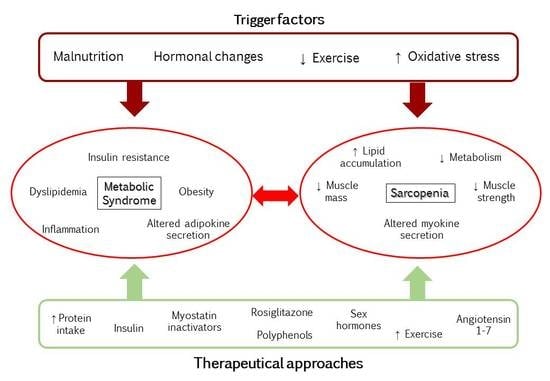
Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures
Abstract
1. Introduction
2. Metabolic Syndrome and Sarcopenia
2.1. Obesity, Inflammation, and Insulin Resistance
2.2. Cross Talk between Muscle and Adipose Tissue
3. Sarcopenia and Sexual Dimorphism
3.1. Sarcopenia in Men
3.2. Sarcopenia in Women
4. Repercussions of MetS-Related Sarcopenia in Other Diseases
5. Therapeutical Approaches
5.1. Diet
5.2. Exercise
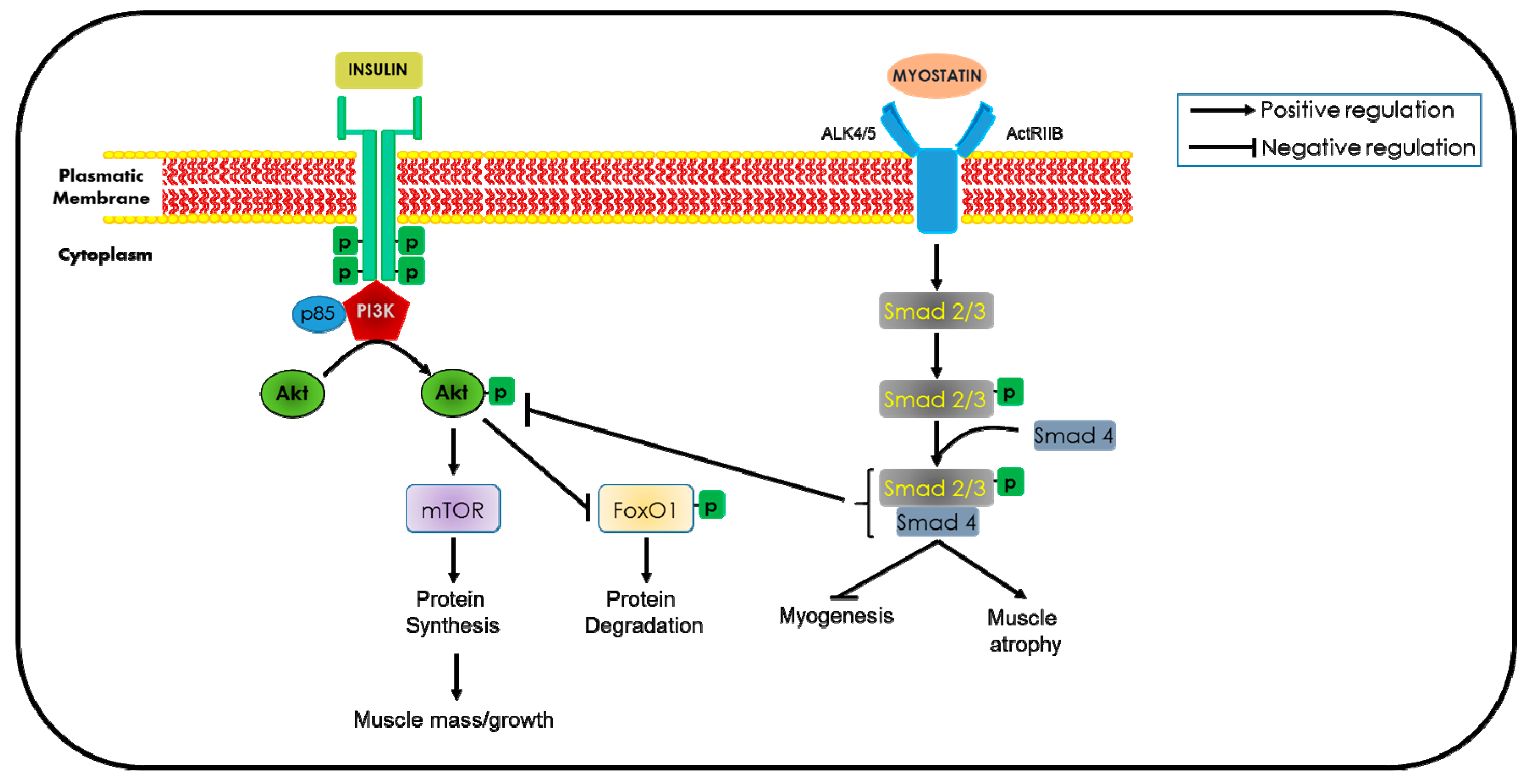
5.3. Insulin and Insulin-Like Growth Factor-1
5.4. Growth Hormone
5.5. Sex Hormones
5.5.1. Androgens
5.5.2. Estrogens
5.6. Myostatin Inactivation
5.7. Urocortins
5.8. Vitamin D
5.9. Angiotensin 1–7 and Angiotensin-Converting Enzyme Inhibitors
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakuma, K.; Yamaguchi, A. Sarcopenic obesity and endocrinal adaptation with age. Int. J. Endocrinol. 2013, 2013, 204164. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Hsiao, P.Y. Obesity in older adults: Relationship to functional limitation. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Waters, D.L.; Gallagher, D.; Morley, J.E.; Garry, P.J. Predictors of skeletal muscle mass in elderly men and women. Mech. Ageing Dev. 1999, 107, 123–136. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J. Ageing and human muscle: Observations from Sweden. Can. J. Appl. Physiol. 1993, 18, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J. Human aging, muscle mass, and fiber type composition. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50. [Google Scholar]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Di Iorio, A.; Di Renzo, D.; Paganelli, R.; Saggini, R.; Abate, G. Frailty in the elderly: The physical dimension. Eura Medicophys. 2007, 43, 407–415. [Google Scholar]
- Gomez-Cabello, A.; Rodriguez, V.; Vila-Maldonado, S.; Casajus, J.A.; Ara, I. Envejecimiento y composicion corporal: La obesidad sarcopenica en Espana. Nutr. Hosp. 2012, 27, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Sanders, K.M.; Aitken, D.; Hayes, A.; Ebeling, P.R.; Jones, G. Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle-aged and older adults. Obesity 2014, 22, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Hunter, G.R.; Livingstone, M.B. Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos. Int. 2006, 17, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.D. Muscle mass, survival, and the elderly ICU patient. Nutrition 1996, 12, 456–458. [Google Scholar] [CrossRef]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R. Sarcopenic obesity: Does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann. N. Y. Acad. Sci. 2000, 904, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Ingles, S.; Ashley, J.M.; Maxwell, M.H.; Lyons, R.F.; Elashoff, R.M. Clinical detection of sarcopenic obesity by bioelectrical impedance analysis. Am. J. Clin. Nutr. 1996, 64, 472S–477S. [Google Scholar] [CrossRef]
- Kim, T.N.; Yang, S.J.; Yoo, H.J.; Lim, K.I.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: The Koreansarcopenic obesity study. Int. J. Obes. 2009, 33, 885–892. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef]
- Zoico, E.; Di Francesco, V.; Guralnik, J.M.; Mazzali, G.; Bortolani, A.; Guariento, S.; Sergi, G.; Bosello, O.; Zamboni, M. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 234–241. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.E.; Jun, J.E.; Lee, Y.B.; Ahn, J.; Bae, J.C.; Jin, S.M.; Hur, K.Y.; Jee, J.H.; Lee, M.K.; et al. Increase in relative skeletal muscle mass over time and its inverse association with metabolic syndrome development: A 7-year retrospective cohort study. Cardiovasc. Diabetol. 2018, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, V.; Rouault, C.; Rodriguez-Cuenca, S.; Albert, V.; Edom-Vovard, F.; Vidal-Puig, A.; Clement, K.; Butler-Browne, G.S.; Lacasa, D. Human adipocytes induce inflammation and atrophy in muscle cells during obesity. Diabetes 2015, 64, 3121–3134. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V. Handbook on Metabolic Syndrome. Classification, Risk Factors and Health Impact; Lopez Garcia, C.M., Perez Gonzalez, P.A., Eds.; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2012; pp. 169–188. [Google Scholar]
- Lim, J.P.; Leung, B.P.; Ding, Y.Y.; Tay, L.; Ismail, N.H.; Yeo, A.; Yew, S.; Chong, M.S. Monocyte chemoattractant protein-1: A proinflammatory cytokine elevated in sarcopenic obesity. Clin. Interv. Aging 2015, 10, 605–609. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.S.; Joe, Y.; Chung, H.T.; Ha, T.Y.; Yu, R. Quercetin reduces tumor necrosis factor alpha induced muscle atrophy by upregulation of Heme Oxygenase-1. J. Med. Food 2018, 21, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.J.; Patel, B.M. TNF-α and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017, 170, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.W.; Yang, Q.; Mody, N.; Graham, T.E.; Hsu, C.H.; Xu, Z.; Houstis, N.E.; Kahn, B.B.; Rosen, E.D. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007, 56, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Preissner, K.T. Adipose tissue-derived PAI-1: A molecular link for thrombo-inflammatory disease states? Thromb. Haemost. 2012, 108, 415. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef]
