Optimization of Early Steps in Oncolytic Adenovirus ONCOS-401 Production in T-175 and HYPERFlasks
Abstract
:1. Introduction
2. Results
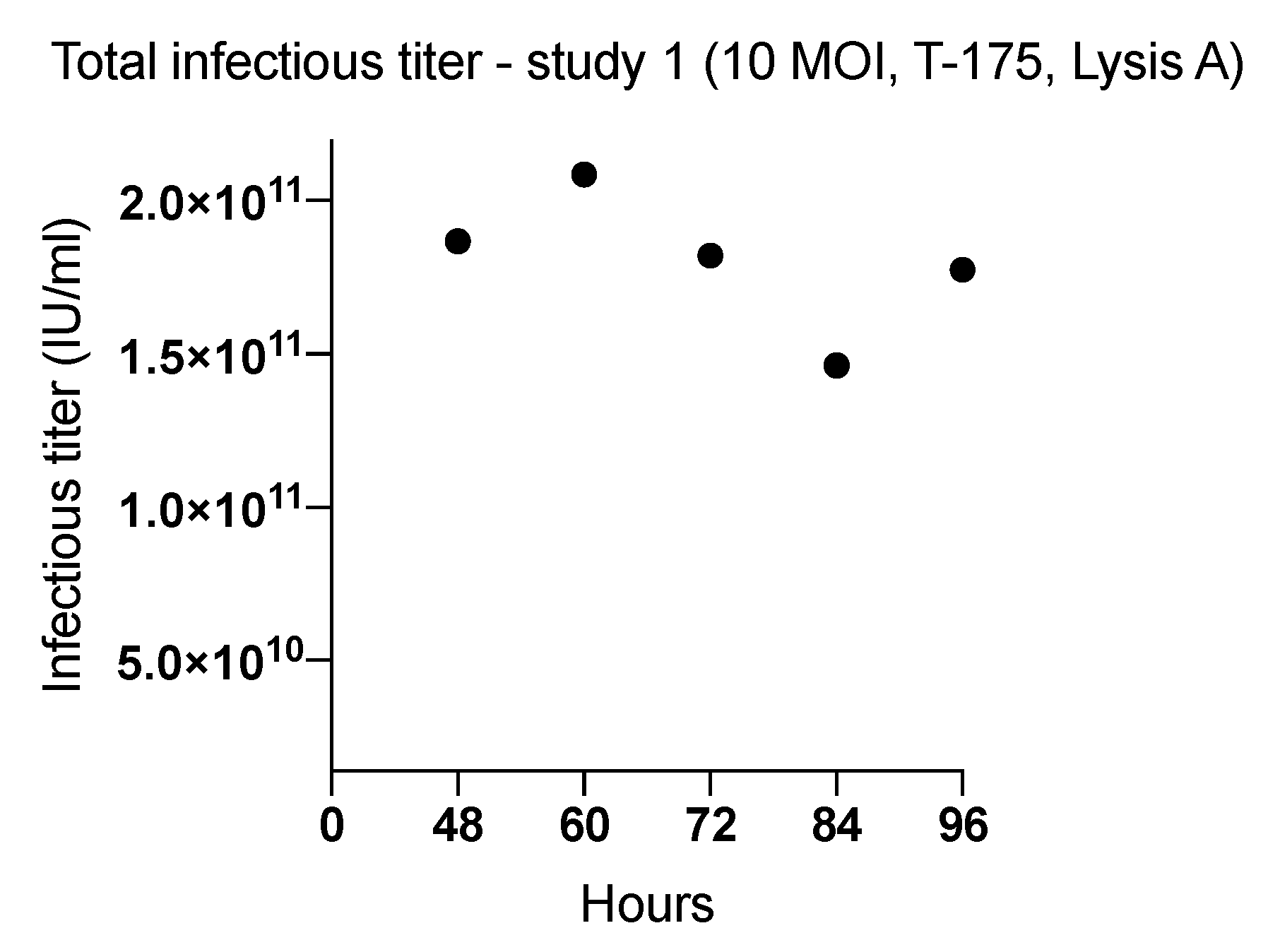
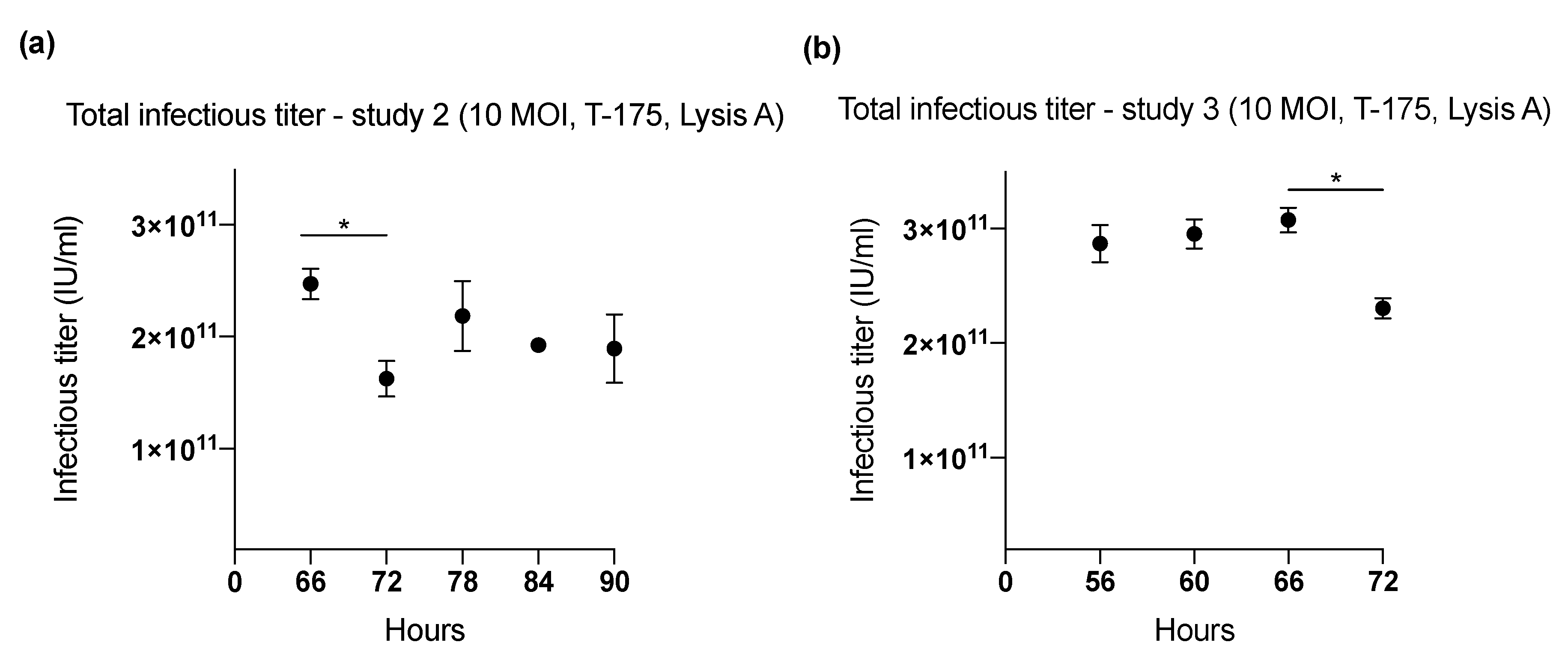
2.1. Effect of Harvesting Time After Infection with MOI of 10
2.2. Effect of Inoculum Size on Quantity of Harvested Viruses
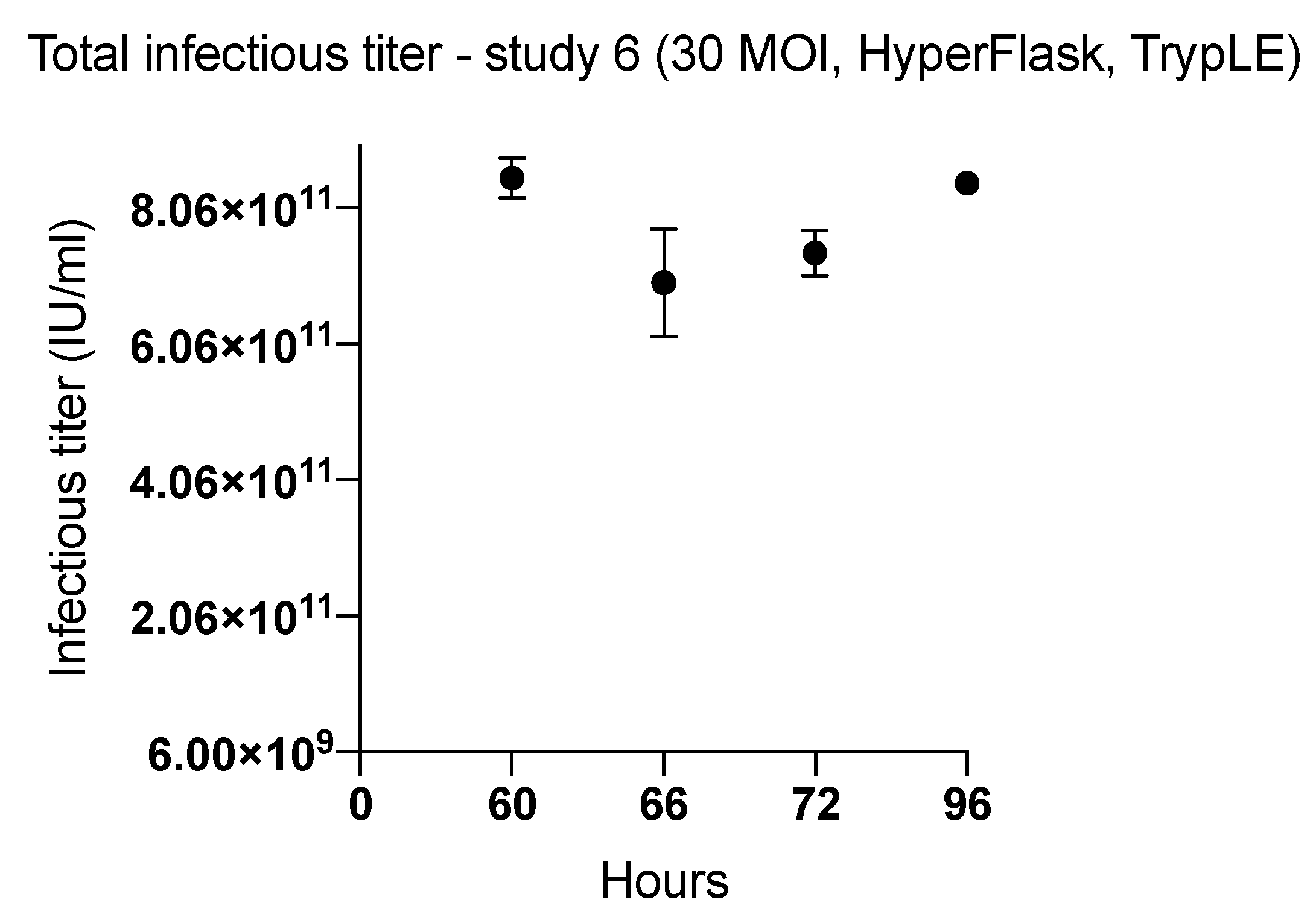
2.3. Factors Affecting Yield in the Harvesting Method
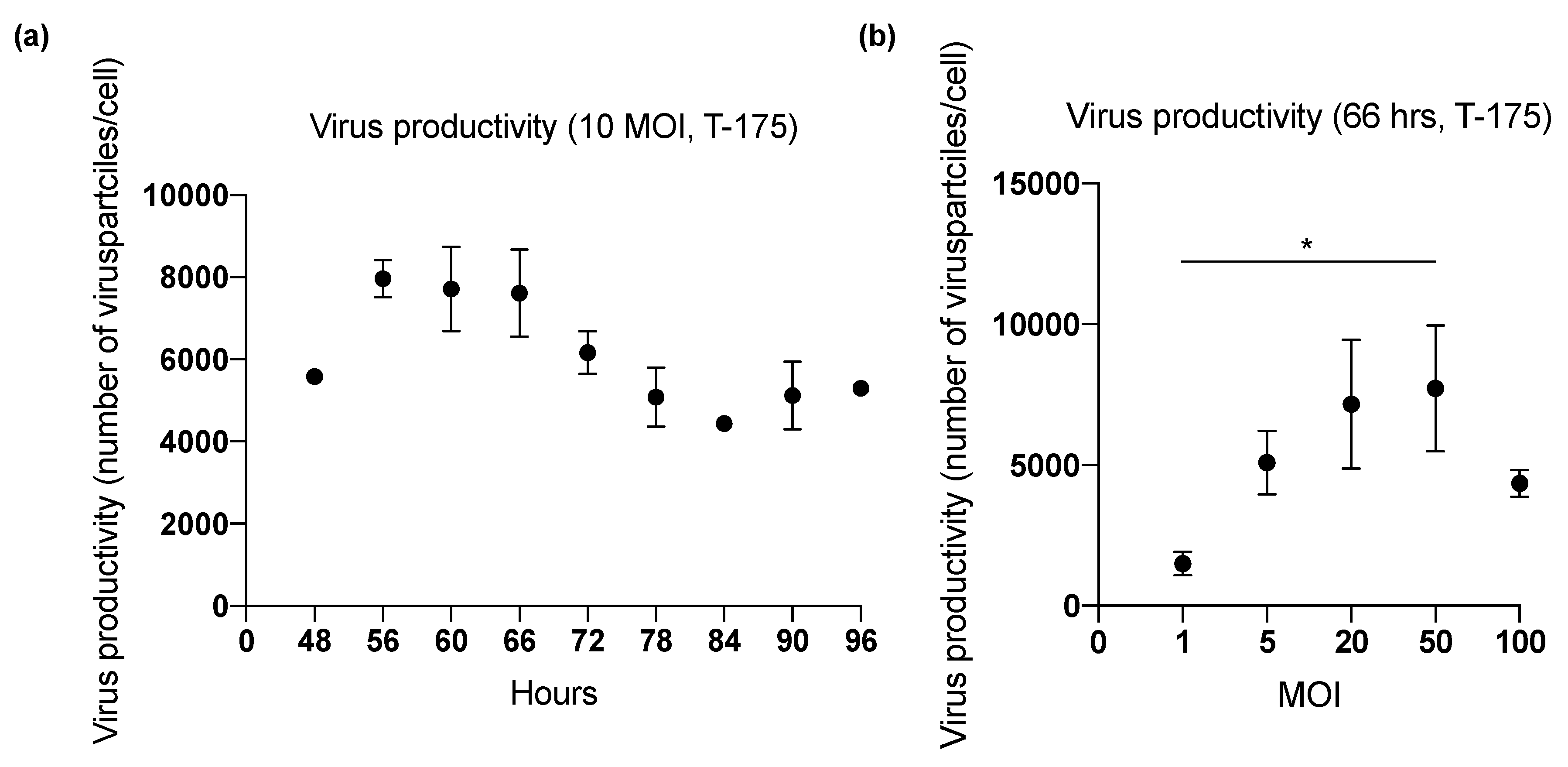
2.4. Viral Productivity Summary
2.5. Glucose and Lactate Measurements
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Lactate/Glucose Measurements
4.3. Virus Titration
4.4. Cell Counting
4.5. Viral Infection
4.6. Harvesting Methods
4.7. Biostatistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DAMP | damage-associated molecular patterns |
| DSG | desmoglein |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| hTERT | human telomerase promoter |
| ICC | immunocytochemistry assay |
| i.v. | intravenously |
| MOI | multiplicity of infection |
| OV | oncolytic viruses |
References
- Kim, M.; Williamson, C.T.; Prudhomme, J.; Bebb, D.G.; Riabowol, K.; Lee, P.W.; Lees-Miller, S.P.; Mori, Y.; Rahman, M.M.; McFadden, G.; et al. The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status. Oncogene 2010, 29, 3990–3996. [Google Scholar] [CrossRef] [Green Version]
- Kuryk, L.; Wieczorek, M.; Diedrich, S.; Boettcher, S.A.; Witek, A.; Litwinska, B. Genetic analysis of poliovirus strains isolated from sewage in Poland. J. Med. Virol. 2014, 86, 1243–1248. [Google Scholar] [CrossRef]
- Kuryk, L. Strategies to Enhance Efficacy of Oncolytic Virotherapy. Ph.D. Dissertation, University of Helsinki, Helsinki, Finland, 2016. [Google Scholar]
