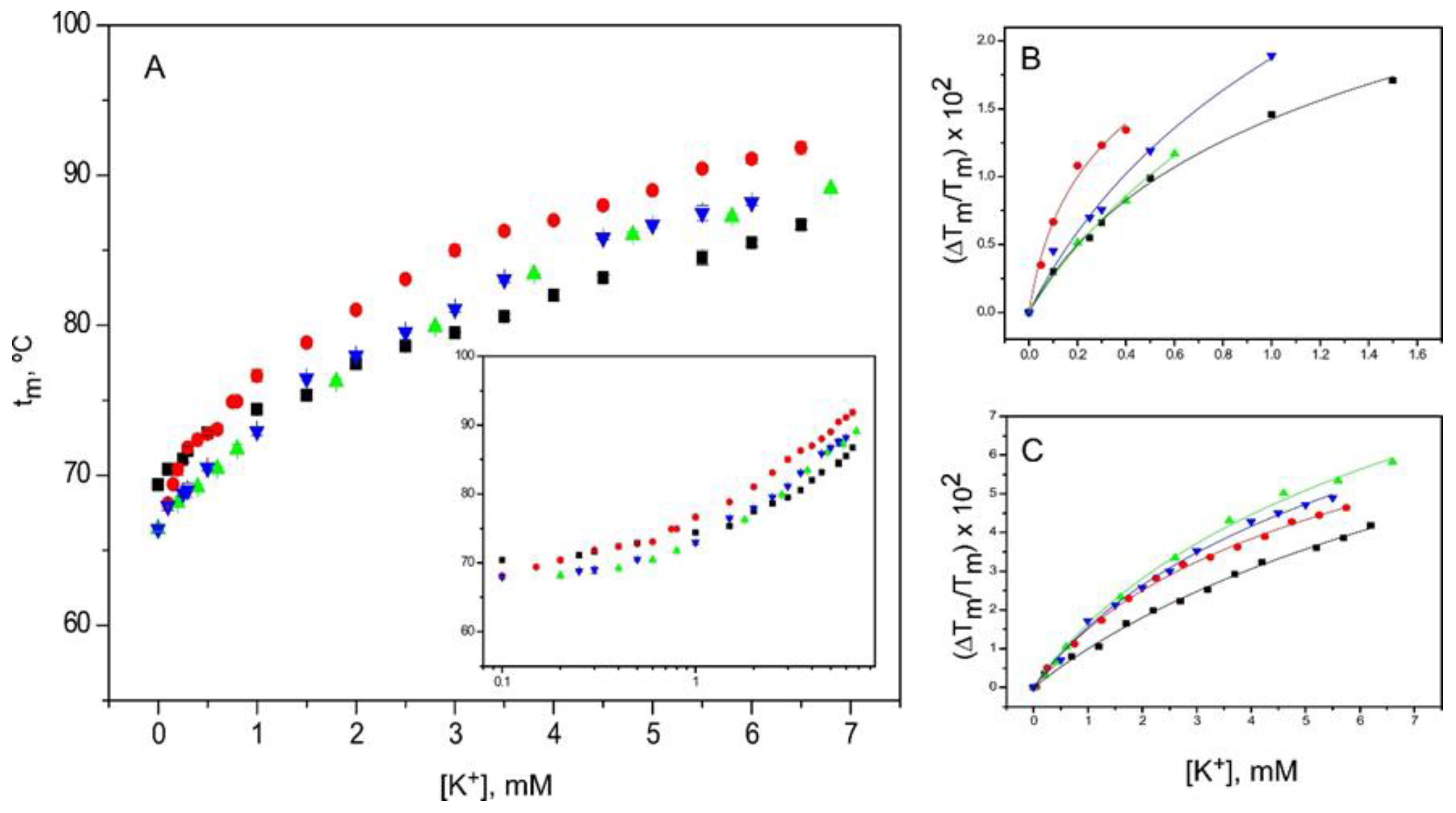
2.4. Binding of K+
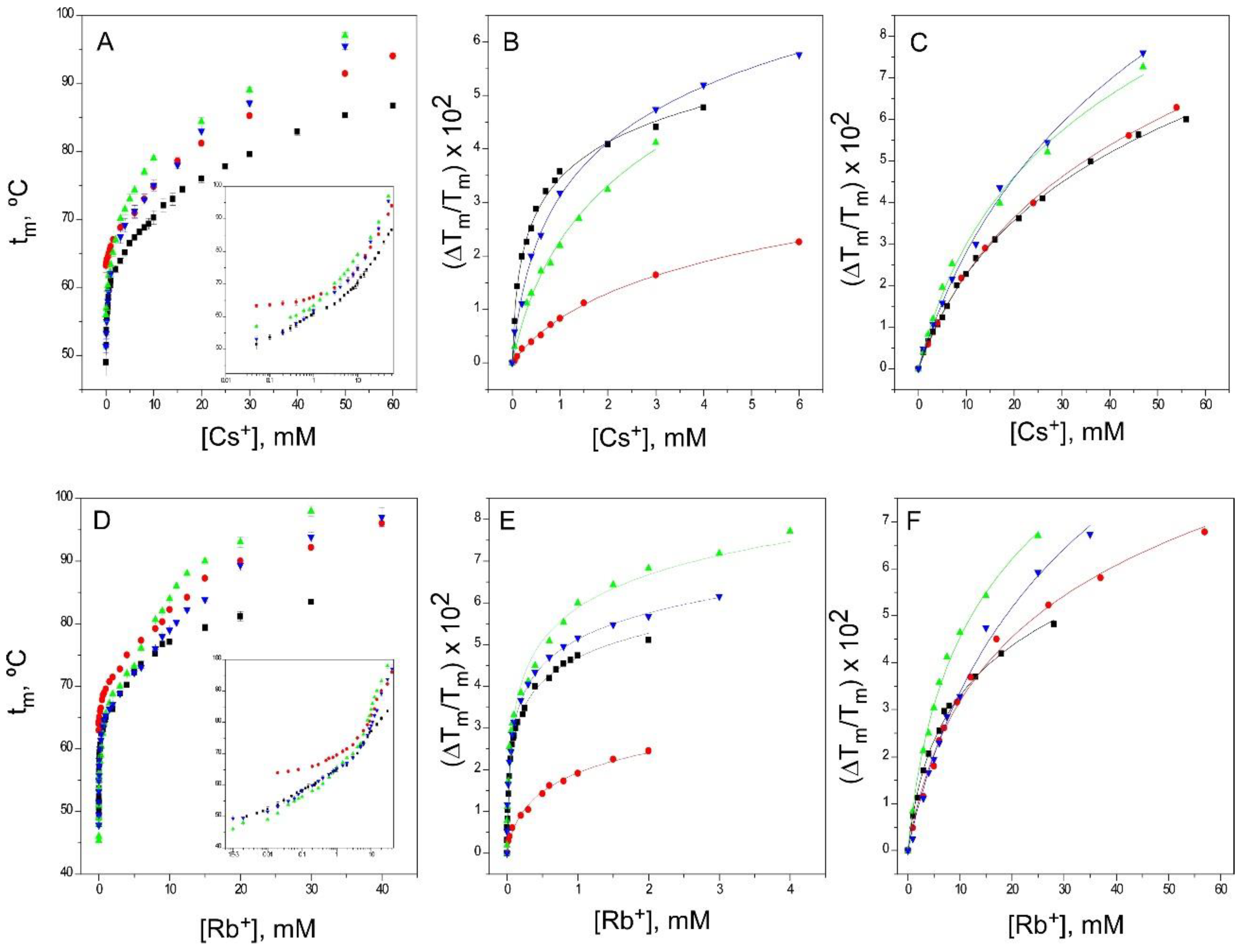
2.5. Binding of Rb+ and Cs+
2.6. Effects of an Acidic pH on the Binding of Cations to the Inactivated Channel Models
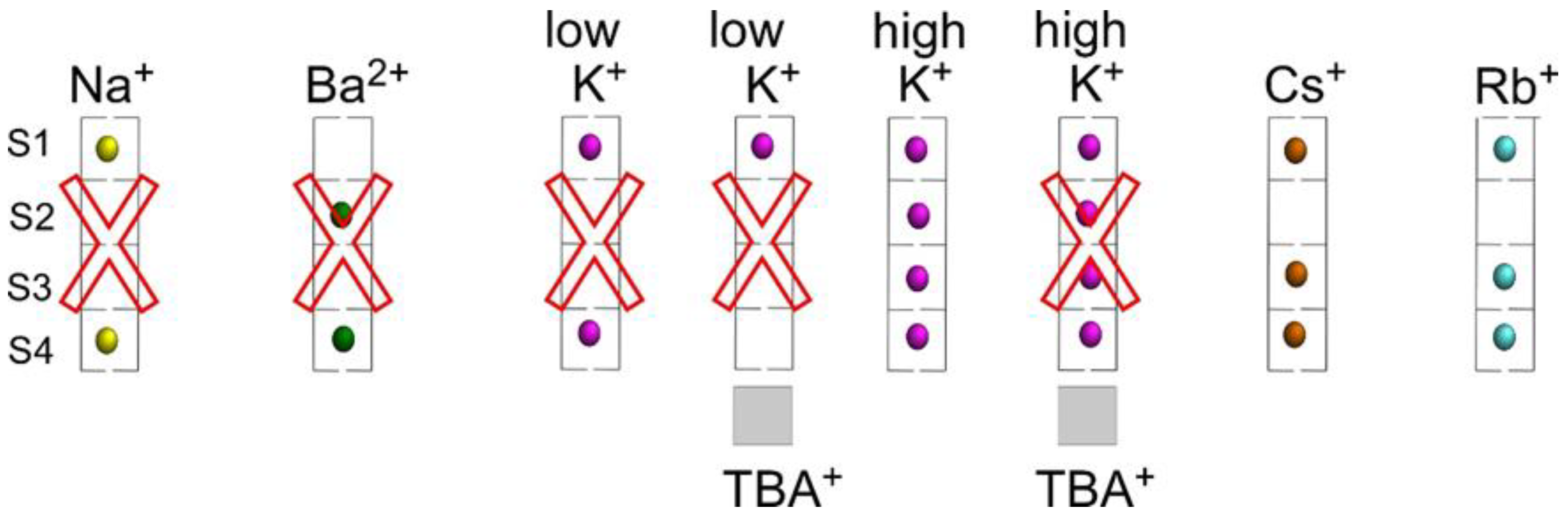
3. Discussion
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. Fluorescence Monitoring of Cation Binding
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| KcsA | potassium channel from Streptomyces lividans |
| 1–125 KcsA | KcsA deletion that lacks the cytoplasmic 125 to 136 residues |
| OPEN | H25R, R117Q, E120Q, R121Q, R122Q and H124Q sextuple mutant channel |
| DDM | dodecyl β-d-maltoside |
| TM | transmembrane segment |
| SDS–PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| tm | midpoint denaturation temperature (in degrees Celsius) |
| Tm | midpoint denaturation temperature (in kelvins) |
| ΔH0 | enthalpy change upon protein unfolding in the absence of ligand |
| KD | dissociation constant |
| TBA+ | tetrabutylammonium cation |
References
- Hille, B. Ion Channels of Excitable Membranes; Sinauer Assoc. Inc.: Sunderland, MA, USA, 2001; p. 5. ISBN 0-87893-321-2. [Google Scholar]
- Doyle, D.A. The Structure of the Potassium Channel: Molecular Basis of K+ Conduction and Selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Vasquez, V.; Tereshko, V.; Esaki, K.; Fellouse, F.A.; Sidhu, S.S.; Koide, S.; Perozo, E.; Kossiakoff, A. Crystal structure of full-length KcsA in its closed conformation. Proc. Natl. Acad. Sci. USA 2009, 106, 6644–6649. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Morais-Cabral, J.H.; Kaufman, A.; Mackinnon, R. Chemistry of ion coordination and hydration revealed by a K+channel-Fab complex at 2.0 Å resolution. Nature 2001, 414, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.W.L.; McCoy, J.G.; Thompson, A.N.; Nichols, C.G.; Nimigean, C.M. Mechanism for selectivity-inactivation coupling in KcsA potassium channels. Proc. Natl. Acad. Sci. USA 2011. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Morales, J.F.; Cuello, L.G.; Zhao, Y.; Jogini, V.; Cortes, D.M.; Roux, B.; Perozo, E. Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 2006, 13, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Morais-Cabral, J.H.; Zhou, Y.; MacKinnon, R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature 2001, 414, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; MacKinnon, R. The occupancy of ions in the K+selectivity filter: Charge balance and coupling of ion binding to a protein conformational change underlie high conduction rates. J. Mol. Biol. 2003, 333, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Ader, C.; Schneider, R.; Hornig, S.; Velisetty, P.; Vardanyan, V.; Giller, K.; Ohmert, I.; Becker, S.; Pongs, O.; Baldus, M. Coupling of activation and inactivation gate in a K-channel: Potassium and ligand sensitivity. EMBO J. 2009, 28, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.A.; Tzitzilonis, C.; Kwiatkowski, W.; Choe, S.; Riek, R. Conformational dynamics of the KcsA potassium channel governs gating properties. Nat. Struct. Mol. Biol. 2007, 14, 1089. [Google Scholar] [CrossRef]
- Bhate, M.P.; Wylie, B.J.; Tian, L.; McDermott, A.E. Conformational dynamics in the selectivity filter of KcsA in response to potassium ion concentration. J. Mol. Biol. 2010, 401, 155–166. [Google Scholar] [CrossRef]
- Imai, S.; Osawa, M.; Takeuchi, K.; Shimada, I. Structural basis underlying the dual gate properties of KcsA. Proc. Natl. Acad. Sci. USA 2010, 107, 6216–6221. [Google Scholar] [CrossRef] [PubMed]
- Wylie, B.J.; Bhate, M.P.; McDermott, A.E. Transmembrane allosteric coupling of the gates in a potassium channel. Proc. Natl. Acad. Sci. USA 2014, 111, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Chill, J.H. NMR study of the tetrameric KcsA potassium channel in detergent micelles. Protein Sci. 2006, 15, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Lockless, S.W.; Zhou, M.; MacKinnon, R. Structural and thermodynamic properties of selective ion binding in a K+ channel. PLoS Biol. 2007, 5, e121. [Google Scholar] [CrossRef] [PubMed]