- Kim, T.N.; Won, J.C.; Kim, Y.J.; Lee, E.J.; Kim, M.K.; Park, M.S.; Lee, S.K.; Kim, J.M.; Ko, K.S.; Rhee, B.D. Serum adipocyte fatty acid-binding protein levels are independently associated with sarcopenic obesity. Diabetes Res. Clin. Pract. 2013, 101, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Z.; Hu, J.; Du, J.; Mitch, W.E. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006, 147, 4160–4168. [Google Scholar] [CrossRef] [PubMed]
- Katta, A.; Kundla, S.; Kakarla, S.K.; Wu, M.; Fannin, J.; Paturi, S.; Liu, H.; Addagarla, H.S.; Blough, E.R. Impaired overload-induced hypertrophy is associated with diminished mTOR signaling in insulin-resistant skeletal muscle of the obese Zucker rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1666–R1675. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Bikman, B.T.; Wang, L.P.; Yuguang, G.; Sargent, K.M.; Bulchand, S.; Knotts, T.A.; Shui, G.; Clegg, D.J.; Wenk, M.R.; et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Investig. 2011, 121, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Badin, P.M.; Louche, K.; Mairal, A.; Liebisch, G.; Schmitz, G.; Rustan, A.C.; Smith, S.R.; Langin, D.; Moro, C. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes 2011, 60, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H. Longevity, lipotoxicity and leptin: The adipocyte defense against feasting and famine. Biochimie 2005, 7, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sishi, B.; Loos, B.; Ellis, B.; Smith, W.; du Toit, E.F.; Engelbrecht, A.M. Diet induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp. Physiol. 2011, 96, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Reid, M.B.; Li, Y.P. Tumor necrosis factor-alpha and muscle wasting: A cellular perspective. Respir. Res. 2001, 2, 269–272. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.F.; Deeg, D.J.H.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Colbert, L.H.; Pahor, M.; Rubin, S.M.; Tylavsky, F.A.; et al. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1183–1189. [Google Scholar] [CrossRef]
- Steinbacher, P.; Eckl, P. Impact of Oxidative Stress on Exercising Skeletal Muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Havekes, B.; Sauerwein, H.P. Adipocyte-myocyte crosstalk in skeletal muscle insulin resistance; is there a role for thyroid hormone? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Drevon, C.A.; Eckel, J. Secreted proteins from adipose tissue and skeletal muscle - adipokines, myokines and adipose/muscle cross-talk. Arch. Physiol. Biochem. 2011, 117, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscles and their myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Thomas, D.R. Sarcopenia. Clin. Geriatr. Med. 2010, 26, 331–346. [Google Scholar] [CrossRef]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef]
- Carson, J.A.; Manolagas, S.C. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone 2015, 80, 67–78. [Google Scholar] [CrossRef]
- Visser, M.; Kritchevsky, S.B.; Goodpaster, B.H.; Newman, A.B.; Nevitt, M.; Stamm, E.; Harris, T.B. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: The health, aging and body composition study. J. Am. Geriatr. Soc. 2002, 50, 897–904. [Google Scholar] [CrossRef]
- Kob, R.; Fellner, C.; Bertsch, T.; Wittmann, A.; Mishura, D.; Sieber, C.C.; Fischer, B.E.; Stroszczynski, C.; Bollheimer, C.L. Gender-specific differences in the development of sarcopenia in the rodent model of the ageing high-fat rat. J. Cachexia Sarcopenia Muscle 2015, 6, 181–191. [Google Scholar] [CrossRef]
- Lafortuna, C.L.; Maffiuletti, N.A.; Agosti, F.; Sartorio, A. Gender variations of body composition, muscle strength and power output in morbid obesity. Int. J. Obes. 2005, 29, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Silva Neto, L.S.; Karnikowiski, M.G.; Tavares, A.B.; Lima, R.M. Association between sarcopenia, sarcopenic obesity, muscle strength and quality of life variables in elderly women. Rev. Bras. Fisioter. 2012, 16, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Visser, M.; De Meersman, R.E.; Sepúlveda, D.; Baumgartner, R.N.; Pierson, R.N.; Harris, T.; Heymsfield, S.B. Appendicular skeletal muscle mass: Effects of age, gender, and ethnicity. J. Appl. Physiol. 1997, 83, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Nicklas, B.J. Age-related changes in fat deposition in mid-thigh muscle in women: Relationships with metabolic cardiovascular disease risk factors. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Gabriely, I.; Atzmon, G.; Suh, Y.; Rothenberg, D.; Bergman, A. Genetic studies reveal the role of the endocrine and metabolic systems in aging. J. Clin. Endocrinol. Metab. 2010, 95, 4493–4500. [Google Scholar] [CrossRef] [PubMed]
