Olanzapine Induced Dysmetabolic Changes Involving Tissue Chromium Mobilization in Female Rats
Abstract
1. Introduction
2. Results
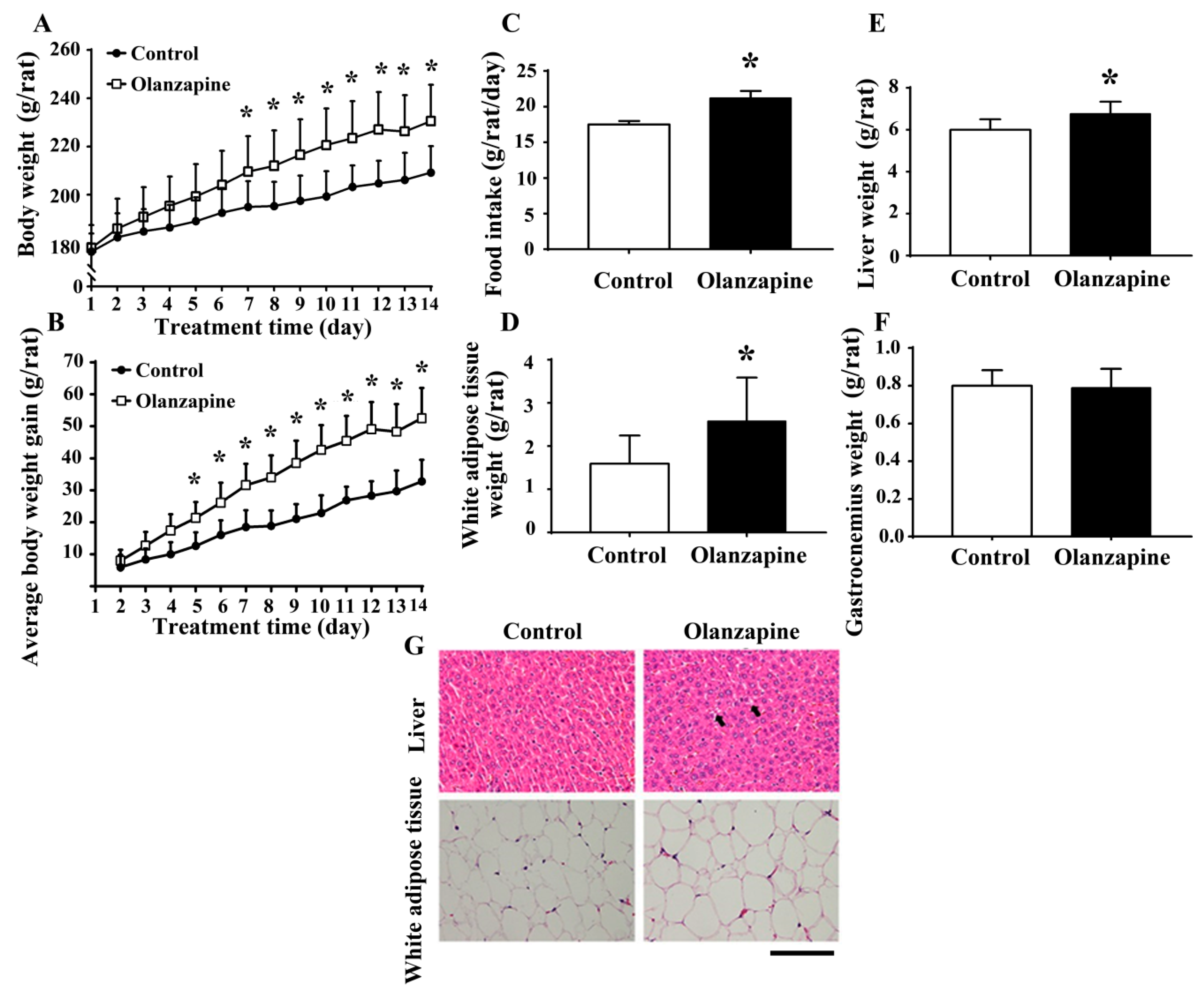
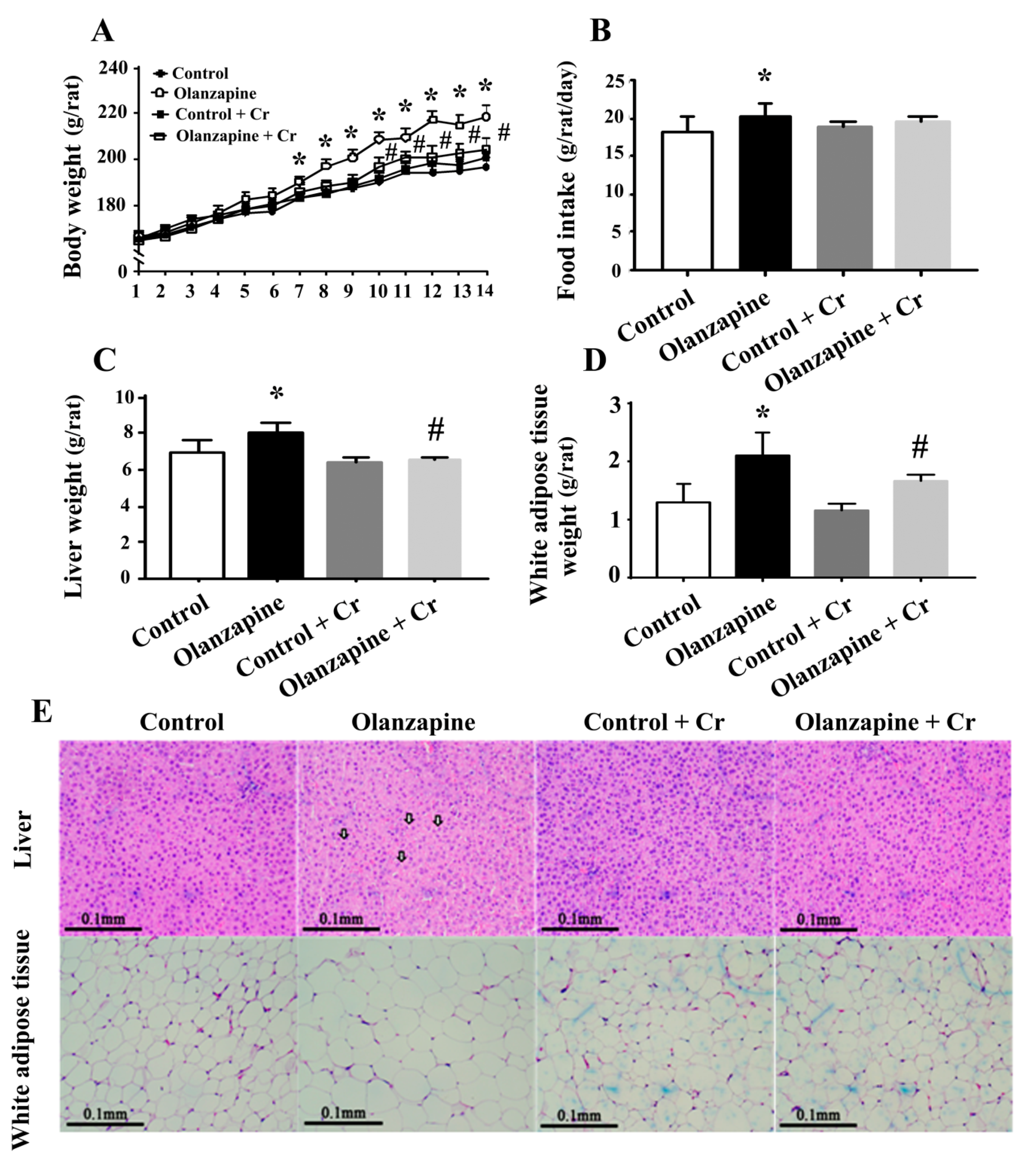
2.1. Olanzapine Caused Weight Gain and Adiposity
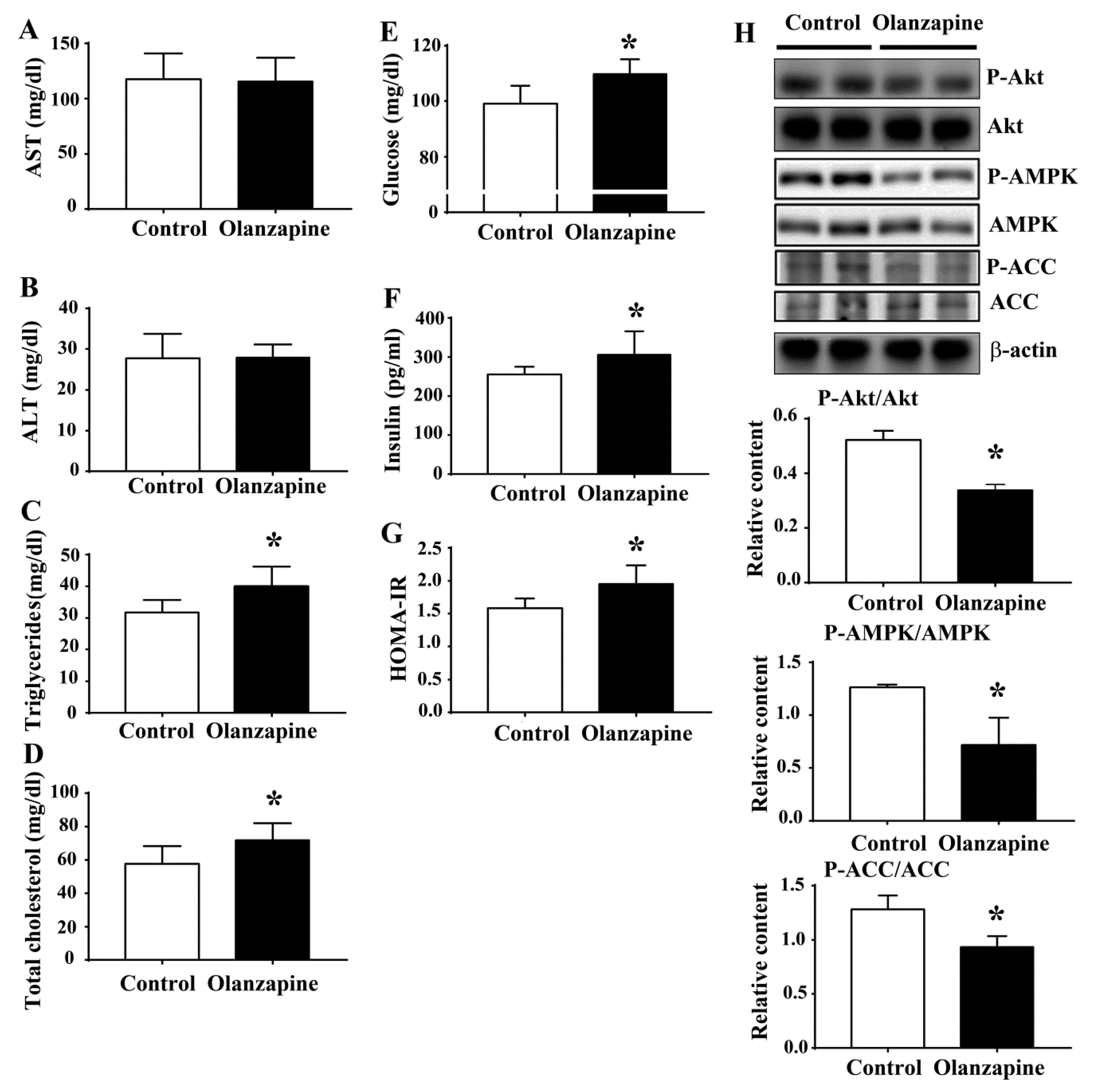
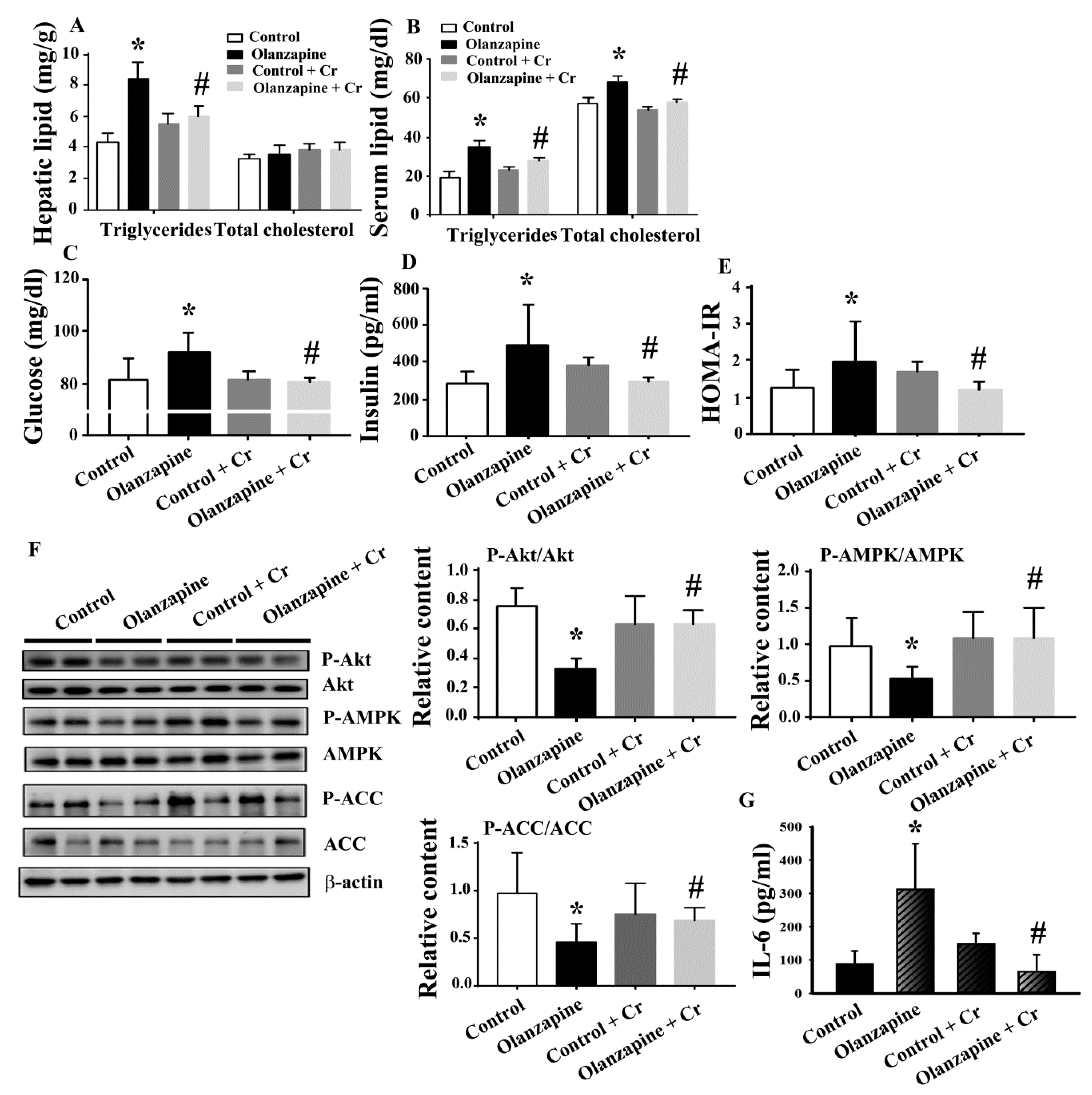
2.2. Olanzapine Caused Metabolic Disturbance
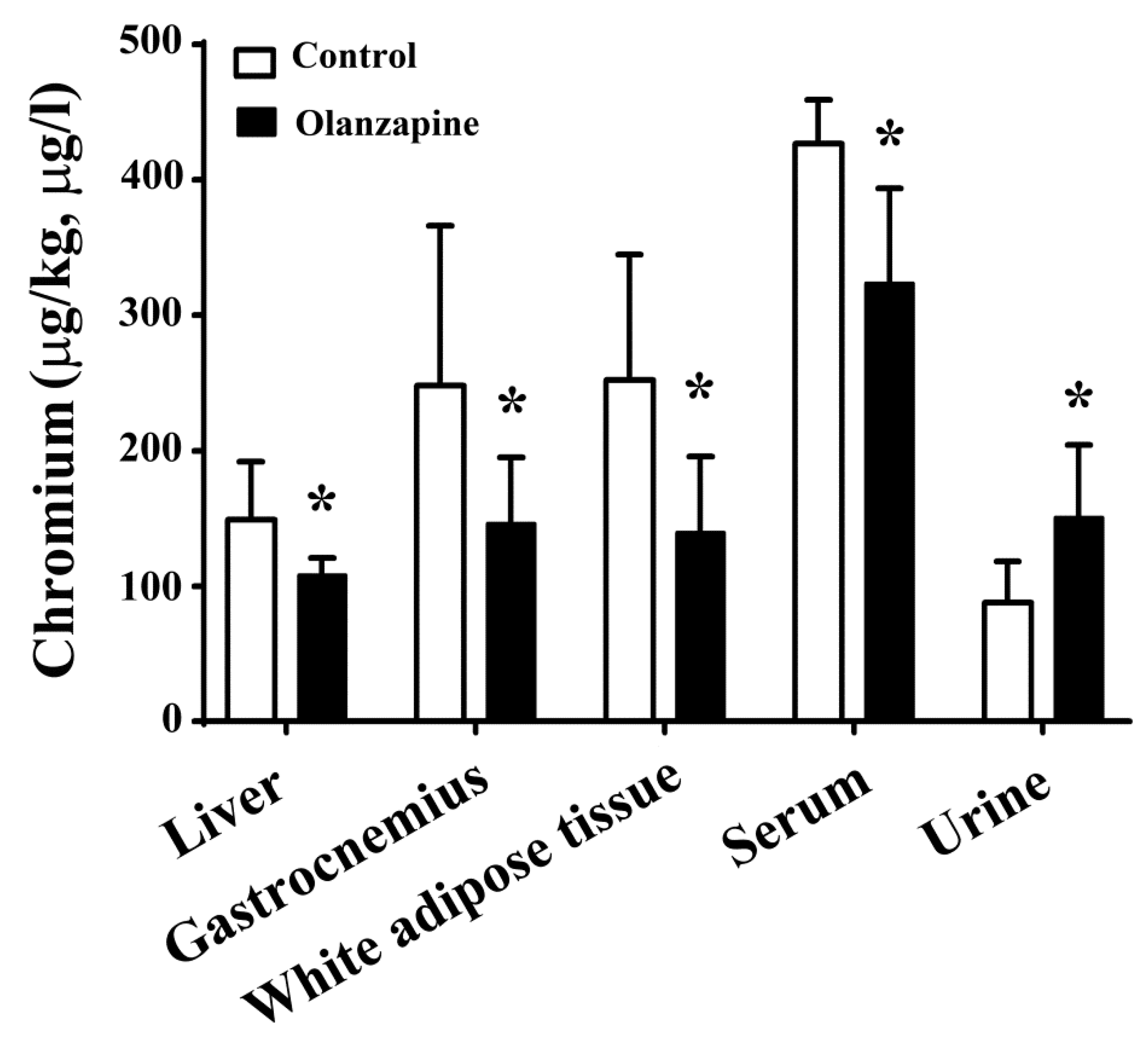
2.3. Olanzapine Caused Tissue Chromium Mobilization
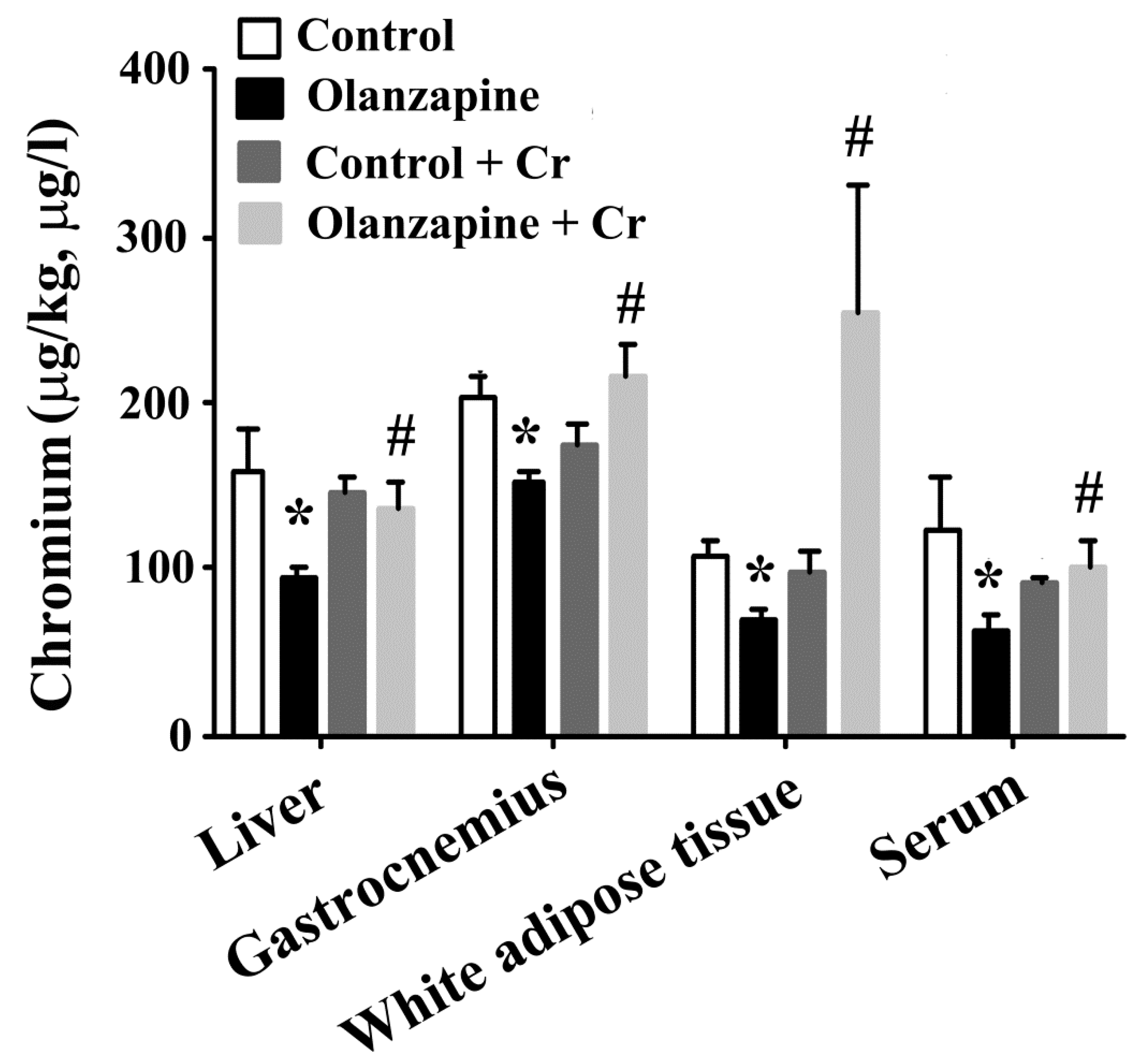
2.4. Chromium Improved Olanzapine-Induced Weight Gain and Metabolic Disturbance
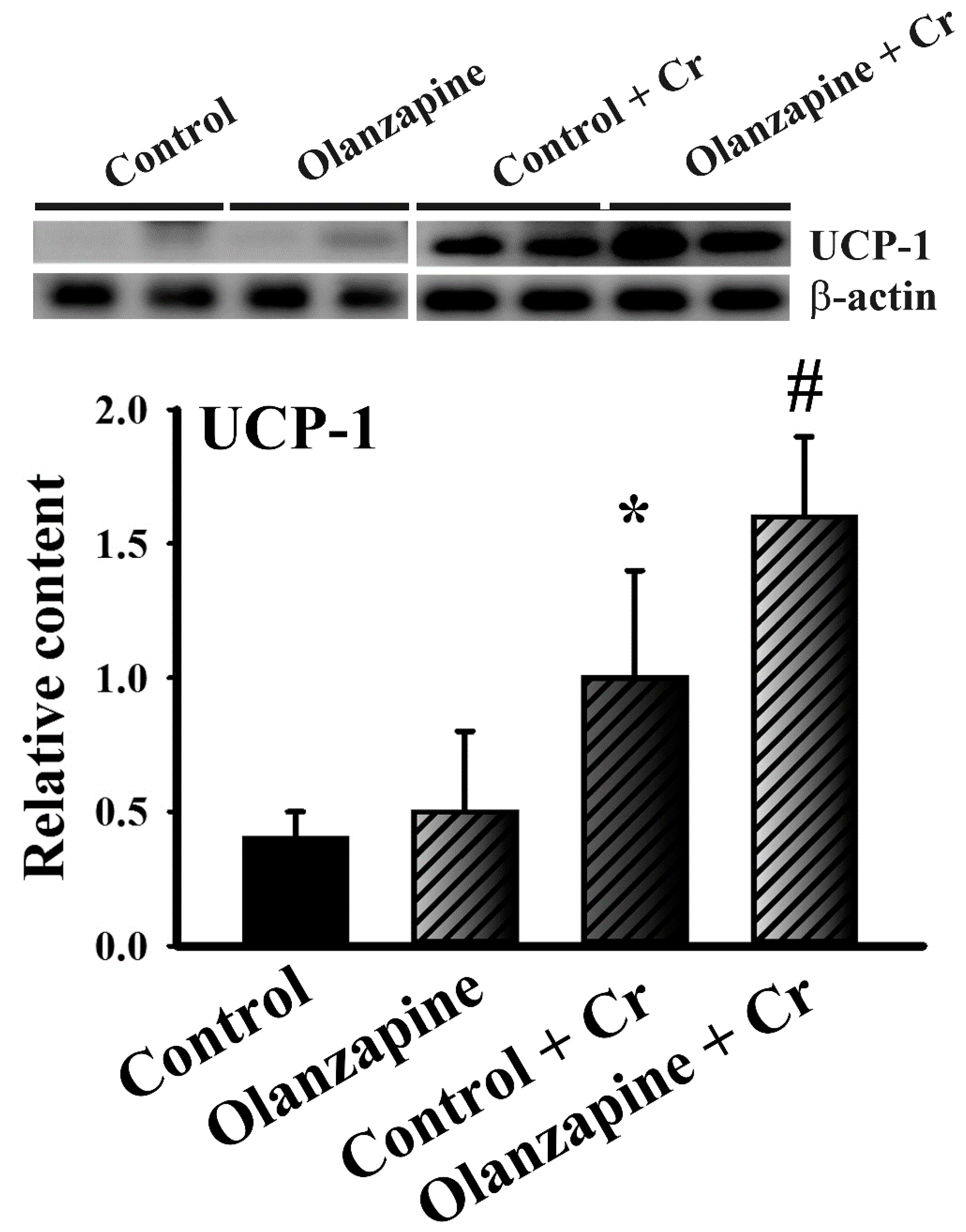
2.5. Chromium Increased Thermogenic Pncoupling protein-1 (UCP-1) Expression in White Adipose Tissues
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animal Experiments
4.3. Drug Treatment
4.4. Biochemical Analyses
4.5. Homeostasis Model Assessment Index
4.6. Trace Element Analysis
4.7. Histological Examination
4.8. Tissue Preparation and Western Blot Analysis
4.9. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Hoertel, N.; Limosin, F.; Leleu, H. Poor longitudinal continuity of care is associated with an increased mortality rate among patients with mental disorders: Results from the French National Health Insurance Reimbursement Database. Eur. Psychiatry 2014, 29, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.; Mangalore, R.; Simon, J. The global costs of schizophrenia. Schizophr. Bull. 2004, 30, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Domecq, J.P.; Prutsky, G.; Leppin, A.; Sonbol, M.B.; Altayar, O.; Undavalli, C.; Wang, Z.; Elraiyah, T.; Brito, J.P.; Mauck, K.F.; et al. Clinical review: Drugs commonly associated with weight