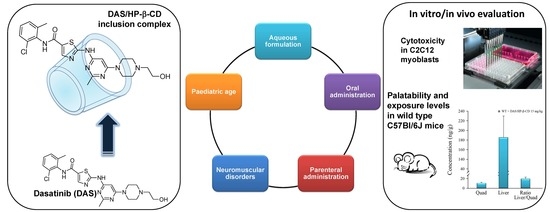
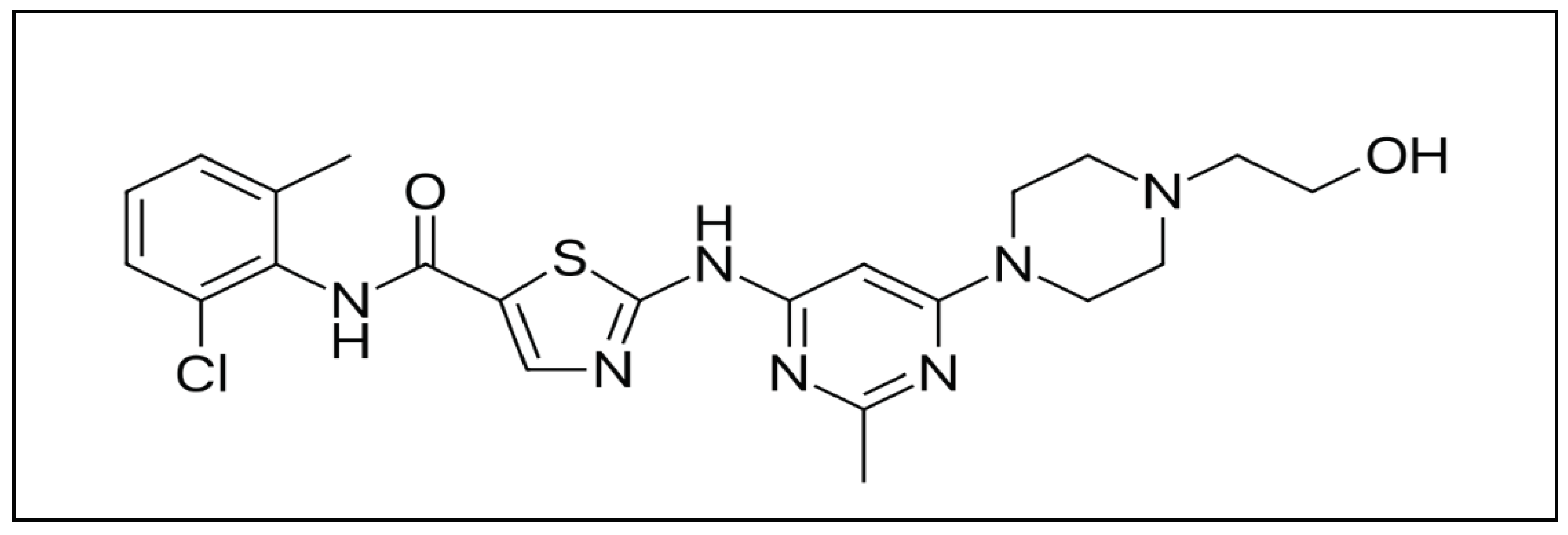
Dasatinib/HP-β-CD Inclusion Complex Based Aqueous Formulation as a Promising Tool for the Treatment of Paediatric Neuromuscular Disorders
Abstract
1. Introduction
2. Results and Discussion
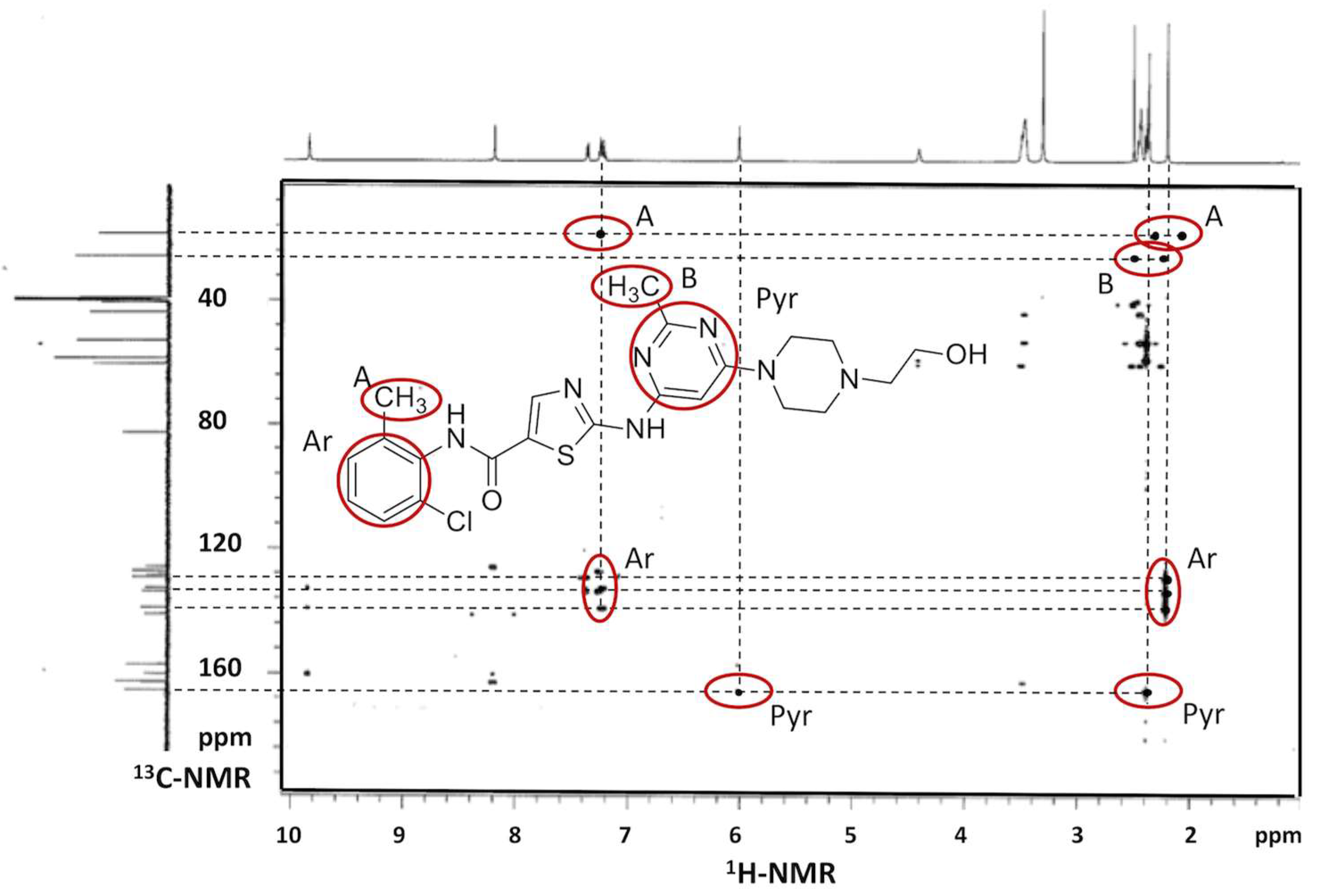
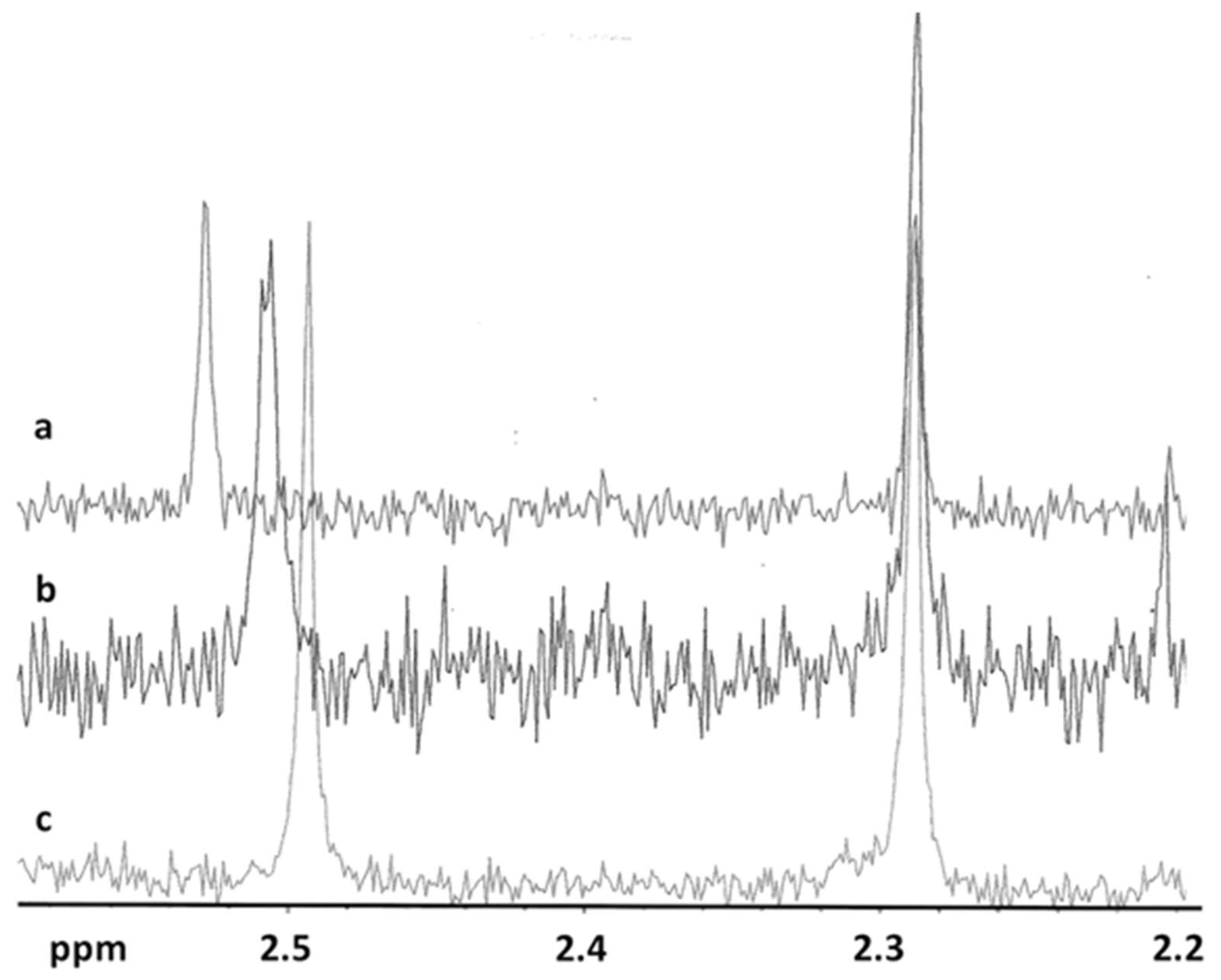
2.1. Evaluation of the Inclusion Complex in Solution
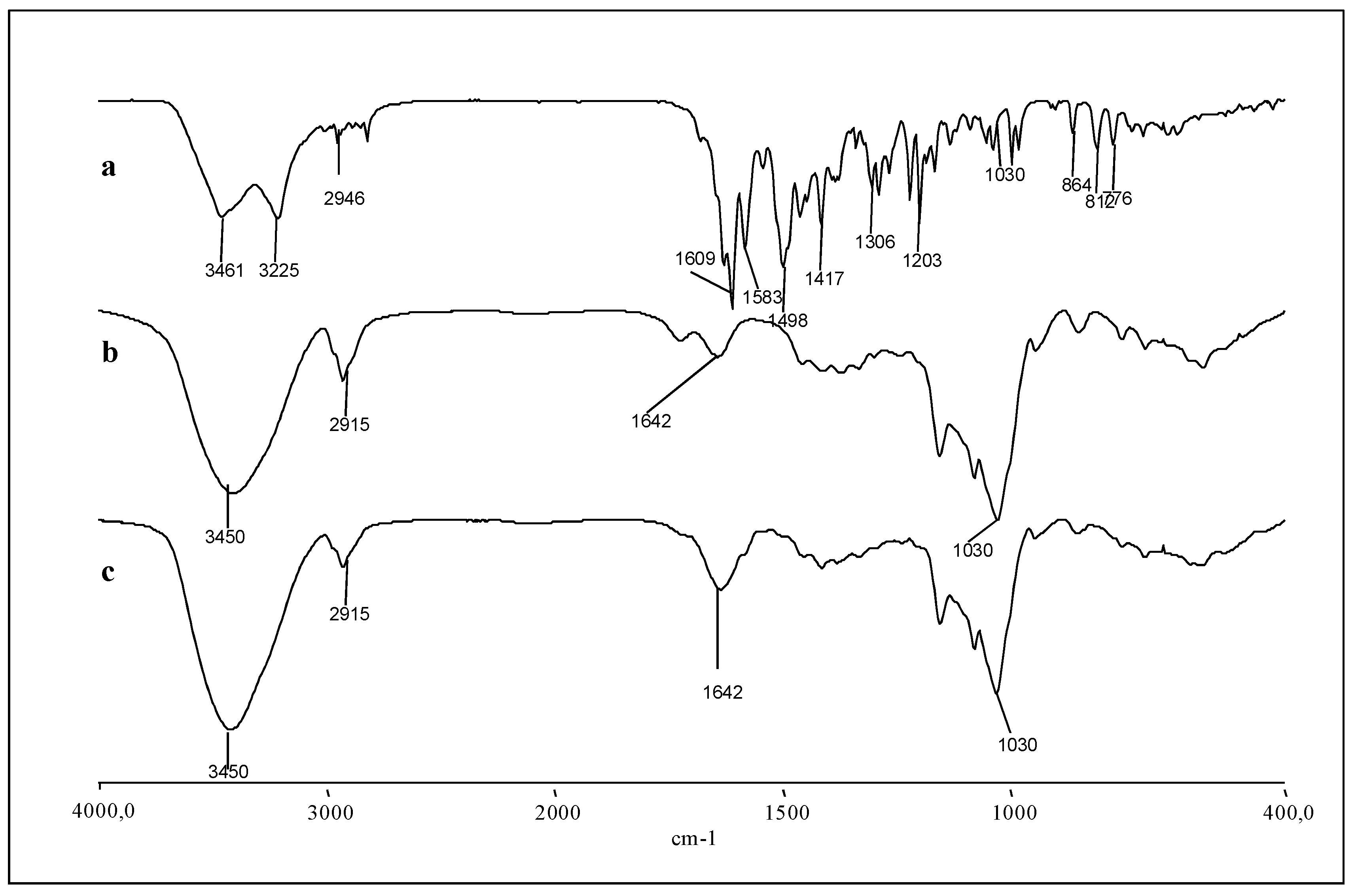
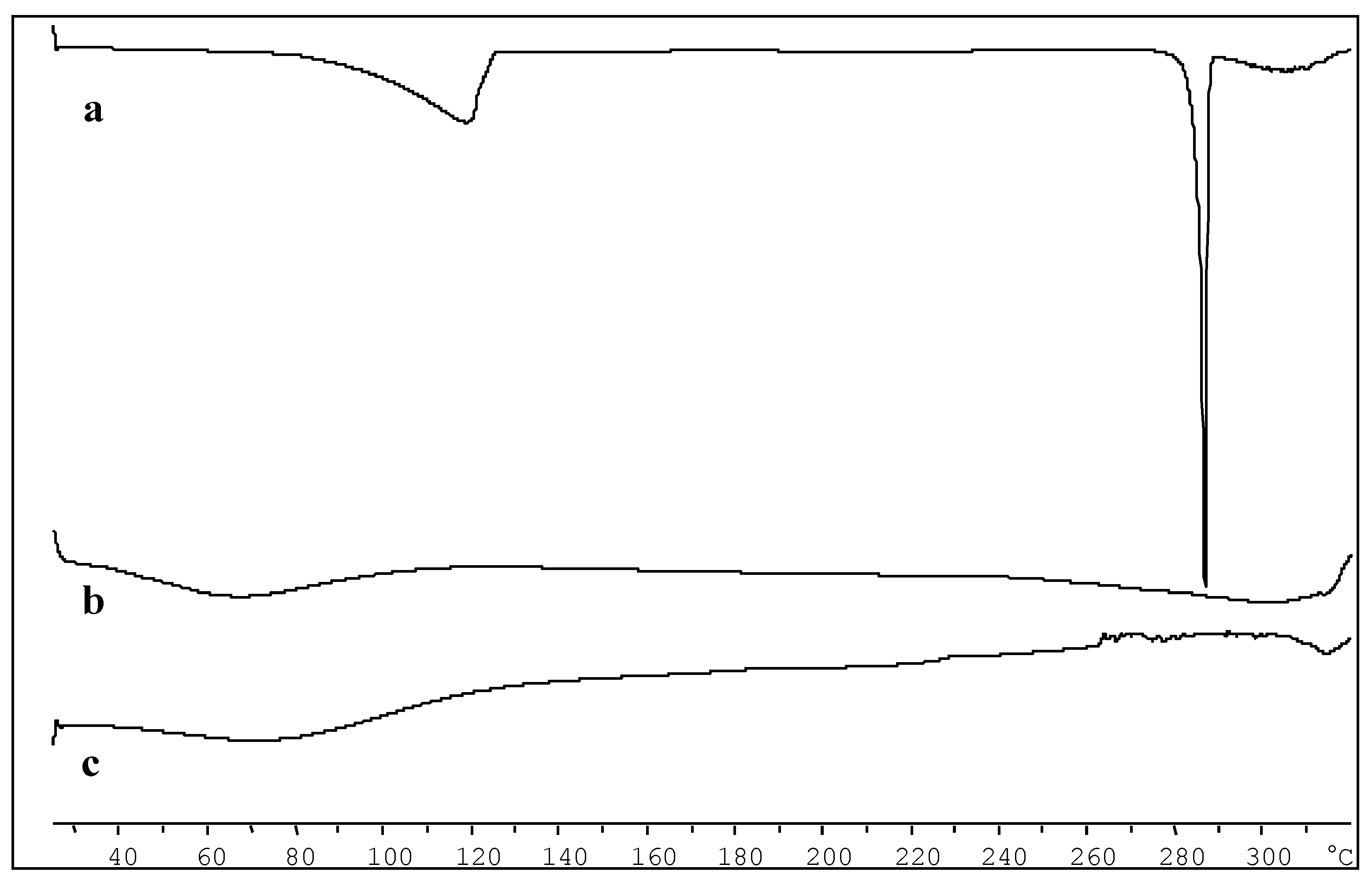
2.2. Characterization of the Inclusion Complex in the Solid State
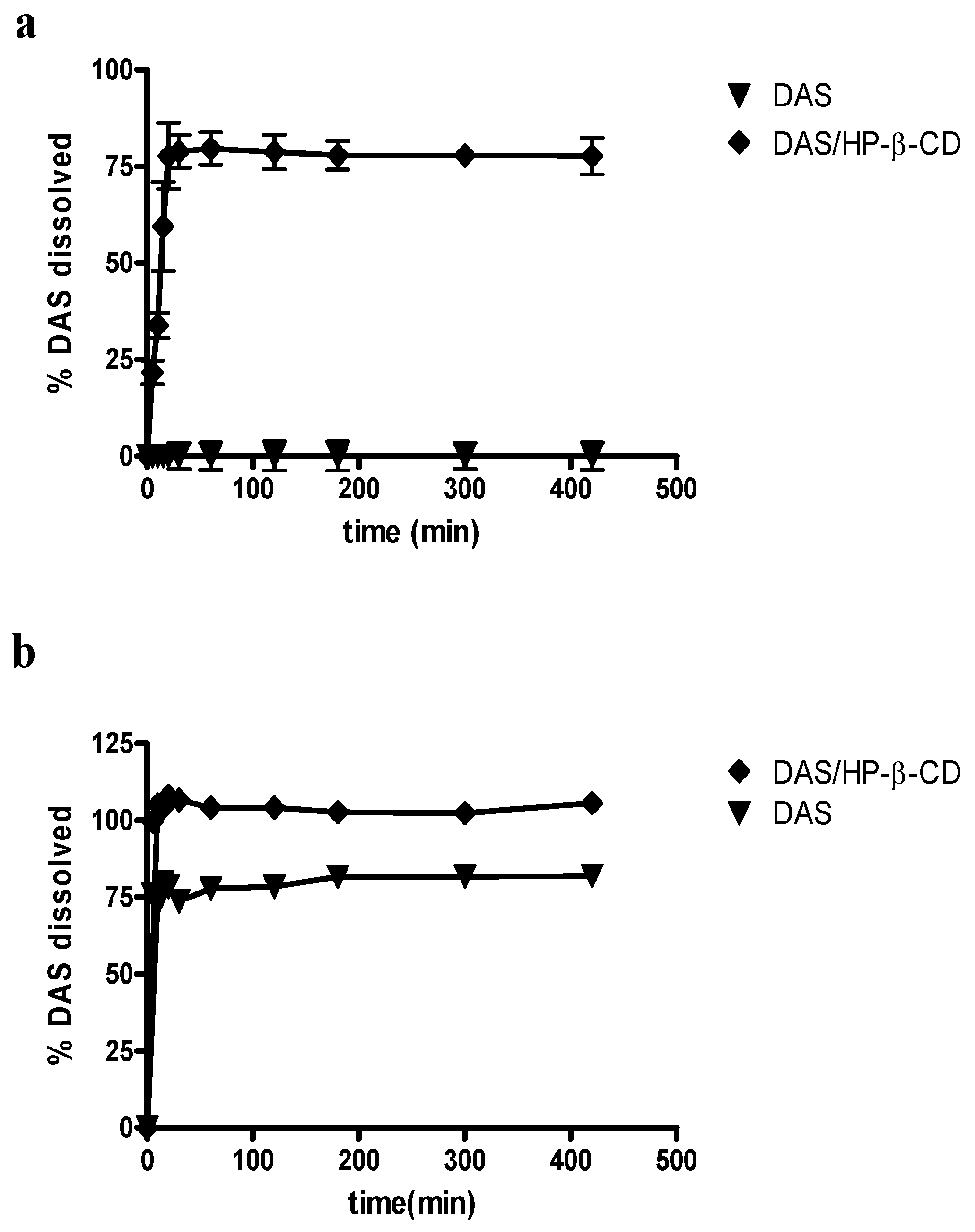
2.3. Dissolution Studies
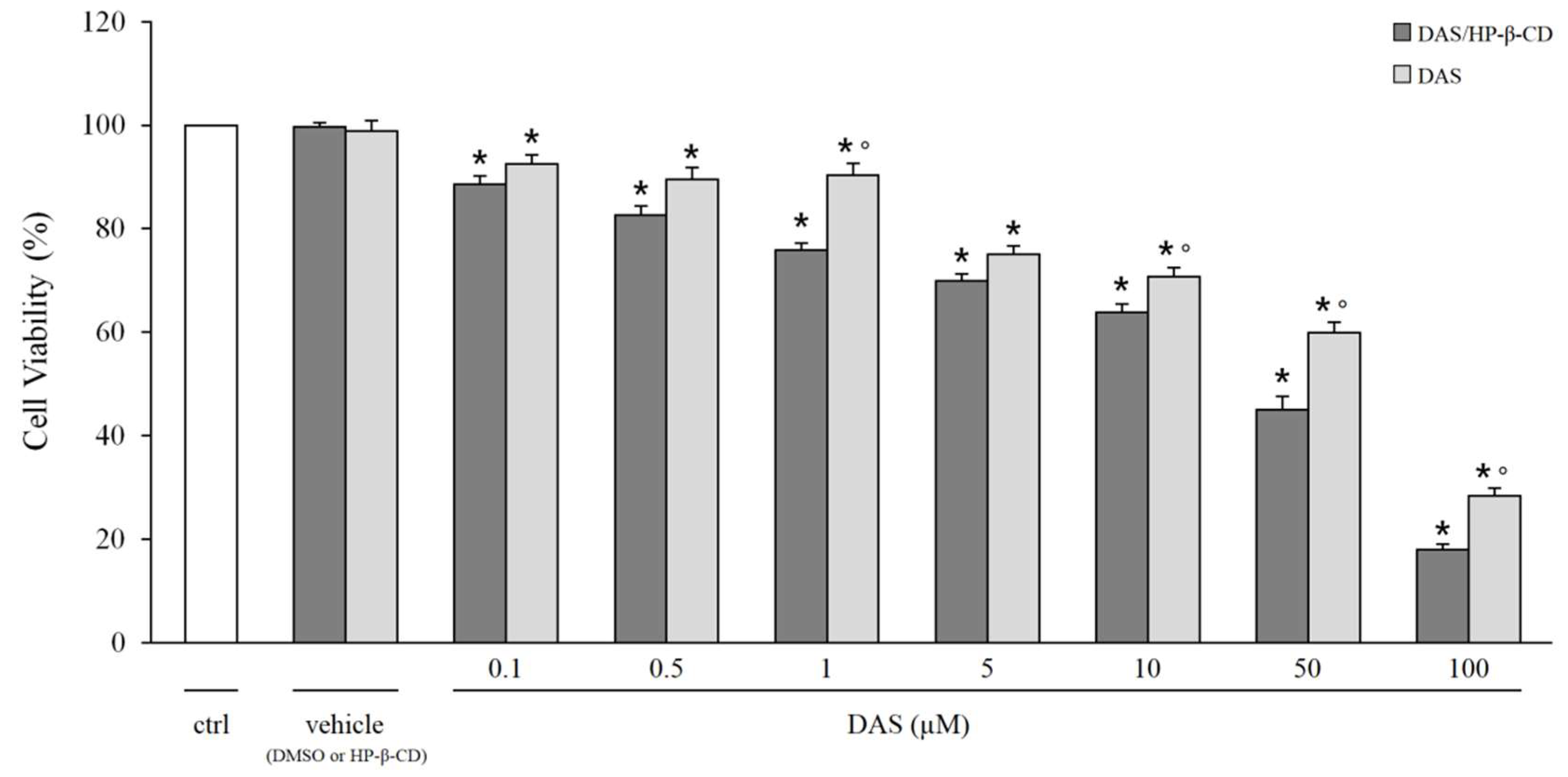
2.4. Cytotoxicity Studies
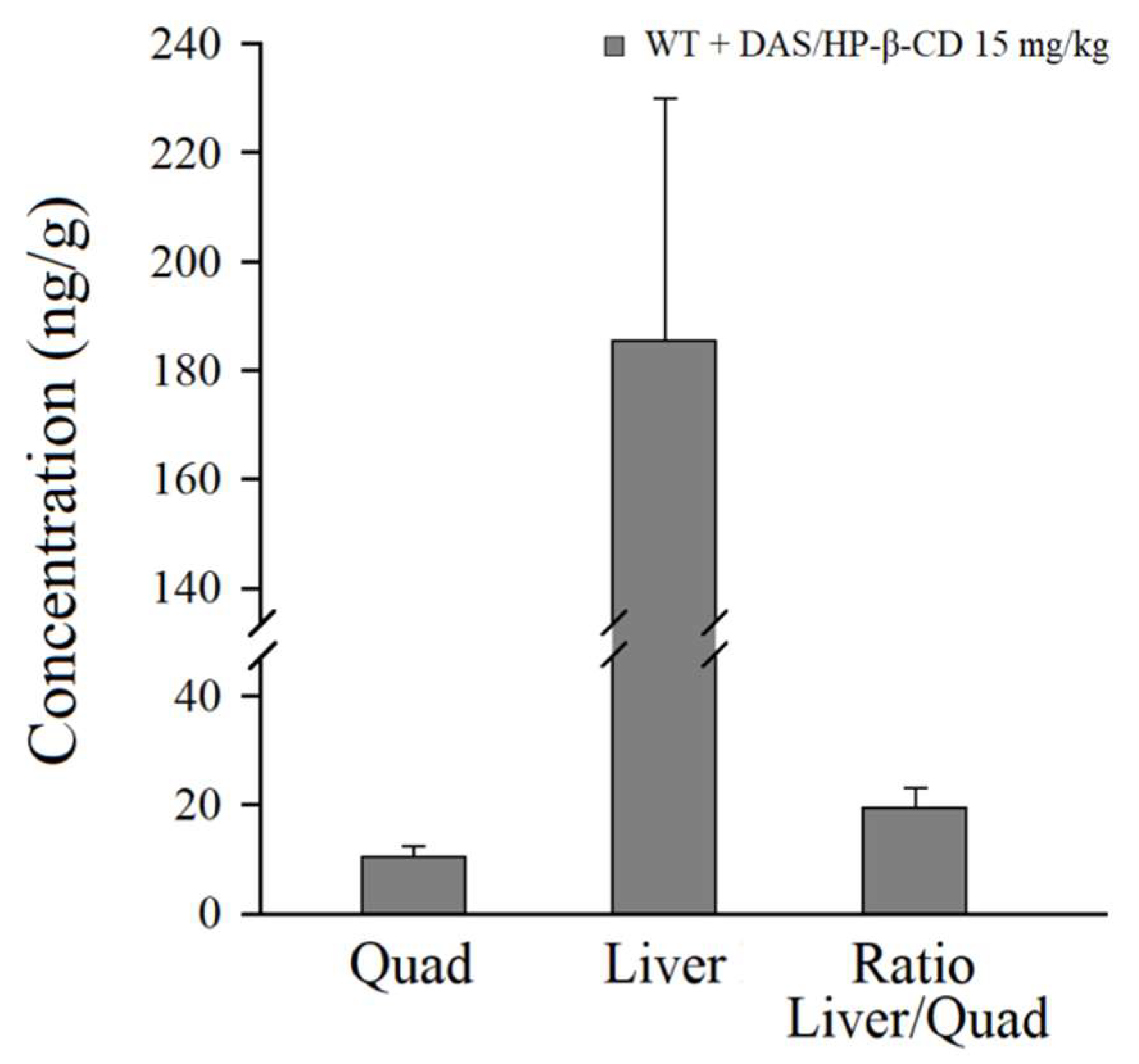
2.5. Pharmacokinetic Results
3. Materials and Methods
3.1. Materials
3.2. Quantitative Analysis of DAS
3.3. Solubility and Phase-Solubility Studies
3.4. Preparation of Solid DAS /HP-β-CD Inclusion Complex
3.5. Determination of DAS Incorporation Degree in the Solid Cyclodextrin Inclusion Complex
3.6. Job’s Plot Method
3.7. 1H-NMR and Heteronuclear Multiple Bond Correlation (HMBC) Spectroscopic Studies
3.8. Fourier Transform Infrared (FT-IR) Spectroscopy
3.9. Differential Scanning Calorimetry (DSC) Analysis
3.10. Dissolution Studies
3.11. Cytotoxicity Studies
3.12. In Vivo Study
3.13. Ex vivo Study: Pharmacokinetic Analysis
3.14. Statistic