- Loskog, A.; Maleka, A.; Mangsbo, S.; Svensson, E.; Lundberg, C.; Nilsson, A.; Krause, J.; Agnarsdottir, M.; Sundin, A.; Ahlstrom, H.; et al. Immunostimulatory AdCD40L gene therapy combined with low-dose cyclophosphamide in metastatic melanoma patients. Br. J. Cancer 2016, 114, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Rosewell Shaw, A.; Suzuki, M. Recent advances in oncolytic adenovirus therapies for cancer. Curr. Opin. Virol. 2016, 21, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Garofalo, M.; Iovine, B.; Kuryk, L.; Capasso, C.; Hirvinen, M.; Vitale, A.; Yliperttula, M.; Bevilacqua, M.A.; Cerullo, V. Oncolytic Adenovirus Loaded with L-carnosine as Novel Strategy to Enhance the Antitumor Activity. Mol. Cancer Ther. 2016, 15, 651–660. [Google Scholar] [CrossRef]
- Kuryk, L.; Møller, A.-S.W.; Garofalo, M.; Cerullo, V.; Pesonen, S.; Alemany, R.; Jaderberg, M. Antitumor-specific T-cell responses induced by oncolytic adenovirus ONCOS-102 (AdV5/3-D24-GM-CSF) in peritoneal mesothelioma mouse model. J. Med. Virol. 2018, 90, 1669–1673. [Google Scholar] [CrossRef]
- Ungerechts, G.; Bossow, S.; Leuchs, B.; Holm, P.S.; Rommelaere, J.; Coffey, M.; Coffin, R.; Bell, J.; Nettelbeck, D.M. Moving oncolytic viruses into the clinic: Clinical-grade production, purification, and characterization of diverse oncolytic viruses. Mol. Ther. Methods Clin. Dev. 2016, 3, 16018. [Google Scholar] [CrossRef]
- Capasso, C.; Magarkar, A.; Cervera-Carascon, V.; Fusciello, M.; Feola, S.; Muller, M.; Garofalo, M.; Kuryk, L.; Tahtinen, S.; Pastore, L.; et al. A novel in silico framework to improve MHC-I epitopes and break the tolerance to melanoma. Oncoimmunology 2017, 6, e1319028. [Google Scholar] [CrossRef] [Green Version]
- Capasso, C.; Hirvinen, M.; Garofalo, M.; Romaniuk, D.; Kuryk, L.; Sarvela, T.; Vitale, A.; Antopolsky, M.; Magarkar, A.; Viitala, T.; et al. Oncolytic adenoviruses coated with MHC-I tumor epitopes increase the antitumor immunity and efficacy against melanoma. Oncoimmunology 2015, 5, e1105429. [Google Scholar] [CrossRef]
- Garofalo, M.; Saari, H.; Somersalo, P.; Crescenti, D.; Kuryk, L.; Aksela, L.; Capasso, C.; Madetoja, M.; Koskinen, K.; Oksanen, T.; et al. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J. Control. Release Off. J. Control. Release Soc. 2018, 283, 223–234. [Google Scholar] [CrossRef]