change: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2015, 100, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, J.W.; Haupt, D.W. The metabolic effects of antipsychotic medications. Can. J. Psychiatry 2006, 51, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, J.W. Second-generation (atypical) antipsychotics and metabolic effects: A comprehensive literature review. CNS Drugs 2005, 19, 1–93. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.A.; van Winkel, R.; Van Eyck, D.; Hanssens, L.; Wampers, M.; Scheen, A. Prevalence of the metabolic syndrome in patients with schizophrenia treated with antipsychotic medication. Schizophr. Res. 2006, 83, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Deng, C. Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol. Metab. Clin. North. Am. 2013, 42, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Lian, J.; Hu, C.H.; Huang, X.F.; Deng, C. Time-dependent changes and potential mechanisms of glucose-lipid metabolic disorders associated with chronic clozapine or olanzapine treatment in rats. Sci. Rep. 2017, 7, 2762. [Google Scholar] [CrossRef]
- Manu, P.; Dima, L.; Shulman, M.; Vancampfort, D.; De Hert, M.; Correll, C.U. Weight gain and obesity in schizophrenia: Epidemiology, pathobiology, and management. Acta. Psychiatr. Scand. 2015, 132, 97–108. [Google Scholar] [CrossRef]
- Fonseka, T.M.; Müller, D.J.; Kennedy, S.H. Inflammatory cytokines and antipsychotic-induced weight gain: Review and clinical implications. Mol. Neuropsychiatry 2016, 2, 1–14. [Google Scholar] [CrossRef]