- Cuello, L.G.; Cortes, D.M.; Jogini, V.; Sompornpisut, A.; Perozo, E. A molecular mechanism for proton-dependent gating in KcsA. FEBS Lett. 2010, 584, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Onishi, Y.; Yanagida, T.; Ide, T. Role of the KcsA channel cytoplasmic domain in pH-dependent gating. Biophys. J. 2011, 101, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Posson, D.J.; Thompson, A.N.; McCoy, J.G.; Nimigean, C.M. Molecular interactions involved in proton-dependent gating in KcsA potassium channels. J. Gen. Physiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Mi, X.; Paajanen, V.; Wang, K.; Fan, Z. Activation-coupled inactivation in the bacterial potassium channel KcsA. Proc. Natl. Acad. Sci. USA 2005, 102, 17630–17635. [Google Scholar] [CrossRef]
- Hoshi, T.; Zagotta, W.N.; Aldrich, R.W. Two types of inactivation in Shaker K+channels: Effects of alterations in the carboxy-terminal region. Neuron 1991, 7, 547–556. [Google Scholar] [CrossRef]
- Liu, Y.; Jurman, M.E.; Yellen, G. Dynamic rearrangement of the outer mouth of a K+channel during gating. Neuron 1996, 16, 859–867. [Google Scholar] [CrossRef]
- Li, J.; Ostmeyer, J.; Cuello, L.G.; Perozo, E.; Roux, B. Rapid constriction of the selectivity filter underlies C-type inactivation in the KcsA potassium channel. J. Gen. Physiol. 2018, 150, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Cuello, L.G.; Cortes, D.M.; Perozo, E. The gating cycle of a K+ channel at atomic resolution. eLife 2017, 6, e28032. [Google Scholar] [CrossRef] [PubMed]
- Cuello, L.G.; Jogini, V.; Cortes, D.M.; Perozo, E. Structural mechanism of C-type inactivation in K+ channels. Nature 2010, 466, 203. [Google Scholar] [CrossRef]
- Devaraneni, P.K.; Komarov, A.G.; Costantino, C.A.; Devereaux, J.J.; Matulef, K.; Valiyaveetil, F.I. Semisynthetic K+ channels show that the constricted conformation of the selectivity filter is not the C-type inactivated state. Proc. Natl. Acad. Sci. USA 2013, 110, 15698–15703. [Google Scholar] [CrossRef] [PubMed]
