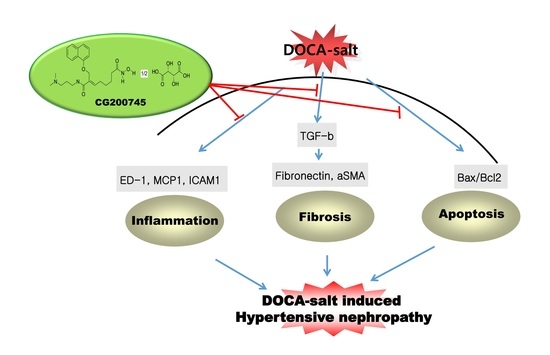
Renoprotective Effect of the Histone Deacetylase Inhibitor CG200745 in DOCA-Salt Hypertensive Rats
Abstract
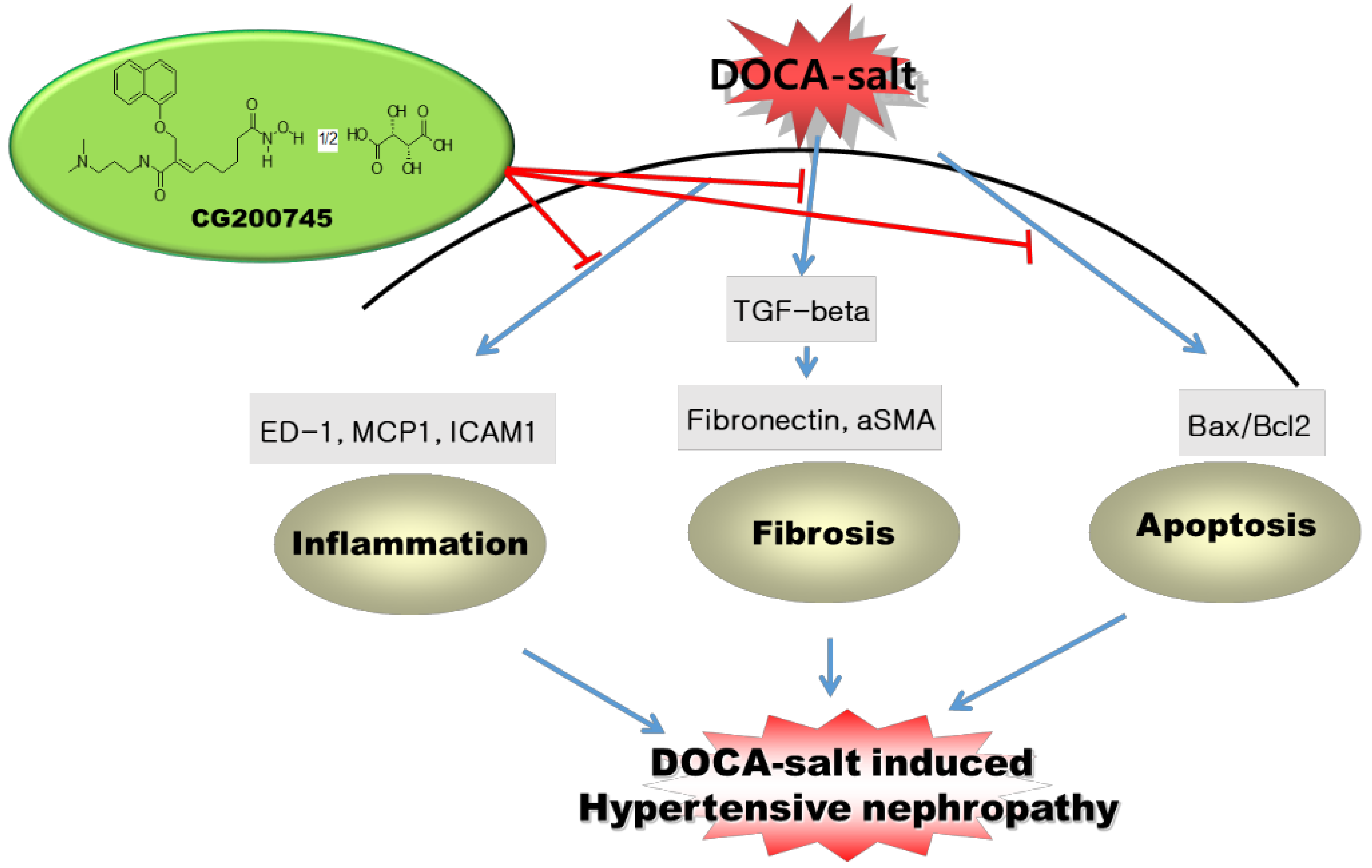
:1. Introduction
2. Results
2.1. Blood Pressure and Renal Function
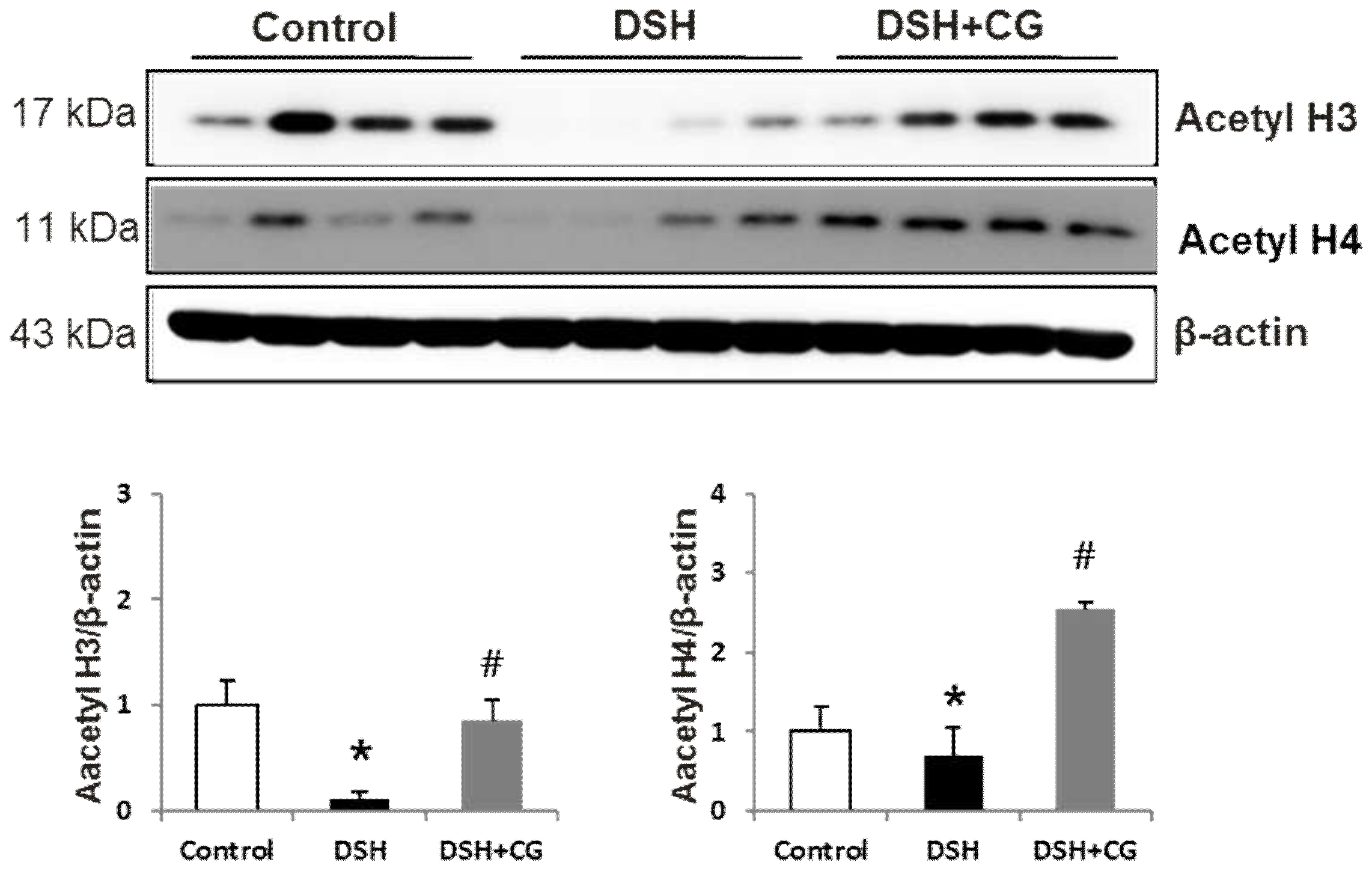
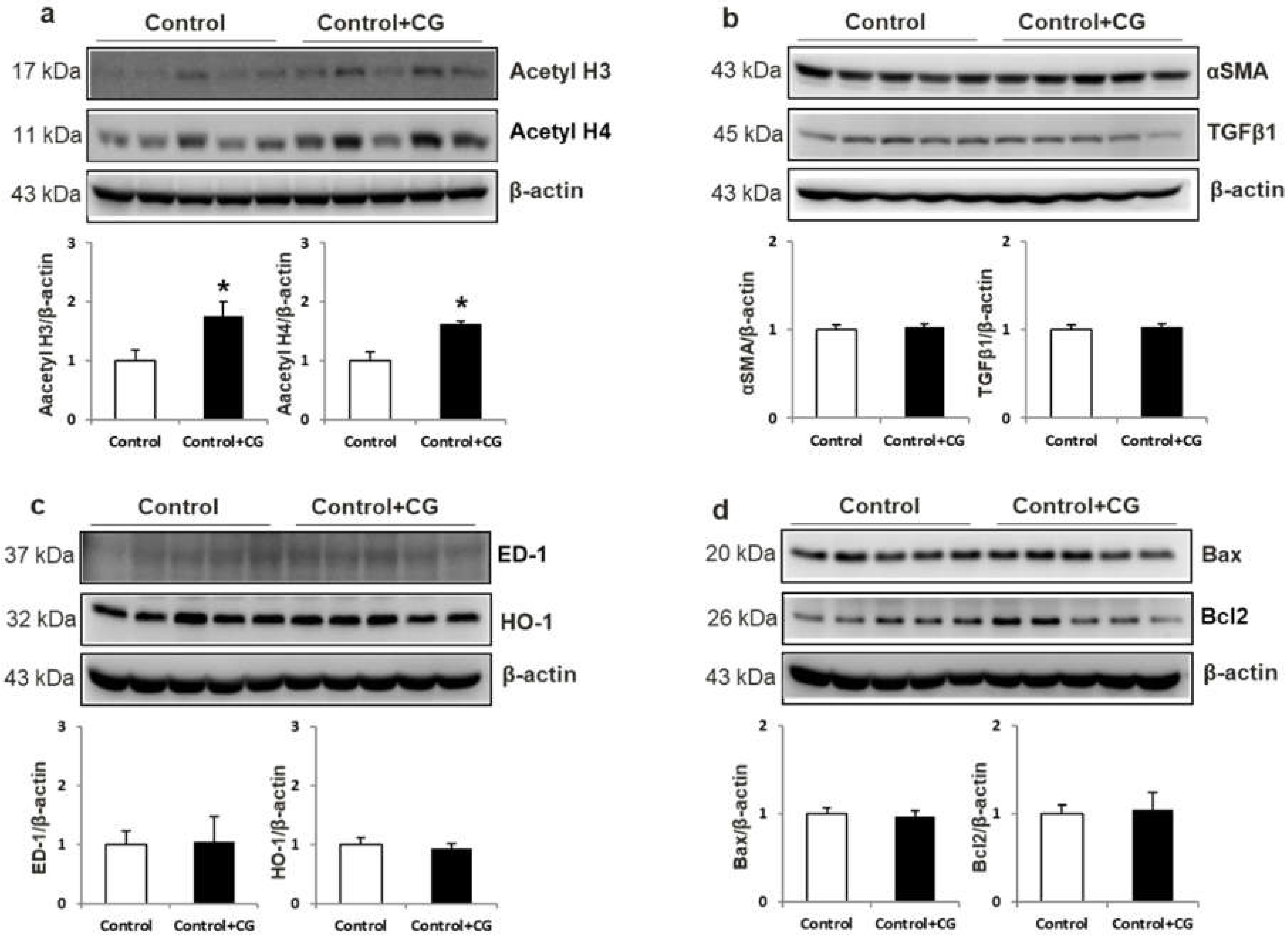
2.2. Effect of CG200745 on Acetylation in DSH Rats
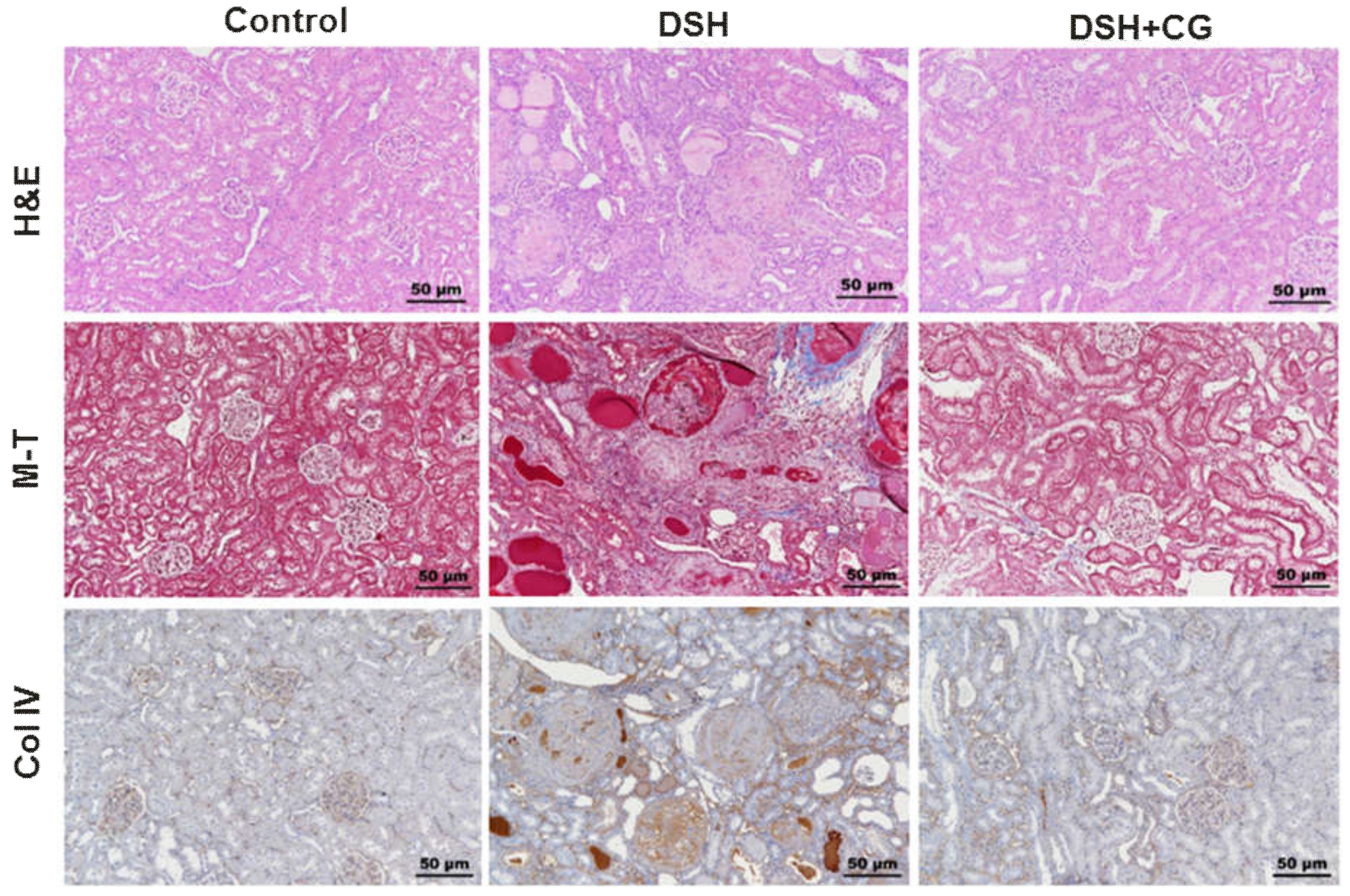
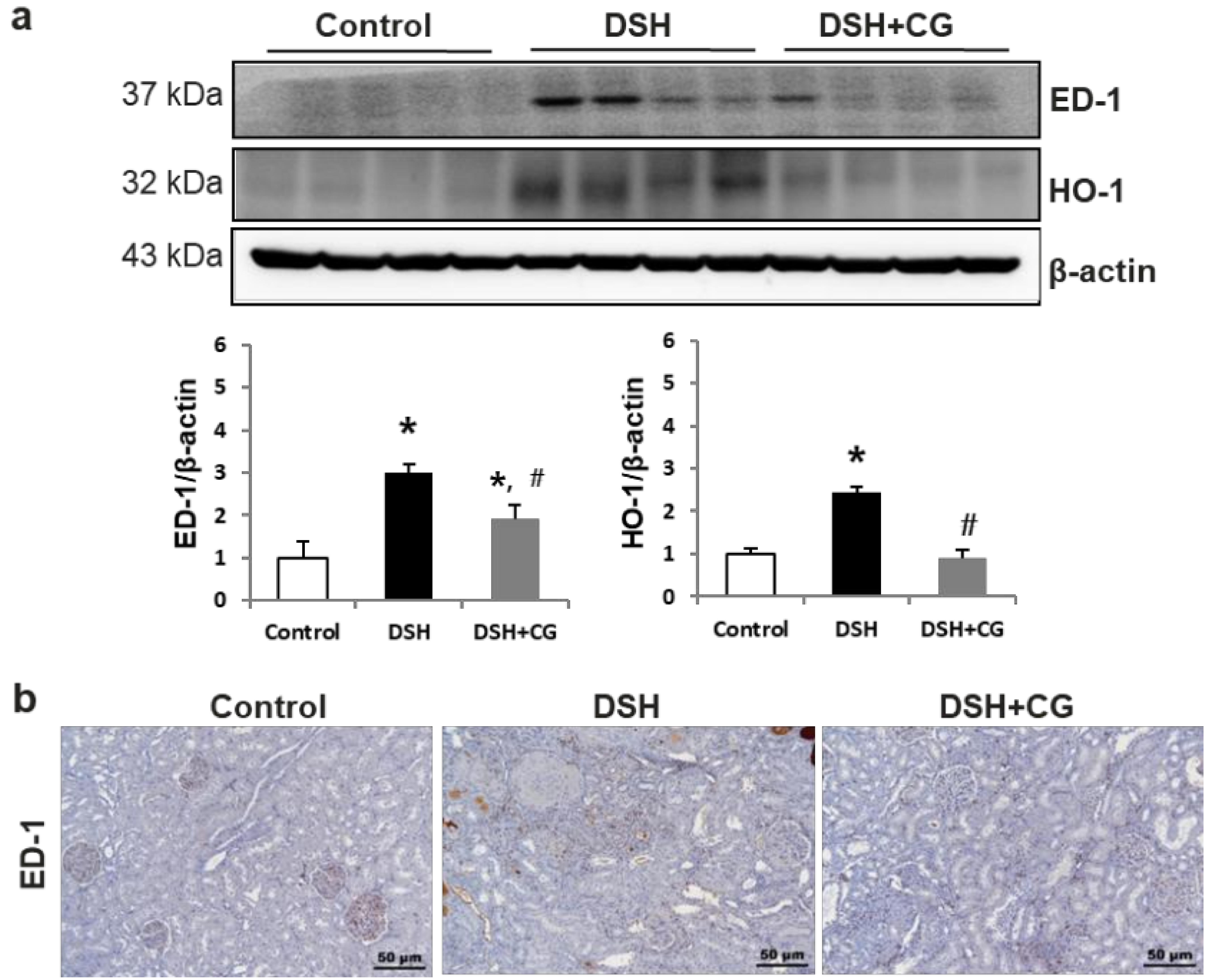
2.3. Effect of CG200745 on Morphological Changes in DSH Rats
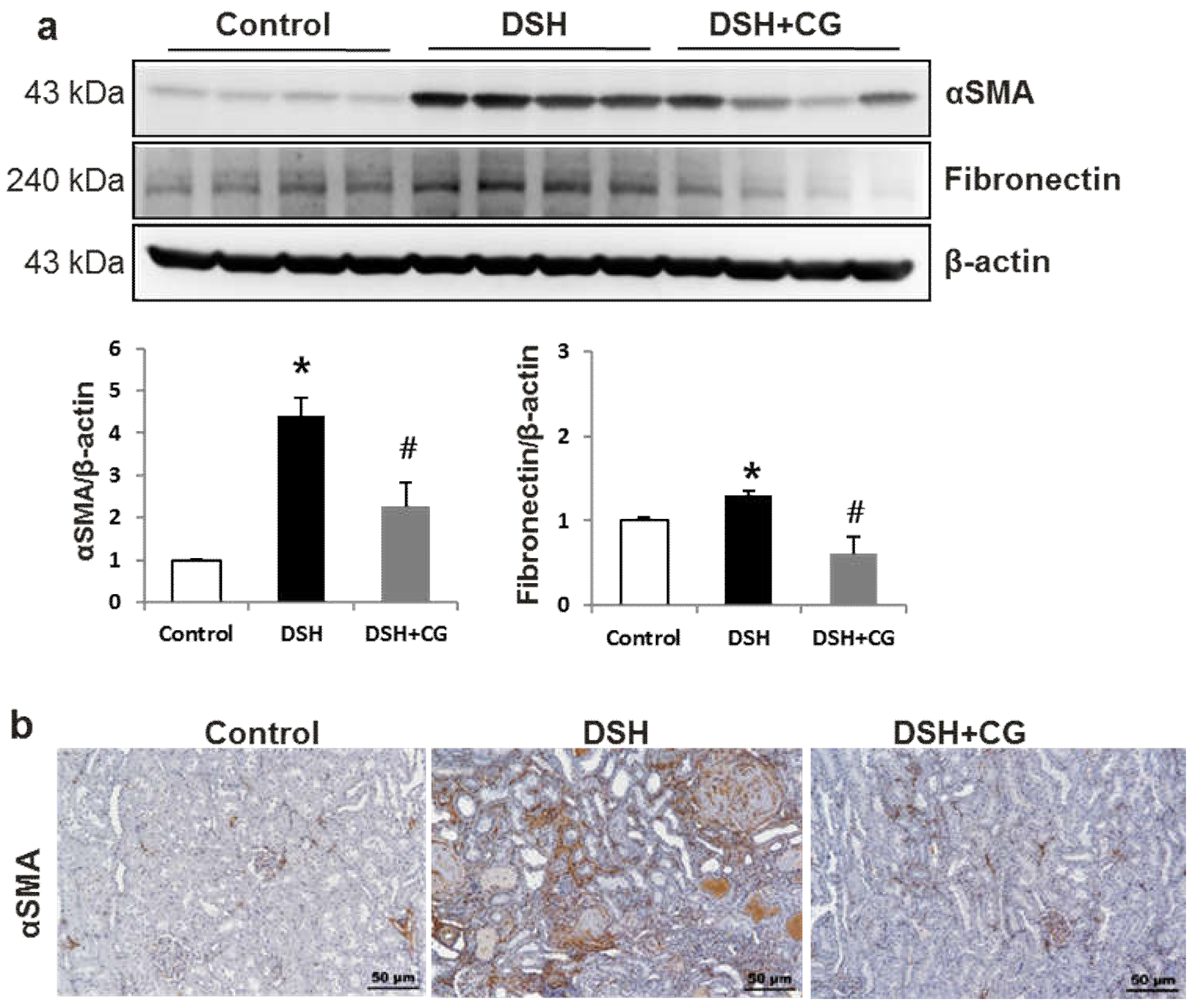
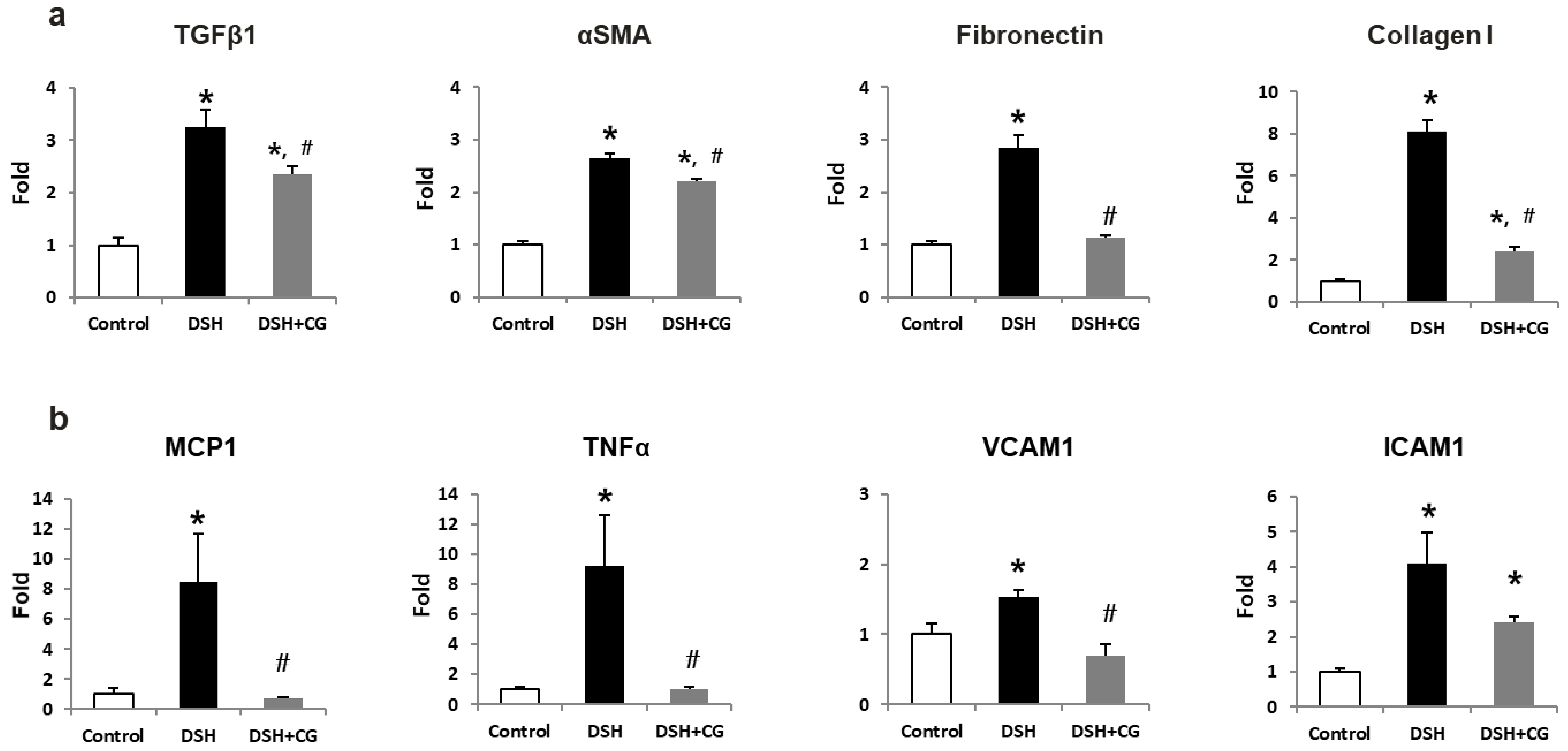
2.4. Effects of CG200745 on Kidney Fibrosis in DSH Rats
2.5. Effects of CG200745 on TGF-β–Smad Signaling in DSH Rats
2.6. Effects of CG200745 on Inflammatory Markers in DSH Rats
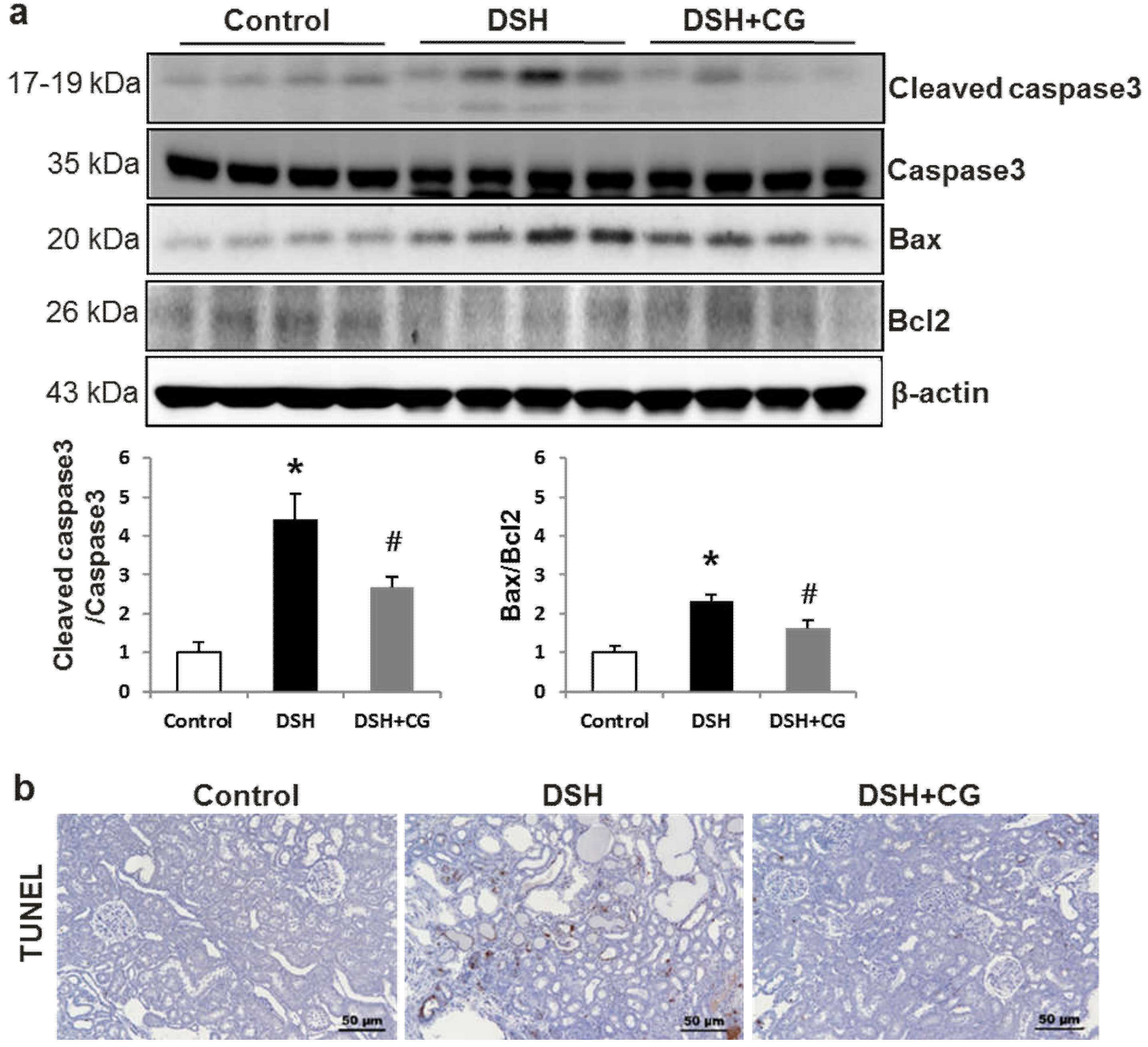
2.7. Effects of CG200745 on Apoptosis Markers in DSH Rats
2.8. Effects of CG200745 on Control Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Semiquantitative Immunoblotting
4.3. Immunohistochemistry
4.4. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Assay
4.5. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HDAC | Histone deacetylase |
| DOCA | Deoxycorticosterone acetate |
| HO-1 | Heme oxygenase-1 |
| MCP-1 | Monocyte chemoattractant protein 1 |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling |
| TNFα | Tumor necrosis factor α |