- Horska, K.; Ruda-Kucerova, J.; Babinska, Z.; Karpisek, M.; Demlova, R.; Opatrilova, R.; Suchy, P.; Kotolova, H. Olanzapine-depot administration induces time-dependent changes in adipose tissue endocrine function in rats. Psychoneuroendocrinology 2016, 73, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Gonçalves, V.F.; Harripaul, R.; Cuperfain, A.B.; Rollins, B.; Tiwari, A.K.; Zai, C.C.; Maciukiewicz, M.; Müller, D.J.; Vawter, M.P.; et al. A comprehensive analysis of mitochondrial genes variants and their association with antipsychotic-induced weight gain. Schizophr. Res. 2017, 187, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Nimura, S.; Yamaguchi, T.; Ueda, K.; Kadokura, K.; Aiuchi, T.; Kato, R.; Obama, T.; Itabe, H. Olanzapine promotes the accumulation of lipid droplets and the expression of multiple perilipins in human adipocytes. Biochem. Biophys. Res. Commun. 2015, 467, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Sezlev-Bilecen, D.; Ak, M.; Yanik, T. Dysregulation of hypothalamic modulation in olanzapine treated male rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, A.; Verty, A.N.; Allen, A.M.; Owens, N.C.; Cowley, M.A.; Oldfield, B.J. The role of thermogenesis in antipsychotic drug-induced weight gain. Obesity 2009, 17, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, A.; Watt, M.J.; Cowley, M.A.; Oldfield, B.J. Prevention of the adverse effects of olanzapine on lipid metabolism with the antiepileptic zonisamide. Neuropharmacology 2017, 123, 55–66. [Google Scholar] [CrossRef]
- Tsuneyama, N.; Suzuki, Y.; Sawamura, K.; Sugai, T.; Fukui, N.; Watanabe, J.; Ono, S.; Saito, M.; Someya, T. Effect of serum leptin on weight gain induced by olanzapine in female patients with schizophrenia. PLoS ONE 2016, 11, e0149518. [Google Scholar] [CrossRef]
- Zhang, Q.; He, M.; Deng, C.; Wang, H.; Huang, X.F. Effects of olanzapine on the elevation of macrophage infiltration and pro-inflammatory cytokine expression in female rats. J. Psychopharmacol. 2014, 28, 1161–1169. [Google Scholar] [CrossRef]
