Steam Reforming of Model Bio-Oil Aqueous Fraction Using Ni-(Cu, Co, Cr)/SBA-15 Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Fresh Catalysts
2.2. Steam Reforming of Bio-Oil Aqueous Fraction Model Compounds
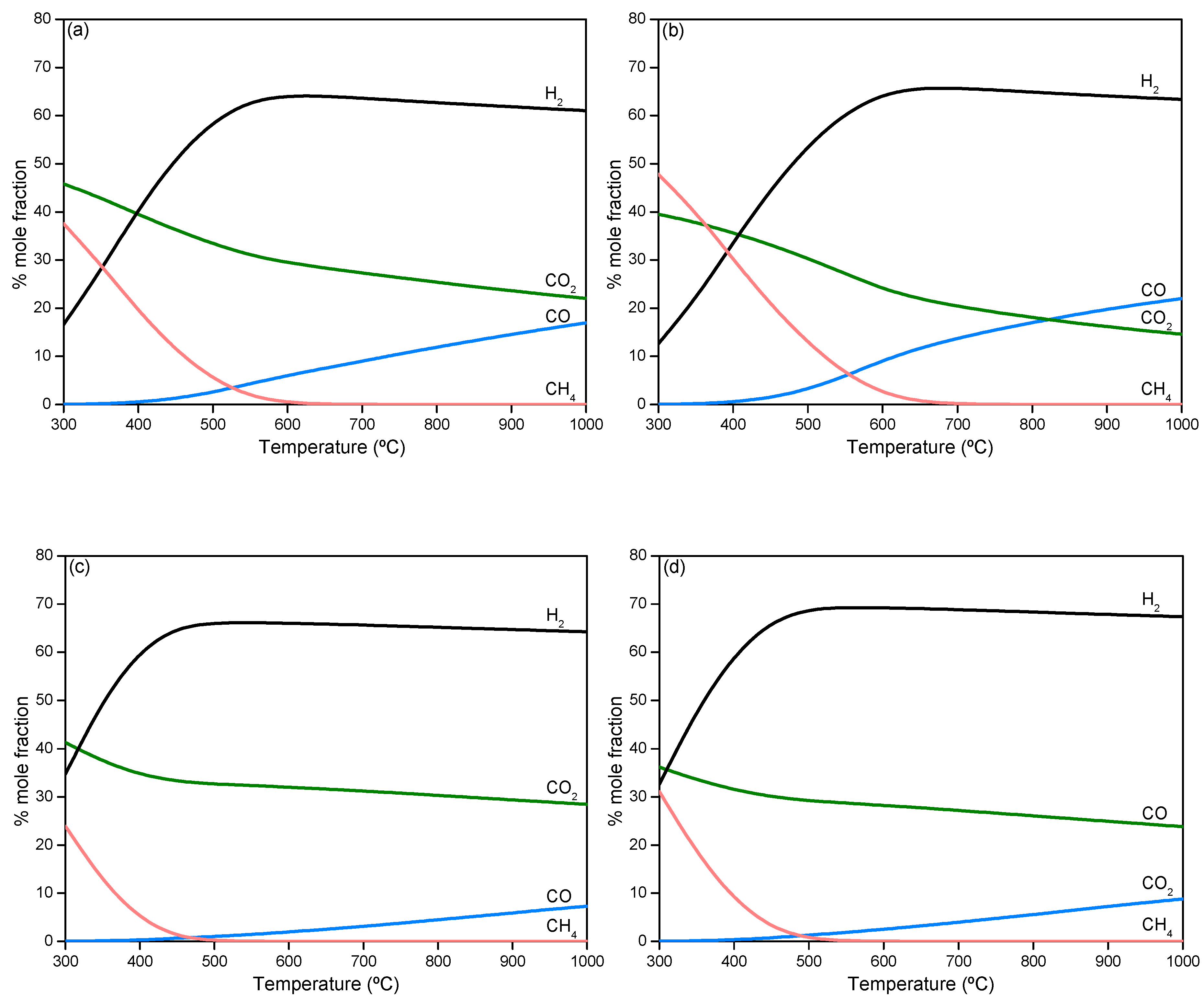
2.2.1. Thermodynamic Analysis of Steam Reforming of Model Compounds
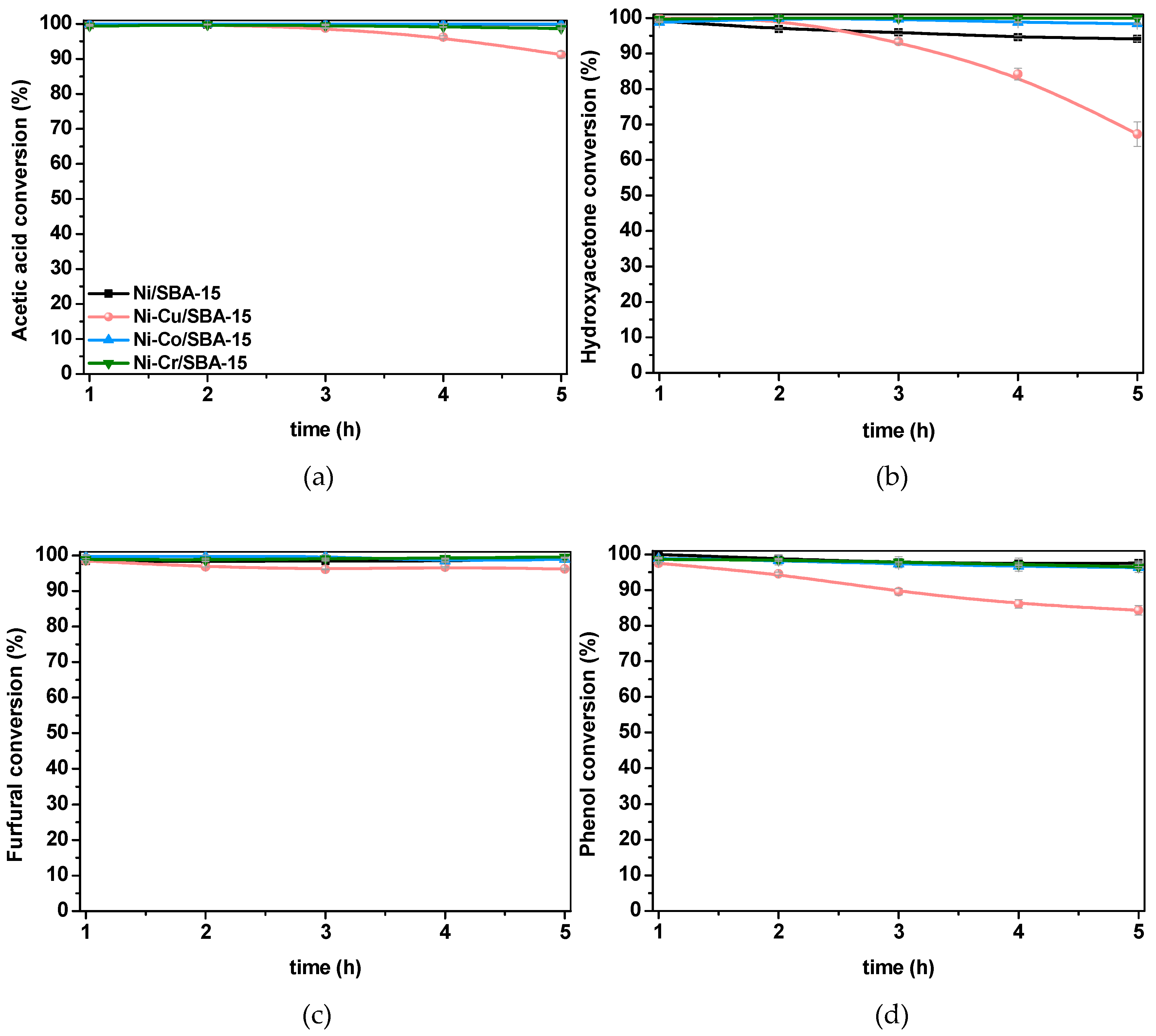
2.2.2. Steam Reforming of Model Compounds on Ni-M/SBA-15 Catalysts
2.2.3. Coke Formation during the Steam Reforming of Model Compounds on Ni-M/SBA-15 Catalysts
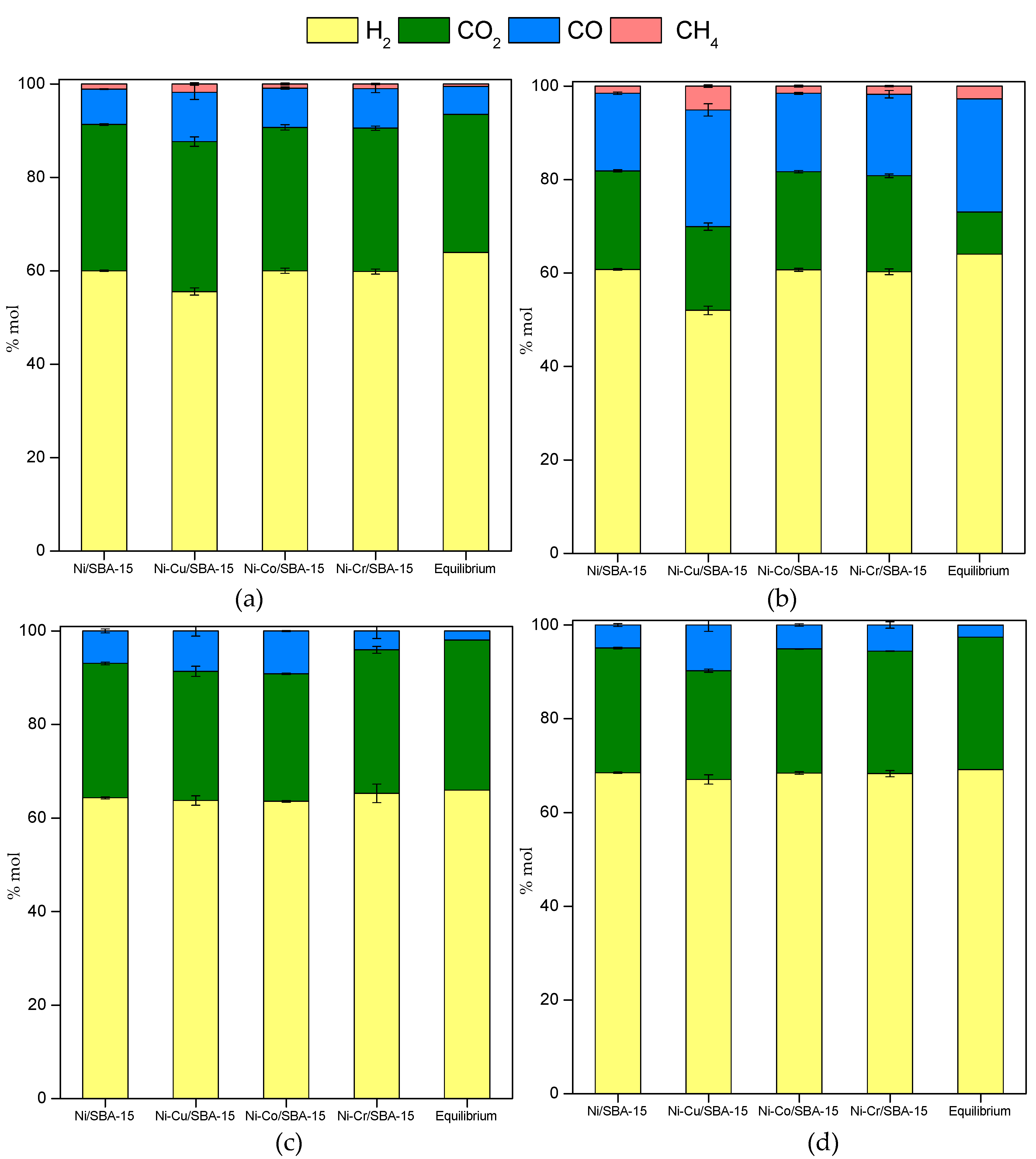
2.3. Steam Reforming of Model Bio-Oil Aqueous Fraction
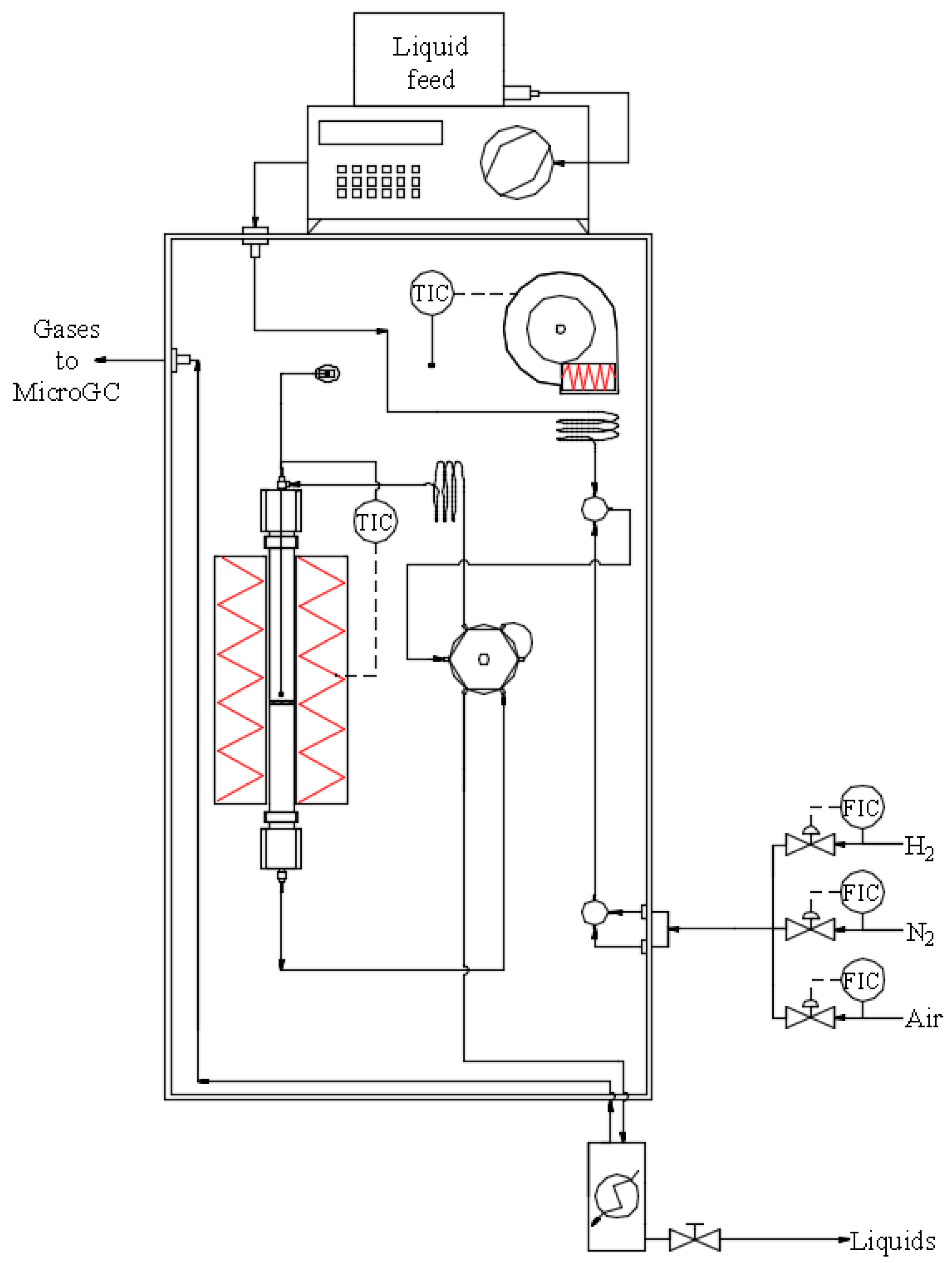
3. Experimental
3.1. Catalyst Synthesis and Characterization
3.2. Catalytic Tests
3.3. Thermodynamic Calculations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nashawi, I.S.; Malallah, A.; Al-Bisharah, M. Forecasting world crude oil production using multicyclic Hubbert model. Energy Fuels 2010, 24, 1788–1800. [Google Scholar] [CrossRef]
- Lior, N. Sustainable energy development: The present (2009) situation and possible paths to the future. Energy 2010, 35, 3976–3994. [Google Scholar] [CrossRef]
- Marino, C.; Nucara, A.; Pietrafesa, M.; Pudao, A. An energy self-sufficient public building using integrated renewable sources and hydrogen storage. Energy 2013, 57, 95–105. [Google Scholar] [CrossRef]
- Ajanovic, A. Renewable fuels–A comparative assessment from economic, energetic and ecological point-of-view up to 2050 in EU-countries. Renew. Energy 2013, 60, 733–738. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Tang, L.; Zhang, H.; Zhang, G.; Yang, X.; Liu, P.; Mao, Z. Establishment and assessment of a novel cleaner production process of corn grain fuel ethanol. Bioresour. Technol. 2013, 148, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Gökgöz, F.; Güvercin, M.T. Energy security and renewable energy efficiency in EU. Renew. Sustain. Energy Rev. 2018, 96, 226–239. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Hu, K.; Hu, C.; Zhang, Z.; Liu, Q.; Hu, S.; Xiang, J.; Wang, Y.; Zhang, S. Progress in the reforming of bio-oil derived carboxylic acids for hydrogen generation. J. Power Sources 2018, 403, 137–156. [Google Scholar] [CrossRef]