- Yuki, A.; Otsuka, R.; Kozakai, R.; Kitamura, I.; Okura, T.; Ando, F.; Shimokata, H. Relationship between low free testosterone levels and loss of muscle mass. Sci. Rep. 2013, 3, 1818. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1140–1152. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Sheffield-Moore, M.; Yeckel, C.W.; Gilkison, C.; Jiang, J.; Achacosa, A.; Lieberman, S.A.; Tipton, K.; Wolfe, R.R.; Urban, R.J. Testosterone administration to older men improves muscle function: Molecular and physiological mechanisms. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E601–E607. [Google Scholar] [CrossRef]
- Iannuzzi-Sucich, M.; Prestwood, K.M. Kenny, A.M. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M772–M777. [Google Scholar] [CrossRef] [PubMed]
- Naftolin, F.; Ryan, K.J.; Petro, Z. Aromatization of androstenedione by limbic system tissue from human foetuses. J. Endocrinol. 1971, 51, 795–796. [Google Scholar] [CrossRef] [PubMed]
- van den Beld, A.W.; de Jong, F.H.; Grobbee, D.E.; Pols, H.A.; Lamberts, S.W. Measures of.bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J. Clin. Endocrinol. Metab. 2000, 85, 3276–3282. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.D.; Launer, L.J.; Rodriguez, B.L.; Ross, G.W.; Wilson, P.W.; Masaki, K.H.; Strozyk, D.; Curb, J.D.; Yano, K.; Popper, J.S.; et al. Serum estradiol and risk of stroke in elderly men. Neurology 2007, 68, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Enns, D.L.; Tiidus, P.M. The influence of estrogen on skeletal muscle: Sex matters. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Baltgalvis, K.A.; Greising, S.M.; Warren, G.L.; Lowe, D.A. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS ONE 2010, 5, e10164. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, R.; Niclos, B.B.; Rutherford, O.M. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J. Physiol. 1996, 493, 267–272. [Google Scholar] [CrossRef]
- Sipilä, S. Body composition and muscle performance during menopause and hormone replacement therapy. J. Endocrinol. Investig. 2003, 26, 893–901. [Google Scholar] [CrossRef]
- Sirola, J.; Rikkonen, T. Muscle performance after the menopause. J. Br. Menopause Soc. 2005, 11, 45–50. [Google Scholar] [CrossRef]
- Bai, H.J.; Sun, J.Q.; Chen, M.; Xu, D.F.; Xie, H.; Yu, Z.W.; Bao, Z.J.; Chen, J.; Pan, Y.R.; Lu, D.J.; et al. Age-related decline in skeletal muscle mass and function among elderly men and women in Shanghai, China: A cross sectional study. Asia Pac. J. Clin. Nutr. 2016, 25, 326–332. [Google Scholar] [CrossRef]
- Dutra, M.T.; Avelar, B.P.; Souza, V.C.; Bottaro, M.; Oliveira, R.J.; Nóbrega, O.T.; Moreno Lima, R. Relationship between sarcopenic obesity-related phenotypes and inflammatory markers in postmenopausal women. Clin. Physiol. Funct. Imaging 2017, 37, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.A.J.; Armstrong, S.T. Androgens in the ovarian micro environment. Semin. Reprod. Endocrinol. 1986, 4, 89–100. [Google Scholar] [CrossRef]
- van Geel, T.A.; Geusens, P.P.; Winkens, B.; Sels, J.P.; Dinant, G.J. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscles trength and bone mineral density in postmenopausal women: A cross-sectional study. Eur. J. Endocrinol. 2009, 160, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Zumoff, B.; Strain, G.W.; Miller, L.K.; Rosner, W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J. Clin. Endocrinol. Metab. 1995, 80, 1429–1430. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Feng, X.; Zhou, J.; Gong, H.; Xia, S.; Wei, Q.; Hu, X.; Tao, R.; Li, L.; Qian, F.; et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci. Rep. 2016, 6, 38937. [Google Scholar] [CrossRef]
- Mori, H.; Kuroda, A.; Araki, M.; Suzuki, R.; Taniguchi, S.; Tamaki, M.; Akehi, Y.; Matsuhisa, M. Advanced glycation end-products are a risk for muscle weakness in Japanese patients with type 1 diabetes. J. Diabetes Investig. 2016, 11. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. The RAGE axis and endothelial dysfunction: Maladaptive roles in the diabetic vasculature and beyond. Trends. Cardiovasc. Med. 2005, 15, 237–243. [Google Scholar] [CrossRef]
- Dalal, M.; Ferrucci, L.; Sun, K.; Beck, J.; Fried, L.P.; Semba, R.D. Elevated serum advanced glycation end products and poor grip strength in older community-dwelling women. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Snijder, M.B.; Twisk, J.W.; van Mechelen, W.; Kemper, H.C.; Seidell, J.C.; Stehouwer, C.D. Central fat mass versus peripheral fat and lean mass: Opposite (adverse versus favorable) associations with arterial stiffness? The Amsterdam Growth and Health Longitudinal Study. J. Clin. Endocrinol. Metab. 2004, 89, 2632–2639. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M.; Jebb, S.A. Beyond body mass index. Obes. Rev. 2001, 2, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K.; Correia, M.L.; Haynes, W.G.; Mark, A.L. Obesity-associated hypertension: New insights into mechanisms. Hypertension 2005, 45, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ischaki, E.; Papatheodorou, G.; Gaki, E.; Papa, I.; Koulouris, N.; Loukides, S. Body mass and fat-free mass indices in COPD: Relation with variables expressing disease severity. Chest 2007, 132, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.; Broekhuizen, R.; Weling-Scheepers, C.A.; Wouters, E.F. Body composition and mortality in chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2005, 82, 53–59. [Google Scholar] [CrossRef]