- Zhang, Q.; Lian, J.; He, M.; Deng, C.; Wang, H.; Huang, X.F. Olanzapine reduced brown adipose tissue thermogenesis and locomotor activity in female rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014, 51, 172–180. [Google Scholar] [CrossRef]
- Lazzari, P.; Serra, V.; Marcello, S.; Pira, M.; Mastinu, A. Metabolic side effects induced by olanzapine treatment are neutralized by CB1 receptor antagonist compounds co-administration in female rats. Eur. Neuropsychopharmacology 2017, 27, 667–678. [Google Scholar] [CrossRef]
- Lian, J.; Huang, X.F.; Pai, N.; Deng, C. Ameliorating antipsychotic-induced weight gain by betahistine: Mechanisms and clinical implications. Pharmacol. Res. 2016, 106, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lian, J.; Hu, C.H.; Deng, C. Betahistine co-treatment ameliorates dyslipidemia induced by chronic olanzapine treatment in rats through modulation of hepatic AMPKα-SREBP-1 and PPARα-dependent pathways. Pharmacol. Res. 2015, 100, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Mao, F.C.; Liu, C.H.; Kuan, Y.H.; Lai, N.W.; Wu, C.C.; Chen, C.J. Chromium supplementation improved post-stroke brain infarction and hyperglycemia. Metab. Brain Dis. 2016, 31, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, V.; Dasher, R.; Gitlin, M.; Parsi, M. Minimizing weight gain for patients taking antipsychotic medications: The potential role for early use of metformin. Ann. Clin. Psychiatry 2017, 29, 120–124. [Google Scholar] [PubMed]