- Santori, G.; DiNicola, G.; Moglie, M.; Polonara, F. A review analyzing the industrial biodiesel production practice starting from vegetable oil refining. Appl. Energy 2012, 92, 109–132. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of thermochemical routes for hydrogen production from biomass: A review. Energ. Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Hydrogen Production from Bioethanol. In Hydrogen Production: Prospects and Processes; Honnery, D.R., Moriarty, P., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 247–294. ISBN 978-1-62100-246-8. [Google Scholar]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Comparison of ethanol steam reforming using Co and Ni catalysts supported on SBA-15 modified by Ca and Mg. Fuel Process. Technol. 2016, 146, 99–109. [Google Scholar] [CrossRef]
- Carrero, A.; Vizcaíno, A.J.; Calles, J.A.; García-Moreno, L. Hydrogen production through glycerol steam reforming using Co catalysts supported on SBA-15 doped with Zr, Ce and La. J. Energy Chem. 2017, 26, 42–48. [Google Scholar] [CrossRef]
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; dos Santos, R.G. Upgrading the Glycerol from Biodiesel Production as a Source of Energy Carriers and Chemicals—A Technological Review for Three Chemical Pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Fermoso, J.; Hernando, H.; Jiménez-Sánchez, S.; Lappas, A.A.; Heracleous, E.; Pizarro, P.; Coronado, J.M.; Serrano, D.P. Bio-oil production by lignocellulose fast-pyrolysis: Isolating and comparing the effects of indigenous versus external catalysts. Fuel Process. Technol. 2017, 167, 563–574. [Google Scholar] [CrossRef]
- Bimbela, F.; Ábrego, J.; Puerta, R.; García, L.; Arauzo, J. Catalytic steam reforming of the aqueous fraction of bio-oil using Ni-Ce/Mg-Al catalysts. Appl. Catal. B-Environ. 2017, 209, 346–357. [Google Scholar] [CrossRef]
- Lian, X.; Xue, Y.; Zhao, A.; Xu, G.; Han, S.; Yu, H. Progress on upgrading methods of bio-oil: A review. Int. J. Energy Res. 2017, 41, 1798–1816. [Google Scholar] [CrossRef]
- Vagia, E.C.; Lemonidou, A.A. Thermodynamic analysis of hydrogen production via steam reforming of selected components of aqueous bio-oil fraction. Int. J. Hydrogen Energy 2007, 32, 212–223. [Google Scholar] [CrossRef]