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lombardo, L.J.; Lee, F.Y.; Chen, P.; Norris, D.; Barrish, J.C.; Behnia, K.; Castaneda, S.; Cornelius, L.A.; Das, J.; Doweyko, A.M.; et al. Discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)–piperazin-1-yl]-2–methylpyrimidin-4-ylamino] thiazole 5–carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem. 2004, 47, 6658–6661. [Google Scholar] [CrossRef] [PubMed]
- Tokarski, J.S.; Newitt, J.A.; Chang, C.Y.J.; Cheng, J.D.; Wittekind, M.; Kiefer, S.E.; Kish, K.; Lee, F.Y.; Borzillerri, R.; Lombardo, L.J.; et al. The structure of dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res. 2006, 66, 5790–5797. [Google Scholar] [CrossRef]
- Talpaz, M.; Shah, N.P.; Kantarjian, H.; Donato, N.; Nicoll, J.; Paquette, R.; Cortes, J.; O’Brien, S.; Nicaise, C.; Bleickardt, E.; et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2006, 354, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Melville, N.A. Dasatinib in Children: An Effective Alternative to Imatinib. In Proceedings of the European Hematology Association (EHA) 2017 Congress, Madrid, Spain, 22–25 June 2017. [Google Scholar]
- Zwaan, C.M.; Rizzari, C.; Mechinaud, F.; Lancaster, D.I.; Lehrnbecher, T.; Van der Velden, V.H.J.; Beverloo, B.B.; den Boer, M.L.; Pieters, R.; Reinhardt, D.; et al. Dasatinib in Children and Adolescent With Relapsed or Refractory Leukemia: Results of the CA180-018 Phase I Dose-Escalation Study of the innovative Therapies for Children With Cancer Consortium. J. Clin. Oncol. 