- Hu, Y.; Young, A.J.; Ehli, E.A.; Nowotny, D.; Davies, P.S.; Droke, E.A.; Soundy, T.J.; Davies, G.E. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS ONE 2014, 9, e93310. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.R.; Vedtofte, L.; Jakobsen, M.S.L.; Jespersen, H.R.; Jakobsen, M.I.; Svensson, C.K.; Koyuncu, K.; Schjerning, O.; Oturai, P.S.; Kjaer, A.; et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: A randomized clinical trial. JAMA Psychiatry 2017, 74, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.A.; Solhi, M.; Mohammadi, M.R.; Akhondzadeh, S. Melatonin for reducing weight gain following administration of atypical antipsychotic olanzapine for adolescents with bipolar disorder: A randomized, double-blind, placebo-controlled trial. J. Child. Adolesc. Psychopharmacol. 2017, 27, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Lookian, F.; Hosseinzadeh, H. Protective effects of green tea on olanzapine-induced-metabolic syndrome in rats. Biomed. Pharmacother. 2017, 92, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Pechova, A.; Pavlata, L. Chromium as an essential nutrient: A review. Vet. Med. 2007, 52, 1–18. [Google Scholar] [CrossRef]
- Balk, E.M.; Tatsioni, A.; Lichtenstein, A.H.; Lau, J.; Pittas, A.G. Effect of chromium supplementation on glucose metabolism and lipids: A systematic review of randomized controlled trials. Diabetes Care 2007, 30, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chen, C.J.; Liao, J.W.; Mao, F.C. Chromium attenuates hepatic damage in a rat model of chronic cholestasis. Life Sci. 2009, 84, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chen, C.J.; Liu, C.H.; Mao, F.C. Chromium supplementation enhances insulin signaling in skeletal muscle of obese KK/HlJ diabetic mice. Diabetes Obes. Metab. 