- Bimbela, F.; Oliva, M.; Ruiz, J.; García, L.; Arauzo, J. Catalytic steam reforming of model compounds of biomass pyrolysis liquids in fixed bed: Acetol and n-butanol. J. Anal. Appl. Pyrolysis 2009, 85, 204–213. [Google Scholar] [CrossRef]
- Wang, S.; Cai, Q.; Zhang, F.; Li, X.; Zhang, L.; Luo, Z. Hydrogen production via catalytic reforming of the bio-oil model compounds: Acetic acid, phenol and hydroxyacetone. Int. J. Hydrogen Energy 2014, 39, 18675–18687. [Google Scholar] [CrossRef]
- Koike, M.; Li, D.; Watanabe, H.; Nakagawa, K.; Tomishige, K. Comparative study on steam reforming of model aromatic compounds of biomass tar over Ni and Ni–Fe alloy nanoparticles. Appl. Catal. A-Gen. 2015, 506, 151–162. [Google Scholar] [CrossRef]
- Ma, Z.; Xiao, R.; Zhang, H. Catalytic steam reforming of bio-oil model compounds for hydrogen-rich gas production using bio-char as catalyst. Int. J. Hydrogen Energy 2017, 42, 3579–3585. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Xie, H.; Duan, W.; Han, Z.; Liu, S.; Qin, Q. The production of hydrogen through steam reforming of bio-oil model compounds recovering waste heat from blast furnace slag. J. Therm. Anal. Calorim. 2018, 131, 2951–2962. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Jiang, X.; Han, X.; Ji, H. Compositional analysis of bio-oil derived from pyrolysis of seaweed. Energy Conv. Manag. 2013, 68, 273–280. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, T.; Ren, Z. Upgrading of liquid fuel from the pyrolysis of biomass. Bioresour. Technol. 2005, 96, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lu, G. Inhibition of methane formation in steam reforming reactions through modification of Ni catalyst and the reactants. Green Chem. 2009, 11, 724–732. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Ethanol steam reforming on Mg-and Ca-modified Cu–Ni/SBA-15 catalysts. Catal. Today 2009, 146, 63–70. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; Vizcaíno, A.J. Effect of Mg and Ca addition on coke deposition over Cu–Ni/SiO2 catalysts for ethanol steam reforming. Chem. Eng. J. 2010, 163, 395–402. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; García-Moreno, L. Hydrogen production by glycerol steam reforming over SBA-15-supported nickel catalysts: Effect of alkaline earth promoters on activity and stability. Catal. Today 2014, 227, 198–206. [Google Scholar] [CrossRef]
