A Retrospective Analysis of the Cartilage Kunitz Protease Inhibitory Proteins Identifies These as Members of the Inter-α-Trypsin Inhibitor Superfamily with Potential Roles in the Protection of the Articulatory Surface
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Identity of the Ovine Cartilage SPIs
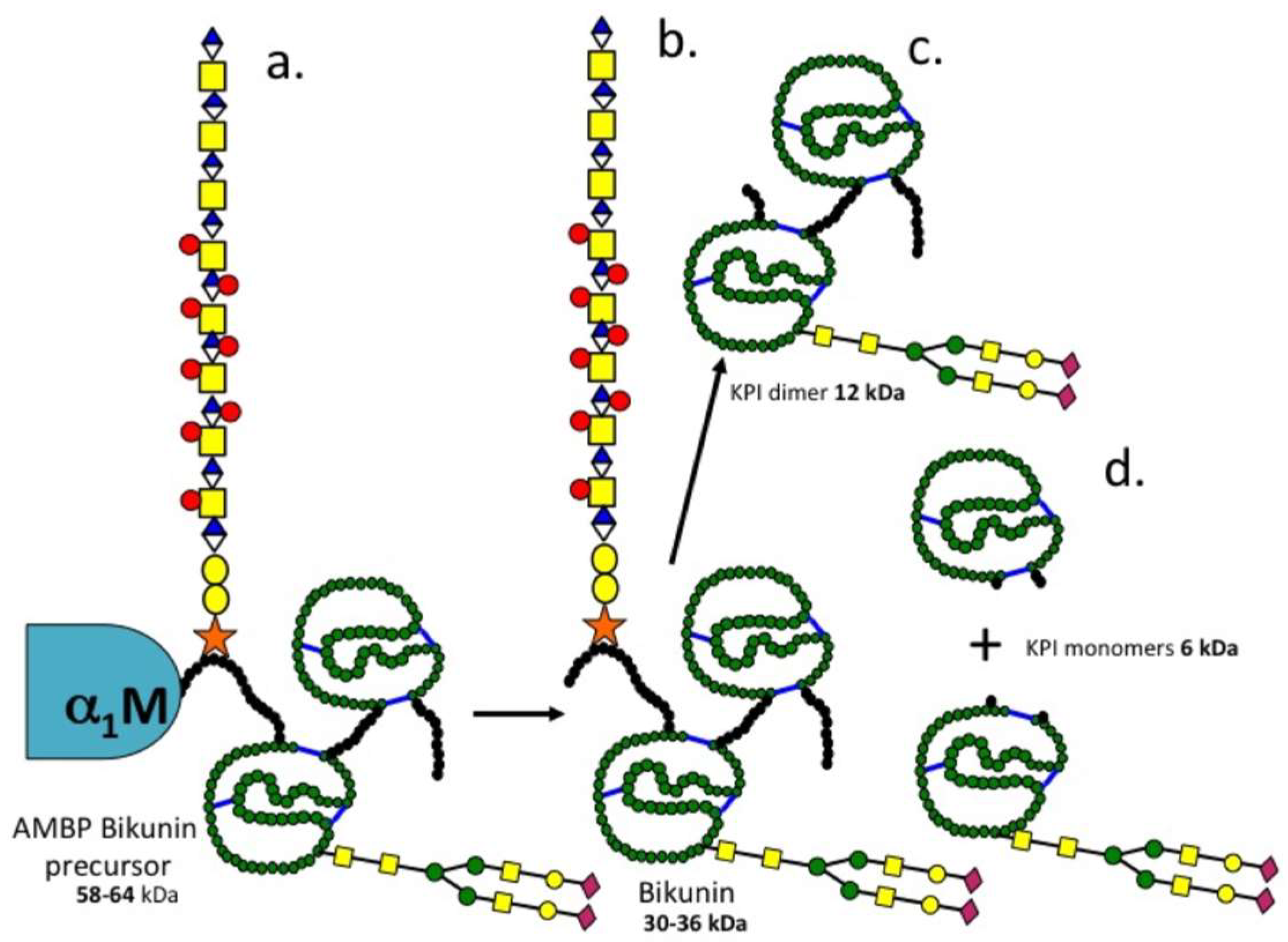
3.2. The Structural Organisation of Bikunin
3.3. Inter-Relationships between Ovine Articular Cartilage SPI Species and Bikunin/ITI
3.4. ITI/Bikunin Is A Multifunctional Protein
3.5. Detrimental Aspects of HC-HA Complex Transfer in Tissues
3.6. Tissue Isoforms of ITI
3.7. Beneficial Aspects of HC-HA Transfer in Connective Tissues
3.8. Protective Roles for ITI KPIs in Connective Tissues
3.9. KPIs in Meniscus, AC and IVD
3.10. Bikunin As A Cell Regulatory Proteoglycan
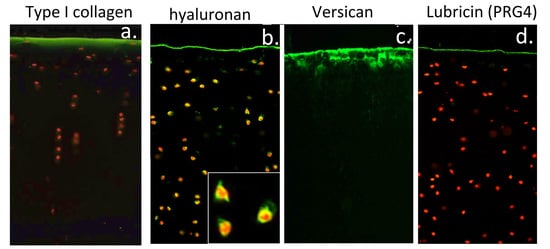
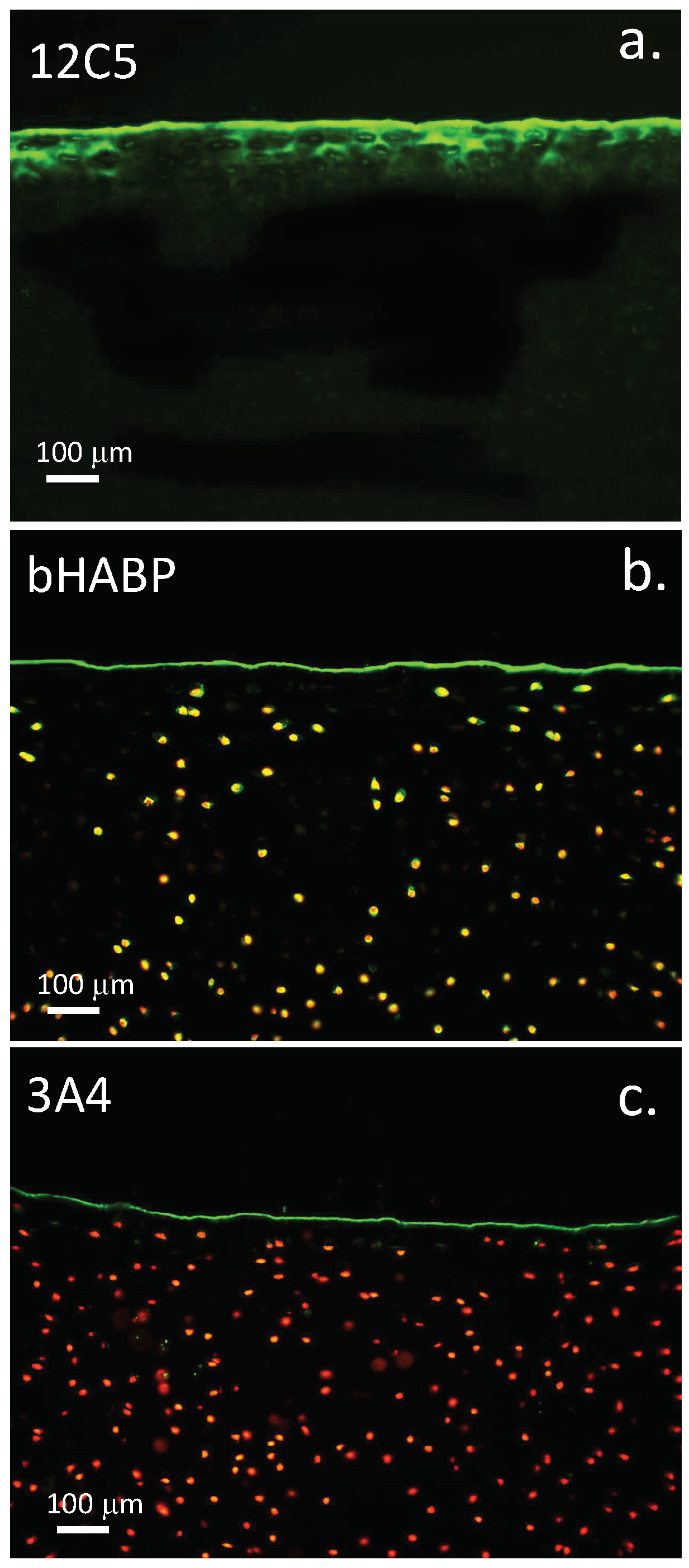
3.11. Localisation of HA and ITI SPIs at the Articular Surface Is of Physiological Significance
4. Materials and Methods
4.1. Tissues
4.2. Visualisation of Cartilage Surface Components
4.3. Preparation of Biotinylated Trypsin
4.4. Preparation of Immobilised Hyaluronan
4.5. Determination of Trypsin Inhibitory Activity
4.6. Measurement of the Relative Protease Inhibitory Activity of Bikunin KPI-1, KPI-2 and BPTI
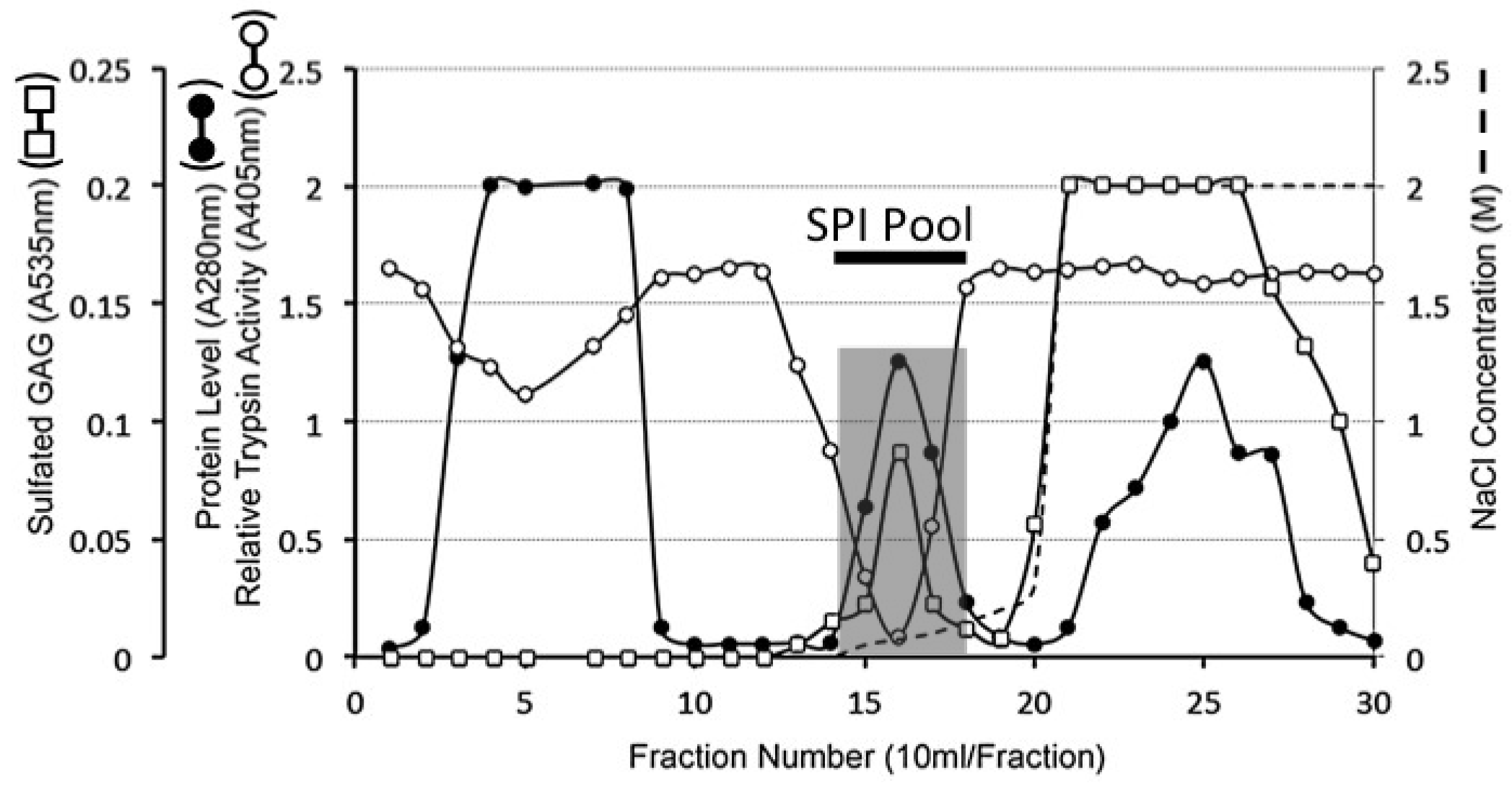
4.7. Extraction of Serine Proteinase Inhibitory Proteins from Articular Cartilage
4.8. DEAE Sepharose 4B Anion Exchange Chromatography
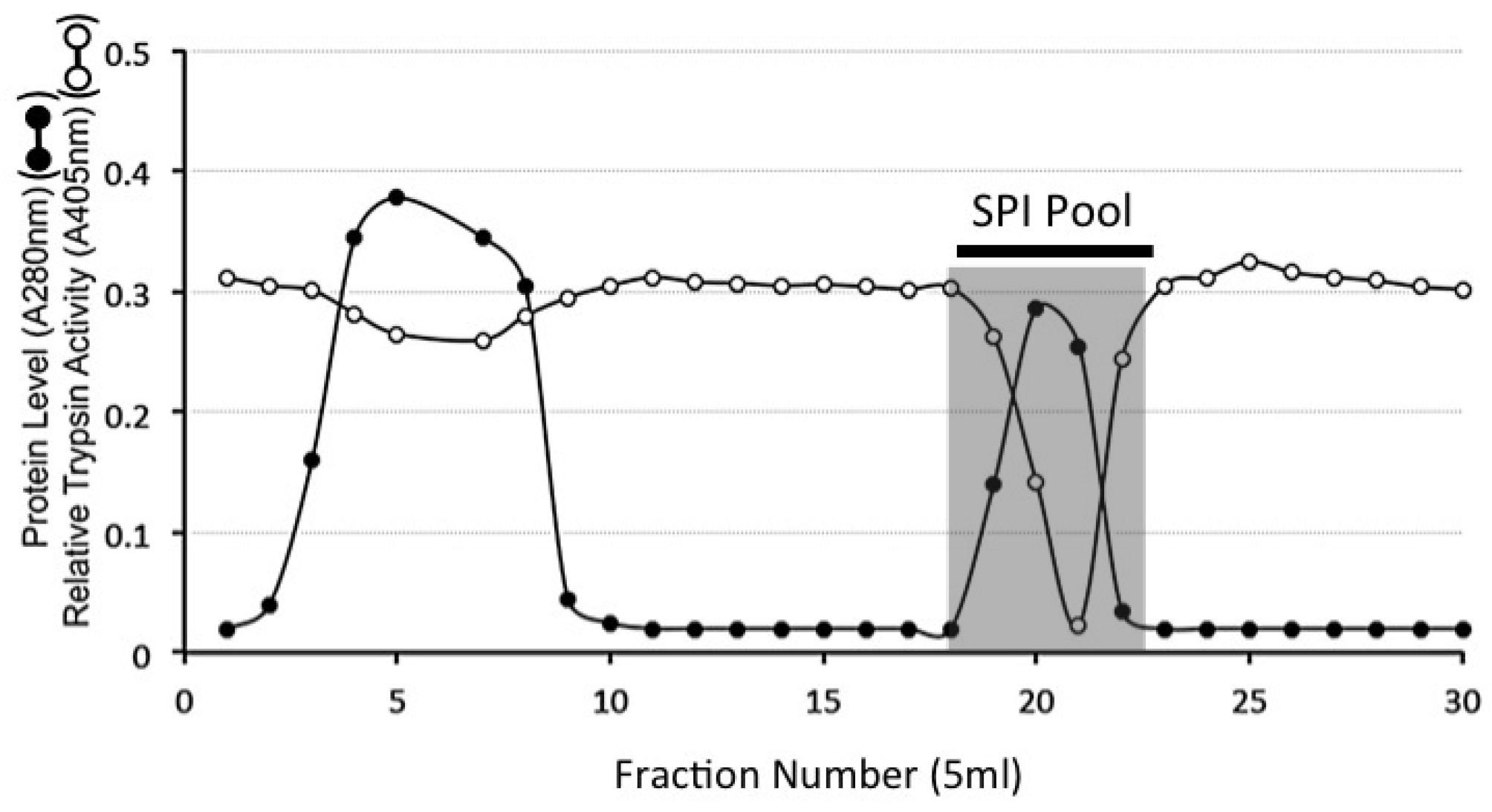
4.9. HA Affinity Chromatography of the DEAE SPI Pools
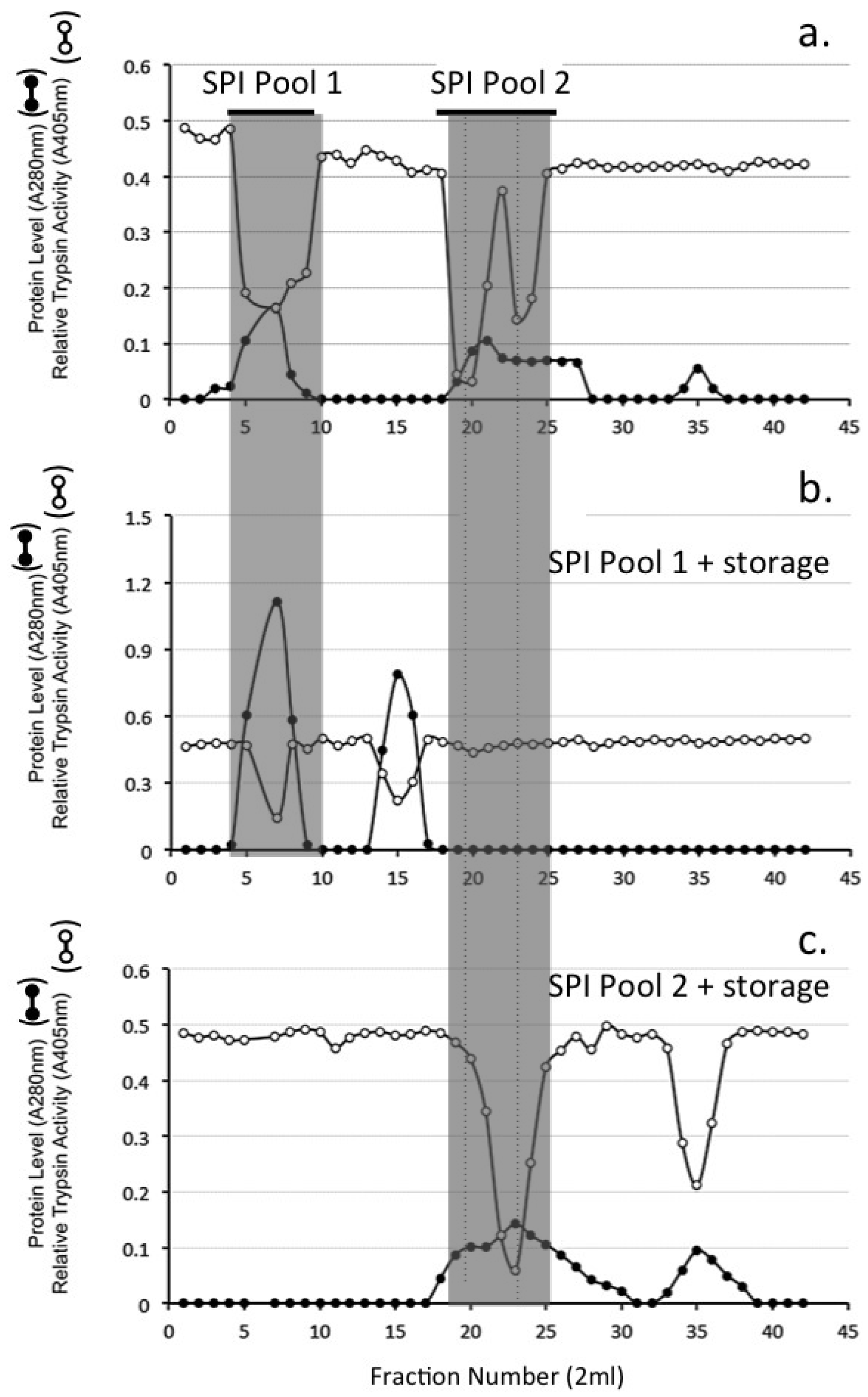
4.10. Sephadex G100 Gel Permeation Chromatography of HA Affinity Purified SPI Samples
4.11. Chymotrypsin Affinity Chromatography
4.12. Concanavalin A Lectin Affinity Chromatography of SPI Samples
4.13. Detection of Active SPIs Using Biotinylated Trypsin and Affinity Blotting
4.14. Development of SPI Western Blots with Antibodies to Chondroitin-4 Sulfate Stub Epitope, Bikunin, TSG-6 and α1-Microglobulin
4.15. Sulfated GAG Analysis
4.16. Cartilage Histological Processing and Confocal Microscopy
4.17. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | articular cartilage |
| ITI | inter-α-trypsin inhibitor |
| Pre-α-TI | pre-α-trypsin inhibitor |
| SPI | serine proteinase inhibitor |
| KPI | Kunitz protease inhibitor |
| CS | Chondroitin sulfate |
| HC | heavy chain (ITI) |
| OA | osteoarthritis |
| RA | rheumatoid arthritis |
| HA | hyaluronan |
| SLPI | secretory leucocyte proteinase inhibitor |
| TOM | Trappin ovine molecule |
| bT | biotinylated trypsin |
| TSG-6 | Tumour necrosis factor-stimulated gene-6 |
| GAG | glycosaminoglycan |
| KPI | Kunitz protease inhibitor |
| KS | Kaposi sarcoma |
| UTS | urinary trypsin inhibitor |
| BPTI | basic pancreatic trypsin inhibitor |
| IVD | intervertebral disc |
| ChD | chondrodystrophoid |
| Non-ChD | non-chondrodystrophoid |
References
- Steinbuch, M.; Loeb, J. Isolation of an alpha2-globulin from human plasma. Nature 1961, 192, 1196. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, K.; Albrecht, G.; Schonberger, O.L.; Wachter, E. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor, VII. Characterization of the bovine inhibitor as double-headed trypsin-elastase inhibitor. Hoppe Seylers Z. Physiol. Chem. 1983, 364, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, K.; Wachter, E.; Albrecht, G.J.; Reisinger, P. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor, X. The amino-acid sequences of the trypsin-released inhibitors from horse and pig inter-alpha-trypsin inhibitors. Biol. Chem. Hoppe Seyler 1985, 366, 473–478. [Google Scholar] [CrossRef]
- Wachter, E.; Hochstrasser, K. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor, III. Sequence of the two Kunitz-type domains inside the native inter-alpha-trypsin inhibitor, its biological aspects and also of its cleavage products. Hoppe Seylers Z. Physiol. Chem. 1979, 360, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Salier, J.P. Inter-alpha-trypsin inhibitor: Emergence of a family within the Kunitz-type protease inhibitor superfamily. Trends Biochem. Sci. 1990, 15, 435–439. [Google Scholar] [CrossRef]
- Nishimura, H.; Kakizaki, I.; Muta, T.; Sasaki, N.; Pu, P.X.; Yamashita, T.; Nagasawa, S. cDNA and deduced amino acid sequence of human PK-120, a plasma kallikrein-sensitive glycoprotein. FEBS Lett. 1995, 357, 207–211. [Google Scholar] [CrossRef]
- Saguchi, K.; Tobe, T.; Hashimoto, K.; Sano, Y.; Nakano, Y.; Miura, N.H.; Tomita, M. Cloning and characterization of cDNA for inter-alpha-trypsin inhibitor family heavy chain-related protein (IHRP), a novel human plasma glycoprotein. J. Biochem. 1995, 117, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, K.; Schonberger, O.L.; Rossmanith, I.; Wachter, E. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor, V. Attachments of carbohydrates in the human urinary trypsin inhibitor isolated by affinity chromatography. Hoppe Seylers Z. Physiol. Chem. 1981, 362, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Wolff, J.J.; Laremore, T.N.; Restaino, O.F.; Xie, J.; Schiraldi, C.; Toida, T.; Amster, I.J.; Linhardt, R.J. Structural analysis of bikunin glycosaminoglycan. J. Am. Chem. Soc. 2008, 130, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Day, A.J.; Youssef, P.; Zhuo, L.; Watanabe, H.; Caterson, B.; Whitelock, J.M. Sulfation of the bikunin chondroitin sulfate chain determines heavy chain.hyaluronan complex formation. J. Biol. Chem. 2013, 288, 22930–22941. [Google Scholar] [CrossRef] [PubMed]