- Lipiec, A.; Kuryk, L. Onkolityczne Wektory Wirusowe w Immunoterapii Nowotworów; Immunoterapia, Wydawnictwo Lekarskie, PZWL: Warszawa, Poland, 2018. [Google Scholar]
- Kuryk, L.; Møller, A.-S.W.; Jaderberg, M. Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. Oncoimmunology 2018, 1–11. [Google Scholar] [CrossRef]
- Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Mazzaferro, V.; Ciana, P. Systemic Administration and Targeted Delivery of Immunogenic Oncolytic Adenovirus Encapsulated in Extracellular Vesicles for Cancer Therapies. Viruses 2018, 10, 558. [Google Scholar] [CrossRef]
- Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Rinner, B.; Cerullo, V.; Yliperttula, M.; Mazzaferro, V.; Ciana, P. Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J. Control. Release 2018. [Google Scholar] [CrossRef]
- Bramante, S.; Kaufmann, J.K.; Veckman, V.; Liikanen, I.; Nettelbeck, D.M.; Hemminki, O.; Vassilev, L.; Cerullo, V.; Oksanen, M.; Heiskanen, R.; et al. Treatment of melanoma with a serotype 5/3 chimeric oncolytic adenovirus coding for GM-CSF: Results in vitro, in rodents and in humans. Int. J. Cancer 2015, 137, 1775–1783. [Google Scholar] [CrossRef]
- Kuryk, L.; Haavisto, E.; Garofalo, M.; Capasso, C.; Hirvinen, M.; Pesonen, S.; Ranki, T.; Vassilev, L.; Cerullo, V. Synergistic anti-tumor efficacy of immunogenic adenovirus ONCOS-102 (Ad5/3-D24-GM-CSF) and standard of care chemotherapy in preclinical mesothelioma model. Int. J. Cancer 2016, 139, 1883–1893. [Google Scholar] [CrossRef]
- Malmstrom, P.U.; Loskog, A.S.; Lindqvist, C.A.; Mangsbo, S.M.; Fransson, M.; Wanders, A.; Gardmark, T.; Totterman, T.H. AdCD40L immunogene therapy for bladder carcinoma—The first phase I/IIa trial. Clin. Cancer Res. 2010, 16, 3279–3287. [Google Scholar] [CrossRef]
- Pesonen, S.; Diaconu, I.; Kangasniemi, L.; Ranki, T.; Kanerva, A.; Pesonen, S.K.; Gerdemann, U.; Leen, A.M.; Kairemo, K.; Oksanen, M.; et al. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: Assessment of safety and immunologic responses in patients. Cancer Res. 2012, 72, 1621–1631. [Google Scholar] [CrossRef]
