Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review
Abstract
1. Introduction
2. Types of Vascular Calcification
3. Matrix Gla Protein
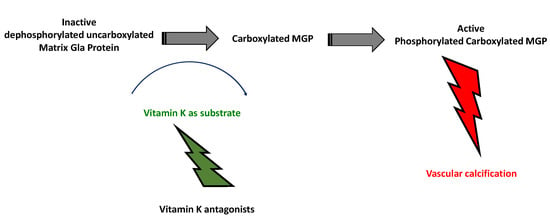
3.1. Role and Activation
3.2. MGP Forms
3.3. MGP and VC
3.4. Uncarboxylated Form of MGP: An Early VC Biomarker
3.5. Dephosphorylated, Uncarboxylated MGP: the Fully Inactive Circulating Form of MGP
3.6. DpucMGP and Renal Function
4. DpucMGP and Arterial Calcification/Stiffness
4.1. General Population
4.2. Patients with Diabetes Mellitus
4.3. Patients with Hypertension
4.4. CKD and HD Patients
5. DpucMGP and Mortality/CV Events
5.1. General Population
5.2. Patients with Diabetes Mellitus
5.3. CKD and HD Patients
5.4. Patients with High CVD Risk and Heart Failure
6. MGP Gene Polymorphisms, VC, Mortality and CVD
7. Vitamin K Affects Activation of MGP
8. Effect of Vitamin K Supplementation on MGP Forms in Human Interventional Studies
8.1. General Population
8.2. CKD and HD Patients
8.3. Patients with High CVD Risk and Heart Failure
8.4. Ongoing Clinical Trials
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, N.X.; Moe, S.M. Arterial calcification in diabetes. Curr. Diab. Rep. 2003, 3, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative stress in hemodialysis patients: A review of the literature. Oxid. Med. Cell Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2018, 32, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; Moe, S.M. Vascular calcification in chronic kidney disease. Semin. Nephrol. 2004, 24, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI—Atheromatous affection of arteries. 1858. Nutr. Rev. 1989, 47, 23–25. [Google Scholar] [CrossRef]
- Doherty, T.M.; Detrano, R.C. Coronary arterial calcification as an active process: A new perspective on an old problem. Calcif. Tissue Int. 1994, 54, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, R.J.; Kessels, A.G.; Schurgers, L.J.; van Engelshoven, J.M.; de Leeuw, P.W.; Kroon, A.A. Vascular calcifications as a marker of increased cardiovascular risk: A meta-analysis. Vasc. Health Risk Manag. 2009, 5, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Shaw, L.J.; Berman, D.S.; Callister, T.Q. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J. Am. Coll. Cardiol. 2004, 43, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Marchais, S.J.; Guerin, A.P.; Metivier, F.; Adda, H. Arterial structure and function in end-stage renal disease. Nephrol. Dial Transplant. 2002, 17, 1713–1724. [Google Scholar] [CrossRef]
- Demer, L.L.; Tintut, Y. Vascular calcification: Pathobiology of a multifaceted disease. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A. Gla-containing proteins of bone. Connect. Tissue Res. 1989, 21, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Urist, M.R.; Otawara, Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem. Biophys. Res. Commun. 1983, 117, 765–771. [Google Scholar] [CrossRef]
- Sato, M.; Yasui, N.; Nakase, T.; Kawahata, H.; Sugimoto, M.; Hirota, S.; Kitamura, Y.; Nomura, S.; Ochi, T. Expression of bone matrix proteins mRNA during distraction osteogenesis. J. Bone Miner. Res. 1998, 13, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Munroe, P.B.; Olgunturk, R.O.; Fryns, J.P.; Van Maldergem, L.; Ziereisen, F.; Yuksel, B.; Gardiner, R.M.; Chung, E. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat. Genet. 1999, 21, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Teebi, A.S.; Lambert, D.M.; Kaye, G.M.; Al-Fifi, S.; Tewfik, T.L.; Azouz, E.M. Keutel syndrome: Further characterization and review. Am. J. Med. Genet. 1998, 78, 182–187. [Google Scholar] [CrossRef]
- Shanahan, C.M. Mechanisms of vascular calcification in renal disease. Clin. Nephrol. 2005, 63, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Teunissen, K.J.; Knapen, M.H.; Kwaijtaal, M.; van Diest, R.; Appels, A.; Reutelingsperger, C.P.; Cleutjens, J.P.; Vermeer, C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: Undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.M.; Cary, N.R.; Salisbury, J.R.; Proudfoot, D.; Weissberg, P.L.; Edmonds, M.E. Medial localization of mineralization-regulating proteins in association with Mönckeberg’s sclerosis: Evidence for smooth muscle cell–mediated vascular calcification. Circulation 1999, 100, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, K.; Watson, K.E.; Horn, S.; Wortham, C.; Herman, I.M.; Demer, L.L. Bone morphogenetic protein expression in human atherosclerotic lesions. J. Clin. Invest. 1993, 91, 1800–1809. [Google Scholar] [CrossRef]
- Zebboudj, A.F.; Imura, M.; Bostrom, K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J. Biol. Chem. 2002, 277, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Shea, C.M.; Edgar, C.M.; Einhorn, T.A.; Gerstenfeld, L.C. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J. Cell Biochem. 2003, 90, 1112–1127. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, A.; Sane, D.C.; Hutson, S.M.; Wallin, R. Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats. J. Thromb. Haemost. 2003, 1, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.E.; Nishimoto, S.K. Matrix Gla protein binding to hydroxyapatite is dependent on the ionic environment: calcium enhances binding affinity but phosphate and magnesium decrease affinity. Bone 2002, 31, 296–302. [Google Scholar] [CrossRef]
- Murshed, M.; Schinke, T.; McKee, M.D.; Karsenty, G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J. Cell Biol. 2004, 165, 625–630. [Google Scholar] [CrossRef]
- Shearer, M.J. Vitamin K. Lancet 1995, 345, 229–234. [Google Scholar] [CrossRef]
- Wallin, R.; Cain, D.; Hutson, S.M.; Sane, D.C.; Loeser, R. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2). Thromb. Haemost. 2000, 84, 1039–1044. [Google Scholar]
- Schurgers, L.J.; Spronk, H.M.; Skepper, J.N.; Hackeng, T.M.; Shanahan, C.M.; Vermeer, C.; Weissberg, P.L.; Proudfoot, D. Post-translational modifications regulate matrix Gla protein function: importance for inhibition of vascular smooth muscle cell calcification. J. Thromb. Haemost. 2007, 5, 2503–2511. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Cranenburg, E.C.; Vermeer, C. Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost. 2008, 100, 593–603. [Google Scholar]