- Ly, M.; Leach, F.E., 3rd; Laremore, T.N.; Toida, T.; Amster, I.J.; Linhardt, R.J. The proteoglycan bikunin has a defined sequence. Nat. Chem. Biol. 2011, 7, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.; Salustri, A.; Kimata, K. A physiological function of serum proteoglycan bikunin: The chondroitin sulfate moiety plays a central role. Glycoconj. J. 2002, 19, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.; Hascall, V.C.; Kimata, K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J. Biol. Chem. 2004, 279, 38079–38082. [Google Scholar] [CrossRef] [PubMed]
- Hamm, A.; Veeck, J.; Bektas, N.; Wild, P.J.; Hartmann, A.; Heindrichs, U.; Kristiansen, G.; Werbowetski-Ogilvie, T.; Del Maestro, R.; Knuechel, R.; et al. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: A systematic expression analysis. BMC Cancer 2008, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Huth, S.; Heise, R.; Vetter-Kauczok, C.S.; Skazik, C.; Marquardt, Y.; Czaja, K.; Knuchel, R.; Merk, H.F.; Dahl, E.; Baron, J.M. Inter-alpha-trypsin inhibitor heavy chain 5 (ITIH5) is overexpressed in inflammatory skin diseases and affects epidermal morphology in constitutive knockout mice and murine 3D skin models. Exp. Dermatol. 2015, 24, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, Y.; Plaas, A.; Osborn, B.; Margulis, A.; Nelson, F.; Stewart, M.; Rugg, M.S.; Milner, C.M.; Day, A.J.; Nemoto, K.; et al. Superficial zone chondrocytes in normal and osteoarthritic human articular cartilages synthesize novel truncated forms of inter-alpha-trypsin inhibitor heavy chains which are attached to a chondroitin sulfate proteoglycan other than bikunin. Osteoarthr. Cartil. 2008, 16, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Lamkin, E.; Cheng, G.; Calabro, A.; Hascall, V.C.; Joo, E.J.; Li, L.; Linhardt, R.J.; Lauer, M.E. Heavy chain transfer by tumor necrosis factor-stimulated gene 6 to the bikunin proteoglycan. J. Biol. Chem. 2015, 290, 5156–5166. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Melrose, J.; Ghosh, P. Purification and characterisation of 6 and 58 kDa forms of the endogenous serine proteinase inhibitory proteins of ovine articular cartilage. Biol. Chem. 1996, 377, 837–845. [Google Scholar]
- Melrose, J.; Smith, S.; Ghosh, P. Synthesis of a Kunitz-type serine proteinase inhibitory protein that shares homology with bovine pancreatic trypsin inhibitor by ovine intervertebral disc cells in serum-free alginate bead culture. J. Spinal Disord. Tech. 2002, 15, 164–171. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Melrose, J.; Ghosh, P. Biotin-labeled potato chymotrypsin inhibitor-1: A useful probe for the detection and quantitation of chymotrypsin-like serine proteinases on western blots and its application in the detection of a serine proteinase synthesised by articular chondrocytes. Anal. Biochem. 1995, 227, 129–134. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Melrose, J.; Ghosh, P. Biotinylated trypsin and its application as a sensitive, versatile probe for the detection and characterisation of an ovine chondrocyte serine proteinase inhibitor using Western blotting. Electrophoresis 1996, 17, 213–218. [Google Scholar] [CrossRef]
- Rasp, G.; Hochstrasser, K.; Wachter, E.; Reisinger, P.W. The amino-acid sequence of the trypsin-released inhibitor from sheep inter-alpha-trypsin inhibitor. Biol. Chem. Hoppe Seyler 1987, 368, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Rodgers, K. Preparation and use of biotinylated probes for the detection and characterisation of serine proteinases and serine proteinase inhibitory proteins. In A Laboratory Guide to Biotin Labelling in Biomolecule Analysis; Meier, T., Fahrenholz, F., Eds.; Birkhauser Publishin: Basel, Switzerland, 1996. [Google Scholar]
- Melrose, J.; Rodgers, K.; Ghosh, P. The preparation and use of biotinylated trypsin in western blotting for the detection of trypsin inhibitory proteins. Anal. Biochem. 1994, 222, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.L.; Melrose, J.; Ghosh, P. A comparative study of the low-molecular mass serine proteinase inhibitors of human connective tissues. Biol. Chem. Hoppe Seyler 1992, 373, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Ghosh, P. Development of an avidin-biotin competitive inhibition assay and validation of its use for the quantitation of human intervertebral disc serine proteinase inhibitory proteins. Anal. Biochem. 1992, 204, 372–382. [Google Scholar] [CrossRef]
- Melrose, J.; Ghosh, P.; Taylor, T.K.; Andrews, J.L. The serine proteinase inhibitory proteins of the human intervertebral disc: Their isolation, characterization and variation with ageing and degeneration. Matrix 1992, 12, 456–470. [Google Scholar] [CrossRef]
- Melrose, J.; Shen, B.; Ghosh, P. Affinity and Western blotting reveal homologies between ovine intervertebral disc serine proteinase inhibitory proteins and bovine pancreatic trypsin inhibitor. Proteomics 2001, 1, 1529–1533. [Google Scholar] [CrossRef]