- Diaconu, I.; Cerullo, V.; Hirvinen, M.L.; Escutenaire, S.; Ugolini, M.; Pesonen, S.K.; Bramante, S.; Parviainen, S.; Kanerva, A.; Loskog, A.S.; et al. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012, 72, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Schiza, A.; Wenthe, J.; Mangsbo, S.; Eriksson, E.; Nilsson, A.; Totterman, T.H.; Loskog, A.; Ullenhag, G. Adenovirus-mediated CD40L gene transfer increases Teffector/Tregulatory cell ratio and upregulates death receptors in metastatic melanoma patients. J. Transl. Med. 2017, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Moller, A.W.; Jaderberg, M. Quantification and functional evaluation of CD40L production from the adenovirus vector ONCOS-401. Cancer Gene Ther. 2018. [Google Scholar] [CrossRef]
- Choi, I.K.; Lee, J.S.; Zhang, S.N.; Park, J.; Sonn, C.H.; Lee, K.M.; Yun, C.O. Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rbeta2 or IL-18Ralpha. Gene Ther. 2011, 18, 898–909. [Google Scholar] [CrossRef]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimaki, A.; et al. Phase I study with ONCOS-102 for the treatment of solid tumors—An evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer 2016, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.Y.; Liu, Y.; Persson, J.; Beyer, I.; Moller, T.; Koyuncu, D.; Drescher, M.R.; Strauss, R.; Zhang, X.B.; et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2011, 17, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Koyama-Nasu, R.; Takahashi, R.; Yanagida, S.; Nasu-Nishimura, Y.; Oyama, M.; Kozuka-Hata, H.; Haruta, R.; Manabe, E.; Hoshino-Okubo, A.; Omi, H.; et al. The cancer stem cell marker CD133 interacts with plakoglobin and controls desmoglein-2 protein levels. PLoS ONE 2013, 8, e53710. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, L.J.; Meseck, M.; Derecho, I.; Lopez, P.; Knoblauch, C.; McMahon, R.; Anderson, J.; Dunphy, N.; Quezada, V.; Khan, R.; et al. Current good manufacturing practice production of an oncolytic recombinant vesicular stomatitis viral vector for cancer treatment. Hum. Gene Ther. 2011, 22, 489–497. [Google Scholar] [CrossRef]