2009, 11, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chen, C.J.; Liu, C.H.; Mao, F.C. Chromium attenuates high-fat diet-induced nonalcoholic fatty liver disease in KK/HlJ mice. Biochem. Biophys. Res. Commun. 2010, 397, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Hu, F.B. Role of chromium in human health and in diabetes. Diabetes Care 2014, 27, 2741–2751. [Google Scholar] [CrossRef]
- Li, R.M.; Chen, S.Q.; Zeng, N.X.; Zheng, S.H.; Guan, L.; Liu, H.M.; Zhou, L.Q.; Xu, J.W. Browning of abdominal aorta perivascular adipose tissue inhibits adipose tissue inflammation. Metab Syndr Relat Disord. 2017, 15, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitz, H.; Friedensohn, A.; Leibovitz, A.; Gabay, G.; Rocas, C.; Habot, B. Effect of chromium supplementation on blood glucose and lipid levels in type 2 diabetes mellitus elderly patients. Int. J. Vitam. Nutr. Res. 2004, 74, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Abdourahman, A.; Edwards, J.G. Chromium supplementation improves glucose tolerance in diabetic Goto-Kakizaki rats. IUBMB Life 2008, 60, 541–548. [Google Scholar] [CrossRef]
- Hou, P.H.; Chang, G.R.; Chen, C.P.; Lin, Y.L.; Chao, I.S.; Shen, T.T.; Mao, F.C. Long-term administration of olanzapine induces adiposity and increases hepatic fatty acid desaturation protein in female C57BL/6J mice. Iran J. Basic Med. Sci. 2018, 21, 495–501. [Google Scholar]
- Weston-Green, K.; Babic, I.; de Santis, M.; Pan, B.; Montgomery, M.K.; Mitchell, T.; Huang, X.F.; Nealon, J. Disrupted sphingolipid metabolism following acute clozapine and olanzapine administration. J. Biomed. Sci. 2018, 25, 40. [Google Scholar] [CrossRef]