- Melrose, J.; Smith, S.; Rodgers, K.; Little, C.; Burkhardt, D.; Ghosh, P. Immunolocalisation of BPTI-like serine proteinase inhibitory proteins in mast cells, chondrocytes and intervertebral disc fibrochondrocytes of ovine and bovine connective tissues. An immunohistochemical and biochemical study. Histochem. Cell Biol. 2000, 114, 137–146. [Google Scholar]
- Melrose, J.; Taylor, T.K.; Ghosh, P. Variation in intervertebral disc serine proteinase inhibitory proteins with ageing in a chondrodystrophoid (beagle) and a non-chondrodystrophoid (greyhound) canine breed. Gerontology 1996, 42, 322–329. [Google Scholar] [CrossRef]
- Melrose, J.; Taylor, T.K.; Ghosh, P. The serine proteinase inhibitory proteins of the chondrodystrophoid (beagle) and non-chondrodystrophoid (greyhound) canine intervertebral disc. Electrophoresis 1997, 18, 1059–1063. [Google Scholar] [CrossRef]
- Fries, E.; Blom, A.M. Bikuni—Not just a plasma proteinase inhibitor. Int. J. Biochem. Cell Biol. 2000, 32, 125–137. [Google Scholar] [CrossRef]
- Kanayama, S.; Yamada, Y.; Onogi, A.; Shigetomi, H.; Ueda, S.; Tsuji, Y.; Haruta, S.; Kawaguchi, R.; Yoshida, S.; Sakata, M.; et al. Molecular structure and function analysis of bikunin on down-regulation of tumor necrosis factor-alpha expression in activated neutrophils. Cytokine 2008, 42, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S. Bikunin and its emerging role in the modulation of tumor invasion and metastasis. J. Surg. Res. 2013, 183, 982. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Suzuki, M.; Hirashima, Y.; Terao, T. The protease inhibitor bikunin, a novel anti-metastatic agent. Biol. Chem. 2003, 384, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Suzuki, M.; Tanaka, Y.; Kanayama, N.; Terao, T. A Kunitz-type protease inhibitor, bikunin, inhibits ovarian cancer cell invasion by blocking the calcium-dependent transforming growth factor-beta 1 signaling cascade. J. Biol. Chem. 2003, 278, 7790–7799. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Kobayashi, H.; Yagyu, T.; Wakahara, K.; Kondo, T.; Kurita, N.; Sekino, H.; Inagaki, K.; Suzuki, M.; Kanayama, N.; et al. Bikunin inhibits lipopolysaccharide-induced tumor necrosis factor alpha induction in macrophages. Clin. Diagn. Lab. Immunol. 2004, 11, 1140–1147. [Google Scholar] [CrossRef]
- Roberts, S.; Evans, H.; Menage, J.; Urban, J.P.; Bayliss, M.T.; Eisenstein, S.M.; Rugg, M.S.; Milner, C.M.; Griffin, S.; Day, A.J. TNFalpha-stimulated gene product (TSG-6) and its binding protein, IalphaI, in the human intervertebral disc: New molecules for the disc. Eur. Spine J. 2005, 14, 36–42. [Google Scholar] [CrossRef]
- Briggs, D.C.; Birchenough, H.L.; Ali, T.; Rugg, M.S.; Waltho, J.P.; Ievoli, E.; Jowitt, T.A.; Enghild, J.J.; Richter, R.P.; Salustri, A.; et al. Metal Ion-dependent Heavy Chain Transfer Activity of TSG-6 Mediates Assembly of the Cumulus-Oocyte Matrix. J. Biol. Chem. 2015, 290, 28708–28723. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S. Ovulation: New factors that prepare the oocyte for fertilization. Mol. Cell. Endocrinol. 2005, 234, 75–79. [Google Scholar] [CrossRef]
- Melrose, J.; Shu, C.; Whitelock, J.M.; Lord, M.S. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biol. 2016, 52–54, 363–383. [Google Scholar] [CrossRef]
- Sallenave, J.M. Antimicrobial activity of antiproteinases. Biochem. Soc. Trans. 2002, 30, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Sallenave, J.M. Secretory leukocyte protease inhibitor and elafin/trappin-2: Versatile mucosal antimicrobials and regulators of immunity. Am. J. Respir. Cell Mol. Biol. 2010, 42, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Quabius, E.S.; Gorogh, T.; Fischer, G.S.; Hoffmann, A.S.; Gebhard, M.; Evert, M.; Beule, A.; Maune, S.; Knecht, R.; Ovari, A.; et al. The antileukoprotease secretory leukocyte protease inhibitor (SLPI) and its role in the prevention of HPV-infections in head and neck squamous cell carcinoma. Cancer Lett. 2015, 357, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Quabius, E.S.; Moller, P.; Haag, J.; Pfannenschmidt, S.; Hedderich, J.; Gorogh, T.; Rocken, C.; Hoffmann, M. The role of the antileukoprotease SLPI in smoking-induced human papillomavirus-independent head and neck squamous cell carcinomas. Int. J. Cancer 2014, 134, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.M.; McNeely, T.B.; Janoff, E.N.; Shugars, D.; Worley, P.; Tucker, C.; Orenstein, J.M. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-I. Oral Dis. 1997, 3 (Suppl. 1), S64–S69. [Google Scholar] [CrossRef] [PubMed]
- McNeely, T.B.; Dealy, M.; Dripps, D.J.; Orenstein, J.M.; Eisenberg, S.P.; Wahl, S.M. Secretory leukocyte protease inhibitor: A human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J. Clin. Investig. 1995, 96, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yoshida, R.; Kanada, Y.; Fukuda, Y.; Yagyu, T.; Inagaki, K.; Kondo, T.; Kurita, N.; Suzuki, M.; Kanayama, N.; et al. Suppression of lipopolysaccharide-induced cytokine production of gingival fibroblasts by a soybean, Kunitz trypsin inhibitor. J. Periodontal. Res. 2005, 40, 461–468. [Google Scholar] [CrossRef]
- Wakahara, K.; Kobayashi, H.; Yagyu, T.; Matsuzaki, H.; Kondo, T.; Kurita, N.; Sekino, H.; Inagaki, K.; Suzuki, M.; Kanayama, N.; et al. Bikunin suppresses lipopolysaccharide-induced lethality through down-regulation of tumor necrosis factor- alpha and interleukin-1 beta in macrophages. J. Infect. Dis. 2005, 191, 930–938. [Google Scholar] [CrossRef]