| TGFβ | Tumor growth factor β |
| SMA | Smooth muscle actin |
References
- Klahr, S.; Schreiner, G.; Ichikawa, I. The progression of renal disease. N. Engl. J. Med. 1988, 318, 1657–1666. [Google Scholar] [CrossRef]
- Iyer, A.; Fenning, A.; Lim, J.; Le, G.T.; Reid, R.C.; Halili, M.A.; Fairlie, D.P.; Brown, L. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. Br. J. Pharmacol. 2010, 159, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, J.P.; Sriramula, S.; Pariaut, R.; Guggilam, A.; Mariappan, N.; Elks, C.M.; Francis, J. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension 2010, 56, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Ma, L.; Liu, N.; Ponnusamy, M.; Zhao, T.C.; Yan, H.; Zhuang, S. Histone deacetylase 1/2 mediates proliferation of renal interstitial fibroblasts and expression of cell cycle proteins. J. Cell. Biochem. 2011, 112, 2138–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglarz, M.; Touyz, R.M.; Viel, E.C.; Amiri, F.; Schiffrin, E.L. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction wtih the renin-angiotension system. Am. J. Hypertens. 2004, 17, 597–603. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Lu, L.; Chen, S.S.; Quinn, M.T.; Weber, K.T. Aldosterone-induced inflammation in the rat heart: Role of oxidative stress. Am. J. Pathol. 2002, 161, 1773–1781. [Google Scholar] [CrossRef]