2013, 31, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Prins, K.W.; Humston, J.L.; Mehta, A.; Tate, V.; Ralston, E.; Ervasti, J.M. Dystrophin is a microtubule-associated protein. J. Cell Biol. 2009, 186, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P.; Dressman, D. Molecular pathophysiology and targeted therapeutics for muscular dystrophy. Trends Pharm. Sci. 2001, 22, 465–470. [Google Scholar] [CrossRef]
- De Luca, A. Pre-clinical drug tests in the mdx mouse as a model of dystrophinopathies: An overview. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2012, 31, 40–47. [Google Scholar]
- Camerino, G.M.; Cannone, M.; Giustino, A.; Massari, A.M.; Capogrosso, R.F.; Cozzoli, A.; De Luca, A. Gene expression in mdx mouse muscle in relation to age and exercise: Aberrant mechanical-metabolic coupling and implications for pre-clinical studies in Duchenne muscular dystrophy. Hum. Mol. Genet. 2014, 23, 5720–5732. [Google Scholar] [CrossRef]
- Paletta-Silva, R.; Rocco-Machado, N.; Meyer-Fernandes, J.R. NADPH oxidase biology and the regulation of tyrosine kinase receptor signaling and cancer drug cytotoxicity. Int. J. Mol. Sci. 2013, 14, 3683–3704. [Google Scholar] [CrossRef]
- Lipscomb, L.; Piggot, R.W.; Emmerson, T.; Winder, S.J. Dasatinib as a treatment for Duchenne muscular dystrophy. Hum. Mol. Genet. 2016, 25, 266–274. [Google Scholar] [CrossRef]
- Lopalco, A.; Curci, A.; Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Franco, M.; Denora, N. Pharmaceutical preformulation studies and paediatric oral formulations of sodium dichloroacetate. Eur. J. Pharm. Sci. 2019, 127, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Korashy, H.M.; Rahman, A.F.M.M.; Kassem, G.M. «Dasatinib» in