- LeMasurier, M.; Heginbotham, L.; Miller, C. KcsA: It’s a potassium channel. J. Gen. Physiol. 2001, 118, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, S.; Cordero-Morales, J.F.; Perozo, E. A quantitative description of KcsA gating I: Macroscopic currents. J. Gen. Physiol. 2007, 130, 465–478. [Google Scholar] [CrossRef]
- Liu, S.; Focke, P.J.; Matulef, K.; Bian, X.; Moënne-Loccoz, P.; Valiyaveetil, F.I.; Lockless, S.W. Ion-binding properties of a K + channel selectivity filter in different conformations. Proc. Natl. Acad. Sci. USA 2015, 112, 15096–15100. [Google Scholar] [CrossRef]
- Thompson, A.N.; Posson, D.J.; Parsa, P.V.; Nimigean, C.M. Molecular mechanism of pH sensing in KcsA potassium channels. Proc. Natl. Acad. Sci. USA 2008, 105, 6900–6905. [Google Scholar] [CrossRef]
- Renart, M.L.; Triano, I.; Poveda, J.A.; Encinar, J.A.; Fernández, A.M.; Ferrer-Montiel, A.V.; Gómez, J.; González Ros, J.M. Ion binding to KcsA: Implications in ion selectivity and channel gating. Biochemistry 2010, 49, 9480–9487. [Google Scholar] [CrossRef]
- Triano, I.; Barrera, F.N.; Renart, M.L.; Molina, M.L.; Fernandez-Ballester, G.; Poveda, J.A.; Fernandez, A.M.; Encinar, J.A.; Ferrer-Montiel, A.V.; Otzen, D.; et al. Occupancy of nonannular lipid binding sites on KcsA greatly increases the stability of the tetrameric protein. Biochemistry 2010, 49, 5397–5404. [Google Scholar] [CrossRef]
- Montoya, E.; Lourdes Renart, M.; Marcela Giudici, A.; Poveda, J.A.; Fernández, A.M.; Morales, A.; González-Ros, J.M. Differential binding of monovalent cations to KcsA: Deciphering the mechanisms of potassium channel selectivity. Biochim. Biophys. Acta Biomembr. 2017, 1859, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Renart, M.L.; Montoya, E.; Giudici, A.M.; Poveda, J.A.; Fernández, A.M.; Morales, A.; González-Ros, J.M. Selective exclusion and selective binding both contribute to ion selectivity in KcsA, a model potassium channel. J. Biol. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Yohannan, S.; Hu, Y.; Zhou, Y. Crystallographic Study of the Tetrabutylammonium Block to the KcsA K+Channel. J. Mol. Biol. 2007, 366, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Cuello, L.G.; Jogini, V.; Cortes, D.M.; Pan, A.C.; Gagnon, D.G.; Dalmas, O.; Cordero-Morales, J.F.; Chakrapani, S.; Roux, B.; Perozo, E. Structural basis for the coupling between activation and inactivation gates in K+channels. Nature 2010, 466, 272. [Google Scholar] [CrossRef] [PubMed]