- Wallin, R.; Wajih, N.; Greenwood, G.T.; Sane, D.C. Arterial calcification: A review of mechanisms, animal models, and the prospects for therapy. Med. Res. Rev. 2001, 21, 274–301. [Google Scholar] [CrossRef]
- Khavandgar, Z.; Roman, H.; Li, J.; Lee, S.; Vali, H.; Brinckmann, J.; Davis, E.C.; Murshed, M. Elastin haploinsufficiency impedes the progression of arterial calcification in MGP-deficient mice. J. Bone Miner. Res. 2014, 29, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.M.; Cary, N.R.; Metcalfe, J.C.; Weissberg, P.L. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J. Clin. Invest. 1994, 93, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Dhore, C.R.; Cleutjens, J.P.; Lutgens, E.; Cleutjens, K.B.; Geusens, P.P.; Kitslaar, P.J.; Tordoir, J.H.; Spronk, H.M.; Vermeer, C.; Daemen, M.J. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1998–2003. [Google Scholar] [CrossRef]
- Braam, L.A.; Dissel, P.; Gijsbers, B.L.; Spronk, H.M.; Hamulyak, K.; Soute, B.A.; Debie, W.; Vermeer, C. Assay for human matrix gla protein in serum: Potential applications in the cardiovascular field. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Dissel, P.E.; Spronk, H.M.; Soute, B.A.; Dhore, C.R.; Cleutjens, J.P.; Vermeer, C. Role of vitamin K and vitamin K-dependent proteins in vascular calcification. Z. Kardiol. 2001, 90, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Roijers, R.B.; Debernardi, N.; Cleutjens, J.P.; Schurgers, L.J.; Mutsaers, P.H.; van der Vusse, G.J. Microcalcifications in early intimal lesions of atherosclerotic human coronary arteries. Am. J. Pathol. 2011, 178, 2879–2887. [Google Scholar] [CrossRef]
- Chatrou, M.L.; Cleutjens, J.P.; van der Vusse, G.J.; Roijers, R.B.; Mutsaers, P.H.; Schurgers, L.J. Intra-section analysis of human coronary arteries reveals a potential role for micro-calcifications in macrophage recruitment in the early stage of atherosclerosis. PLoS ONE 2015, 10, e0142335. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Spronk, H.M.; Soute, B.A.; Schiffers, P.M.; DeMey, J.G.; Vermeer, C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood 2007, 109, 2823–2831. [Google Scholar] [CrossRef]
- Lomashvili, K.A.; Wang, X.; Wallin, R.; O’Neill, W.C. Matrix Gla protein metabolism in vascular smooth muscle and role in uremic vascular calcification. J. Biol. Chem. 2011, 286, 28715–28722. [Google Scholar] [CrossRef]
- Rennenberg, R.J.; de Leeuw, P.W.; Kessels, A.G.; Schurgers, L.J.; Vermeer, C.; van Engelshoven, J.M.; Kemerink, G.J.; Kroon, A.A. Calcium scores and matrix Gla protein levels: Association with vitamin K status. Eur. J. Clin. Invest. 2010, 40, 344–349. [Google Scholar] [CrossRef]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.J.; Vermeer, C.; Elias, S.G.; Velthuis, B.K.; de Jong, P.A.; Beulens, J.W. Circulating species of matrix Gla protein and the risk of vascular calcification in healthy women. Int. J. Cardiol. 2013, 168, e168–e170. [Google Scholar] [CrossRef]
- Parker, B.D.; Schurgers, L.J.; Brandenburg, V.M.; Christenson, R.H.; Vermeer, C.; Ketteler, M.; Shlipak, M.G.; Whooley, M.A.; Ix, J.H. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul study. Ann. Intern. Med. 2010, 152, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Nigwekar, S.U.; Bloch, D.B.; Nazarian, R.M.; Vermeer, C.; Booth, S.L.; Xu, D.; Thadhani, R.I.; Malhotra, R. Vitamin K-dependent carboxylation of matrix Gla protein influences the risk of calciphylaxis. J. Am. Soc. Nephrol. 2017, 28, 1717–1722. [Google Scholar] [CrossRef]
- Hermans, M.M.; Vermeer, C.; Kooman, J.P.; Brandenburg, V.; Ketteler, M.; Gladziwa, U.; Rensma, P.L.; Leunissen, K.M.; Schurgers, L.J. Undercarboxylated matrix GLA protein levels are decreased in dialysis patients and related to parameters of calcium-phosphate metabolism and aortic augmentation index. Blood Purif. 2007, 25, 395–401. [Google Scholar] [CrossRef]
- Cranenburg, E.C.; Brandenburg, V.M.; Vermeer, C.; Stenger, M.; Muhlenbruch, G.; Mahnken, A.H.; Gladziwa, U.; Ketteler, M.; Schurgers, L.J. Uncarboxylated matrix Gla protein (ucMGP) is associated with coronary artery calcification in haemodialysis patients. Thromb. Haemost. 2009, 101, 359–366. [Google Scholar] [PubMed]
- Cranenburg, E.C.; Vermeer, C.; Koos, R.; Boumans, M.L.; Hackeng, T.M.; Bouwman, F.G.; Kwaijtaal, M.; Brandenburg, V.M.; Ketteler, M.; Schurgers, L.J. The circulating inactive form of matrix Gla protein (ucMGP) as a biomarker for cardiovascular calcification. J. Vasc. Res. 2008, 45, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.C.; Shah, V.; Hiorns, M.P.; Schoppet, M.; Hofbauer, L.C.; Hawa, G.; Schurgers, L.J.; Singhal, A.; Merryweather, I.; Brogan, P.; et al. The circulating calcification inhibitors, fetuin-A and osteoprotegerin, but not matrix Gla protein, are associated with vascular stiffness and calcification in children on dialysis. Nephrol. Dial. Transplant. 2008, 23, 3263–3271. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.D.; Price, P.A. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla protein. Implications for the possible functions of matrix Gla protein and for the tissue distribution of the gamma-carboxylase. J. Biol. Chem. 1988, 263, 11033–11036. [Google Scholar]
- Koos, R.; Krueger, T.; Westenfeld, R.; Kuhl, H.P.; Brandenburg, V.; Mahnken, A.H.; Stanzel, S.; Vermeer, C.; Cranenburg, E.C.; Floege, J.; et al. Relation of circulating Matrix Gla-Protein and anticoagulation status in patients with aortic valve calcification. Thromb. Haemost. 2009, 101, 706–713. [Google Scholar]
- O’Donnell, C.J.; Shea, M.K.; Price, P.A.; Gagnon, D.R.; Wilson, P.W.; Larson, M.G.; Kiel, D.P.; Hoffmann, U.; Ferencik, M.; Clouse, M.E.; et al. Matrix Gla protein is associated with risk factors for atherosclerosis but not with coronary artery calcification. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2769–2774. [Google Scholar] [CrossRef]
- Jono, S.; Ikari, Y.; Vermeer, C.; Dissel, P.; Hasegawa, K.; Shioi, A.; Taniwaki, H.; Kizu, A.; Nishizawa, Y.; Saito, S. Matrix Gla protein is associated with coronary artery calcification as assessed by electron-beam computed tomography. Thromb. Haemost. 2004, 91, 790–794. [Google Scholar] [PubMed]