Profiles of Drug Substances, Excipients, and Related Methodology; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cutrignelli, A.; Lopedota, A.; Denora, N.; Iacobazzi, R.M.; Fanizza, E.; Laquintana, V.; Perrone, M.; Maggi, V.; Franco, M. A new complex of curcumin with sulfobutylether-β-cyclodextrin: Characterization studies and in vitro evaluation of cytotoxic and antioxidant activity on HepG-2 cells. J. Pharm. Sci. 2014, 103, 3932–3940. [Google Scholar] [CrossRef] [PubMed]
- Cutrignelli, A.; Lopedota, A.; Denora, N.; Laquintana, V.; Tongiani, S.; Franco, M. Characterization and release studies of liposomal gels containing glutathione/cyclodextrins complexes potentially useful for cutaneous administration. J. Pharm. Sci. 2014, 103, 1246–1254. [Google Scholar] [CrossRef]
- Lopedota, A.; Trapani, A.; Cutrignelli, A.; Laquintana, V.; Denora, N.; Franco, M.; Trapani, G.; Liso, G. Effect of cyclodextrins on physico-chemical and release properties of Eudragit RS 100 microparticles containing glutathione. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 425–432. [Google Scholar] [CrossRef]
- Tricarico, D.; Maqoud, F.; Curci, A.; Camerino, G.; Zizzo, N.; Denora, N.; Cutrignelli, A.; Laquintana, V.; Lopalco, A.; la Forgia, F.; et al. Characterization of minoxidil/hydroxypropyl-β-cyclodextrin inclusion complex in aqueous alginate gel useful for alopecia management: Efficacy evaluation in male rat. Eur. J. Pharm. Biopharm. 2018, 122, 146–157. [Google Scholar] [CrossRef]