- Kratochvil, H.T.; Maj, M.; Matulef, K.; Annen, A.W.; Ostmeyer, J.; Perozo, E.; Roux, B.; Valiyaveetil, F.I.; Zanni, M.T. Probing the Effects of Gating on the Ion Occupancy of the K+ Channel Selectivity Filter Using Two-Dimensional Infrared Spectroscopy. J. Am. Chem. Soc. 2017, 139, 8837–8845. [Google Scholar] [CrossRef] [PubMed]
- Matulef, K.; Annen, A.W.; Nix, J.C.; Valiyaveetil, F.I. Individual Ion Binding Sites in the K+ Channel Play Distinct Roles in C-type Inactivation and in Recovery from Inactivation. Structure 2016, 24, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Baukrowitz, T.; Yellen, G. Modulation of K+current by frequency and external [K+]: A tale of two inactivation mechanisms. Neuron 1995, 15, 951–960. [Google Scholar] [CrossRef]
- Xu, Y.; Bhate, M.P.; McDermott, A.E. Transmembrane allosteric energetics characterization for strong coupling between proton and potassium ion binding in the KcsA channel. Proc. Natl. Acad. Sci. USA 2017, 14, 8788–8793. [Google Scholar] [CrossRef]
- López-Barneo, J.; Hoshi, T.; Heinemann, S.H.; Aldrich, R.W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Recept. Channels 1993, 1, 61–71. [Google Scholar]
- Hoshi, T.; Zagotta, W.N.; Aldrich, R.W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 1990, 250, 533–538. [Google Scholar] [CrossRef]
- Fedida, D.; Maruoka, N.D.; Lin, S. Modulation of slow inactivation in human cardiac Kv1.5 channels by extra- and intracellular permeant cations. J. Physiol. 1999, 515, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, R.L.; Morales, M.J.; Castellino, R.C.; Zhang, Y.; Campbell, D.L.; Strauss, H.C. C-type inactivation controls recovery in a fast inactivating cardiac K+ channel (Kv1.4) expressed in Xenopus oocytes. J. Physiol. 