- Bello, J.R.; Miller, R.; Khandker, R.; Bourgeois, N.; Galwey, N.; Clark, R.V. Association between low muscle mass, functional limitations and hospitalisation in heart failure: NHANES 1999-2004. Age Ageing 2015, 44, 948–954. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Shen, X.; Yan, S. Sarcopenia associated with renal function in the patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 118, 121–129. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Ângulo, P.; Meza-Junco, J.; Prado, C.M.; Sawyer, M.B.; Beaumont, C.; Esfandiari, N.; Ma, M.; Baracos, V.E. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachexia Sarcopenia Muscle 2016, 7, 126–135. [Google Scholar] [CrossRef]
- Meza-Junco, J.; Montano-Loza, A.; Prado, C.M.; Lieffers, J.R.; Baracos, V.E.; Bain, V.; Spratlin, J.L.; Sawyer, M.B. Sarcopenia in patients with hepatocellular carcinoma (HCC). J. Clin. Oncol. 2011, 29, e14570. [Google Scholar] [CrossRef]
- Song, D.S.; Chang, U.I.; Choi, S.; Jung, Y.D.; Han, K.; Ko, S.H.; Ahn, Y.B.; Yang, J.M. Heavy alcohol consumption with alcoholic liver disease accelerates sarcopenia in elderly Korean males: The Korean National Health and Nutrition Examination Survey 2008–2010. PLoS ONE 2016, 11, e0163222. [Google Scholar] [CrossRef] [PubMed]
- Steffl, M.; Bohannon, R.W.; Petr, M.; Kohlikova, E.; Holmerova, I. Alcohol consumption as a risk factor for sarcopenia—A meta-analysis. BMC Geriatr. 2016, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.L.; Billings, F.T., 4th; Bojanowski, M.T.; Gilliam, L.A.; Yu, C.; Roshanravan, B.; Roberts, L.J., 2nd; Himmelfarb, J.; Ikizler, T.A.; Brown, N.J. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol. Rep. 2016, 4, e12780. [Google Scholar] [CrossRef] [PubMed]
- Bouchonville, M.F.; Villareal, D.T. Sarcopenic obesity: How do we treat it? Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Chapman, I. Preventig sarcopenia in older people. Maturitas 2010, 66, 383–388. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.L.; Baumgartner, R.N.; Garry, P.J.; Vellas, B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: An update. Clin. Interv. Aging 2010, 5, 259–270. [Google Scholar] [CrossRef]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef]
- Muscariello, E.; Nasti, G.; Siervo, M.; Di Maro, M.; Lapi, D.; D’Addio, G.; Colantuoni, A. Dietary protein intake in sarcopenic obese older women. Clin. Interv. Aging 2016, 11, 133–140. [Google Scholar] [CrossRef]
- Anand, I.; Chandrashekhan, Y.; De Giuli, F.; Pasini, E.; Mazzoletti, A.; Confortini, R.; Ferrari, R. Chronic effects of propionyl-L-carnitine on the hemodynamics, exercise capacity, and hormones in patients with congestive heart failure. Cardiovasc. Drugs Ther. 1998, 12, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; van Loon, L.J. Leucine as a pharmaconutrient to prevent and treat sarcopenia and type 2 diabetes. Nutr. Rev. 2011, 69, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Rasmussen, B.B. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signaling and human skeletal muscle protein synthesis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Solerte, S.B.; Gazzaruso, C.; Bonacasa, R.; Rondanelli, M.; Zamboni, M.; Basso, C.; Locatelli, E.; Schifino, N.; Giustina, A.; Fioravanti, M. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am. J. Cardiol. 2008, 101, 69E–77E. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ruiz, M.E.; El Hafidi, M.; Pérez-Torres, I.; Baños, G.; Guarner, V. Medicinal agents and metabolic syndrome. Curr. Med. Chem. 2013, 20, 2626–2640. [Google Scholar] [CrossRef]
- Peredo-Escárcega, A.E.; Guarner-Lans, V.; Pérez-Torres, I.; Ortega-Ocampo, S.; Carreón-Torres, E.; Castrejón-Tellez, V.; Díaz-Díaz, E.; Rubio-Ruiz, M.E. The combination of resveratrol and quercetin attenuates Metabolic Syndrome in rats by modifying the serum fatty acid composition and by upregulating SIRT 1 and SIRT 2 expression in white adipose tissue. Evid. Based Complement. Alternat. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Le, N.H.; Kim, C.S.; Park, T.; Park, J.H.; Sung, M.K.; Lee, D.G.; Hong, S.M.; Choe, S.Y.; Goto, T.; Kawada, T.; et al. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediat. Inflamm. 2014, 2014, 834294. [Google Scholar] [CrossRef]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Tanno, M.; Miura, T.; Shimamoto, K.; Horio, Y. Resveratrol ameliorates muscular pathology in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. J. Pharmacol. Exp. Ther. 2011, 338, 784–794. [Google Scholar] [CrossRef]