- Lopedota, A.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Lopalco, A.; Tricarico, D.; Maqoud, F.; Curci, A.; Mastrodonato, M.; la Forgia, F.; et al. Alginate-Based Hydrogel Containing Minoxidil/Hydroxypropyl-β-Cyclodextrin Inclusion Complex for Topical Alopecia Treatment. J. Pharm. Sci. 2018, 107, 1046–1054. [Google Scholar] [CrossRef]
- Lopedota, A.; Cutrignelli, A.; Denora, N.; Laquintana, V.; Lopalco, A.; Selva, S.; Ragni, L.; Tongiani, S.; Franco, M. New ethanol and propylene glycol free gel formulations containing a minoxidil-methyl-β-cyclodextrin complex as promising tools for alopecia treatment. Drug Dev. Ind. Pharm. 2015, 41, 728–736. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Ventura, C.A.; Tommasini, S.; Falcone, A.; Giannone, I.; Paolino, D.; Sdrafkakis, V.; Mondello, M.R.; Puglisi, G. Influence of modified cyclodextrins on solubility and percutaneous absorption of celecoxib through human skin. Int. J. Pharm. 2006, 314, 37–45. [Google Scholar] [CrossRef]
- Purohit, P.; Rampalli, S.; Murali, M.S.V.; Upalla, L.; Pothana, P. Process for Preparing Dasatinib Monohydrate. U.S. Patent No 9,145.406 B2, 29 September 2015. [Google Scholar]
- Yan, R.; Yang, H.; Xu, Y. Polymorphs of Dasatinib, Preparation Methods and Pharmaceutical Composition Thereof. U.S. Patent No 2012/0309968 A1, 6 December 2012. [Google Scholar]
- Duckett, D.R.; Cameron, M.D. Metabolism considerations for kinase inhibitors in cancer treatment. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1175–1193. [Google Scholar] [CrossRef]