- Parker, B.D.; Schurgers, L.J.; Vermeer, C.; Schiller, N.B.; Whooley, M.A.; Ix, J.H. The association of uncarboxylated matrix Gla protein with mitral annular calcification differs by diabetes status: The Heart and Soul study. Atherosclerosis 2010, 210, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Renard, C.; Magdeleyns, E.J.; Vermeer, C.; Choukroun, G.; Massy, Z.A. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: A preliminary report. Clin. J. Am. Soc. Nephrol. 2010, 5, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Zwakenberg, S.R.; van der Schouw, Y.T.; Vermeer, C.; Pasterkamp, G.; den Ruijter, H.M.; Beulens, J.W.J. Matrix Gla protein, plaque stability, and cardiovascular events in patients with severe atherosclerotic disease. Cardiology 2018, 141, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Barrett, H.; O’Keeffe, M.; Kavanagh, E.; Walsh, M.; O’Connor, E.M. Is matrix gla protein associated with vascular calcification? A systematic review. Nutrients 2018, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.; Koos, R.; Schurgers, L.J.; Magdeleyns, E.J.; Schoonbrood, T.H.; Landewe, R.B.; Brandenburg, V.M.; Bekers, O.; Vermeer, C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb. Haemost. 2010, 104, 811–822. [Google Scholar] [CrossRef]
- Delanaye, P.; Krzesinski, J.M.; Warling, X.; Moonen, M.; Smelten, N.; Medart, L.; Pottel, H.; Cavalier, E. Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol. 2014, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Obeid, R. Vitamins in the Prevention of Human Diseases; Walter de Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Wei, F.F.; Trenson, S.; Monney, P.; Yang, W.Y.; Pruijm, M.; Zhang, Z.Y.; Bouatou, Y.; Huang, Q.F.; Ponte, B.; Martin, P.Y.; et al. Epidemiological and histological findings implicate matrix Gla protein in diastolic left ventricular dysfunction. PLoS ONE 2018, 13, e0193967. [Google Scholar] [CrossRef] [PubMed]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Vermeer, C.; Magdeleyns, E.J.; Schurgers, L.J.; Beulens, J.W. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J. Nutr. Biochem. 2013, 24, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Boxma, P.Y.; van den Berg, E.; Geleijnse, J.M.; Laverman, G.D.; Schurgers, L.J.; Vermeer, C.; Kema, I.P.; Muskiet, F.A.; Navis, G.; Bakker, S.J.; et al. Vitamin K intake and plasma desphospho-uncarboxylated matrix Gla-protein levels in kidney transplant recipients. PLoS ONE 2012, 7, e47991. [Google Scholar] [CrossRef]
- Caluwe, R.; Vandecasteele, S.; Van Vlem, B.; Vermeer, C.; De Vriese, A.S. Vitamin K2 supplementation in haemodialysis patients: A randomized dose-finding study. Nephrol. Dial. Transplant. 2013, 29, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.J.; Vermeer, C.; Verschuren, W.M.; Boer, J.M.; Beulens, J.W. Matrix Gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care 2013, 36, 3766–3771. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Dahl, C.P.; Gullestad, L.; Aakhus, S.; Broch, K.; Skardal, R.; Vermeer, C.; Aukrust, P.; Schurgers, L.J. Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clin. Sci. (London) 2011, 121, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.C.; McNair, R.; Figg, N.; Skepper, J.N.; Schurgers, L.; Gupta, A.; Hiorns, M.; Donald, A.E.; Deanfield, J.; Rees, L.; et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 2008, 118, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: A randomized trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Fain, M.E.; Kapuku, G.K.; Paulson, W.D.; Williams, C.F.; Raed, A.; Dong, Y.; Knapen, M.H.J.; Vermeer, C.; Pollock, N.K. Inactive matrix gla protein, arterial stiffness, and endothelial function in African American hemodialysis patients. Am. J. Hypertens. 2018, 31, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Schlieper, G.; Westenfeld, R.; Kruger, T.; Cranenburg, E.C.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J. Am. Soc. Nephrol. 2011, 22, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Hallajzadeh, J.; Ghorbanihaghjo, A.; Argani, H.; Dastmalchi, S.; Rashtchizadeh, N. Growth arrest-specific 6 protein and matrix gla protein in hemodialysis patients. Iran. J. Kidney Dis. 2015, 9, 249–255. [Google Scholar] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Panagoutsos, S.; Giannakopoulou, E.; Papanas, N.; Manolopoulos, V.G.; Passadakis, P.; Tavridou, A. Matrix gla protein T-138C polymorphism is associated with carotid intima media thickness and predicts mortality in patients with diabetic nephropathy. J. Diabetes Complicat. 2017, 31, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.F.; Trenson, S.; Thijs, L.; Huang, Q.F.; Zhang, Z.Y.; Yang, W.Y.; Moliterno, P.; Allegaert, K.; Boggia, J.; Janssens, S.; et al. Desphospho-uncarboxylated matrix Gla protein is a novel circulating biomarker predicting deterioration of renal function in the general population. Nephrol. Dial. Transplant. 2017, 33, 1122–1128. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefanczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Plasma desphospho-uncarboxylated matrix Gla protein as a marker of kidney damage and cardiovascular risk in advanced stage of chronic kidney disease. Kidney Blood Press. Res. 2016, 41, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Puzantian, H.; Akers, S.R.; Oldland, G.; Javaid, K.; Miller, R.; Ge, Y.; Ansari, B.; Lee, J.; Suri, A.; Hasmath, Z.; et al. Circulating dephospho-uncarboxylated matrix Gla-protein is associated with kidney dysfunction and arterial stiffness. Am. J. Hypertens. 2018, 31, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Sardana, M.; Vasim, I.; Varakantam, S.; Kewan, U.; Tariq, A.; Koppula, M.R.; Syed, A.A.; Beraun, M.; Drummen, N.E.; Vermeer, C. Inactive matrix Gla-protein and arterial stiffness in type 2 diabetes mellitus. Am. J. Hypertens. 2017, 30, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Bourron, O.; Vemeer, C.; Theuwissen, E.; Magdeleyns, E.; Aubert, C.E.; Brazier, M.; Mentaverri, R.; Hartemann, A.; Massy, Z.A. Vascular calcification in patients with type 2 diabetes: The involvement of matrix Gla protein. Cardiovasc. Diabetol. 2014, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.D.; Ix, J.H.; Cranenburg, E.C.; Vermeer, C.; Whooley, M.A.; Schurgers, L.J. Association of kidney function and uncarboxylated matrix Gla protein: Data from the Heart and Soul Study. Nephrol. Dial. Transplant. 2009, 24, 2095–20101. [Google Scholar] [CrossRef] [PubMed]