- Bae, E.H.; Kim, I.J.; Park, J.W.; Ma, S.K.; Lee, J.U.; Kim, S.W. Renoprotective effect of rosuvastatin in DOCA-salt hypertensive rats. Nephrol. Dial. Transplant. 2010, 25, 1051–1059. [Google Scholar] [CrossRef]
- Oh, E.T.; Park, M.T.; Choi, B.H.; Ro, S.; Choi, E.K.; Jeong, S.Y.; Park, H.J. Novel histone deacetylase inhibitor CG200745 induces clonogenic cell death by modulating acetylation of p53 in cancer cells. Investig. New Drugs 2012, 30, 435–442. [Google Scholar] [CrossRef]
- Hwang, J.J.; Kim, Y.S.; Kim, T.; Kim, M.J.; Jeong, I.G.; Lee, J.H.; Choi, J.; Jang, S.; Ro, S.; Kim, C.S. A novel histone deacetylase inhibitor, CG200745, potentiates anticancer effect of docetaxel in prostate cancer via decreasing Mcl-1 and Bcl-XL. Investig. New Drugs 2012, 30, 1434–1442. [Google Scholar] [CrossRef]
- Chun, S.M.; Lee, J.Y.; Choi, J.; Lee, J.H.; Hwang, J.J.; Kim, C.S.; Suh, Y.A.; Jang, S.J. Epigenetic modulation with HDAC inhibitor CG200745 induces anti-proliferation in non-small cell lung cancer cells. PLoS ONE 2015, 10, e0119379. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, S.B.; Kim, S.A.; Kwon, S.K.; Cha, H.; Lee, D.Y.; Ro, S.; Cho, J.M.; Song, S.Y. A novel HDAC inhibitor, CG200745, inhibits pancreatic cancer cell growth and overcomes gemcitabine resistance. Sci. Rep. 2017, 7, 41615. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.E.; Park, S.B.; Kim, K.; Kim, C.; Song, S.Y. CG200745, an HDAC inhibitor, induces anti-tumour effects in cholangiocarcinoma cell lines via miRNAs targeting the Hippo pathway. Sci. Rep. 2017, 7, 10921. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Song, M.J.; Lee, H.A.; Kang, S.H.; Kim, M.; Yang, E.K.; Lee, D.Y.; Ro, S.; Cho, J.M.; Kim, I. Histone deacetylase inhibitor, CG200745, attenuates cardiac hypertrophy and fibrosis in DOCA-induced hypertensive rats. Korean J. Physiol. Pharmacol. 2016, 20, 477–485. [Google Scholar] [CrossRef]
- Kee, H.J.; Kook, H. Kruppel-like factor 4 mediates histone deacetylase inhibitor-induced prevention of cardiac hypertrophy. J. Mol. Cell. Cardiol. 2009, 47, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.J.; Kwon, J.S.; Shin, S.; Ahn, Y.; Jeong, M.H.; Kook, H. Trichostatin A prevents neointimal hyperplasia via activation of Kruppel like factor 4. Vascul. Pharmacol. 2011, 55, 127–134. [Google Scholar] [CrossRef]
- Liu, N.; He, S.; Tolbert, E.; Gong, R.; Bayliss, G.; Zhuang, S. Suramin alleviates glomerular injury and inflammation in the remnant kidney. PLoS ONE 2012, 7, e36194. [Google Scholar] [CrossRef]
- Nicoletti, A.; Michel, J.B. Cardiac fibrosis and inflammation: Interaction with hemodynamic and hormonal factors. Cardiovasc. Res. 1999, 41, 532–543. [Google Scholar] [CrossRef]