- Majchrzak-Gorecka, M.; Majewski, P.; Grygier, B.; Murzyn, K.; Cichy, J. Secretory leukocyte protease inhibitor (SLPI), a multifunctional protein in the host defense response. Cytokine Growth Factor Rev. 2016, 28, 79–93. [Google Scholar] [CrossRef]
- Scott, A.; Weldon, S.; Taggart, C.C. SLPI and elafin: Multifunctional antiproteases of the WFDC family. Biochem. Soc. Trans. 2011, 39, 1437–1440. [Google Scholar] [CrossRef]
- Chen, X.; Rivard, L.; Naqvi, S.; Nakada, S.; Padbury, J.F.; Sanchez-Esteban, J.; Stopa, E.G.; Lim, Y.P.; Stonestreet, B.S. Expression and localization of Inter-alpha Inhibitors in rodent brain. Neuroscience 2016, 324, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, R.; Peigneur, S.; Pons, T.; Alvarez, C.; Gonzalez, L.; Chavez, M.A.; Tytgat, J. The Kunitz-Type Protein ShPI-1 Inhibits Serine Proteases and Voltage-Gated Potassium Channels. Toxins (Basel) 2016, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, F.; Rehemtulla, A.; Van Nostrand, W.E.; Bajaj, S.P.; Schmaier, A.H. Protease nexin-2/Amyloid beta-protein precursor regulates factor VIIa and the factor VIIa-tissue factor complex. Thromb. Res. 2000, 99, 267–276. [Google Scholar] [CrossRef]
- Peigneur, S.; Billen, B.; Derua, R.; Waelkens, E.; Debaveye, S.; Beress, L.; Tytgat, J. A bifunctional sea anemone peptide with Kunitz type protease and potassium channel inhibiting properties. Biochem. Pharm. 2011, 82, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Schweitz, H.; Bruhn, T.; Guillemare, E.; Moinier, D.; Lancelin, J.M.; Beress, L.; Lazdunski, M. Kalicludines and kaliseptine. Two different classes of sea anemone toxins for voltage sensitive K+ channels. J. Biol. Chem. 1995, 270, 25121–25126. [Google Scholar] [CrossRef] [PubMed]
- Schweitz, H.; Heurteaux, C.; Bois, P.; Moinier, D.; Romey, G.; Lazdunski, M. Calcicludine, a venom peptide of the Kunitz-type protease inhibitor family, is a potent blocker of high-threshold Ca2+ channels with a high affinity for L-type channels in cerebellar granule neurons. Proc. Natl. Acad. Sci. USA 1994, 91, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, J.; Wang, B.; Cao, Z.; Li, W.; Wu, Y.; Chen, Z. BF9, the first functionally characterized snake toxin peptide with Kunitz-type protease and potassium channel inhibiting properties. J. Biochem. Mol. Toxicol. 2014, 28, 76–83. [Google Scholar] [CrossRef]
- Jacoby, A.S.; Melrose, J.; Robinson, B.G.; Hyland, V.J.; Ghosh, P. Secretory leucocyte proteinase inhibitor is produced by human articular cartilage chondrocytes and intervertebral disc fibrochondrocytes. Eur. J. Biochem. 1993, 218, 951–957. [Google Scholar] [CrossRef]
- Ohlsson, S.; Tufvesson, B.; Polling, A.; Ohlsson, K. Distribution of the secretory leucocyte proteinase inhibitor in human articular cartilage. Biol. Chem. 1997, 378, 1055–1058. [Google Scholar] [CrossRef]
- Brown, T.I.; Mistry, R.; Collie, D.D.; Tate, S.; Sallenave, J.M. Trappin ovine molecule (TOM), the ovine ortholog of elafin, is an acute phase reactant in the lung. Physiol. Genom. 2004, 19, 11–21. [Google Scholar] [CrossRef]
- Brown, T.I.; Mistry, R.; Gray, R.; Imrie, M.; Collie, D.D.; Sallenave, J.M. Characterization of the ovine ortholog of secretory leukoprotease inhibitor. Mamm. Genome 2005, 16, 621–630. [Google Scholar] [CrossRef]
- Baranger, K.; Zani, M.L.; Labas, V.; Dallet-Choisy, S.; Moreau, T. Secretory leukocyte protease inhibitor (SLPI) is, like its homologue trappin-2 (pre-elafin), a transglutaminase substrate. PLoS ONE 2011, 6, e20976. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, G.J.; Hochstrasser, K.; Schonberger, O.L. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor, IX. Isolation and characterization of the inhibitory parts of inter-alpha-trypsin inhibitors from several mammalian sera. Hoppe Seylers Z. Physiol. Chem. 1983, 364, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, W.; Schreitmuller, T.; Vetr, H.; Wachter, E.; Hochstrasser, K. Complementary DNA and deduced amino acid sequences of procine alpha 1-microglobulin and bikunin. FEBS Lett. 1990, 269, 32–36. [Google Scholar] [CrossRef]
- Glant, T.T.; Kamath, R.V.; Bardos, T.; Gal, I.; Szanto, S.; Murad, Y.M.; Sandy, J.D.; Mort, J.S.; Roughley, P.J.; Mikecz, K. Cartilage-specific constitutive expression of TSG-6 protein (product of tumor necrosis factor alpha-stimulated gene 6) provides a chondroprotective, but not antiinflammatory, effect in antigen-induced arthritis. Arthritis Rheum. 2002, 46, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Fouladi-Nashta, A.A.; Raheem, K.A.; Marei, W.F.; Ghafari, F.; Hartshorne, G.M. Regulation and roles of the hyaluronan system in mammalian reproduction. Reproduction 2017, 153, R43–R58. [Google Scholar] [CrossRef] [PubMed]
- Hamasuna, R.; Kataoka, H.; Meng, J.Y.; Itoh, H.; Moriyama, T.; Wakisaka, S.; Koono, M. Reduced expression of hepatocyte growth factor activator inhibitor type-2/placental bikunin (HAI-2/PB) in human glioblastomas: Implication for anti-invasive role of HAI-2/PB in glioblastoma cells. Int. J. Cancer 2001, 93, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kobayashi, H.; Fujie, M.; Nishida, T.; Takigawa, M.; Kanayama, N.; Terao, T. Kunitz-type protease inhibitor bikunin disrupts phorbol ester-induced oligomerization of CD44 variant isoforms containing epitope v9 and subsequently suppresses expression of urokinase-type plasminogen activator in human chondrosarcoma cells. J. Biol. Chem. 2002, 277, 8022–8032. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.J.; Adams, D.J.; Talbot, S.J. Ribonuclease inhibits Kaposi’s sarcoma. Nature 1997, 390, 568. [Google Scholar] [CrossRef]