- Thamratnopkoon, S.; Susantitaphong, P.; Tumkosit, M.; Katavetin, P.; Tiranathanagul, K.; Praditpornsilpa, K.; Eiam-Ong, S. Correlations of plasma desphosphorylated uncarboxylated matrix Gla protein with vascular calcification and vascular stiffness in chronic kidney disease. Nephron 2017, 135, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.F.; Drummen, N.E.; Schutte, A.E.; Thijs, L.; Jacobs, L.; Petit, T.; Yang, W.Y.; Smith, W.; Zhang, Z.Y.; Gu, Y.M. Vitamin K dependent protection of renal function in multi-ethnic population studies. EBioMedicine 2016, 4, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.F.; Thijs, L.; Zhang, Z.Y.; Jacobs, L.; Yang, W.Y.; Salvi, E.; Citterio, L.; Cauwenberghs, N.; Kuznetsova, T.; Drummen, N.E.A.; et al. The risk of nephrolithiasis is causally related to inactive matrix Gla protein, a marker of vitamin K status: A Mendelian randomization study in a Flemish population. Nephrol. Dial. Transplant. 2018, 33, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Toledo, C.; Thomas, G.; Schold, J.D.; Arrigain, S.; Gornik, H.L.; Nally, J.V.; Navaneethan, S.D. Renal resistive index and mortality in chronic kidney disease. Hypertension 2015, 66, 382–388. [Google Scholar] [CrossRef]
- Pivin, E.; Pruijm, M.; Ackermann, D.; Guessous, I.; Ehret, G.; Pechere-Bertschi, A.; Paccaud, F.; Mohaupt, M.; Vermeer, C.; Staessen, J.A.; et al. 1d.03: Inactive matrix Gla protein is associated with renal resistive index in a population-based study. J. Hypertens. 2015, 33. [Google Scholar] [CrossRef][Green Version]
- Rennenberg, R.J.; Schurgers, L.J.; Vermeer, C.; Scholte, J.B.; Houben, A.J.; de Leeuw, P.W.; Kroon, A.A. Renal handling of matrix Gla-protein in humans with moderate to severe hypertension. Hypertens. Res. 2008, 31, 1745–1751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyata, K.N.; Nast, C.C.; Dai, T.; Dukkipati, R.; LaPage, J.A.; Troost, J.P.; Schurgers, L.J.; Kretzler, M.; Adler, S.G. Renal matrix Gla protein expression increases progressively with CKD and predicts renal outcome. Exp. Mol. Pathol. 2018, 105, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Stamou, A.; Panagoutsos, S.; Theodoridis, M.; Kantartzi, K.; Tavridou, A.; Passadakis, P. The inactive dephosphorylated uncarboxylated form of matrix Gla protein is an indepedent predictor of renal function deterioration in diabetic nephropathy. Nephrol. Dial. Transplant. 2018, 33, 441–442. [Google Scholar] [CrossRef]
- Cranenburg, E.C.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.; Dalmeijer, G.W.; Westerhuis, R.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; Laverman, G.D. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; Booth, S.L.; Hopman, W.M.; Holden, R.M. Assessment of potential biomarkers of subclinical vitamin K deficiency in patients with end-stage kidney disease. Can. J. Kidney Health Dis. 2014, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Holden, R.M.; Morton, A.R.; Garland, J.S.; Pavlov, A.; Day, A.G.; Booth, S.L. Vitamins K and D status in stages 3–5 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M. Matrix Gla-protein (MGP) not only inhibits calcification in large arteries but also may be renoprotective: Connecting the dots. EBioMedicine 2016, 4, 16–17. [Google Scholar] [CrossRef][Green Version]
- Ueland, T.; Gullestad, L.; Dahl, C.P.; Aukrust, P.; Aakhus, S.; Solberg, O.G.; Vermeer, C.; Schurgers, L.J. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J. Intern. Med. 2010, 268, 483–492. [Google Scholar] [CrossRef]
- Shea, M.K.; O’Donnell, C.J.; Vermeer, C.; Magdeleyns, E.J.; Crosier, M.D.; Gundberg, C.M.; Ordovas, J.M.; Kritchevsky, S.B.; Booth, S.L. Circulating uncarboxylated matrix gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J. Nutr. 2011, 141, 1529–1534. [Google Scholar] [CrossRef]
- Pivin, E.; Ponte, B.; Pruijm, M.; Ackermann, D.; Guessous, I.; Ehret, G.; Liu, Y.P.; Drummen, N.E.; Knapen, M.H.; Pechere-Bertschi, A.; et al. Inactive matrix Gla-protein is associated with arterial stiffness in an adult population-based study. Hypertension 2015, 66, 85–92. [Google Scholar] [CrossRef]
- Knapen, M.H.; Braam, L.A.; Drummen, N.E.; Bekers, O.; Hoeks, A.P.; Vermeer, C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb. Haemost. 2015, 113, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O., Jr.; Seidlerova, J.; Wohlfahrt, P.; Filipovsky, J.; Vanek, J.; Cifkova, R.; Windrichova, J.; Topolcan, O.; Knapen, M.H.; Drummen, N.E.; et al. Desphospho-uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. J. Hum. Hypertens. 2016, 30, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Aoun, M.; Makki, M.; Azar, H.; Matta, H.; Chelala, D.N. High dephosphorylated-uncarboxylated MGP in hemodialysis patients: Risk factors and response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol. 2017, 18, 191. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, R.J.; van Varik, B.J.; Schurgers, L.J.; Hamulyak, K.; Ten Cate, H.; Leiner, T.; Vermeer, C.; de Leeuw, P.W.; Kroon, A.A. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood 2010, 115, 5121–5123. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Sardana, M.; Syed, A.A.; Koppula, M.R.; Varakantam, S.; Vasim, I.; Oldland, H.G.; Phan, T.S.; Drummen, N.E.A.; Vermeer, C.; et al. Aldosterone, inactive matrix Gla-protein, and large artery stiffness in hypertension. J. Am. Soc. Hypertens. 2018, 12, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, E.G.; van Schoor, N.M.; Lips, P.; Magdeleyns, E.J.; Deeg, D.J.; Vermeer, C.; den Heijer, M. Circulating uncarboxylated matrix Gla protein, a marker of vitamin K status, as a risk factor of cardiovascular disease. Maturitas 2014, 77, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Gu, Y.M.; Thijs, L.; Knapen, M.H.; Salvi, E.; Citterio, L.; Petit, T.; Carpini, S.D.; Zhang, Z.; Jacobs, L.; et al. Inactive matrix Gla protein is causally related to adverse health outcomes: A Mendelian randomization study in a Flemish population. Hypertension 2015, 65, 463–470. [Google Scholar] [CrossRef]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.J.; Vermeer, C.; Verschuren, W.M.; Boer, J.M.; Beulens, J.W. Circulating desphospho-uncarboxylated matrix gamma-carboxyglutamate protein and the risk of coronary heart disease and stroke. J. Thromb. Haemost. 2014, 12, 1028–1034. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Panagoutsos, S.; Giannakopoulou, E.; Papanas, N.; Manolopoulos, V.; Tavridou, A.; Passadakis, P. Matrix Gla Protein T 138C polymorphism and its inactive dephosphorylated uncarboxylated form predict mortality in patients with diabetic nephropathy. Nephrol. Dial. Transplant. 2017, 32, 607–608. [Google Scholar] [CrossRef]