- Rao, S.; Larroque-Lombard, A.L.; Peyrard, L.; Thauvin, C.; Rachid, Z.; Williams, C.; Jean-Claude, B.J. Target modulation by a kinase inhibitor engineered to induce a tandem blockade of the epidermal growth factor receptor (EGFR) and c-Src: The concept of type III combi-targeting. PLoS ONE 2015, 10, e0117215. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Martinez-Becerra, F.; Antonio, L.; Johnson, R.T.; de Campos, R.P.S.; Siegel, J.M.; Wijesinghe, M.B.; Lazzarino, G.; Lunte, S.M. Carnosine modulates nitric oxide in stimulated murine RAW 264.7 macrophages. Mol. Cell. Biochem. 2017, 431, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Stella, V.J. Effect of Molecular Structure on the Relative Hydrogen Peroxide Scavenging Ability of Some α-Keto Carboxylic Acids. J. Pharm. Sci. 2016, 105, 2879–2885. [Google Scholar] [CrossRef]
- Lopalco, A.; Dalwadi, G.; Niu, S.; Schowen, R.L.; Douglas, J.; Stella, V.J. Mechanism of Decarboxylation of Pyruvic Acid in the Presence of Hydrogen Peroxide. J. Pharm. Sci. 2016, 105, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Douglas, J.; Denora, N.; Stella, V.J. Determination of pKa and Hydration Constants for a Series of α-Keto-Carboxylic Acids Using Nuclear Magnetic Resonance Spectrometry. J. Pharm. Sci. 2016, 105, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Margiotta, N.; Laquintana, V.; Lopedota, A.; Cutrignelli, A.; Losacco, M.; Franco, M.; Natile, G. Synthesis, characterization, and in vitro evaluation of a new TSPO-selective bifunctional chelate ligand. ACS Med. Chem. Lett. 2014, 5, 685–689. [Google Scholar]