1995, 489, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, R.L.; Morales, M.J.; Wang, S.; Liu, S.; Campbell, D.L.; Brahmajothi, M.V.; Strauss, H.C. Inactivation of voltage-gated cardiac K+ channels. Circ. Res. 1998, 82, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Swenson, R.P.; Armstrong, C.M. K+channels close more slowly in the presence of external K+ and Rb+. Nature 1981, 291, 427. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Syeda, R.; Montal, M. Stabilization of the conductive conformation of a voltage-gated K + (Kv) channel: The lid mechanism. J. Biol. Chem. 2013, 288, 16619–16628. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, S.; Cordero-Morales, J.F.; Perozo, E. A quantitative description of KcsA gating II: Single-channel currents. J. Gen. Physiol. 2007, 130, 479–496. [Google Scholar] [CrossRef]
- Chakrapani, S.; Cordero-Morales, J.F.; Jogini, V.; Pan, A.C.; Cortes, D.M.; Roux, B.; Perozo, E. On the structural basis of modal gating behavior in K(+) channels. Nat. Struct. Mol. Biol. 2011, 18, 67–74. [Google Scholar] [CrossRef]
- Liu, S.; Bian, X.; Lockless, S.W. Preferential binding of K+ ions in the selectivity filter at equilibrium explains high selectivity of K+ channels. J. Gen. Physiol. 2012, 140, 671–679. [Google Scholar] [CrossRef]
- Hirano, M.; Ide, T. Electrostatic state of the cytoplasmic domain influences inactivation at the selectivity filter of the KcsA potassium channel. Biochim. Biophys. Acta Biomembr. 2019, 1861, 220–227. [Google Scholar] [CrossRef]
- Starkus, J.G.; Kuschel, L.; Rayner, M.D.; Heinemann, S.H. Ion conduction through C-type inactivated Shaker channels. J. Gen. Physiol. 1997, 110, 539–550. [Google Scholar] [CrossRef]
- Kiss, L.; LoTurco, J.; Korn, S.J. Contribution of the selectivity filter to inactivation in potassium channels. Biophys. J. 1999, 76, 253–263. [Google Scholar] [CrossRef]