- Ikegami, M.; Ikeda, H.; Ohashi, T.; Ohsawa, M.; Ishikawa, Y.; Kai, M.; Kamei, A.; Kamei, J. Olanzapine increases hepatic glucose production through the activation of hypothalamic adenosine 5’-monophosphate-activated protein kinase. Diabetes Obes. Metab. 2013, 15, 1128–1135. [Google Scholar] [CrossRef]
- Bush, N.D.; Townsend, L.K.; Wright, D.C. AICAR prevents acute olanzapine-induced disturbances in glucose homeostasis. J. Pharmacol. Exp. Ther. 2018, 365, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A. Chromium, glucose intolerance and diabetes. J. Am. Coll. Nutr. 1998, 17, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C.; Prohaska, C.; Pomazal, K.; Steffan, I.; Schernthaner, G.; Marktl, W. Concentrations of seven trace elements in different hematological matrices in patients with type 2 diabetes compared to healthy controls. Biol. Trace Elem. Res. 2001, 7, 205–219. [Google Scholar] [CrossRef]
- Padmavathi, I.J.; Rao, K.R.; Venu, L.; Ganeshan, M.; Kumar, K.A.; Rao, C.N.; Harishankar, N.; Ismail, A.; Raghunath, M. Chronic maternal dietary chromium restriction modulates visceral adiposity: Probable underlying mechanisms. Diabetes 2010, 59, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Bryden, N.A.; Polansky, M.M.; Thorp, J.W. Effect of carbohydrate loading and underwater exercise on circulating cortisol, insulin and urinary losses of chromium and zinc. Eur. J. Appl. Occup. Physiol. 1991, 63, 146–150. [Google Scholar] [CrossRef]
- Hahn, M.K.; Wolever, T.M.; Arenovich, T.; Teo, C.; Giacca, A.; Powell, V.; Clarke, L.; Fletcher, P.; Cohn, T.; McIntyre, R.S.; et al. Acute effects of single-dose olanzapine on metabolic, endocrine, and inflammatory markers in healthy controls. J. Clin. Psychopharmacol. 2013, 33, 740–746. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Belviranli, M. Serum levels of calcium, selenium, magnesium, phosphorus, chromium, copper and iron--their relation to zinc in rats with induced hypothyroidism. Acta Clin. Croat. 2013, 52, 151–156. [Google Scholar]