- Rutschow, S.; Li, J.; Schultheiss, H.P.; Pauschinger, M. Myocardial proteases and matrix remodeling in inflammatory heart disease. Cardiovasc. Res. 2006, 69, 646–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoni, F.; Zaliani, A.; Bertolini, G.; Porro, G.; Pagani, P.; Pozzi, P.; Dona, G.; Fossati, G.; Sozzani, S.; Azam, T.; et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc. Natl. Acad. Sci. USA 2002, 99, 2995–3000. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, F.; Chipoy, C. Histone deacetylase inhibitors: New drugs for the treatment of inflammatory diseases? Drug Discov. Today 2005, 10, 197–204. [Google Scholar] [CrossRef]
- Murphy, M.; Crean, J.; Brazil, D.P.; Sadlier, D.; Martin, F.; Godson, C. Regulation and consequences of differential gene expression in diabetic kidney disease. Biochem. Soc. Trans. 2008, 36 Pt 5, 941–945. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, X.R.; Lan, H.Y. Smad3 mediates ANG II-induced hypertensive kidney disease in mice. Am. J. Physiol. Ren. Physiol. 2012, 302, F986–F997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagami, S.; Border, W.A.; Miller, D.E.; Noble, N.A. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J. Clin. Investig. 1994, 93, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Kikuta, T.; Kobayashi, T.; Inoue, T.; Kanno, Y.; Takigawa, M.; Sugaya, T.; Kopp, J.B.; Suzuki, H. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J. Am. Soc. Nephrol. 2005, 16, 133–143. [Google Scholar] [CrossRef]