- Hendrickx, R.; Stichling, N.; Koelen, J.; Kuryk, L.; Lipiec, A.; Greber, U.F. Innate immunity to adenovirus. Hum. Gene Ther. 2014, 25, 265–284. [Google Scholar] [CrossRef]
- Diaconu, I.; Cerullo, V.; Escutenaire, S.; Kanerva, A.; Bauerschmitz, G.J.; Hernandez-Alcoceba, R.; Pesonen, S.; Hemminki, A. Human adenovirus replication in immunocompetent Syrian hamsters can be attenuated with chlorpromazine or cidofovir. J. Gene Med. 2010, 12, 435–445. [Google Scholar] [CrossRef]
- Kovesdi, I.; Hedley, S.J. Adenoviral Producer Cells. Viruses 2010, 2, 1681–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, M.F.; Silva, M.M.; Giroux, D.; Hashimura, Y.; Wesselschmidt, R.; Lee, B.; Roldao, A.; Carrondo, M.J.; Alves, P.M.; Serra, M. Production of oncolytic adenovirus and human mesenchymal stem cells in a single-use, Vertical-Wheel bioreactor system: Impact of bioreactor design on performance of microcarrier-based cell culture processes. Biotechnol. Prog. 2015, 31, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Dormond, E.; Kamen, A.A. Manufacturing of adenovirus vectors: Production and purification of helper dependent adenovirus. Methods Mol. Biol. 2011, 737, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Vellinga, J.; Smith, J.P.; Lipiec, A.; Majhen, D.; Lemckert, A.; van Ooij, M.; Ives, P.; Yallop, C.; Custers, J.; Havenga, M. Challenges in manufacturing adenoviral vectors for global vaccine product deployment. Hum. Gene Ther. 2014, 25, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Lesch, H.P.; Heikkila, K.M.; Lipponen, E.M.; Valonen, P.; Muller, A.; Rasanen, E.; Tuunanen, T.; Hassinen, M.M.; Parker, N.; Karhinen, M.; et al. Process Development of Adenoviral Vector Production in Fixed Bed Bioreactor: From Bench to Commercial Scale. Hum. Gene Ther. 2015, 26, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Eglon, M.N.; Duffy, A.M.; O’Brien, T.; Strappe, P.M. Purification