- Cutrignelli, A.; Lopedota, A.; Trapani, A.; Boghetich, G.; Franco, M.; Denora, N.; Laquintana, V.; Trapani, G. Relationship between dissolution efficiency of Oxazepam/carrier blends and drug and carrier molecular descriptors using multivariate regression analysis. Int. J. Pharm. 2008, 358, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Denora, N.; Iacobazzi, R.M.; Perrone, M.; Fanizza, E.; Mastrodonato, M.; Mentino, D.; Lopalco, A.; et al. Spray Dried Chitosan Microparticles for Intravesical Delivery of Celecoxib: Preparation and Characterization. Pharm. Res. 2016, 33, 2195–2208. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, R.F.; Mantuano, P.; Uaesoontrachoon, K.; Cozzoli, A.; Giustino, A.; Dow, T.; Srinivassane, S.; Filipovic, M.; Bell, C.; Vandermeulen, J.; et al. Ryanodine channel complex stabilizer compound S48168/ARM210 as a disease modifier in dystrophin-deficient mdx mice: Proof-of-concept study and independent validation of efficacy. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 1025–1043. [Google Scholar] [CrossRef] [PubMed]

| Environment | DAS Solubility (mg/mL) | DAS Solubility (M) |
|---|---|---|
| HCl 0.05 M pH 1.2 | 4.31 × 10−2 | 8.84 × 10−5 |
| Phosphate Buffer 0.05 M pH 7.4 | 7.65 × 10−4 | 1.56 × 10−6 |
| Water | 6.49 × 10−4 | 1.33 × 10−6 |
| Molar Ratio DAS: HP-β-CD | δ CH3 (Pyrimidine) | δ CH3 (Benzene) |
|---|---|---|
| 1:0 | 2.498 | 2.292 |
| 1:1 | 2.513 | 2.293 |