- Das, A.; Ockaili, R.; Salloum, F.; Kukreja, R.C. Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1455–H1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikumar, P.; Dong, Z.; Patel, Y.; Hall, K.; Hopfer, U.; Weinberg, J.M.; Venkatachalam, M.A. Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 1998, 17, 3401–3415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikumar, P.; Dong, Z.; Weinberg, J.M.; Venkatachalam, M.A. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene 1998, 17, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Waller, H.L.; Harper, S.J.; Hosgood, S.A.; Bagul, A.; Kay, M.D.; Kaushik, M.; Yang, B.; Bicknell, G.R.; Nicholson, M.L. Differential expression of cytoprotective and apoptotic genes in an ischaemia-reperfusion isolated organ perfusion model of the transplanted kidney. Transpl. Int. 2007, 20, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Schou, U.K.; Peters, C.D.; de Seigneux, S.; Kwon, T.H.; Knepper, M.A.; Jonassen, T.E.; Frokiaer, J.; Nielsen, S. Increased apical targeting of renal epithelial sodium channel subunits and decreased expression of type 2 11beta-hydroxysteroid dehydrogenase in rats with CCl4-induced decompensated liver cirrhosis. J. Am. Soc. Nephrol. 2005, 16, 3196–3210. [Google Scholar] [CrossRef]