- Liu, C.J.; Latham, N.K. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009, 8, CD002759. [Google Scholar] [CrossRef]
- Marini, M.; Sarchielli, E.; Brogi, L.; Lazzeri, R.; Salerno, R.; Sgambati, E.; Monaci, M. Role of adapted physical activity to prevent the adverse effects of the sarcopenia. A pilot study. Ital. J. Anat. Embryol. 2008, 113, 217–225. [Google Scholar] [PubMed]
- Baar, K. Involvement of PPAR gamma co-activator-1, nuclear respiratory factors 1 and 2, and PPAR alpha in the adaptive response to endurance exercise. Proc. Nutr. Soc. 2004, 63, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Law, T.D.; Clark, L.A.; Clark, B.C. Resistance exercise to prevent and manage sarcopenia and dynapenia. Annu. Rev. Gerontol. Geriatr. 2016, 36, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Kiilerich, K.; Pilegaard, H. PGC-1alpha-mediated adaptations in skeletal muscle. Pflug. Arch. 2010, 460, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.M.; Gordon, S.E. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J. Appl. Physiol. 2005, 98, 557–564. [Google Scholar] [CrossRef]
- Nakashima, K.; Yakabe, Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci. Biotechnol. Biochem. 2007, 71, 1650–1656. [Google Scholar] [CrossRef]
- Bolster, D.R.; Crozier, S.J.; Kimball, S.R.; Jefferson, L.S. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J. Biol. Chem. 2002, 277, 23977–23980. [Google Scholar] [CrossRef]
- Shan, T.; Zhang, P.; Liang, X.; Bi, P.; Yue, F.; Kuang, S. Lkb1 is indispensable for skeletal muscle development, regeneration, and satellite cell homeostasis. Stem Cells. 2014, 32, 2893–2907. [Google Scholar] [CrossRef]
- Bostrom, E.A.; Choi, J.H.; Long, J.Z.; Kajimura, S.; Zingaretti, M.C.; Vind, B.F.; Tu, H.; Cinti, S.; Højlund, K.; Gygi, S.P.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisloff, U.; Tjonna, A.E.; Raastad, T.; et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef]
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A.; et al. Irisin—A myth rather than an exercise-inducible myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef] [PubMed]
- Campins, L.; Camps, M.; Riera, A.; Pleguezuelos, E.; Yebenes, J.C.; Serra-Prat, M. Oral drugs related with muscle wasting and sarcopenia. A review. Pharmacology 2017, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Umpleby, A.M.; Russell-Jones, D.L. The hormonal control of protein metabolism. Baillieres Clin. Endocrinol. Metab. 1996, 10, 551–570. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Guillet, C.; Zangarelli, A.; Gachon, P.; Morio, B.; Giraudet, C.; Rousset, P.; Boirie, Y. Whole body protein breakdown is less inhibited by insulin, but still responsive to amino acid, in nondiabetic elderly subjects. J. Clin. Endocrinol. Metab. 2004, 89, 6017–6024. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, E.A.; Selby, A.L.; Atherton, P.J.; Patel, R.; Rankin, D.; Smith, K.; Rennie, M.J. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am. J. Clin. Nutr. 2009, 90, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Rasmussen, B.B.; Cadenas, J.G.; Grady, J.J.; Volpi, E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E745–E754. [Google Scholar] [CrossRef] [PubMed]
- Guillet, C.; Delcourt, I.; Rance, M.; Giraudet, C.; Walrand, S.; Bedu, M.; Duche, P.; Boirie, Y. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J. Clin. Endocrinol. Metab. 2009, 94, 3044–3050. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Dobson, J.P.; Greene, N.P.; Wiggs, M.P.; Shimkus, K.L.; Wudeck, E.V.; Davis, A.R.; Laureano, M.L.; Fluckey, J.D. Abnormal protein turnover and anabolic resistance to exercise in sarcopenic obesity. FASEB J. 2003, 27, 3905–3916. [Google Scholar] [CrossRef]
- Murton, A.J.; Marimuthu, K.; Mallinson, J.E.; Selby, A.L.; Smith, K.; Rennie, M.J.; Greenhaff, P.L. Obesity appears to be associated with altered muscle protein synthetic and breakdown responses to increased nutrient delivery in older men, but not reduced muscle mass or contractile function. Diabetes 2015, 64, 3160–3171. [Google Scholar] [CrossRef]
- Fujita, S.; Rasmussen, B.B.; Cadenas, J.G.; Drummond, M.J.; Glynn, E.L.; Sattler, F.R.; Volpi, E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 2007, 56, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef] [PubMed]
- Florini, J.R.; Ewton, D.Z.; Coolican, S.A. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr. Rev. 1996, 17, 481–517. [Google Scholar] [CrossRef] [PubMed]
- Hermann, M.; Berger, P. Hormonal changes in aging men: A therapeutic indication? Exp. Gerontol. 2001, 36, 1075–1082. [Google Scholar] [CrossRef]
- Ryall, J.G.; Schertzer, J.D.; Lynch, G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008, 9, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Johannsson, G.; Christiansen, J.S.; Kopchick, J.J.; Thorner, M.O. The aging population—Is there a role for endocrine interventions? Growth Horm. IGF Res. 2009, 19, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A. Molecular mechanisms in aging and current strategies to counteract sarcopenia. Curr. Aging Sci. 2010, 3, 90–101. [Google Scholar] [CrossRef]