| 1:10 | 2.534 | 2.294 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutrignelli, A.; Sanarica, F.; Lopalco, A.; Lopedota, A.; Laquintana, V.; Franco, M.; Boccanegra, B.; Mantuano, P.; De Luca, A.; Denora, N. Dasatinib/HP-β-CD Inclusion Complex Based Aqueous Formulation as a Promising Tool for the Treatment of Paediatric Neuromuscular Disorders. Int. J. Mol. Sci. 2019, 20, 591. https://doi.org/10.3390/ijms20030591
Cutrignelli A, Sanarica F, Lopalco A, Lopedota A, Laquintana V, Franco M, Boccanegra B, Mantuano P, De Luca A, Denora N. Dasatinib/HP-β-CD Inclusion Complex Based Aqueous Formulation as a Promising Tool for the Treatment of Paediatric Neuromuscular Disorders. International Journal of Molecular Sciences. 2019; 20(3):591. https://doi.org/10.3390/ijms20030591
Chicago/Turabian StyleCutrignelli, Annalisa, Francesca Sanarica, Antonio Lopalco, Angela Lopedota, Valentino Laquintana, Massimo Franco, Brigida Boccanegra, Paola Mantuano, Annamaria De Luca, and Nunzio Denora. 2019. "Dasatinib/HP-β-CD Inclusion Complex Based Aqueous Formulation as a Promising Tool for the Treatment of Paediatric Neuromuscular Disorders" International Journal of Molecular Sciences 20, no. 3: 591. https://doi.org/10.3390/ijms20030591
APA StyleCutrignelli, A., Sanarica, F., Lopalco, A., Lopedota, A., Laquintana, V., Franco, M., Boccanegra, B., Mantuano, P., De Luca, A., & Denora, N. (2019). Dasatinib/HP-β-CD Inclusion Complex Based Aqueous Formulation as a Promising Tool for the Treatment of Paediatric Neuromuscular Disorders. International Journal of Molecular Sciences, 20(3), 591. https://doi.org/10.3390/ijms20030591