- Jeon, J.; Nam, S.; Ko, C.H. Rapid evaluation of coke resistance in catalysts for methane reforming using low steam-to-carbon ratio. Catal. Today 2018, 309, 140–146. [Google Scholar] [CrossRef]
- He, Z.; Jiao, Y.; Wang, J.; Chen, Y. Bi-functional composite oxides M(Na, K)-Ni/La-Al2O3 catalysts for steam reforming of n-decane. Fuel 2018, 212, 193–201. [Google Scholar] [CrossRef]
- Nabgan, W.; Abdullah, T.A.T.; Mat, R.; Nabgan, B.; Gambo, Y.; Moghadamian, K. Acetic acid-phenol steam reforming for hydrogen production: Effect of different composition of La2O3-Al2O3 support for bimetallic Ni-Co catalyst. J. Environ. Chem. Eng. 2016, 4, 2765–2773. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J. Ce and La modification of mesoporous Cu–Ni/SBA-15 catalysts for hydrogen production through ethanol steam reforming. Microporous Mesoporous Mater. 2009, 119, 200–207. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; Lindo, M. Effect of Ce and Zr addition to Ni/SiO2 catalysts for hydrogen production through ethanol steam reforming. Catalysts 2015, 5, 58–76. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Hydrogen production by ethanol steam reforming over Cu–Ni supported catalysts. Int. J. Hydrogen Energy 2007, 32, 1450–1461. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; Vizcaíno, A.J. Hydrogen production by ethanol steam reforming over Cu-Ni/SBA-15 supported catalysts prepared by direct synthesis and impregnation. Appl. Catal. A-Gen. 2007, 327, 82–94. [Google Scholar] [CrossRef]
- Song, K.; Lu, M.; Xu, S.; Chen, C.; Zhan, Y.; Li, D.; Au, C.; Jiang, L.; Tomishige, K. Effect of alloy composition on catalytic performance and coke-resistance property of Ni-Cu/Mg(Al)O catalysts for dry reforming of methane. Appl. Catal. B Environ. 2018, 239, 324–333. [Google Scholar] [CrossRef]
- Pant, K.K.; Mohanty, P.; Agarwal, S.; Dalai, A.K. Steam reforming of acetic acid for hydrogen production over bifunctional Ni–Co catalysts. Catal. Today 2013, 207, 36–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Zhang, S.; Xu, Q.; Yan, Y. Steam Reforming of Bio-Oil for Hydrogen Production: Effect of Ni-Co Bimetallic Catalysts. Chem. Eng. Technol. 2012, 35, 302–308. [Google Scholar] [CrossRef]