- van Dam, P.S.; Smid, H.E.C.; de Vries, W.R.; Niesink, M.; Bolscher, E.; Waasdorp, E.J.; Dieguez, C.; Casanueva, F.F.; Koppeschaar, H.P. Reduction of free fatty acids by acipimox enhances the growth hormone (GH) responses to GH-releasing peptide 2 in elderly men. J. Clin. Endocrinol. Metab. 2000, 85, 4706–4711. [Google Scholar] [CrossRef] [PubMed]
- Weltman, A.; Weltman, J.Y.; Veldhuis, J.D.; Hartman, M.L. Body composition, physical exercise, growth hormone and obesity. Eat. Weight Disord. 2001, 6, 28–37. [Google Scholar]
- Waters, D.L.; Qualls, C.R.; Dorin, R.I.; Veldhuis, J.D.; Baumgartner, R.N. Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 536–541. [Google Scholar] [CrossRef]
- Makimura, H.; Feldpausch, M.N.; Rope, A.M.; Hemphill, L.C.; Torriani, M.; Lee, H.; Grinspoon, S.K. Metabolic effects of a growth hormone-releasing factor in obese subjects with reduced growth hormone secretion: A randomized controlled trial. J. Clin. Endocrinol. Metab. 2012, 97, 4769–4779. [Google Scholar] [CrossRef] [PubMed]
- Gabriely, I.; Ma, X.H.; Yang, X.M.; Atzmon, G.; Rajala, M.W.; Berg, A.H.; Scherer, P.; Rossetti, L.; Barzilai, N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: An adipokine-mediated process? Diabetes 2002, 51, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Urban, R.J.; Bodenburg, Y.H.; Gilkison, C.; Foxworth, J.; Coggan, A.R.; Wolfe, R.R.; Ferrando, A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am. J. Physiol. Endocrinol. Metab. 1995, 269, E820–E826. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Woodhouse, L.; Storer, T.W. Proof of the effect of testosterone on skeletal muscle. J. Endocrinol. 2001, 170, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.A.; Strauss, B.J.G.; McLachlan, R.I. Body composition, Metabolic syndrome and testosterone in ageing men. Int. J. Impot. Res. 2007, 19, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Perry, H.M. Androgens and women at the menopause and beyond. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M409–M416. [Google Scholar] [CrossRef] [PubMed]
- Cappola, A.R.; Bandeen-Roche, K.; Wand, G.S.; Volpato, S.; Fried, L.P. Association of IGF-1 levels with muscle strength and mobility in older women. J. Clin. Endocrinol. Metab. 2001, 86, 4139–4146. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Pluijm, S.M.F.; Smitt, J.H.; van Schoor, N.M.; Visser, M.; Gooren, L.J.; Lips, P. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin. Endocrinol. 2005, 63, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.W.; Tam, S.; Kung, A.W.C.; Lo, S.; Fan, S.; Wong, R.L.; Morley, J.E.; Lam, K.S. Serum total and bioavailable testosterone levels, central obesity, and muscle strength changes with aging in healthy Chinese men. J. Am. Geriatr. Soc. 2008, 56, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, I.G.; Balagopal, P.; Nair, K.S. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men—A clinical research center study. J. Clin. Endocrinol. Metab. 1996, 81, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Woodhouse, L.; Casaburi, R.; Singh, A.B.; Bhasin, D.; Berman, N.; Chen, X.; Yarasheski, K.E.; Magliano, L.; Dzekov, C.; et al. Testosterone dose-response relationships in healthy young men. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1172–E1181. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Hikim, I.; Cornford, M.; Gaytan, H.; Lee, M.L.; Bhasin, S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J. Clin. Endocrinol. Metab. 2006, 91, 3024–3033. [Google Scholar] [CrossRef] [PubMed]
- Comhaire, F. Hormone replacement therapy and longevity. Andrologia 2016, 48, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Morgentaler, A. Words of wisdom. Re: Adverse events associated with testosterone administration. Eur. Urol. 2011, 59, 465. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Smith, G.I.; Patterson, B.W.; Reeds, D.N.; Kampelman, J.; Magkos, F.; Mittendorfer, B. Testosterone increases the muscle protein synthesis rate but does not affect very-low-density lipoprotein metabolism in obese premenopausal women. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E740–E746. [Google Scholar] [CrossRef]
- Shores, M.M.; Moceri, V.M.; Gruenewald, D.A.; Brodkin, K.I.; Matsumoto, A.M.; Kivlahan, D.R. Low testosterone is associated with decreased function and increased mortality risk: A preliminary study of men in a geriatric rehabilitation unit. J. Am. Geriatr. Soc. 2004, 52, 2077–2081. [Google Scholar] [CrossRef]
- Pérez Torres, I.; El Hafidi, M.; Zamora-González, J.; Infante, O.; Chavira, R.; Baños, G. Modulation of aortic vascular reactivity by sex hormones in a male rat model of Metabolic syndrome. Life Sci. 2007, 80, 2170–2180. [Google Scholar] [CrossRef]