- Renart, M.L.; Montoya, E.; Fernández, A.M.; Molina, M.L.; Poveda, J.A.; Encinar, J.A.; Ayala, J.L.; Ferrer-Montiel, A.V.; Gómez, J.; Morales, A.; et al. Contribution of ion binding affinity to ion selectivity and permeation in KcsA, a model potassium channel. Biochemistry 2012, 51, 3891–3900. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.L.; Encinar, J.A.; Barrera, F.N.; Fernández-Ballester, G.; Riquelme, G.; González-Ros, J.M. Influence of C-terminal protein domains and protein-lipid interactions on tetramerization and stability of the potassium channel KcsA. Biochemistry 2004, 43, 14924–14931. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Scholtz, J.M. Measuring the Conformational Stability of a Protein. In Protein Structure: A Practical Approach; Oxford University Press Inc.: New York, NY, USA, 1997. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.L.; Giudici, A.M.; Poveda, J.A.; Fernández-Ballester, G.; Montoya, E.; Renart, M.L.; Fernández, A.M.; Encinar, J.A.; Riquelme, G.; Morales, A.; et al. Competing lipid-protein and protein-protein interactions determine clustering and gating patterns in the potassium channel from streptomyces lividans (KcsA). J. Biol. Chem. 2015. [Google Scholar] [CrossRef]
- Sehgal, P.; Otzen, D.E. Thermodynamics of unfolding of an integral membrane protein in mixed micelles. Protein Sci. 2006, 15, 890–899. [Google Scholar] [CrossRef]


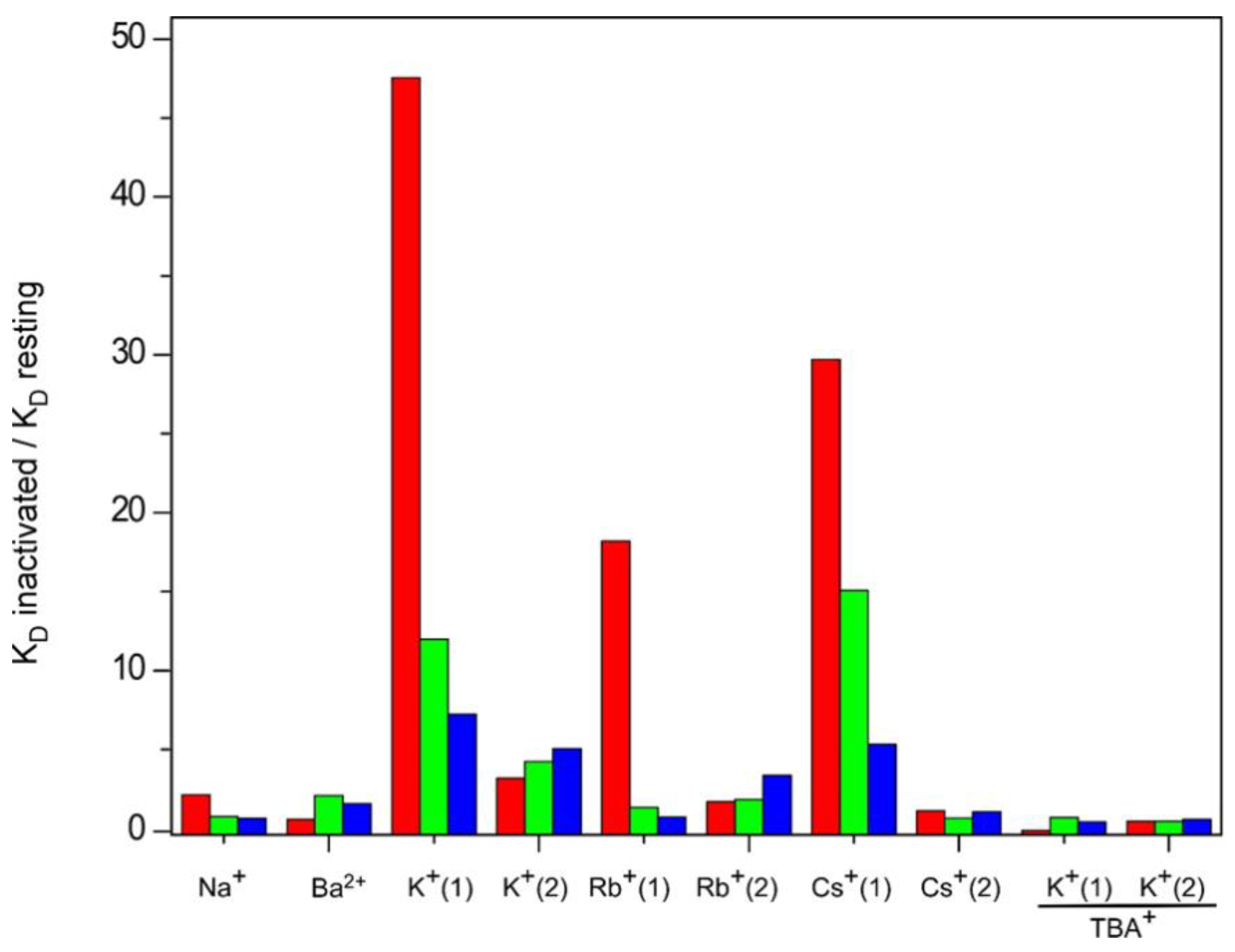
| Tested Cations | Sets of Binding Sites Detected | Resting Channel | Inactivated Channel Models | ||||||
|---|---|---|---|---|---|---|---|---|---|
| WT KcsA, pH 7 | WT KcsA, pH 4 | 1–125 KcsA | OPEN KcsA | ||||||
| KD (M) | 95% CI | KD (M) | 95% CI | KD (M) | 95% CI | KD (M) | 95% CI | ||
| Na+ | 1 | 3.3 × 10−3 | (2.5–4.3) × 10−3 | 7.7 × 10−3 | (6.7–8.9) × 10−3 a | 3.1 × 10−3 | (2.9–3.3) × 10−3 | 2.7 × 10−3 | (2.5–2.9) × 10−3 |
| Ba2+ | 1 | 4.0 × 10−9 | (2.7–6.0) × 10−9 | 3.0 × 10−9 | (1.3–6.9) × 10−9 | 9.0 × 10−9 | (3.0–27) × 10−9 | 7.0 × 10−9 | (2.9–17) × 10−9 |
| K+ | 2 | 1.9 × 10−6 | (1.2–2.9) × 10−6 | 9.0 × 10−5 | (7.4–11) × 10−5 a | 2.3 × 10−5 | (2.0–2.7) × 10−5 a | 1.4 × 10−5 | (1.1–1.8) × 10−5 a |
| 2.5 × 10−3 | (1.8–3.5) × 10−3 | 8.4 × 10−3 | (7.5–9.4) × 10−3 a | 1.1 × 10−2 | (9.3–13) × 10−3 a | 1.3 × 10−2 | (1.1–1.5) × 10−2 a | ||