- Keyzer, C.A.; Vermeer, C.; Joosten, M.M.; Knapen, M.H.; Drummen, N.E.; Navis, G.; Bakker, S.J.; de Borst, M.H. Vitamin K status and mortality after kidney transplantation: A cohort study. Am. J. Kidney Dis. 2015, 65, 474–483. [Google Scholar] [CrossRef]
- Capoulade, R.; Cote, N.; Mathieu, P.; Chan, K.L.; Clavel, M.A.; Dumesnil, J.G.; Teo, K.K.; Tam, J.W.; Fournier, D.; Despres, J.P.; et al. Circulating levels of matrix Gla protein and progression of aortic stenosis: A substudy of the Aortic Stenosis Progression Observation: Measuring Effects of rosuvastatin (ASTRONOMER) trial. Can. J. Cardiol. 2014, 30, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O., Jr.; Seidlerova, J.; Bruthans, J.; Filipovsky, J.; Timoracka, K.; Vanek, J.; Cerna, L.; Wohlfahrt, P.; Cifkova, R.; Theuwissen, E.; et al. Desphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis 2014, 235, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O., Jr.; Seidlerova, J.; Vanek, J.; Karnosova, P.; Bruthans, J.; Filipovsky, J.; Wohlfahrt, P.; Cifkova, R.; Windrichova, J.; Knapen, M.H.; et al. The abnormal status of uncarboxylated matrix Gla protein species represents an additional mortality risk in heart failure patients with vascular disease. Int. J. Cardiol. 2016, 203, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, S.; Ede, J.; Schurgers, L.; Vermeer, C.; Kander, T.; Klarin, B.; Schott, U. Desphospho-uncarboxylated matrix-Gla protein is increased postoperatively in cardiovascular risk patients. Nutrients 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, L.; Bu, C. Vitamin K status and cardiovascular events or mortality: A meta-analysis. Eur. J. Prev. Cardiol. 2018, 0, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.N.; El Hallek, M.; Hopkin, R.J.; Sund, K.L.; Henrickson, M.; Del Gaudio, D.; Yuksel, A.; Acar, G.O.; Bober, M.B.; Kim, J.; et al. Keutel syndrome: Report of two novel MGP mutations and discussion of clinical overlap with arylsulfatase E deficiency and relapsing polychondritis. Am. J. Med. Genet. A 2014, 164A, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.; Van Spaendonck-Zwarts, K.Y.; Bonafe, L.; Mittaz Crettol, L.; Rodiger, L.A.; Dikkers, F.G.; Van Essen, A.J.; Superti-Furga, A.; Alexandrakis, E.; Vermeer, C.; et al. Circulating matrix gamma-carboxyglutamate protein (MGP) species are refractory to vitamin K treatment in a new case of Keutel syndrome. J. Thromb. Haemost. 2011, 9, 1225–1235. [Google Scholar] [CrossRef]
- Cozzolino, M.; Biondi, M.L.; Galassi, A.; Cusi, D.; Brancaccio, D.; Gallieni, M. Vascular calcification and cardiovascular outcome in dialysis patients: The role of gene polymorphisms. Blood Purif. 2010, 29, 347–351. [Google Scholar] [CrossRef]
- Herrmann, S.M.; Whatling, C.; Brand, E.; Nicaud, V.; Gariepy, J.; Simon, A.; Evans, A.; Ruidavets, J.B.; Arveiler, D.; Luc, G. Polymorphisms of the human matrix Gla protein (MGP) gene, vascular calcification, and myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2386–2393. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Abe, H.; Tominaga, T.; Nakamura, M.; Kishi, S.; Matsuura, M.; Nagai, K.; Tsuchida, K.; Minakuchi, J.; Doi, T. Polymorphism in the human matrix Gla protein gene is associated with the progression of vascular calcification in maintenance hemodialysis patients. Clin. Exp. Nephrol. 2013, 17, 882–889. [Google Scholar] [CrossRef]
- Karsli Ceppioglu, S.; Yurdun, T.; Canbakan, M. Assessment of matrix Gla protein, Klotho gene polymorphisms, and oxidative stress in chronic kidney disease. Ren. Fail. 2011, 33, 866–874. [Google Scholar] [CrossRef]
- Najafi, M.; Roustazadeh, A.; Amirfarhangi, A.; Kazemi, B. Matrix Gla protein (MGP) promoter polymorphic variants and its serum level in stenosis of coronary artery. Mol. Biol. Rep. 2014, 41, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Kitazawa, R.; Maeda, S.; Schurgers, L.; Kitazawa, S. T-138C polymorphism of matrix Gla protein promoter alters its expression but is not directly associated with atherosclerotic vascular calcification. Kobe J. Med. Sci. 2004, 50, 69–81. [Google Scholar] [PubMed]
- Wang, Y.; Chen, J.; Zhang, Y.; Yu, W.; Zhang, C.; Gong, L.; Shao, L.; Lu, J.; Gao, Y.; Chen, X.; et al. Common genetic variants of MGP are associated with calcification on the arterial wall but not with calcification present in the atherosclerotic plaques. Circ. Cardiovasc. Genet. 2013, 6, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Honda, H.; Qureshi, A.; Pecoits-Filho, R.; Axelsson, J.; Nordfors, L.; Schalling, M.; Hoff, C.; Holmes, C.; Heimburger, O. The matrix GLA protein-138 genotype is associated with clinical utcome in end-stage renal disease patients. In Proceedings of the ERA-EDTA XLI Congress 2004, Lisbon, Portugal, 15–18 May 2004; pp. 15–18. [Google Scholar]
- Farzaneh-Far, A.; Davies, J.D.; Braam, L.A.; Spronk, H.M.; Proudfoot, D.; Chan, S.W.; O’Shaughnessy, K.M.; Weissberg, P.L.; Vermeer, C.; Shanahan, C.M. A polymorphism of the human matrix gamma-carboxyglutamic acid protein promoter alters binding of an activating protein-1 complex and is associated with altered transcription and serum levels. J. Biol. Chem. 2001, 276, 32466–32473. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, D.; Biondi, M.L.; Gallieni, M.; Turri, O.; Galassi, A.; Cecchini, F.; Russo, D.; Andreucci, V.; Cozzolino, M. Matrix Gla protein gene polymorphisms: Clinical correlates and cardiovascular mortality in chronic kidney disease patients. Am. J. Nephrol. 2005, 25, 548–552. [Google Scholar] [CrossRef]