- Assaf, P.G.; Nogueira, F.G.E.; Assaf, E.M. Ni and Co catalysts supported on alumina applied to steam reforming of acetic acid: Representative compound for the aqueous phase of bio-oil derived from biomass. Catal. Today 2013, 213, 2–8. [Google Scholar] [CrossRef]
- Horlyck, J.; Lawrey, C.; Lovell, E.C.; Amal, R.; Scott, J. Elucidating the impact of Ni and Co loading on the selectivity of bimetallic NiCo catalysts for dry reforming of methane. Chem. Eng. J. 2018, 352, 572–580. [Google Scholar] [CrossRef]
- Garcia, L.; French, R.; Czernik, S.; Chornet, E. Catalytic steam reforming of bio-oils for the production of hydrogen: Effects of catalyst composition. Appl. Catal. A-Gen. 2000, 201, 225–239. [Google Scholar] [CrossRef]
- Xu, L.; Duan, L.; Tang, M.; Liu, P.; Ma, X.; Zhang, Y.; Harris, H.G.; Fan, M. Catalytic CO2 reforming of CH4 over Cr-promoted Ni/char for H2 production. Int. J. Hydrogen Energy 2014, 39, 10141–10153. [Google Scholar] [CrossRef]
- Bangala, D.N.; Abatzoglou, N.; Chornet, E. Steam reforming of naphthalene on Ni–Cr/Al2O3 catalysts doped with MgO, TiO2, and La2O3. AIChE J. 1998, 44, 927–936. [Google Scholar] [CrossRef]
- Bui, Q.T.P.; Kim, Y.; Yoon, S.P.; Hana, J.; Ham, H.C.; Nam, S.W.; Yoon, C.W. Steam reforming of simulated biogas over plate Ni-Cr catalysts: Influence of pre-oxidation on catalytic activity. Appl. Catal. B Environ. 2015, 166–167, 335–344. [Google Scholar] [CrossRef]
- Odedairo, T.; Ma, J.; Chen, J.; Wang, S.; Zhu, Z. Influences of doping Cr/Fe/Ta on the performance of Ni/CeO2 catalyst under microwave irradiation in dry reforming of CH4. J. Solid State Chem. 2016, 233, 166–177. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Wang, Y. Significantly improved catalytic performance of Ni-based MgO catalyst in steam reforming of phenol by inducing mesostructure. Catalysts 2015, 5, 1721–1736. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; García-Moreno, L.; Vizcaíno, A.J. Production of renewable hydrogen from glycerol steam reforming over bimetallic Ni-(Cu,Co,Cr) catalysts supported on SBA-15 silica. Catalysts 2017, 7, 55. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; Wiley-VHC: Weinheim, Germany, 1997; Volume 2, pp. 446–450. ISBN 3-527-29212-8. [Google Scholar]