- Griffiths, S.J.; Bramley, T.A.; Menzies, G.S.; Adams, D.J. Co-purification of a ribonuclease and human chorionic gonadotrophin beta-core protein from human urine and displacement of 125I-human luteinizing hormone from Candida albicans binding sites by ribonucleases. Mol. Cell. Endocrinol. 1997, 134, 69–76. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Huang, P.L.; Sun, Y.; Kung, H.F.; Blithe, D.L.; Chen, H.C. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proc. Natl. Acad. Sci. USA 1999, 96, 2678–2681. [Google Scholar] [CrossRef] [PubMed]
- Lunardi-Iskandar, Y.; Bryant, J.L.; Blattner, W.A.; Hung, C.L.; Flamand, L.; Gill, P.; Hermans, P.; Birken, S.; Gallo, R.C. Effects of a urinary factor from women in early pregnancy on HIV-1, SIV and associated disease. Nat. Med. 1998, 4, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, F.; Bryant, J.L.; Liu, N.; Karp, J.E.; Sabichi, A.L.; Thierry, A.; Lunardi-Iskandar, Y.; Gallo, R.C. Induction of programmed cell death in Kaposi’s sarcoma cells by preparations of human chorionic gonadotropin. J. Natl. Cancer Inst. 1999, 91, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Malatos, S.; Neubert, H.; Kicman, A.T.; Iles, R.K. Identification of placental transforming growth factor-beta and bikunin metabolites as contaminants of pharmaceutical human chorionic gonadotrophin preparations by proteomic techniques. Mol. Cell. Proteom. 2005, 4, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Matuska, B.; Comhair, S.; Farver, C.; Chmiel, J.; Midura, R.J.; Bonfield, T.; Lauer, M.E. Pathological Hyaluronan Matrices in Cystic Fibrosis Airways and Secretions. Am. J. Respir. Cell Mol. Biol. 2016, 55, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, K.A.; Chai, S.; Chen, H.; Danielsen, C.C.; Simonsen, U.; Wogensen, L. Mechanisms involved in extracellular matrix remodeling and arterial stiffness induced by hyaluronan accumulation. Atherosclerosis 2016, 244, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.E.; Majors, A.K.; Comhair, S.; Ruple, L.M.; Matuska, B.; Subramanian, A.; Farver, C.; Dworski, R.; Grandon, D.; Laskowski, D.; et al. Hyaluronan and Its Heavy Chain Modification in Asthma Severity and Experimental Asthma Exacerbation. J. Biol. Chem. 2015, 290, 23124–23134. [Google Scholar] [CrossRef]
- Bogdani, M.; Johnson, P.Y.; Potter-Perigo, S.; Nagy, N.; Day, A.J.; Bollyky, P.L.; Wight, T.N. Hyaluronan and hyaluronan-binding proteins accumulate in both human type 1 diabetic islets and lymphoid tissues and associate with inflammatory cells in insulitis. Diabetes 2014, 63, 2727–2743. [Google Scholar] [CrossRef]
- Bourguignon, J.; Borghi, H.; Sesboue, R.; Diarra-Mehrpour, M.; Bernaudin, J.F.; Metayer, J.; Martin, J.P.; Thiberville, L. Immunohistochemical distribution of inter-alpha-trypsin inhibitor chains in normal and malignant human lung tissue. J. Histochem. Cytochem. 1999, 47, 1625–1632. [Google Scholar] [CrossRef]
- Yoshida, E.; Maruyama, M.; Sugiki, M.; Mihara, H. Immunohistochemical demonstration of bikunin, a light chain of inter-alpha-trypsin inhibitor, in human brain tumors. Inflammation 1994, 18, 589–596. [Google Scholar] [CrossRef]
- Eguchi, Y.; Inoue, M.; Iida, S.; Matsuoka, K.; Noda, S. Heparan sulfate (HS)/heparan sulfate proteoglycan (HSPG) and bikunin are up-regulated during calcium oxalate nephrolithiasis in rat kidney. Kurume Med. J. 2002, 49, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Evan, A.P.; Bledsoe, S.; Worcester, E.M.; Coe, F.L.; Lingeman, J.E.; Bergsland, K.J. Renal inter-alpha-trypsin inhibitor heavy chain 3 increases in calcium oxalate stone-forming patients. Kidney Int. 2007, 72, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, N.; Maehara, K.; She, L.; Belayet, H.M.; Khatun, S.; Tokunaga, N.; Terao, T. Urinary trypsin inhibitor suppresses vascular smooth muscle contraction by inhibition of Ca2+ influx. Biochim. Biophys. Acta 1998, 1381, 139–146. [Google Scholar] [CrossRef]
- Kanayama, N.; Halim, A.; Maehara, K.; Kajiwara, Y.; Fujie, M.; Terao, T. Kunitz-type trypsin inhibitor prevents LPS-induced increase of cytosolic free Ca2+ in human neutrophils and HUVEC cells. Biochem. Biophys. Res. Commun. 1995, 207, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Flo, M.; Margenat, M.; Pellizza, L.; Grana, M.; Duran, R.; Baez, A.; Salceda, E.; Soto, E.; Alvarez, B.; Fernandez, C. Functional diversity of secreted cestode Kunitz proteins: Inhibition of serine peptidases and blockade of cation channels. PLoS Pathog. 2017, 13, e1006169. [Google Scholar] [CrossRef]
- Pangalos, M.N.; Shioi, J.; Efthimiopoulos, S.; Wu, A.; Robakis, N.K. Characterization of appican, the chondroitin sulfate proteoglycan form of the Alzheimer amyloid precursor protein. Neurodegeneration 1996, 5, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, F.; Feng, J.; Yang, W.; Zeng, D.; Zhao, R.; Cao, Z.; Liu, M.; Li, W.; Jiang, L.; et al. Genomic and structural characterization of Kunitz-type peptide LmKTT-1a highlights diversity and evolution of scorpion potassium channel toxins. PLoS ONE 2013, 8, e60201. [Google Scholar] [CrossRef]