- Crosier, M.D.; Booth, S.L.; Peter, I.; Dawson-Hughes, B.; Price, P.A.; O’Donnell, C.J.; Hoffmann, U.; Williamson, M.K.; Ordovas, J.M. Matrix Gla protein polymorphisms are associated with coronary artery calcification in men. J. Nutr. Sci. Vitaminol. (Tokyo) 2009, 55, 59–65. [Google Scholar] [CrossRef]
- Taylor, B.C.; Schreiner, P.J.; Doherty, T.M.; Fornage, M.; Carr, J.J.; Sidney, S. Matrix Gla protein and osteopontin genetic associations with coronary artery calcification and bone density: The CARDIA study. Hum. Genet. 2005, 116, 525–528. [Google Scholar] [CrossRef]
- Ataman, O.V.; Polonikov, O.V.; Harbuzova, V.; Ataman Iu, O.; Matlai, O.I. Analysis of matrix Gla-protein (MGP) G-7A polymorphism association with ischemic atherothrombotic stroke in persons with risk factors. Tsitol. Genet. 2013, 47, 33–40. [Google Scholar]
- Garbuzova, V.Y.; Stroy, D.A.; Dosenko, V.E.; Dubovyk, Y.I.; Borodenko, A.O.; Shimko, K.A.; Obukhova, O.A.; Ataman, O.V. Association of allelic polymorphisms of genes matrix Gla-protein system with ischemic atherothrombotic stroke. Fiziol. Zh. 2015, 61, 19–27. [Google Scholar] [CrossRef]
- Harbuzova, V.; Matlai, O.I.; Ataman Iu, O.; Dubovyk Ie, I.; Borodenko, A.O.; Obukhova, O.A.; Ataman, O.V. The polymorphism of matrix Gla-protein gene in ischemic atherothrombotic stroke patients. Fiziol. Zh. 2012, 58, 14–21. [Google Scholar] [PubMed]
- Garbuzova, V.Y.; Gurianova, V.L.; Stroy, D.A.; Dosenko, V.E.; Parkhomenko, A.N.; Ataman, A.V. Association of matrix Gla protein gene allelic polymorphisms (G(-7)-->A, T(-138)-->C and Thr(83)-->Ala) with acute coronary syndrome in the Ukrainian population. Exp. Clin. Cardiol. 2012, 17, 30–33. [Google Scholar] [PubMed]
- Harbuzova, V.; Hur’ianova, V.L.; Parkhomenko, O.M.; Dosenko, V.; Ataman, O.V. The frequency of allelic polymorphism of matrix Gla-protein gene in acute coronary syndrome patients. Fiziol. Zh. 2011, 57, 16–24. [Google Scholar] [PubMed]
- Tunon-Le Poultel, D.; Cannata-Andia, J.B.; Roman-Garcia, P.; Diaz-Lopez, J.B.; Coto, E.; Gomez, C.; Naves-Diaz, M.; Rodriguez, I. Association of matrix Gla protein gene functional polymorphisms with loss of bone mineral density and progression of aortic calcification. Osteoporos. Int. 2014, 25, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Roustazadeh, A.; Najafi, M.; Amirfarhangi, A.; Nourmohammadi, I. No association between MGP rs1800802 polymorphism and stenosis of the coronary artery. Ann. Saudi. Med. 2013, 33, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Zhang, P.; Lin, W.; Cheng, J.; Li, J.; Chen, J. Association of Matrix Gla protein gene (rs1800801, rs1800802, rs4236) polymorphism with vascular calcification and atherosclerotic disease: A meta-analysis. Sci. Rep. 2017, 7, 8713. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.; van der Meer, I.M.; Hofman, A.; Witteman, J.C. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam Study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.; Bots, M.L.; Atsma, F.; Bartelink, M.L.; Prokop, M.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; van der Schouw, Y.T. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009, 203, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Gast, G.C.; de Roos, N.M.; Sluijs, I.; Bots, M.L.; Beulens, J.W.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; Peeters, P.H.; van der Schouw, Y.T. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Hartley, L.; Clar, C.; Ghannam, O.; Flowers, N.; Stranges, S.; Rees, K. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2015, 9, CD011148. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.; Westenfeld, R.; Ketteler, M.; Schurgers, L.J.; Floege, J. Vitamin K deficiency in CKD patients: A modifiable risk factor for vascular calcification? Kidney Int. 2009, 76, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Pilkey, R.M.; Morton, A.R.; Boffa, M.B.; Noordhof, C.; Day, A.G.; Su, Y.; Miller, L.M.; Koschinsky, M.L.; Booth, S.L. Subclinical vitamin K deficiency in hemodialysis patients. Am. J. Kidney Dis. 2007, 49, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, D.; Veulemans, V.; Horn, P.; Chatrou, M.L.; Potthoff, S.A.; Kelm, M.; Schurgers, L.J.; Westenfeld, R. High-dose menaquinone-7 supplementation reduces cardiovascular calcification in a murine model of extraosseous calcification. Nutrients 2015, 7, 6991–7011. [Google Scholar] [CrossRef] [PubMed]
- Kaesler, N.; Magdeleyns, E.; Herfs, M.; Schettgen, T.; Brandenburg, V.; Fliser, D.; Vermeer, C.; Floege, J.; Schlieper, G.; Kruger, T. Impaired vitamin K recycling in uremia is rescued by vitamin K supplementation. Kidney Int. 2014, 86, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.; Ahmed, N.; Vermeer, C.; Beulens, J.W. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis 2012, 225, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Cranenburg, E.C.; Knapen, M.H.; Magdeleyns, E.J.; Teunissen, K.J.; Schurgers, L.J.; Smit, E.; Vermeer, C. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br. J. Nutr. 2012, 108, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Magdeleyns, E.J.; Braam, L.A.; Teunissen, K.J.; Knapen, M.H.; Binnekamp, I.A.; van Summeren, M.J.; Vermeer, C. Vitamin K status in healthy volunteers. Food Funct. 2014, 5, 229–234. [Google Scholar] [CrossRef]
- Shea, M.K.; O’Donnell, C.J.; Hoffmann, U.; Dallal, G.E.; Dawson-Hughes, B.; Ordovas, J.M.; Price, P.A.; Williamson, M.K.; Booth, S.L. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am. J. Clin. Nutr. 2009, 89, 1799–1807. [Google Scholar] [CrossRef]
- Theuwissen, E.; Teunissen, K.J.; Spronk, H.M.; Hamulyak, K.; Ten Cate, H.; Shearer, M.J.; Vermeer, C.; Schurgers, L.J. Effect of low-dose supplements of menaquinone-7 (vitamin K2) on the stability of oral anticoagulant treatment: Dose-response relationship in healthy volunteers. J. Thromb. Haemost. 2013, 11, 1085–1092. [Google Scholar] [CrossRef]