- Resende, K.A.; Ávila-Neto, C.N.; Rabelo-Neto, R.C.; Noronha, F.B.; Hori, C.E. Thermodynamic analysis and reaction routes of steam reforming of bio-oil aqueous fraction. Renew. Energy 2015, 80, 166–176. [Google Scholar] [CrossRef]
- Montero, C.; Oar-Arteta, L.; Remiro, A.; Arandia, A.; Bilbao, J.; Gayubo, A.G. Thermodynamic comparison between bio-oil and ethanol steam reforming. Int. J. Hydrogen Energy 2015, 40, 15963–15971. [Google Scholar] [CrossRef]
- Sahebdelfar, S. Steam reforming of propionic acid: Thermodynamic analysis of a model compound for hydrogen production from bio-oil. Int. J. Hydrogen Energy 2017, 42, 16386–16395. [Google Scholar] [CrossRef]
- Trane-Restrup, R.; Resasco, D.E.; Jensen, A.D. Steam reforming of light oxygenates. Catal. Sci. Technol. 2013, 3, 3292–3302. [Google Scholar] [CrossRef]
- Lemonidou, A.A.; Vagia, E.C.; Lercher, J.A. Acetic Acid Reforming over Rh Supported on La2O3/CeO2−ZrO2: Catalytic Performance and Reaction Pathway Analysis. ACS Catal. 2013, 3, 1919–1928. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, F.; Cai, Q.; Wang, Y.; Luo, Z. Bio-oil catalytic reforming without steam addition: Application to hydrogen production and studies on its mechanism. Int. J. Hydrogen Energy 2013, 38, 16038–16047. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Zhang, L.; Liu, S.; Lu, G. Steam reforming of acetic acid over Ni/ZrO2 catalysts: Effects of nickel loading and particle size on product distribution and coke formation. Appl. Catal. A Gen. 2012, 417–418, 281–289. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Zhu, Y.; Yang, G.; Zheng, P. DFT study of bio-oil decomposition mechanism on a Co stepped surface: Acetic acid as a model compound. Int. J. Hydrogen Energy 2015, 40, 330–339. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, F.; Cai, Q.; Li, X.; Zhu, L.; Wang, Q.; Luo, Z. Catalytic steam reforming of bio-oil model compounds for hydrogen production over coal ash supported Ni catalyst. Int. J. Hydrogen Energy 2014, 39, 2018–2025. [Google Scholar] [CrossRef]
- Matas Güell, B.; Babich, I.V.; Lefferts, L.; Seshan, K. Steam reforming of phenol over Ni-based catalysts—A comparative study. Appl. Catal. B Environ. 2011, 106, 280–286. [Google Scholar] [CrossRef]