of adenoviral vectors by combined anion exchange and gel filtration chromatography. J. Gene Med. 2009, 11, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Li, H.; Lam, J.; Krasnykh, V.; Curiel, D.; Blackwell, J. Substitution of the Adenovirus Serotype 5 Knob with a Serotype 3 Knob Enhances Multiple Steps in Virus Replication. Cancer Res. 2003, 63. [Google Scholar]
- Doloff, J.C.; Waxman, D.J.; Jounaidi, Y. Human telomerase reverse transcriptase promoter-driven oncolytic adenovirus with E1B-19 kDa and E1B-55 kDa gene deletions. Hum. Gene Ther. 2008, 19, 1383–1400. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, W.; Xu, B.; Zhang, X.; Xia, X.; Sun, H. Single-step concentration and purification of adenoviruses by coxsackievirus-adenovirus receptor-binding capture and elastin-like polypeptide-mediated precipitation. Arch. Virol. 2016, 161, 279–287. [Google Scholar] [CrossRef]
- Kaliberov, S.A.; Kaliberova, L.N.; Buchsbaum, D.J.; Curiel, D.T. Experimental virotherapy of chemoresistant pancreatic carcinoma using infectivity-enhanced fiber-mosaic oncolytic adenovirus. Cancer Gene Ther. 2014, 21, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Kawashita, Y.; Deb, N.J.; Garg, M.K.; Kabarriti, R.; Fan, Z.; Alfieri, A.A.; Roy-Chowdhury, J.; Guha, C. An autologous in situ tumor vaccination approach for hepatocellular carcinoma. 2. Tumor-specific immunity and cure after radio-inducible suicide gene therapy and systemic CD40-ligand and Flt3-ligand gene therapy in an orthotopic tumor model. Radiat. Res. 2014, 182, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Vassilev, L.; Ranki, T.; Hemminki, A.; Karioja-Kallio, A.; Levalampi, O.; Vuolanto, A.; Cerullo, V.; Pesonen, S. Toxicological and bio-distribution profile of a GM-CSF-expressing, double-targeted, chimeric oncolytic adenovirus ONCOS-102—Support for clinical studies on advanced cancer treatment. PLoS ONE 2017, 12, e0182715. [Google Scholar] [CrossRef] [PubMed]