- Traish, A.M.; Saad, F.; Guay, A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J. Androl. 2009, 30, 23–32. [Google Scholar] [CrossRef]
- Dalton, J.T.; Barnette, K.G.; Bohl, C.E.; Hancock, M.L.; Rodriguez, D.; Dodson, S.T.; Morton, R.A.; Steiner, M.S. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: Results of a double-blind, placebo-controlled phase II trial. J. Cachexia Sarcopenia Muscle 2011, 2, 153–161. [Google Scholar] [CrossRef]
- Min, L.; Yanase, T.; Tanaka, T.; Fan, W.; Nomura, M.; Kawate, H.; Okabe, T.; Takayanagi, R.; Nawata, H. A novel synthetic androgen receptor ligand, S42, works as a selective androgen receptor modulator and possesses metabolic effects with little impact on the prostate. Endocrinology 2009, 150, 5606–5616. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Hubal, M.J. Are women less susceptible to exercise-induced muscle damage? Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.B.; Rosenfalck, A.M.; Højgaard, L.; Ottesen, B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes. Res. 2001, 9, 622–626. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Spektor, T.M.; Rice, J.C.; Sattler, F.R.; Schroeder, E.T. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J. Appl. Physiol. 2009, 107, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M.; Lowe, D.A.; Brown, M. Estrogen replacement and skeletal muscle: Mechanisms and population health. J. Appl. Physiol. 2013, 115, 569–578. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef]
- Akpan, I.; Goncalves, M.D.; Dhir, R.; Yin, X.; Pistilli, E.E.; Bogdanovich, S.; Khurana, T.S.; Ucran, J.; Lachey, J.; Ahima, R.S. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int. J. Obes. 2009, 33, 1265–1273. [Google Scholar] [CrossRef]
- Smith, R.C.; Lin, B.K. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr. Opin. Support. Palliat. Care 2013, 7, 352–360. [Google Scholar] [CrossRef]
- Zhang, C.; McFarlane, C.; Lokireddy, S.; Bonala, S.; Ge, X.; Masuda, S.; Gluckman, P.D.; Sharma, M.; Kambadur, R. Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetologia 2011, 54, 1491–1501. [Google Scholar] [CrossRef]
- Zhang, C.; McFarlane, C.; Lokireddy, S.; Masuda, S.; Ge, X.; Gluckman, P.D.; Sharma, M.; Kambadur, R. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia 2012, 55, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Lu-Nguyen, N.B.; Jarmin, S.A.; Saleh, A.F.; Popplewell, L.; Gait, M.J.; Dickson, G. Combination antisense treatment for destructive exon skipping of myostatin and open reading frame rescue of dystrophin in neonatal mdx mice. Mol. Ther. 2015, 23, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.L.; Hittel, D.S.; McPherron, A.C. Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med. Sci. Sports. Exerc. 2011, 43, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.R.; Fleckenstein, J.L.; Amato, A.A.; Barohn, R.J.; Bushby, K.; Escolar, D.M.; Flanigan, K.M.; Pestronk, A.; Tawil, R.; Wolfe, G.I.; et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann. Neurol. 2008, 63, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Krivickas, L.S.; Walsh, R.; Amato, A.A. Single muscle fiber contractile properties in adults with muscular dystrophy treated with MYO-029. Muscle Nerve 2009, 39, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, R.T.; Donnelly, E.; Cody, D.B.; Bauer, M.B.; Isfort, R.J. Urocortin II treatment reduces skeletal muscle mass and function loss during atrophy and increases nonatrophying skeletal muscle mass and function. Endocrinology 2003, 144, 4939–4946. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Brar, B.; Choi, C.S.; Rousso, D.; Vaughan, J.; Kuperman, Y.; Kim, S.N.; Donaldson, C.; Smith, S.M.; Jamieson, P.; et al. Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle. Proc. Natl. Acad. Sci. USA 2006, 103, 16580–16585. [Google Scholar] [CrossRef]
- Bale, T.L.; Anderson, K.R.; Roberts, A.J.; Lee, K.F.; Nagy, T.R.; Vale, W.W. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology 2003, 144, 2580–2587. [Google Scholar] [CrossRef]
- Jamieson, P.M.; Cleasby, M.E.; Kuperman, Y.; Morton, N.M.; Kelly, P.A.; Brownstein, D.G.; Mustard, K.J.; Vaughan, J.M.; Carter, R.N.; Hahn, C.N.; et al. Urocortin 3 transgenic mice exhibit a metabolically favourable phenotype resisting obesity and hyperglycaemia on a high-fat diet. Diabetologia 2011, 54, 2392–2403. [Google Scholar] [CrossRef]
- Roustit, M.M.; Vaughan, J.M.; Jamieson, P.M.; Cleasby, M.E. Urocortin 3 activates AMPK and Akt pathways and enhances glucose disposal in rat skeletal muscle. J. Endocrinol. 2014, 223, 143–154. [Google Scholar] [CrossRef]
- Bates, B.; Bates, C.; Prentice, P.; Swan, G. National Diet and Nutrition Survey Headline Results from Years 1 and 2 (combined) of the Rolling Programme (2008/2009–2009/10); Supplementary Report: Blood Analytes; Department of Health and the Food Standards Agency: London, UK, 2011. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215348/dh_130788.pdf (accessed on 11 January 2019).