- Hennies, H.C. All is balanced: Inter-alpha-trypsin inhibitors as unseen extracellular matrix proteins in epidermal morphology and differentiation. Exp. Dermatol. 2015, 24, 661–662. [Google Scholar] [CrossRef]
- Edqvist, P.H.; Fagerberg, L.; Hallstrom, B.M.; Danielsson, A.; Edlund, K.; Uhlen, M.; Ponten, F. Expression of human skin-specific genes defined by transcriptomics and antibody-based profiling. J. Histochem. Cytochem. 2015, 63, 129–141. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Lindskog, C. The potential clinical impact of the tissue-based map of the human proteome. Expert Rev. Proteom. 2015, 12, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Petryszak, R.; Burdett, T.; Fiorelli, B.; Fonseca, N.A.; Gonzalez-Porta, M.; Hastings, E.; Huber, W.; Jupp, S.; Keays, M.; Kryvych, N.; et al. Expression Atlas update—A database of gene and transcript expression from microarray- and sequencing-based functional genomics experiments. Nucleic Acids Res. 2014, 42, D926–D932. [Google Scholar] [CrossRef] [PubMed]
- Ponten, F.; Jirstrom, K.; Uhlen, M. The Human Protein Atlas—A tool for pathology. J. Pathol. 2008, 216, 387–393. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Kloten, V.; Rose, M.; Kaspar, S.; von Stillfried, S.; Knuchel, R.; Dahl, E. Epigenetic inactivation of the novel candidate tumor suppressor gene ITIH5 in colon cancer predicts unfavorable overall survival in the CpG island methylator phenotype. Epigenetics 2014, 9, 1290–1301. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Y.T.; Chen, S.Y.; He, H.; Tseng, S.C. Constitutive expression of pentraxin 3 (PTX3) protein by human amniotic membrane cells leads to formation of the heavy chain (HC)-hyaluronan (HA)-PTX3 complex. J. Biol. Chem. 2014, 289, 13531–13542. [Google Scholar] [CrossRef]
- Torihashi, S.; Ho, M.; Kawakubo, Y.; Komatsu, K.; Nagai, M.; Hirayama, Y.; Kawabata, Y.; Takenaka-Ninagawa, N.; Wanachewin, O.; Zhuo, L.; et al. Acute and temporal expression of tumor necrosis factor (TNF)-alpha-stimulated gene 6 product, TSG6, in mesenchymal stem cells creates microenvironments required for their successful transplantation into muscle tissue. J. Biol. Chem. 2015, 290, 22771–22781. [Google Scholar] [CrossRef]
- Itoh, H.; Ide, H.; Ishikawa, N.; Nawa, Y. Mast cell protease inhibitor, trypstatin, is a fragment of inter-alpha-trypsin inhibitor light chain. J. Biol. Chem. 1994, 269, 3818–3822. [Google Scholar]
- Dobbertin, A.; Rhodes, K.E.; Garwood, J.; Properzi, F.; Heck, N.; Rogers, J.H.; Fawcett, J.W.; Faissner, A. Regulation of RPTPbeta/phosphacan expression and glycosaminoglycan epitopes in injured brain and cytokine-treated glia. Mol. Cell. Neurosci. 2003, 24, 951–971. [Google Scholar] [CrossRef]
- Faissner, A.; Heck, N.; Dobbertin, A.; Garwood, J. DSD-1-Proteoglycan/Phosphacan and receptor protein tyrosine phosphatase-beta isoforms during development and regeneration of neural tissues. Adv. Exp. Med. Biol. 2006, 557, 25–53. [Google Scholar]
- Garwood, J.; Rigato, F.; Heck, N.; Faissner, A. Tenascin glycoproteins and the complementary ligand DSD-1-PG/ phosphacan--structuring the neural extracellular matrix during development and repair. Restor. Neurol. Neurosci. 2001, 19, 51–64. [Google Scholar] [PubMed]
- Garwood, J.; Schnadelbach, O.; Clement, A.; Schutte, K.; Bach, A.; Faissner, A. DSD-1-proteoglycan is the mouse homolog of phosphacan and displays opposing effects on neurite outgrowth dependent on neuronal lineage. J. Neurosci. 1999, 19, 3888–3899. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hikino, M.; Yajima, Y.; Mikami, T.; Sirko, S.; von Holst, A.; Faissner, A.; Fukui, S.; Sugahara, K. Structural characterization of the epitopes of the monoclonal antibodies 473HD, CS-56, and MO-225 specific for chondroitin sulfate D-type using the oligosaccharide library. Glycobiology 2005, 15, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.U.; Margolis, R.K. Chondroitin sulfate proteoglycans as mediators of axon growth and pathfinding. Cell Tissue Res. 1997, 290, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Hikino, M.; Mikami, T.; Faissner, A.; Vilela-Silva, A.C.; Pavao, M.S.; Sugahara, K. Oversulfated dermatan sulfate exhibits neurite outgrowth-promoting activity toward embryonic mouse hippocampal neurons: Implications of dermatan sulfate in neuritogenesis in the brain. J. Biol. Chem. 2003, 278, 43744–43754. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Mori, Y.; Shiraki, H.; Horie, M.; Okamoto, H.; Narahara, M.; Miyake, M.; Shikimi, T. Detection of bikunin mRNA in limited portions of rat brain. Life Sci. 1999, 65, 757–762. [Google Scholar] [CrossRef]
- Garwood, J.; Heck, N.; Reichardt, F.; Faissner, A. Phosphacan short isoform, a novel non-proteoglycan variant of phosphacan/receptor protein tyrosine phosphatase-beta, interacts with neuronal receptors and promotes neurite outgrowth. J. Biol. Chem. 2003, 278, 24164–24173. [Google Scholar] [CrossRef]
- Yoshida, K.; Suzuki, Y.; Yamamoto, K.; Sinohara, H. Guinea pig alpha 1-microglobulin/bikunin: CDNA sequencing, tissue expression and expression during acute phase. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1999, 122, 165–172. [Google Scholar] [CrossRef]