- Hirvinen, M.; Capasso, C.; Guse, K.; Garofalo, M.; Vitale, A.; Ahonen, M.; Kuryk, L.; Vaha-Koskela, M.; Hemminki, A.; Fortino, V.; et al. Expression of DAI by an oncolytic vaccinia virus boosts the immunogenicity of the virus and enhances antitumor immunity. Mol. Ther. Oncolytics 2016, 3, 16002. [Google Scholar] [CrossRef]
- Wing, A.; Fajardo, C.A.; Posey, A.D., Jr.; Shaw, C.; Da, T.; Young, R.M.; Alemany, R.; June, C.H.; Guedan, S. Improving CART-Cell Therapy of Solid Tumors with Oncolytic Virus-Driven Production of a Bispecific T-cell Engager. Cancer Immunol. Res. 2018, 6, 605–616. [Google Scholar] [CrossRef] [PubMed]

| Optimization Study (Number) | Harvesting Time (h) | MOI | Harvesting Method | Total Virus Particles (IU), (Mean) | Virus Productivity (Number of Virus Particles/Cell), (Mean) |
|---|---|---|---|---|---|
| 1 (T-175) | 48 | 10 | Lysis A | 1.86 × 1011 | 5576 |
| 60 | 2.08 × 1011 | 6226 | |||
| 72 | 1.82 × 1011 | 5438 | |||
| 84 | 1.46 × 1011 | 4365 | |||
| 96 | 1.77 × 1011 | 5299 | |||
| 2 (T-175) | 66 | 10 | Lysis A | 2.47 × 1011 | 6682 |
| 72 | 1.63 × 1011 | 3779 | |||
| 78 | 2.19 × 1011 | 5083 | |||
| 84 | 1.92 × 1011 | 4476 | |||
| 90 | 1.90 × 1011 | 5124 | |||
| 3 (T-175) | 56 | 10 | Lysis A | 2.87 × 1011 | 7966 |
| 60 | 2.95 × 1011 | 8205 | |||
| 66 | 3.07 × 1011 | 8538 | |||
| 72 | 2.30 × 1011 | 6401 | |||
| 4 (T-175) | 66 | 1 | Lysis A | 6.39 × 1010 | 1494 |
| 5 | 2.17 × 1011 | 5085 | |||
| 20 | 3.06 × 1011 | 7153 | |||
| 50 | 3.30 × 1011 | 7715 | |||
| 100 | 1.85 × 1011 | 4338 | |||
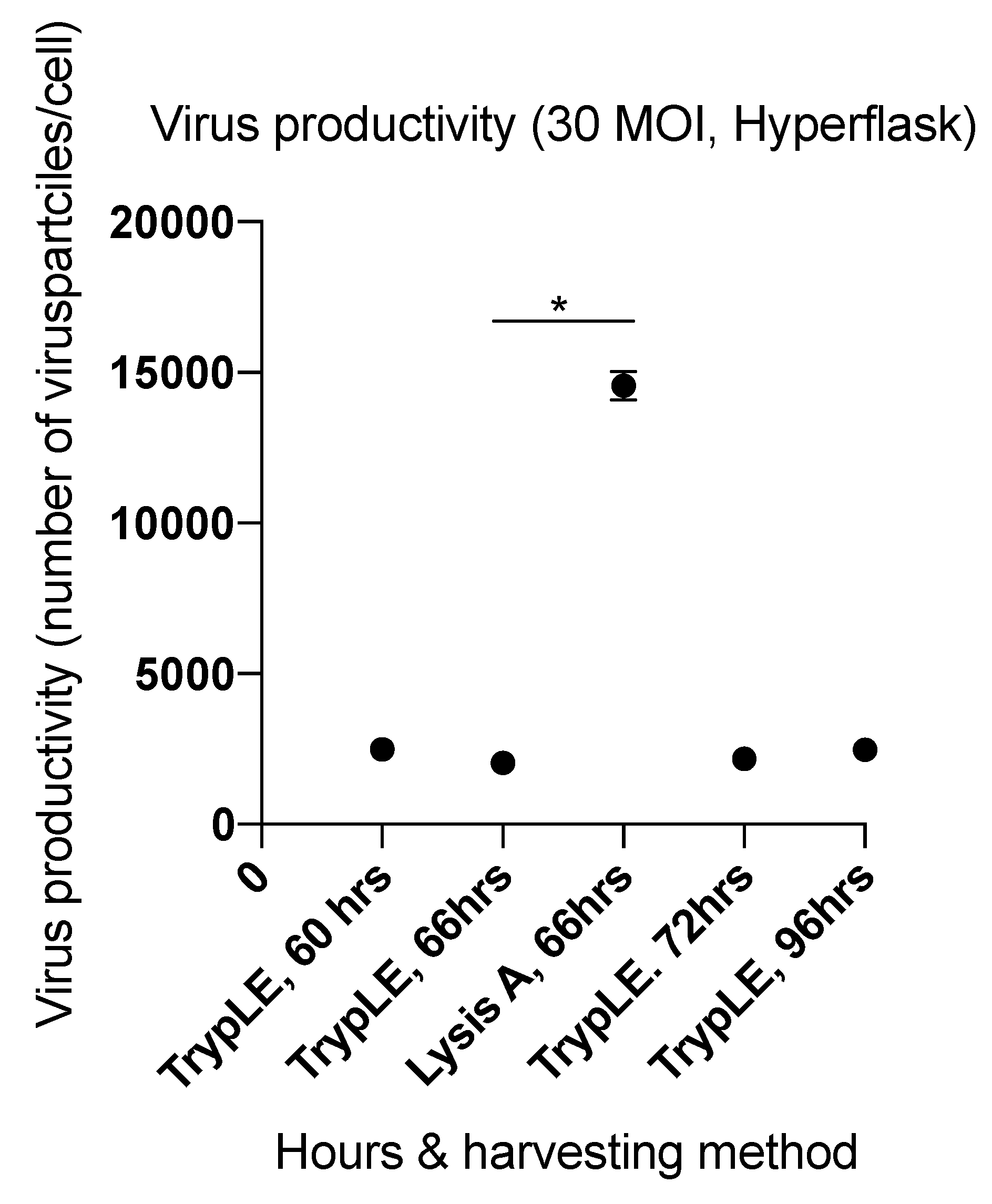
| 5 (HYPERFlask) | 66 | 30 | Lysis A | 3.02 × 1012 | 14,559 |
| 6 (HYPERFlask) | 60 | TrypLE | 8.51 × 1011 | 2497 | |
| 66 | 6.96 × 1011 | 2044 | |||
| 72 | 7.40 × 1011 | 2172 | |||
| 96 | 8.43 × 1011 | 2475 | |||
| 7 (T-175 & HYPERFlask) | 66 | 30 | Lysis A T-175 | 3.72 × 1011 | 10,887 |
| Lysis B T-175 | 1.48 × 1011 | 4331 | |||
| Lysis C T-175 | 1.96 × 1011 | 5741 | |||
| Lysis D HYPERFlask | 4.36 × 1011 | 1279 | |||
| Lysis C HYPERFlask | 8.09 × 1011 | 2374 | |||