- Ma, S.K.; Joo, S.Y.; Choi, H.I.; Bae, E.H.; Nam, K.I.; Lee, J.; Kim, S.W. Activation of G-protein-coupled receptor 40 attenuates the cisplatin-induced apoptosis of hyman proximal tubule epithelial cells. Int. J. Mol. Med. 2014, 34, 1117–1123. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Control | DSH | DSH + CG200745 |
|---|---|---|---|
| Body weight (g) | 387.5 ± 3.33 | 257.5 ± 22.13 * | 280.0 ± 12.25 * |
| Kidney weight (g)/BW (kg) | 5.2 ± 0.3 | 14.2 ± 1.33 * | 11.3 ± 0.8 * |
| LV weight(g)/BW (kg) | 2.0 ± 0.05 | 3.9 ± 0.14 * | 3.3 ± 0.17 *,# |
| SBP (mmHg) | 119.0 ± 3.0 | 207.8 ± 9.7 * | 171.7 ± 9.8 *,# |
| Urine output (mL/24 h) | 21 ± 6.52 | 86.9 ± 16.46 * | 87.0 ± 16.97 * |
| Parameter | Control | DSH | DSH + CG200745 |
|---|---|---|---|
| BUN | 14.8 ± 0.4 | 21.2 ± 5.36 | 19.7 ± 3.41 |
| Creatinine | 0.24 ± 0.05 | 0.31 ± 0.08 | 0.25 ± 0.03 |
| Serum Na | 120.0 ± 4.5 | 118.3 ± 5.5 | 125.5 ± 3.4 |
| Urine Na | 303.0 ± 11.5 | 159.3 ± 10.64 * | 169.5 ± 9.70 * |
| FENa | 2.4 ± 0.67 | 12.3 ± 3.07 * | 9.9 ± 2.68 |
| ACR (mg/gCr) | 297.1 ± 197.3 | 5864.0 ± 662.2 * | 3681.1 ± 267.3 *,# |
| Primers | Sequence |
|---|---|
| TGFβ1 | Sense: GGACTACTACGCCAAAGAAG Antisense: TCAAAAGACAGCCACTCAGG |
| αSMA | Sense: TGTGCTGGACTCTGGAGATG Antisense: GAAGGAATAGCCACGCTCAG |
| Fibronectin | Sense: CATGAAGGGGGTCAGTCCTA Antisense: GTCCATTCCCCTTTTCCATT |
| Collagen I | Sense: CAACCTCAAGAAGTCCCTGC Antisense: ACAAGCGTGCTGTAGGTGAA |