- Pangalos, M.N.; Shioi, J.; Robakis, N.K. Expression of the chondroitin sulfate proteoglycans of amyloid precursor (appican) and amyloid precursor-like protein 2. J. Neurochem. 1995, 65, 762–769. [Google Scholar] [CrossRef]
- Shioi, J.; Pangalos, M.N.; Ripellino, J.A.; Vassilacopoulou, D.; Mytilineou, C.; Margolis, R.U.; Robakis, N.K. The Alzheimer amyloid precursor proteoglycan (appican) is present in brain and is produced by astrocytes but not by neurons in primary neural cultures. J. Biol. Chem. 1995, 270, 11839–11844. [Google Scholar] [CrossRef]
- Tsuchida, K.; Shioi, J.; Yamada, S.; Boghosian, G.; Wu, A.; Cai, H.; Sugahara, K.; Robakis, N.K. Appican, the proteoglycan form of the amyloid precursor protein, contains chondroitin sulfate E in the repeating disaccharide region and 4-O-sulfated galactose in the linkage region. J. Biol. Chem. 2001, 276, 37155–37160. [Google Scholar] [CrossRef] [PubMed]
- Umehara, Y.; Yamada, S.; Nishimura, S.; Shioi, J.; Robakis, N.K.; Sugahara, K. Chondroitin sulfate of appican, the proteoglycan form of amyloid precursor protein, produced by C6 glioma cells interacts with heparin-binding neuroregulatory factors. FEBS Lett. 2004, 557, 233–238. [Google Scholar] [CrossRef]
- Wu, A.; Pangalos, M.N.; Efthimiopoulos, S.; Shioi, J.; Robakis, N.K. Appican expression induces morphological changes in C6 glioma cells and promotes adhesion of neural cells to the extracellular matrix. J. Neurosci. 1997, 17, 4987–4993. [Google Scholar] [CrossRef]
- Kawamura, D.; Funakoshi, T.; Mizumoto, S.; Sugahara, K.; Iwasaki, N. Sulfation patterns of exogenous chondroitin sulfate affect chondrogenic differentiation of ATDC5 cells. J. Orthop. Sci. 2014, 19, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kobayashi, H.; Tanaka, Y.; Hirashima, Y.; Kanayama, N.; Takei, Y.; Saga, Y.; Itoh, H.; Terao, T. Bikunin target genes in ovarian cancer cells identified by microarray analysis. J. Biol. Chem. 2003, 278, 14640–14646. [Google Scholar] [CrossRef] [PubMed]
- Bonnevie, E.D.; Galesso, D.; Secchieri, C.; Cohen, I.; Bonassar, L.J. Elastoviscous Transitions of Articular Cartilage Reveal a Mechanism of Synergy between Lubricin and Hyaluronic Acid. PLoS ONE 2015, 10, e0143415. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, K.; Chichester, C.O.; Jay, G.D. Cathepsin B and neutrophil elastase degrade lubricin and increase friction in excised murine joints. In Proceedings of the 51st Annual Meeting of the Orthopaedic Research Society, Washington, DC, USA, 20–23 February 2005. Post No. 0924. [Google Scholar]
- Elsaid, K.A.; Jay, G.D.; Chichester, C.O. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007, 56, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Jay, G.D.; Waller, K.A. The biology of lubricin: Near frictionless joint motion. Matrix Biol. 2014, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Fujimoto, N.; Tajima, S. Abnormal accumulation of inter-alpha-trypsin inhibitor and hyaluronic acid in lichen sclerosus. J. Cutan. Pathol. 2005, 32, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.C.; Atmuri, V.; Hemming, R.J.; Farley, J.; Mort, J.S.; Byers, S.; Hombach-Klonisch, S.; Csoka, A.B.; Stern, R.; Triggs-Raine, B.L. A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Hum. Mol. Genet. 2008, 17, 1904–1915. [Google Scholar] [CrossRef]
- Melrose, J.; Tammi, M.; Smith, S. Visualisation of hyaluronan and hyaluronan-binding proteins within ovine vertebral cartilages using biotinylated aggrecan G1-link complex and biotinylated hyaluronan oligosaccharides. Histochem. Cell Biol. 2002, 117, 327–333. [Google Scholar] [CrossRef]
- Kao, W.W.; Coulson-Thomas, V.J. Cell Therapy of Corneal Diseases. Cornea 2016, 35 (Suppl. 1), S9–S19. [Google Scholar] [CrossRef] [PubMed]
- Shay, E.; He, H.; Sakurai, S.; Tseng, S.C. Inhibition of angiogenesis by HC.HA, a complex of hyaluronan and the heavy chain of inter-alpha-inhibitor, purified from human amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Alquraini, A.; Garguilo, S.; D’Souza, G.; Zhang, L.X.; Schmidt, T.A.; Jay, G.D.; Elsaid, K.A. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: An anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res. 2015, 17, 353. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Schmidt, T.A.; Krawetz, R.J.; Dufour, A. Proteoglycan 4: From Mere Lubricant to Regulator of Tissue Homeostasis and Inflammation: Does proteoglycan 4 have the ability to buffer the inflammatory response? Bioessays 2019, 41, e1800166. [Google Scholar] [CrossRef]
- Bottazzi, B.; Doni, A.; Garlanda, C.; Mantovani, A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu. Rev. Immunol. 2010, 28, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Inforzato, A.; Jaillon, S.; Moalli, F.; Barbati, E.; Bonavita, E.; Bottazzi, B.; Mantovani, A.; Garlanda, C. The long pentraxin PTX3 at the crossroads between innate immunity and tissue remodelling. Tissue Antigens 2011, 77, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Flowers, S.A.; Zieba, A.; Ornros, J.; Jin, C.; Rolfson, O.; Bjorkman, L.I.; Eisler, T.; Kalamajski, S.; Kamali-Moghaddam, M.; Karlsson, N.G. Lubricin binds cartilage proteins, cartilage oligomeric matrix protein, fibronectin and collagen II at the cartilage surface. Sci. Rep. 2017, 7, 13149. [Google Scholar] [CrossRef]