| Benzonase treatment (pellet 1) HYPERFlask | 4.35 × 1011 | 1275 | |||
| Benzonase treatment (pellet 2) HYPERFlask | 1.08 × 1011 | 315 |
| Steps | Lysis A | TrypLE | Lysis B | Lysis C | Lysis D |
|---|---|---|---|---|---|
| Collected supernatant | Y | Y | Y | Y | Y |
| Chemical lysis buffer with benzonase treatment (1 h) | Y | N | N | N | N |
| Washes (1–2 x) | N | Y | Y | Y | Y |
| TrypLE | N | Y | N | N | N |
| Mechanical disassociation (firmly tapped 5–10 x) | N | Y | Y | Y | Y |
| TrypLE | N | Y | N | N | N |
| Centrifugation | N | 1000 rpm | 1000 rpm | 1000 rpm | 1000 rpm |
| Supernatant removed & discarded | N | Y | Y | Y | Y |
| Pellet saved & resuspended in 8–10 mL | N | Y | Y | Y | Y |
| Chemical lysis buffer with benzonase (1 h) on pellet | N | N | Y | Y | N |
| Pipette-based disruption and homogenization | N | Y | Y | Vortexed | Vortexed |
| Frozen −80 °C, thawed 3 x | N | Y | Y | Y | Y |
| Centrifugation | 3000 rpm | 4000 rpm | 4000 rpm | 4000 rpm | 4000 rpm |
| Supernatant retained, 5% sucrose, & frozen at −80 °C | Y | Y | Y | Y | Y |
| Viral titer by ICC | Y | Y | Y | Y | Y |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuryk, L.; Møller, A.-S.W.; Vuolanto, A.; Pesonen, S.; Garofalo, M.; Cerullo, V.; Jaderberg, M. Optimization of Early Steps in Oncolytic Adenovirus ONCOS-401 Production in T-175 and HYPERFlasks. Int. J. Mol. Sci. 2019, 20, 621. https://doi.org/10.3390/ijms20030621
Kuryk L, Møller A-SW, Vuolanto A, Pesonen S, Garofalo M, Cerullo V, Jaderberg M. Optimization of Early Steps in Oncolytic Adenovirus ONCOS-401 Production in T-175 and HYPERFlasks. International Journal of Molecular Sciences. 2019; 20(3):621. https://doi.org/10.3390/ijms20030621
Chicago/Turabian StyleKuryk, Lukasz, Anne-Sophie W Møller, Antti Vuolanto, Sari Pesonen, Mariangela Garofalo, Vincenzo Cerullo, and Magnus Jaderberg. 2019. "Optimization of Early Steps in Oncolytic Adenovirus ONCOS-401 Production in T-175 and HYPERFlasks" International Journal of Molecular Sciences 20, no. 3: 621. https://doi.org/10.3390/ijms20030621