- Ashok, J.; Subrahmanyam, M.; Venugopal, A. Hydrotalcite structure derived Ni-Cu-Al catalysts for the production of H2 by CH4 decomposition. Int. J. Hydrogen Energy 2008, 33, 2704–2713. [Google Scholar] [CrossRef]
- Artetxe, M.; Nahil, M.A.; Olazar, M.; Williams, P.T. Steam reforming of phenol as biomass tar model compound over Ni/Al2O3 catalyst. Fuel 2016, 184, 629–636. [Google Scholar] [CrossRef]
- Montero, C.; Ochoa, A.; Castaño, P.; Bilbao, J.; Gayubo, A.G. Monitoring Ni0 and coke evolution during the deactivation of a Ni/La2O3–αAl2O3 catalyst in ethanol steam reforming in a fluidized bed. J. Catal. 2015, 331, 181–192. [Google Scholar] [CrossRef]
- Bimbela, F.; Oliva, M.; Ruiz, J.; García, L.; Arauzo, J. Hydrogen production via catalytic steam reforming of the aqueous fraction of bio-oil using nickel-based coprecipitated catalysts. Int. J. Hydrogen Energy 2013, 38, 14476–14487. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Gu, F.; Wang, X.; Lu, X.; Li, H.; Xu, G.; Su, F. CO methanation on ordered mesoporous Ni–Cr–Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Trane-Restrup, R.; Jensen, A.D. Steam reforming of cyclic model compounds of bio-oil over Ni-based catalysts: Product distribution and carbon formation. Appl. Catal. B Env. 2015, 165, 117–127. [Google Scholar] [CrossRef]
- Helveg, S.; Sehested, J.; Rostrup-Nielsen, J.R. Whisker carbon in perspective. Catal. Today 2011, 178, 42–46. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.; Stucky, G. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Remón, J.; Mercado, V.; García, L.; Arauzo, J. Effect of acetic acid, methanol and potassium hydroxide on the catalytic steam reforming of glycerol: Thermodynamic and experimental study. Fuel Process. Technol. 2015, 138, 325–336. [Google Scholar] [CrossRef]
- Remón, J.; Broust, F.; Volle, G.; García, L.; Arauzo, J. Hydrogen production from pine and poplar bio-oils by catalytic steam reforming. Influence of the bio-oil composition on the process. Int. J. Hydrogen Energy 2015, 40, 5593–5608. [Google Scholar] [CrossRef]
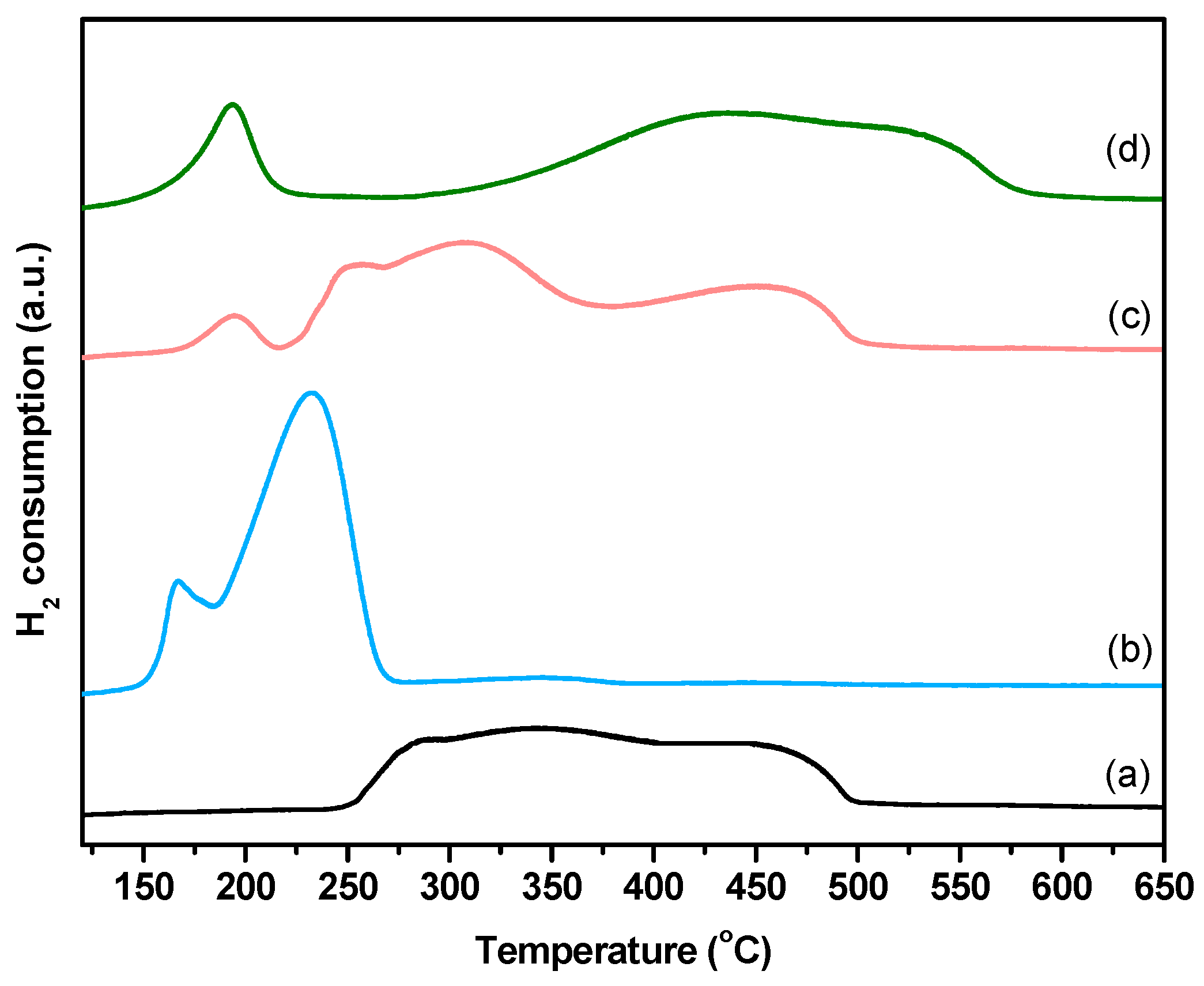
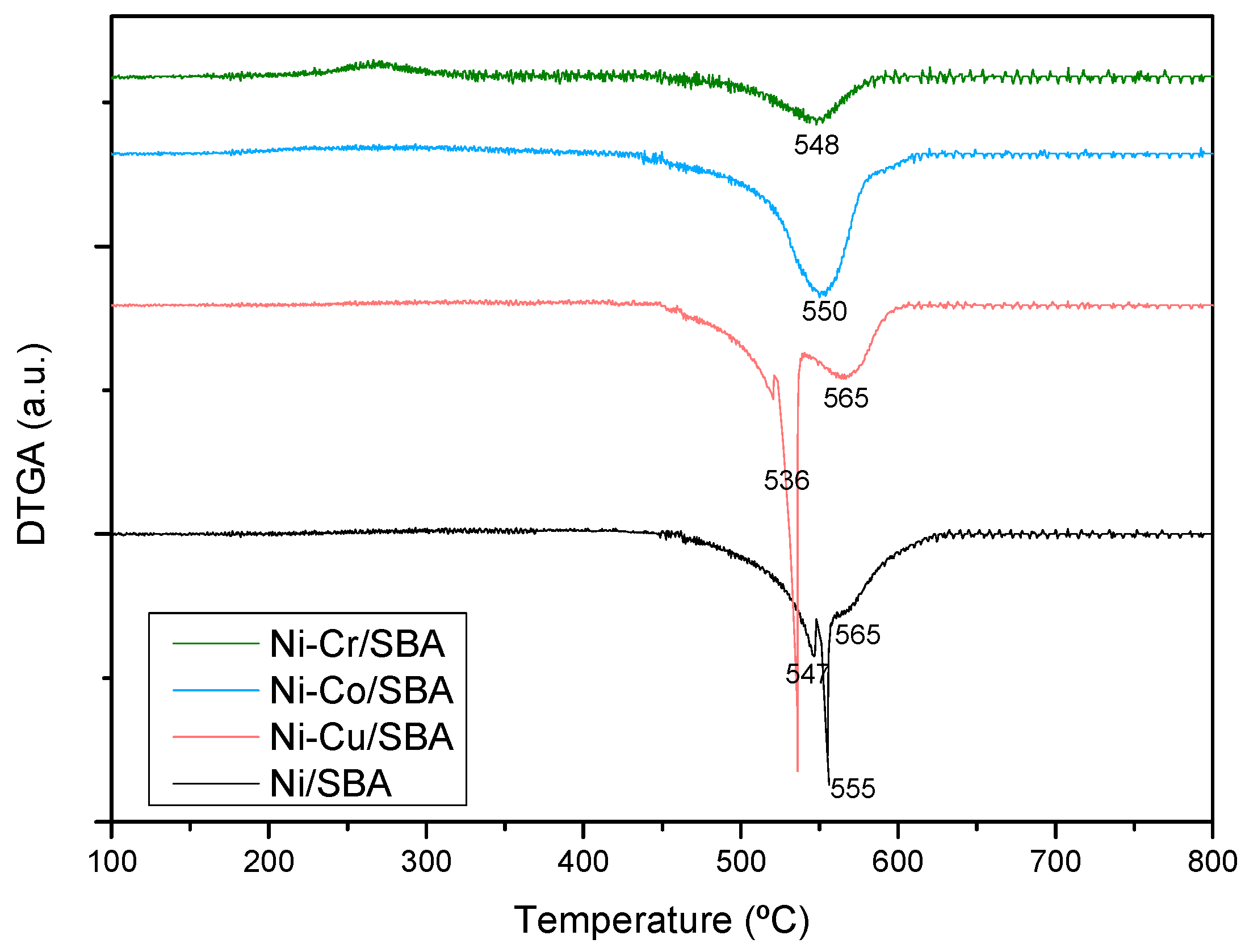
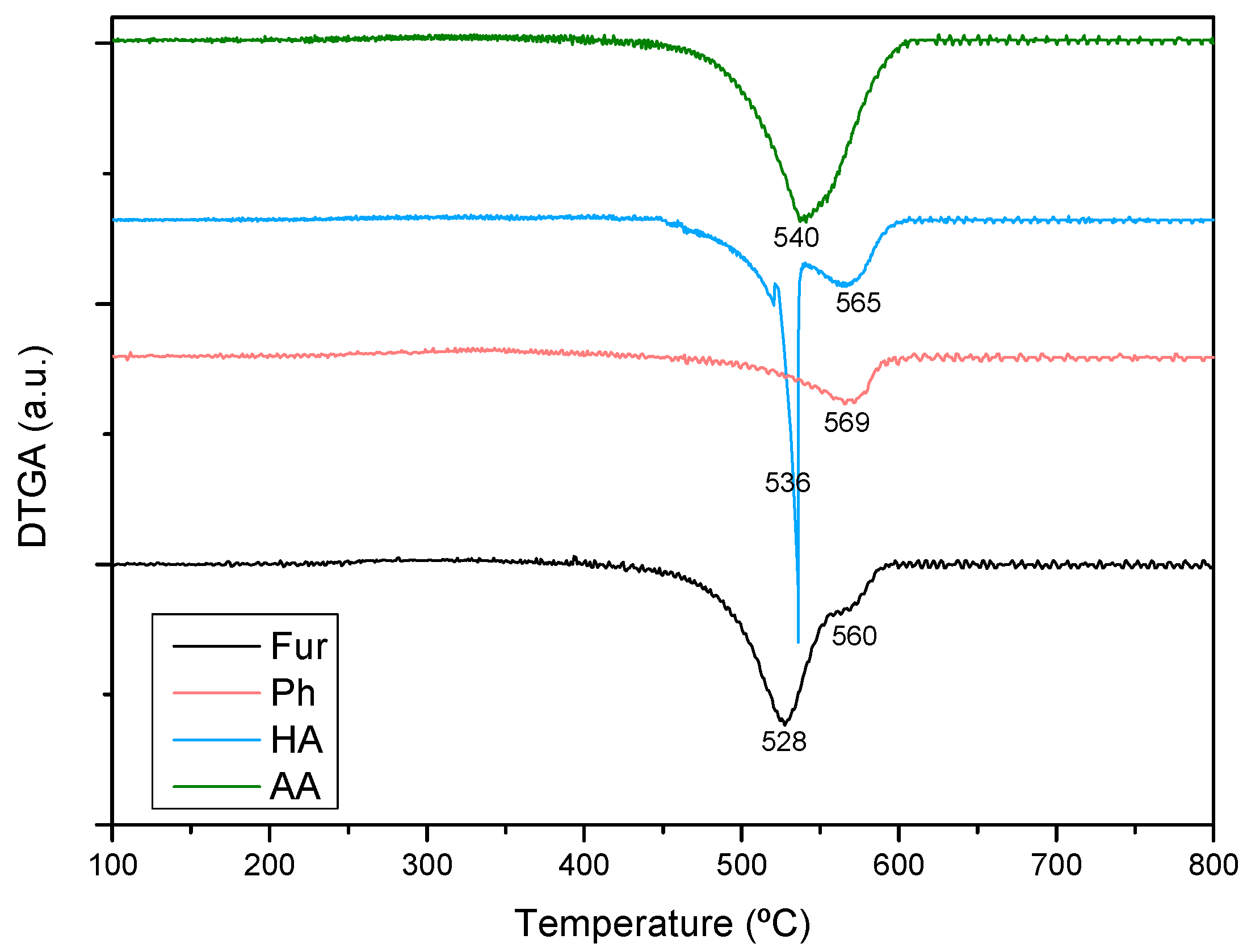
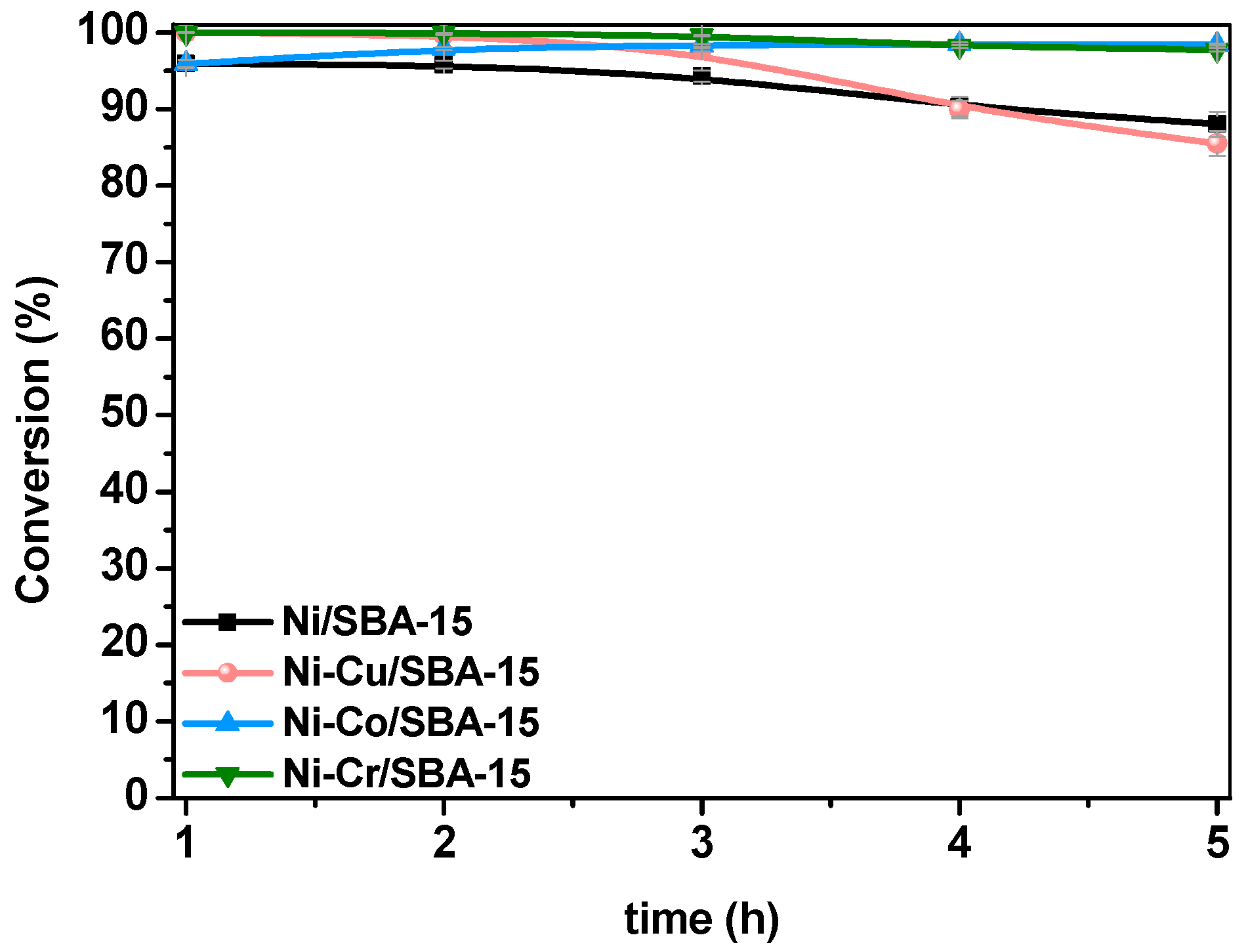
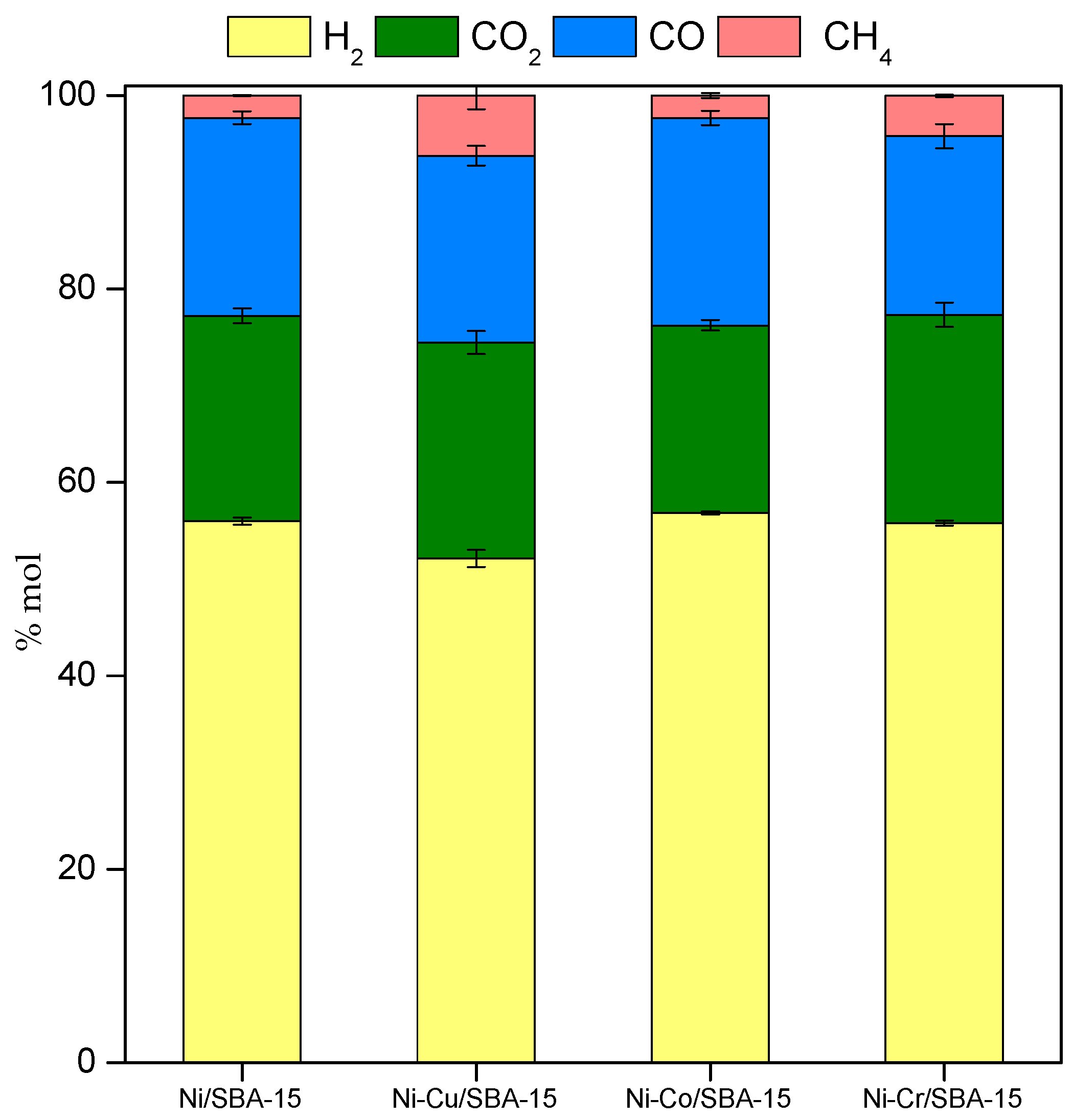
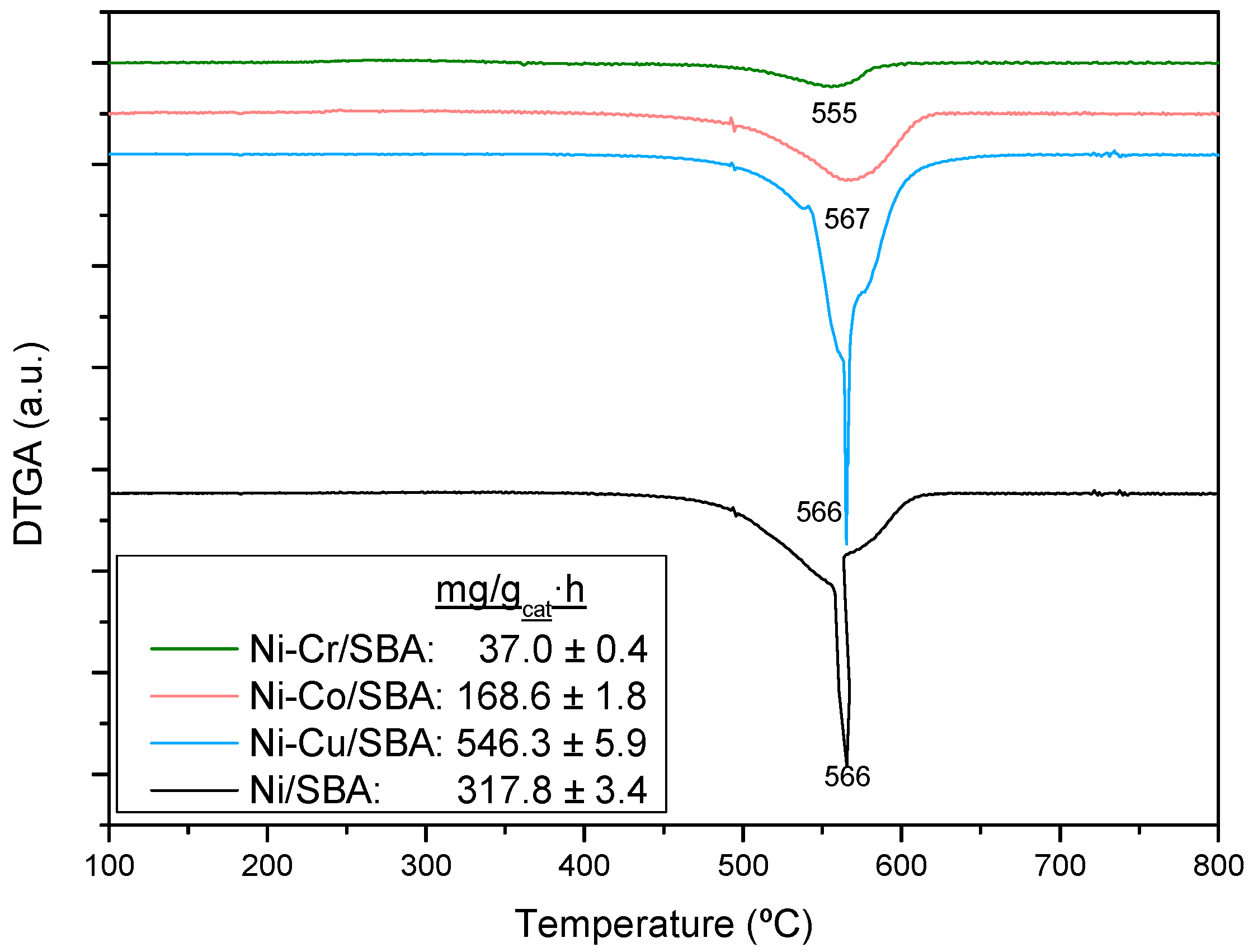
| Sample | Nia (wt %) | Ma,b (wt %) | SBET (m2g−1) | Vporec (cm3g−1) | Dpored (nm) | DNie (nm) | TPR Profile < 400 °C | TPR Profile > 400 °C |