- Majd, S.E.; Kuijer, R.; Kowitsch, A.; Groth, T.; Schmidt, T.A.; Sharma, P.K. Both hyaluronan and collagen type II keep proteoglycan 4 (lubricin) at the cartilage surface in a condition that provides low friction during boundary lubrication. Langmuir 2014, 30, 14566–14572. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.; Hwang, N.S. Regulation of lubricin for functional cartilage tissue regeneration: A review. Biomater. Res. 2018, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Shi, Q.; Chen, J.; Wang, H.; Wu, W.; Zha, Z. Expression and Significance of MMPs in Synovial Fluid, Serum and PBMC Culture Supernatant Stimulated by LPS in Osteoarthritis Patients With or Without Diabetes. Exp. Clin. Endocrinol. Diabetes 2017. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Evans, H.; Wright, K.; van Niekerk, L.; Caterson, B.; Richardson, J.B.; Kumar, K.H.; Kuiper, J.H. ADAMTS-4 activity in synovial fluid as a biomarker of inflammation and effusion. Osteoarthr. Cartil. 2015, 23, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.L.; Ghosh, P.; Lentini, A.; Ternai, B. The interaction of pentosan polysulfate (SP54) with human neutrophil elastase and connective tissue matrix components. Chem. Biol. Interact. 1983, 47, 157–173. [Google Scholar] [CrossRef]
- Baugh, R.J.; Travis, J. Human leukocyte granule elastase: Rapid isolation and characterization. Biochemistry 1976, 15, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. Cartilage and smooth muscle cell proteoglycans detected by affinity blotting using biotinylated hyaluronan. Methods Mol. Biol. 2001, 171, 53–66. [Google Scholar] [PubMed]
- Melrose, J.; Numata, Y.; Ghosh, P. Biotinylated hyaluronan: A versatile and highly sensitive probe capable of detecting nanogram levels of hyaluronan binding proteins (hyaladherins) on electroblots by a novel affinity detection procedure. Electrophoresis 1996, 17, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Somorin, O.; Tokura, S.; Nishi, N.; Noguchi, J. The action of trypsin on synthetic chromogenic arginine substrates. J. Biochem. 1979, 85, 157–162. [Google Scholar] [CrossRef]
- Ascenzi, P.; Menegatti, E.; Guarneri, M.; Amiconi, G. Active-site titration of serine proteinases acting selectively on cationic substrates by N-alpha-carbobenzoxy-L-arginine p-nitrophenyl ester and N-alpha-carbobenzoxy-L-lysine p-nitrophenyl ester; determination of active enzyme concentration. Biochim. Biophys. Acta 1987, 915, 421–425. [Google Scholar] [CrossRef]
- Wenzel, H.R.; Tschesche, H. Cleavage of peptide-4-nitroanilide substrates with varying chain length by human leukocyte elastase. Hoppe Seylers Z. Physiol. Chem. 1981, 362, 829–831. [Google Scholar]
- Farndale, R.W.; Sayers, C.A.; Barrett, A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect. Tissue Res. 1982, 9, 247–248. [Google Scholar] [CrossRef] [PubMed]
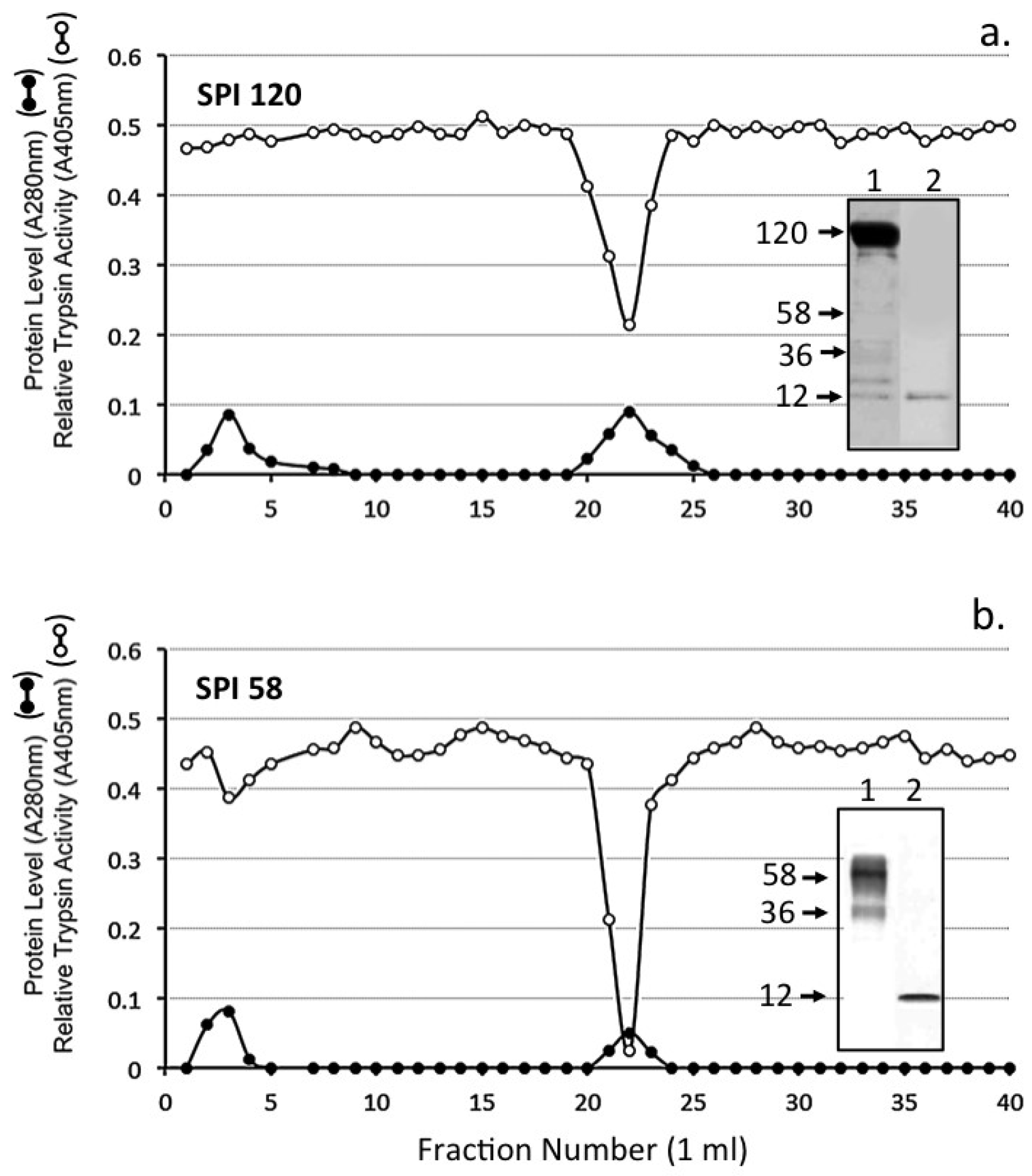
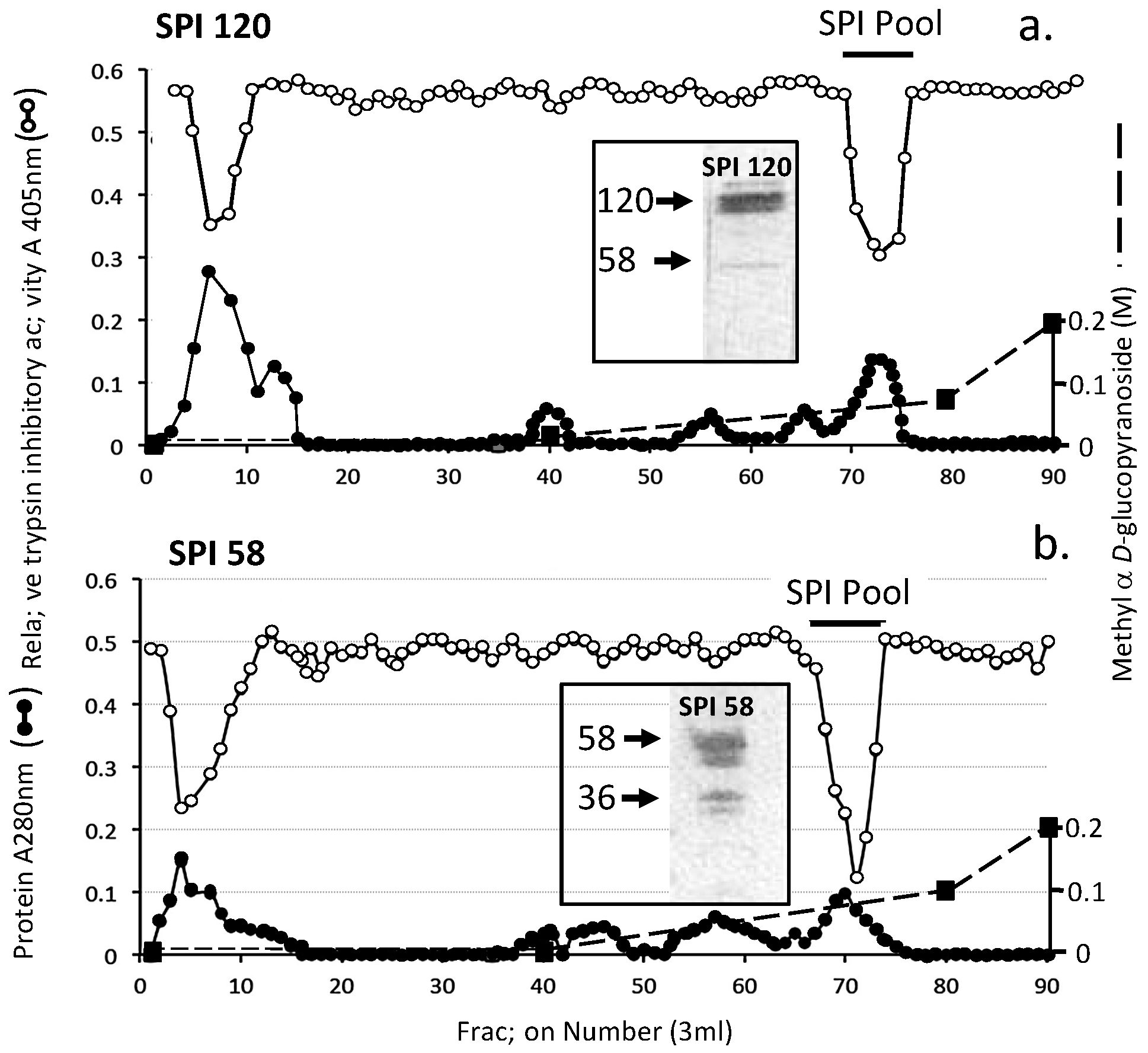
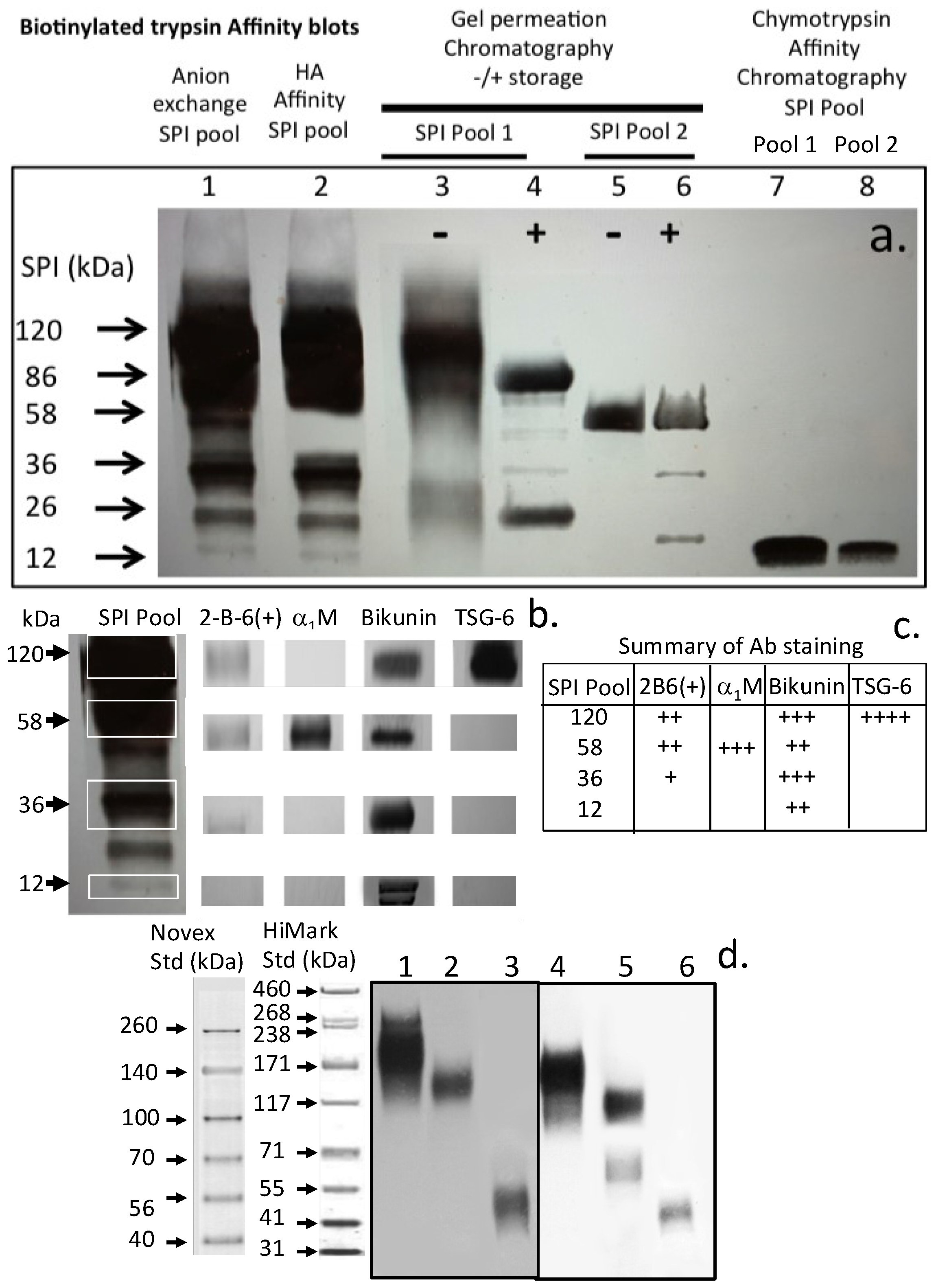
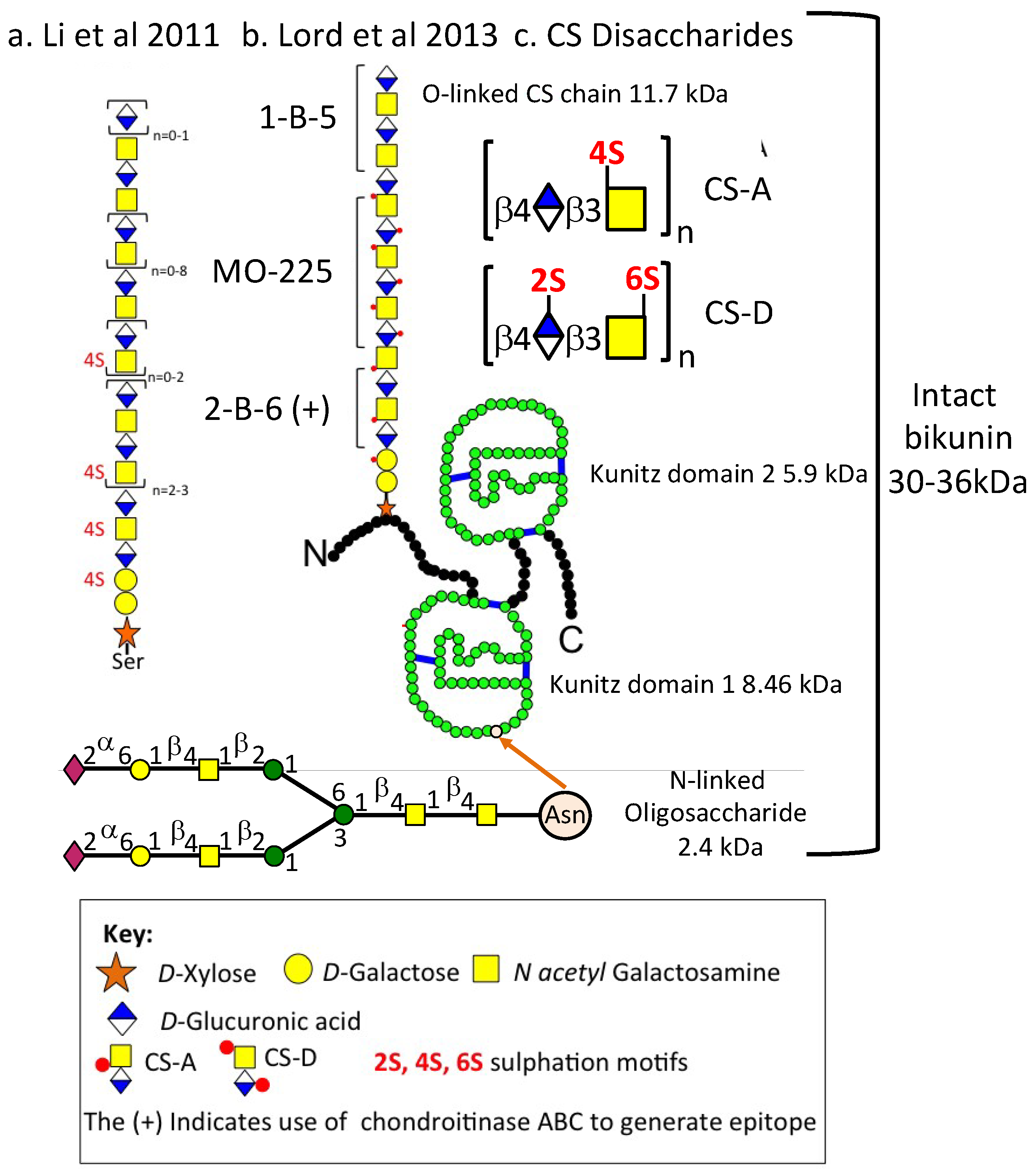
| Proteinase | Substrate * | Mean Relative Inhibitory Activity by 1 Unit of SPI ** (% Inhibition) ± SD (n = 6) | ||
|---|---|---|---|---|
| Sheep Kunitz Domain 1 | Sheep Kunitz Domain 2 | BPTI | ||
| Porcine pancreatic trypsin | ZAPNA | 95 ± 2.61 | 97 ± 1.47 | 96 ± 3.54 |
| Porcine Pancreatic chymotrypsin | AAVANA | 52 ± 4.05 | 55 ± 2.86 | 53 ± 3.73 |
| Human Leucocyte elastase | SAAVNA | 67 ± 4.79 | 69 ± 3.06 | 78 ± 4.07 |
| Human Leucocyte cathepsin G | SAAPPNA | 18 ± 7.09 | 25 ± 5.01 | 28 ± 4.03 |
| Porcine Kallikrein | VLANA | 55 ± 8.84 | 86 ± 4.41 | 92 ± 5.68 |
| Porcine Plasmin | VLLNA | 51 ±4.84 | 85 ± 1.94 | 88 ± 8.84 |
| Proteinase | Statistical Significance | |
|---|---|---|
| KPI-1 vs. BPTI | KPI-2 vs. BPTI | |
| Porcine pancreatic trypsin | NSD | NSD |
| Porcine Pancreatic chymotrypsin | NSD | NSD |
| Human Leucocyte elastase | NSD | NSD |
| Human Leucocyte cathepsin G | KPI-1 < BPTI; p < 0.05 | NSD |
| Porcine Kallikrein | KPI-1 < BPTI; p < 0.05 | NSD |
| Porcine Plasmin | KPI-1 < BPTI; p < 0.05 | NSD |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, S.M.; Melrose, J. A Retrospective Analysis of the Cartilage Kunitz Protease Inhibitory Proteins Identifies These as Members of the Inter-α-Trypsin Inhibitor Superfamily with Potential Roles in the Protection of the Articulatory Surface. Int. J. Mol. Sci. 2019, 20, 497. https://doi.org/10.3390/ijms20030497
Smith SM, Melrose J. A Retrospective Analysis of the Cartilage Kunitz Protease Inhibitory Proteins Identifies These as Members of the Inter-α-Trypsin Inhibitor Superfamily with Potential Roles in the Protection of the Articulatory Surface. International Journal of Molecular Sciences. 2019; 20(3):497. https://doi.org/10.3390/ijms20030497
Chicago/Turabian StyleSmith, Susan M., and James Melrose. 2019. "A Retrospective Analysis of the Cartilage Kunitz Protease Inhibitory Proteins Identifies These as Members of the Inter-α-Trypsin Inhibitor Superfamily with Potential Roles in the Protection of the Articulatory Surface" International Journal of Molecular Sciences 20, no. 3: 497. https://doi.org/10.3390/ijms20030497
APA StyleSmith, S. M., & Melrose, J. (2019). A Retrospective Analysis of the Cartilage Kunitz Protease Inhibitory Proteins Identifies These as Members of the Inter-α-Trypsin Inhibitor Superfamily with Potential Roles in the Protection of the Articulatory Surface. International Journal of Molecular Sciences, 20(3), 497. https://doi.org/10.3390/ijms20030497