- Delanaye, P.; Dubois, B.E.; Lukas, P.; Peters, P.; Krzesinski, J.M.; Pottel, H.; Cavalier, E. Impact of stopping vitamin K antagonist therapy on concentrations of dephospho-uncarboxylated Matrix Gla protein. Clin. Chem. Lab. Med. 2015, 53, e191–e193. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefanczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3–5. Pol. Arch. Med. Wewn. 2015, 125, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.M.; Reinartz, S.; Kaesler, N.; Kruger, T.; Dirrichs, T.; Kramann, R.; Peeters, F.; Floege, J.; Keszei, A.; Marx, N.; et al. Slower progress of aortic valve calcification with vitamin K supplementation: Results from a prospective interventional proof-of-concept study. Circulation 2017, 135, 2081–2083. [Google Scholar] [CrossRef] [PubMed]
- Caluwe, R.; Pyfferoen, L.; De Boeck, K.; De Vriese, A.S. The effects of vitamin K supplementation and vitamin K antagonists on progression of vascular calcification: Ongoing randomized controlled trials. Clin. Kidney J. 2016, 9, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Holden, R.M.; Booth, S.L.; Day, A.G.; Clase, C.M.; Zimmerman, D.; Moist, L.; Shea, M.K.; McCabe, K.M.; Jamal, S.A.; Tobe, S. Inhibiting the progression of arterial calcification with vitamin K in HemoDialysis patients (iPACK-HD) trial: Rationale and study design for a randomized trial of vitamin K in patients with end stage kidney disease. Can. J. Kidney Health Dis. 2015, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.; Schlieper, G.; Schurgers, L.; Cornelis, T.; Cozzolino, M.; Jacobi, J.; Jadoul, M.; Ketteler, M.; Rump, L.C.; Stenvinkel, P.; et al. Vitamin K1 to slow vascular calcification in haemodialysis patients (VitaVasK trial): A rationale and study protocol. Nephrol. Dial. Transplant. 2014, 29, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Peeters, F.; van Mourik, M.J.W.; Meex, S.J.R.; Bucerius, J.; Schalla, S.M.; Gerretsen, S.C.; Mihl, C.; Dweck, M.R.; Schurgers, L.J.; Wildberger, J.E.; et al. Bicuspid aortic valve stenosis and the effect of vitamin K2 on calcification using (18)F-sodium fluoride positron emission tomography/magnetic resonance: The BASIK2 rationale and trial design. Nutrients 2018, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Vossen, L.M.; Schurgers, L.J.; van Varik, B.J.; Kietselaer, B.L.; Vermeer, C.; Meeder, J.G.; Rahel, B.M.; van Cauteren, Y.J.; Hoffland, G.A.; Rennenberg, R.J.; et al. Menaquinone-7 supplementation to reduce vascular calcification in patients with coronary artery disease: Rationale and study protocol (VitaK-CAC Trial). Nutrients 2015, 7, 8905–8915. [Google Scholar] [CrossRef]
| Population, Age, Study, Design | MGP Form | Calcification Dcore | Result |
|---|---|---|---|
| General Population | |||
| 438 general population adults, 68 y, [90], cross-sectional | DpcMGP (pmol/L) | CAC | - |
| 571 general population women, 57.3 y, [41], observational, prospective | DpucMGP (pmol/L) | CAC | ↑ |
| UcMGP (nmol/L) | - | ||
| DpcMGP (pmol/L) | - | ||
| 1001 general population subjects, 46.5 y, [91], multicenter, family-based, cross-sectional | DpucMGP (pmol/L) | Aortic PWV | ↑ |
| 244 healthy post-menopausal women, 59.5 y, [92], sross-sectional | DpucMGP (pmol/L) | cIMT | ↑ |
| Carotid–femoral PWV | ↑ | ||
| Endothelial dysfunction score | ↑ | ||
| 1087 general population subjects, 54.8 y, [93], cross-sectional | DpucMGP (pmol/L) | Femoro–popliteal PWV | ↑ |
| CKD and HD Patients | |||
| 120 HD patients, 61 y, [44], cross-sectional, multicenter | UcMGP (nmol/L) | Aortic augmentation index | ↓ |
| PWV | - | ||
| 61 HD children, 13.4 y, [47], cross-sectional | UcMGP (nmol/L) | cIMT | - |
| Carotid–femoral PWV | -- | ||
| Aortic augmentation index | - | ||
| CAC | - | ||
| 40 HD patients, 67 y, [45], cross-sectional | UcMGP (nmol/L) | CAC | ↓ |
| 107 patients with CKD stages 2–5, 67 y, [53], cross-sectional, prospective | DpucMGP (pmol/L) | Aortic calcification score | ↑ |
| 188 HD patients,59 y, [68], cross-sectional | DpucMGP (pmol/L) | cIMT | - |
| PWV | - | ||
| DpcMGP (pmol/L) | cIMT | - | |
| PWV | - | ||
| 136 HD patients, 74 y, [57], cross-sectional | DpucMGP (pmol/L) | Abdominal aortic calcification score | ↑ |
| 83 patients with CKD stages 3–5, 62.9 y, [77], cross-sectional | DpucMGP (pmol/L) | Abdominal aortic calcification | ↑ |
| Cardio–ankle vascular index | - | ||
| PWV | - | ||
| 50 HD patients, 71.5 y, [94], cross-sectional | DpucMGP (pmol/L) | Abdominal aortic calcification score | ↑ |
| 137 patients with various degrees of CKD, 60.7 y, [73], cross-sectional | DpucMGP (pmol/L) | Carotid–femoral PWV | ↑ |
| 37 HD patients with CKD stages 2–5, 47.7 y, [67], cross-sectional | DpucMGP (pmol/L) | Carotid–femoral PWV | ↑ |
| Brachial artery flow-mediated dilation | ↑ | ||
| High CVD Risk Patients | |||
| 191 aortic valve disease patients, 71 y, [49], observational | UcMGP (nmol/L) | Aortic valve calcification | - |
| 19 subjects treated with vitamin K antagonist, 48 y, [95], observational | DpcMGP (pmol/L) | Femoral artery calcification | ↑ |
| Population, Age, Study, Design | MGP Form | End-Point | Result |
|---|---|---|---|
| General Population | |||
| 1406 general population subjects, >49 y, [99], prospective, obseravtional | DpucMGP (pmol/L) | Stroke | - |
| CHD | - | ||
| DpucMGP (pmol/L) | ↑ | ||
| 577 general population subjects, >55 y, [97], cross-sectional | DpcMGP (pmol/L) | CV event | - |
| 2318 general population subjects, 43.5 y, [98], cross-sectional | DpucMGP (pmol/L) | Overall mortality | ↑ |
| Non-cancer mortality | ↑ | ||
| CVD mortality | ↑ | ||
| Coronary events | ↓ | ||
| 1054 general population subjects, 49.5 y, [59], population-based, cross-sectional | DpucMGP (μg/L) | Left ventricular dysfunction | ↑ |
| Patients with diabetes | |||
| DpucMGP (pmol/L) | CV events | ↑ | |
| PAD | ↑ | ||
| HF | ↑ | ||
| CHD | - | ||
| Stroke | - | ||
| DpcMGP (pmol/L) | CV events | - | |
| PAD | - | ||
| HF | - | ||
| CHD | - | ||
| Stroke | - | ||
| 518 T2DM patients, 58.1 y, [63], prospective, observational | UcMGP (nmol/L) | CV events | - |
| PAD | - | ||
| HF | - | ||
| CHD | - | ||
| Stroke | - | ||
| DpucMGP (pmol/L) | ↑ | ||
| 198 T2DM patients with various degrees of CKD, 64 y, [75], cross-sectional | UcMGP (nmol/L) | PAD | - |
| CKD and HD patients | |||
| 107 CKD patients, stages 2–5, 67 y, [53], prospective, cross-sectional | DpucMGP (pmol/L) | Overall mortality | ↑ |