- Ugwuja, E.I.; Ugwu, N.C.; Aloke, C.; Idenyi, J.N.; Nwibo, A.N.; Ibiam, U.A.; Ezenkwa, U.S. Effects of glycaemic status on plasma levels of calcium, chromium, copper, iron, magnesium, selenium and zinc in diabetic rats. Int. J. Diabetes Res. 2012, 1, 92–95. [Google Scholar]
- Matthews, D.R.; Hoske, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-P.; Wang, Y.-Y.; Lin, S.-Y.; Hong, Y.-J.; Liao, K.-Y.; Hsieh, S.-K.; Pan, P.-H.; Chen, C.-J.; Chen, W.-Y. Olanzapine Induced Dysmetabolic Changes Involving Tissue Chromium Mobilization in Female Rats. Int. J. Mol. Sci. 2019, 20, 640. https://doi.org/10.3390/ijms20030640
Yang C-P, Wang Y-Y, Lin S-Y, Hong Y-J, Liao K-Y, Hsieh S-K, Pan P-H, Chen C-J, Chen W-Y. Olanzapine Induced Dysmetabolic Changes Involving Tissue Chromium Mobilization in Female Rats. International Journal of Molecular Sciences. 2019; 20(3):640. https://doi.org/10.3390/ijms20030640
Chicago/Turabian StyleYang, Ching-Ping, Ya-Yu Wang, Shih-Yi Lin, Yi-Jheng Hong, Keng-Ying Liao, Sheng-Kuo Hsieh, Ping-Ho Pan, Chun-Jung Chen, and Wen-Ying Chen. 2019. "Olanzapine Induced Dysmetabolic Changes Involving Tissue Chromium Mobilization in Female Rats" International Journal of Molecular Sciences 20, no. 3: 640. https://doi.org/10.3390/ijms20030640
APA StyleYang, C.-P., Wang, Y.-Y., Lin, S.-Y., Hong, Y.-J., Liao, K.-Y., Hsieh, S.-K., Pan, P.-H., Chen, C.-J., & Chen, W.-Y. (2019). Olanzapine Induced Dysmetabolic Changes Involving Tissue Chromium Mobilization in Female Rats. International Journal of Molecular Sciences, 20(3), 640. https://doi.org/10.3390/ijms20030640