| MCP1 | Sense: CTGCTACTCATTCACTGGC Antisense: CTTCTGGACCCATTCCTTAT |
| TNFα | Sense: GTCGTAGCAAACCACCAAGC Antisense: CTCCTGGTATGAAATGGCAA |
| VCAM1 | Sense: GGGGGCCAAGTCCGTTCTGA Antisense: GGGGGCCACTGAATTGAATC |
| ICAM1 | Sense: AAGGTGTGATATCCGGTAGA Antisense: CCTTCTAAGTGGTTGGAACA |
| GAPDH | Sense: ATCAAATGGGGTGATGCTGGTGCTG Antisense: CAGGTTTCTCCAGGCGGCATGTCAG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, E.H.; Kim, I.J.; Song, J.H.; Choi, H.S.; Kim, C.S.; Eom, G.H.; Kim, I.; Cha, H.; Cho, J.M.; Ma, S.K.; et al. Renoprotective Effect of the Histone Deacetylase Inhibitor CG200745 in DOCA-Salt Hypertensive Rats. Int. J. Mol. Sci. 2019, 20, 508. https://doi.org/10.3390/ijms20030508
Bae EH, Kim IJ, Song JH, Choi HS, Kim CS, Eom GH, Kim I, Cha H, Cho JM, Ma SK, et al. Renoprotective Effect of the Histone Deacetylase Inhibitor CG200745 in DOCA-Salt Hypertensive Rats. International Journal of Molecular Sciences. 2019; 20(3):508. https://doi.org/10.3390/ijms20030508
Chicago/Turabian StyleBae, Eun Hui, In Jin Kim, Ji Hong Song, Hong Sang Choi, Chang Seong Kim, Gwang Hyeon Eom, Inkyeom Kim, Hyunju Cha, Joong Myung Cho, Seong Kwon Ma, and et al. 2019. "Renoprotective Effect of the Histone Deacetylase Inhibitor CG200745 in DOCA-Salt Hypertensive Rats" International Journal of Molecular Sciences 20, no. 3: 508. https://doi.org/10.3390/ijms20030508