| DpucMGP (pmol/L) | Overall mortality | ↓ | |
| CV mortality | ↓ | ||
| 188 HD patients, 59 y, [68], | DpcMGP (pmol/L) | Overall mortality | ↓ |
| cross-sectional | CV mortality | ↓ | |
| 518 kidney transplant recipients, 51 y, [101], observational with longitudinal design | DpucMGP (pmol/L) | Overall mortality | ↑ |
| Transplant failure | ↑ | ||
| 57 T2DM patients in CKD stages 1–5 and 10 T2DM controls, 68.6 y, [100], prospective, cross-sectional | DpucMGP (pmol/L) | Overall mortality | ↑ |
| CV mortality | ↑ | ||
| CV events | ↑ | ||
| High CVD risk and HF patients | |||
| DpucMGP (pmol/L) | Overall mortality | ↑ | |
| HF | ↑ | ||
| 147 aortic stenosis patients, 74 y, [89], cross-sectional | DpcMGP (pmol/L) | Overall mortality | - |
| HF | - | ||
| 833 CHD patients, 67 y, [42], observational, multicenter | UcMGP (nmol/L) | Overall mortality | ↓ |
| CV events | ↓ | ||
| 615 CVD non-diabetic patients, 68 y, [52], cross-sectional | ↓ | ||
| 221 CVD, diabetic patients, 68 y, [52], cross-sectional | UcMGP (nmol/L) | Mitral annular calcification | ↑ |
| DpucMGP (pmol/L) | ↑ | ||
| 179 HF patients, 56 y, [64], cross-sectional | DpcMGP (pmol/L) | HF mortality | - |
| 215 aortic stenosis patients, 18–82 y, [102], cross-sectional | DpMGP | AS progression | ↑ |
| (nmol/L) | |||
| DpucMGP (pmol/L) | Overall mortality | ↑ | |
| CV mortality | ↑ | ||
| DpcMGP (pmol/L) | Overall mortality | ↑ | |
| CV mortality | ↑ | ||
| 799 CVD patients, 65.1 y, [103,104], prospective, cross-sectional, multicenter | UcMGP (nmol/L) | Overall mortality | ↓ |
| CV mortality | ↓ | ||
| Population, Age, Study, Design | Study Groups | Intervention Time | End-Points | Result | |
|---|---|---|---|---|---|
| General Population | |||||
| 500 mg/day K1 | 3 years | MGP (ng/mL) | ↑ 3.5% | ||
| 388 healthy men and postmenopausal women, 68 y, [140], randomized, double-blind, placebo-controlled | placebo | ↓ 4% | |||
| 500 mg/day K1 + 10 μg vitamin D | ↓ 80% | ||||
| 374 general population subjects, 60–80 y, [90], randomized, placebo-controlled | Placebo + 10 μg vitamin D | 3 years | UcMGP (pmol/L) | ↓ 4% | |
| DpucMGP (pmol/L) | ↓ 31% | ||||
| DpcMGP (pmol/L) | - | ||||
| 180 μg/day MK-7 | UcMGP (nmol/L) | - | |||
| DpucMGP (pmol/L) | ↓ 46% | ||||
| DpcMGP (pmol/L) | - | ||||
| 360 μg/day MK-7 | UcMGP (nmol/L) | - | |||
| DpucMGP (pmol/L) | - | ||||
| 60 general population subjects, 40–65 y, [137], | DpcMGP (pmol/L) | - | |||
| randomized, double-blind, placebo-controlled | placebo | 12 weeks | UcMGP (nmol/L) | - | |
| 10 μg/day MK-7 | ↓ 12.1% | ||||
| 20 μg/day MK-7 | ↓ 10.9% | ||||
| 45 μg/day MK-7 | ↓ 12.3% | ||||
| 90 μg/day MK-7 | ↓ 33.6% | ||||
| 180 μg/day MK-7 | ↓ 39.7% | ||||
| 360 μg/day MK-7 | ↓ 56% | ||||
| 44 general population subjects, 18–45 y, [138], randomized, double-blind, placebo-controlled | placebo | 3 months | DpucMGP (pmol/L) | ↑ 16.8% | |
| 10 μg/day MK-7 | - | ||||
| 20 μg/day MK-7 | 6 weeks | DpucMGP (pmol/L) | - | ||
| 18 healthy subjects treated with VKA for 4 weeks, 29 y, [141], observational | 45 μg/day MK-7 | - | |||
| 45 μg/day MK-7 | ↓ 38% | ||||
| 42 healthy children, 6–10 y, [139], randomized, placebo-controlled | placebo | 2 months | DpucMGP (pmol/L) | - | |
| 90 μg/day MK-7 | ↓ 36% | ||||
| 69 healthy subjects, 20–40 y, [139], randomized, placebo-controlled | placebo | 7 weeks | DpucMGP (pmol/L) | - | |
| 244 healthy, postmenopausal women, 59.5 y, [92], randomized, double-blind, placebo-controlled | 180 μg/day MK-7 | ↓ 32% | |||
| placebo | 3 years | DpucMGP (pmol/L) | ↑ 22% | ||
| CKD and HD patients | |||||
| DpucMGP (pmol/L) | ↓ 27% | ||||
| 17 HD patients, 59 y, [68], non- placebo controlled | 135 μg/day MK-7 | 6 weeks | DpcMGP (pmol/L) | - | |
| 45 μg/day MK-7 | ↓ 17.9% | ||||
| 135 μg/day MK-7 | ↓ 36.7% | ||||
| 53 HD patients, 64.6 y, [66], randomized, non- placebo controlled | 360 μg/day MK-7 | 6 weeks | DpucMGP (pmol/L) | ↓ 61.1% | |
| 360 μg, thrice weekly MK-7 | ↓ 17% | ||||
| 720 μg, thrice weekly MK-7 | ↓ 33% | ||||
| 200 HD patients, 70.8 y, [62], randomized, prospective, single-blind | 1080 μg, thrice weekly MK-7 | 8 weeks | DpucMGP (pmol/L) | ↓ 46% | |
| 7 HD patients, 75 y, [142], observational | Stop VKA | 5 days | DpucMGP (pmol/L) | ↓ 40% | |
| 90 μg/day MK-7 | DpucMGP (pmol/L) | ↓ 19% | |||
| +10 μg/day | MGP (pg/mL) | ↑ | |||
| vitamin D | |||||
| 10 μg/day vitamin D | DpucMGP (pmol/L) | ↑ | |||
| 42 CKD patients, stages 3–5, 58y, [143], Randomized, non-placebo controlled | 9 months | MGP (pg/mL) | ↓ | ||
| 90 μg/day MK-7 | ↓ 10.7% | ||||
| +10 μg/day | |||||
| vitamin D | |||||
| 42 CKD patients, stages 4–5, 58 y, [72], randomized, double-blind | 10 μg/day vitamin D | 270 days | DpucMGP (pmol/L) | ↑ | |
| 50 HD patients, 64.6 y, [94], pre–post intervention | 360 μg/day MK-7 | 4 weeks | DpucMGP (pmol/L) | ↓ 86% | |
| High CVD risk and HF patients | |||||
| DpMGP (nmol/L) | |||||
| PMGP (nmol/L) | |||||
| UcMGP (pmol/L) | |||||
| 1 Keutel syndrome patient, 21 y, [108], case-report, observational | 10 mg/day K1 | 3 months | CMGP (pmol/L) | - | |
| ↓ 45% | |||||
| 2 mg/day K1 | |||||
| 72 AVC patients, 69.1 y, [144], single-center, open-label | placebo | 12 months | DpucMGP (pmol/L) | ↑ | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roumeliotis, S.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review. Int. J. Mol. Sci. 2019, 20, 628. https://doi.org/10.3390/ijms20030628
Roumeliotis S, Dounousi E, Eleftheriadis T, Liakopoulos V. Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review. International Journal of Molecular Sciences. 2019; 20(3):628. https://doi.org/10.3390/ijms20030628
Chicago/Turabian StyleRoumeliotis, Stefanos, Evangelia Dounousi, Theodoros Eleftheriadis, and Vassilios Liakopoulos. 2019. "Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review" International Journal of Molecular Sciences 20, no. 3: 628. https://doi.org/10.3390/ijms20030628
APA StyleRoumeliotis, S., Dounousi, E., Eleftheriadis, T., & Liakopoulos, V. (2019). Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review. International Journal of Molecular Sciences, 20(3), 628. https://doi.org/10.3390/ijms20030628