|---|---|---|---|---|---|---|---|---|
| Area (%) | Area (%) | |||||||
| Ni/SBA-15 | 13.5 ± 0.1 | - | 521 ± 4 | 0.77 ± 0.02 | 8.3 ± 0.1 | 10.6 ± 0.5 | 69.8 | 30.2 |
| Ni-Cu/SBA-15 | 15.0 ± 0.1 | 4 ± 0.1 | 485 ± 1 | 0.71 ± 0.01 | 8.1 ± 0.1 | 9.9 ± 0.2 | 99.0 | 1.0 |
| Ni-Co/SBA-15 | 14.5 ± 0.1 | 4 ± 0.1 | 486 ± 1 | 0.72 ± 0.02 | 8.0 ± 0.1 | 9.0 ± 0.3 | 67.7 | 32.3 |
| Ni-Cr/SBA-15 | 14.3 ± 0.1 | 3.6 ± 0.1 | 482 ± 3 | 0.68 ± 0.03 | 8.3 ± 0.1 | 5.8 ± 0.1 | 16.5 | 83.5 |
| Catalyst | AA | HA | Fur | Ph |
|---|---|---|---|---|
| Ni/SBA-15 | 44.8 ± 0.5 | 96.3 ± 1.0 | 21.2 ± 0.2 | 14.1 ± 0.2 |
| Ni-Cu/SBA-15 | 154.0 ± 1.6 | 165.0 ± 1.8 | 96.7 ± 1.1 | 19.8 ± 0.2 |
| Ni-Co/SBA-15 | 35.8 ± 0.4 | 77.0 ± 0.8 | 9.2 ± 0.1 | 9.0 ± 0.2 |
| Ni-Cr/SBA-15 | 3.5 ± 0.1 | 18.3 ± 0.2 | 2.6 ± 0.1 | 7.0 ± 0.2 |
| Catalyst | AA | HA | Fur | Ph |
|---|---|---|---|---|
| Ni/SBA-15 | 87.6 ± 1.4 | 99.5 ± 0.0 | 77.4 ± 3.0 | 69.9 ± 2.6 |
| Ni-Cu/SBA-15 | 80.4 ± 2.2 | 96.5 ± 0.4 | 84.7 ± 2.1 | 74.0 ± 2.3 |
| Ni-Co/SBA-15 | 99.5 ± 0.1 | 100 ± 0.0 | 97.9 ± 0.3 | 89.5 ± 0.9 |
| Ni-Cr/SBA-15 | 98.4 ± 0.2 | 99.9 ± 0.0 | 95.5 ± 0.6 | 91.5 ± 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; García-Moreno, L.; Megía, P.J. Steam Reforming of Model Bio-Oil Aqueous Fraction Using Ni-(Cu, Co, Cr)/SBA-15 Catalysts. Int. J. Mol. Sci. 2019, 20, 512. https://doi.org/10.3390/ijms20030512
Calles JA, Carrero A, Vizcaíno AJ, García-Moreno L, Megía PJ. Steam Reforming of Model Bio-Oil Aqueous Fraction Using Ni-(Cu, Co, Cr)/SBA-15 Catalysts. International Journal of Molecular Sciences. 2019; 20(3):512. https://doi.org/10.3390/ijms20030512
Chicago/Turabian StyleCalles, José A., Alicia Carrero, Arturo J. Vizcaíno, Lourdes García-Moreno, and Pedro J. Megía. 2019. "Steam Reforming of Model Bio-Oil Aqueous Fraction Using Ni-(Cu, Co, Cr)/SBA-15 Catalysts" International Journal of Molecular Sciences 20, no. 3: 512. https://doi.org/10.3390/ijms20030512
APA StyleCalles, J. A., Carrero, A., Vizcaíno, A. J., García-Moreno, L., & Megía, P. J. (2019). Steam Reforming of Model Bio-Oil Aqueous Fraction Using Ni-(Cu, Co, Cr)/SBA-15 Catalysts. International Journal of Molecular Sciences, 20(3), 512. https://doi.org/10.3390/ijms20030512