- Schubert, L.; DeLuca, H.F. Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch. Biochem. Biophys. 2010, 500, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Talaei, A.; Mohamadi, M.; Adgi, Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2013, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Wongwiwatthananukit, S.; Sansanayudh, N.; Phetkrajaysang, N.; Krittiyanunt, S. Effects of vitamin D(2) supplementation on insulin sensitivity and metabolic parameters in Metabolic syndrome patients. J. Endocrinol. Investig. 2013, 36, 558–563. [Google Scholar] [CrossRef]
- Ceglia, L.; Niramitmahapanya, S.; da Silva Morais, M.; Rivas, D.A.; Harris, S.S.; Bischoff-Ferrari, H.; Fielding, R.A.; Dawson-Hughes, B. A randomized study on the effect of vitamin D (3) supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J. Clin. Endocrinol. Metab. 2013, 98, E1927–E1935. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyere, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Burne, T.H.; Johnston, A.N.; McGrath, J.J.; Mackay-Sim, A. Swimming behavior and post-swimming activity in Vitamin D receptor knockout mice. Brain Res. Bull. 2006, 69, 74–78. [Google Scholar] [CrossRef]
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 2003, 144, 5138–5144. [Google Scholar] [CrossRef]
- Narvaez, C.J.; Matthews, D.; Broun, E.; Chan, M.; Welsh, J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 2009, 150, 651–661. [Google Scholar] [CrossRef]
- Bruyère, O.; Cavalier, E.; Buckinx, F.; Reginster, J.Y. Relevance of vitamin D in the pathogenesis and therapy of frailty. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 26–29. [Google Scholar] [CrossRef]
- Anagnostis, P.; Dimopoulou, C.; Karras, S.; Lambrinoudaki, I.; Goulis, D.G. Sarcopenia in post-menopausal women: Is there any role for vitamin D? Pflug. Arch. 2010, 460, 153–162. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Morales, M.G.; Rivera, J.C.; Cabrera, D.; Simon, F. Renin-angiotensin system: An old player with novel functions in skeletal muscle. Med. Res. Rev. 2015, 35, 437–463. [Google Scholar] [CrossRef] [PubMed]
- Ábrigo, J.; Simon, F.; Cabrera, D.; Cabello-Verrugio, C. Angiotensin-(1-7) prevents skeletal muscle atrophy induced by transforming growth factor type beta (TGF-β) via Mas receptor activation. Cell Physiol. Biochem. 2016, 40, 27–38. [Google Scholar] [CrossRef]
- Morales, M.G.; Abrigo, J.; Acuña, M.J.; Santos, R.A.; Bader, M.; Brandan, E.; Simon, F.; Olguin, H.; Cabrera, D.; Cabello-Verrugio, C. Angiotensin-(1-7) attenuates disuse skeletal muscle atrophy in mice via its receptor, Mas. Dis. Model Mech. 2016, 9, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y.; Shefer, G.; Sasson, K.; Kohen, F.; Limor, R.; Pappo, O.; Nevo, N.; Biton, I.; Bach, M.; Berkutzki, T.; et al. Angiotensin 1-7 as means to prevent the metabolic syndrome: Lessons from the fructose-fed rat model. Diabetes 2013, 62, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Onder, G.; Kritchevsky, S.B.; Pahor, M. Angiotensin-converting enzyme inhibition intervention in elderly persons: Effects on body composition and physical performance. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Sartiani, L.; Spinelli, V.; Laurino, A.; Blescia, S.; Raimondi, L.; Cerbai, E.; Mugelli, A. Pharmacological perspectives in sarcopenia: A potential role for renin-angiotensin system blockers? Clin. Cases Miner. Bone Metab. 2015, 12, 135–138. [Google Scholar] [CrossRef] [PubMed]
| Therapeutical Agent | Effects/Molecular Target | References |
|---|---|---|
| Protein intake | ↑protein turnover ↑oxygen acquirement | Volpi et al. [100] Anad et al. [102] |
| Leucine | ↑energy-sensing signaling ↑insulin sensitivity | Paddon-Jones et al. [98] Drummond et al. [105] Solerte et al. [106] |
| Flavonoids and polyphenols | ↓muscular atrophy ↑Sirt1 | Le et al. [109] Hori et al. [110] |
| Resistance exercise | ↑Metabolic fitness: ↑PGC-1α ↓insulin resistance: ↑AMPk ↓muscular hypertrophy | Law, et al. [114] Thompson, et al. [116] |
| Insulin | ↑muscle mass and metabolism ↑MAPk ↑mTOR/p70S6k | Guillet et al. [126] Fuijita et al. [128] |
| Rosiglitazone | ↑muscle mass ↑Akt ↑mTOR | Sandri et al. [133] |
| Sex hormones | ↑muscle size and force ↑insulin sensitivity | Sinha-Hikim et al. [154] Traish et al. [160] Dalton et al. [161] Tiidus et al. [166] |
| Myostatin inactivators | ↑lean mass ↓fat mass ↑glucose homeostasis | Sakuma et al. [1] Zhang et al. [171] Zhang et al. [172] |
| Urocortins | ↑muscle mass and metabolism ↑HPA axis ↑insulin signaling pathway | Hinkle et al. [173] Roustit et al. [177] |
| Vitamin D | ↑muscle mass/force ↑insulin sensitivity | Bates et al. [182] Ceglia et al. [186] Narvaez et al. [190] |
| Angiotensin 1–7 | ↓catabolic pathway ↓insulin resistance: ↑Akt ↑IGF-1 ↓hypertriglyceridemia | Morales et al. [195] Marcus et al. [196] Carter et al. [197] Sartiani et al. [198] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. Int. J. Mol. Sci. 2019, 20, 647. https://doi.org/10.3390/ijms20030647
Rubio-Ruiz ME, Guarner-Lans V, Pérez-Torres I, Soto ME. Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. International Journal of Molecular Sciences. 2019; 20(3):647. https://doi.org/10.3390/ijms20030647
Chicago/Turabian StyleRubio-Ruiz, María Esther, Verónica Guarner-Lans, Israel Pérez-Torres, and María Elena Soto. 2019. "Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures" International Journal of Molecular Sciences 20, no. 3: 647. https://doi.org/10.3390/ijms20030647
APA StyleRubio-Ruiz, M. E., Guarner-Lans, V., Pérez-Torres, I., & Soto, M. E. (2019). Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. International Journal of Molecular Sciences, 20(3), 647. https://doi.org/10.3390/ijms20030647