| K+, 10 mM TBA | 2 | 8.0 × 10−4 | (6.4–9.9) × 10−4 b | 6.0 × 10−5 | (3.6–9.9) × 10−5 a | 7.0 × 10−4 | (4.5–11) × 10−4 b | 4.9 × 10−4 | (4.5–5.3) × 10−4 a,b |
| 3.2 × 10−3 | (2.4–4.2) × 10−3 | 2.1 × 10−3 | (1.8–2.5) × 10−3 b | 2.1 × 10−3 | (1.8–2.5) × 10−3 b | 2.4 × 10−3 | (2.1–2.8) × 10−3 | ||
| Rb+ | 2 | 4.0 × 10−6 | (3.5–4.6) × 10−6 | 7.3 × 10−5 | (7.1–7.5) × 10−5 a | 6.0 × 10−6 | (4.5–8.0) × 10−6 | 3.7 × 10−6 | (3.0–4.5) × 10−6 |
| 3.4 × 10−3 | (2.8–4.2) × 10−3 | 7.0 × 10−3 | (6.1–8.0) × 10−3 a | 7.5 × 10−3 | (6.9–8.1) × 10−3 a | 7.4 × 10−3 | (6.7–8.2) × 10−3 a | ||
| Cs+ | 2 | 3.1 × 10−5 | (2.5–3.9) × 10−5 | 9.2 × 10−4 | (8.8–9.6) × 10−4 a | 4.7 × 10−4 | (3.5–6.3) × 10−4 a | 1.7 × 10−4 | (1.4–2.1) × 10−4 a |
| 1.3 × 10−2 | (1.1–1.5) × 10−2 | 1.7 × 10−2 | (1.5–1.9) × 10−2 a | 1.1 × 10−2 | (9.3–13) × 10−3 | 1.6 × 10−2 | (1.3–2.0) × 10−2 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giudici, A.M.; Renart, M.L.; Díaz-García, C.; Morales, A.; Poveda, J.A.; González-Ros, J.M. Accessibility of Cations to the Selectivity Filter of KcsA in the Inactivated State: An Equilibrium Binding Study. Int. J. Mol. Sci. 2019, 20, 689. https://doi.org/10.3390/ijms20030689
Giudici AM, Renart ML, Díaz-García C, Morales A, Poveda JA, González-Ros JM. Accessibility of Cations to the Selectivity Filter of KcsA in the Inactivated State: An Equilibrium Binding Study. International Journal of Molecular Sciences. 2019; 20(3):689. https://doi.org/10.3390/ijms20030689
Chicago/Turabian StyleGiudici, Ana Marcela, Maria Lourdes Renart, Clara Díaz-García, Andrés Morales, José Antonio Poveda, and José Manuel González-Ros. 2019. "Accessibility of Cations to the Selectivity Filter of KcsA in the Inactivated State: An Equilibrium Binding Study" International Journal of Molecular Sciences 20, no. 3: 689. https://doi.org/10.3390/ijms20030689
APA StyleGiudici, A. M., Renart, M. L., Díaz-García, C., Morales, A., Poveda, J. A., & González-Ros, J. M. (2019). Accessibility of Cations to the Selectivity Filter of KcsA in the Inactivated State: An Equilibrium Binding Study. International Journal of Molecular Sciences, 20(3), 689. https://doi.org/10.3390/ijms20030689