Cadmium and Lead Decrease Cell–Cell Aggregation and Increase Migration and Invasion in Renca Mouse Renal Cell Carcinoma Cells
Abstract
1. Introduction
2. Results
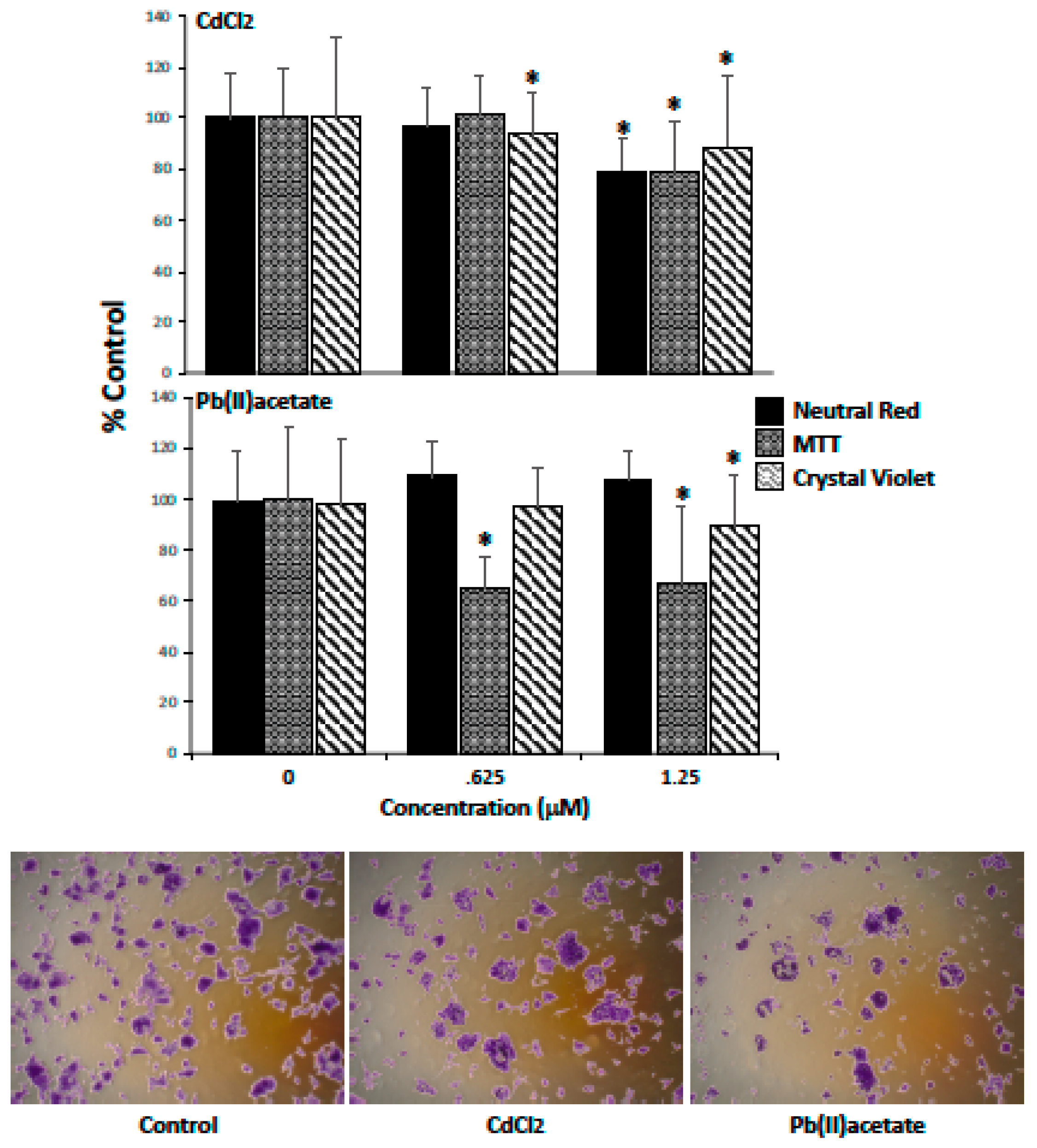
2.1. Impact of Cadmium and Lead on Renca Cell Viability
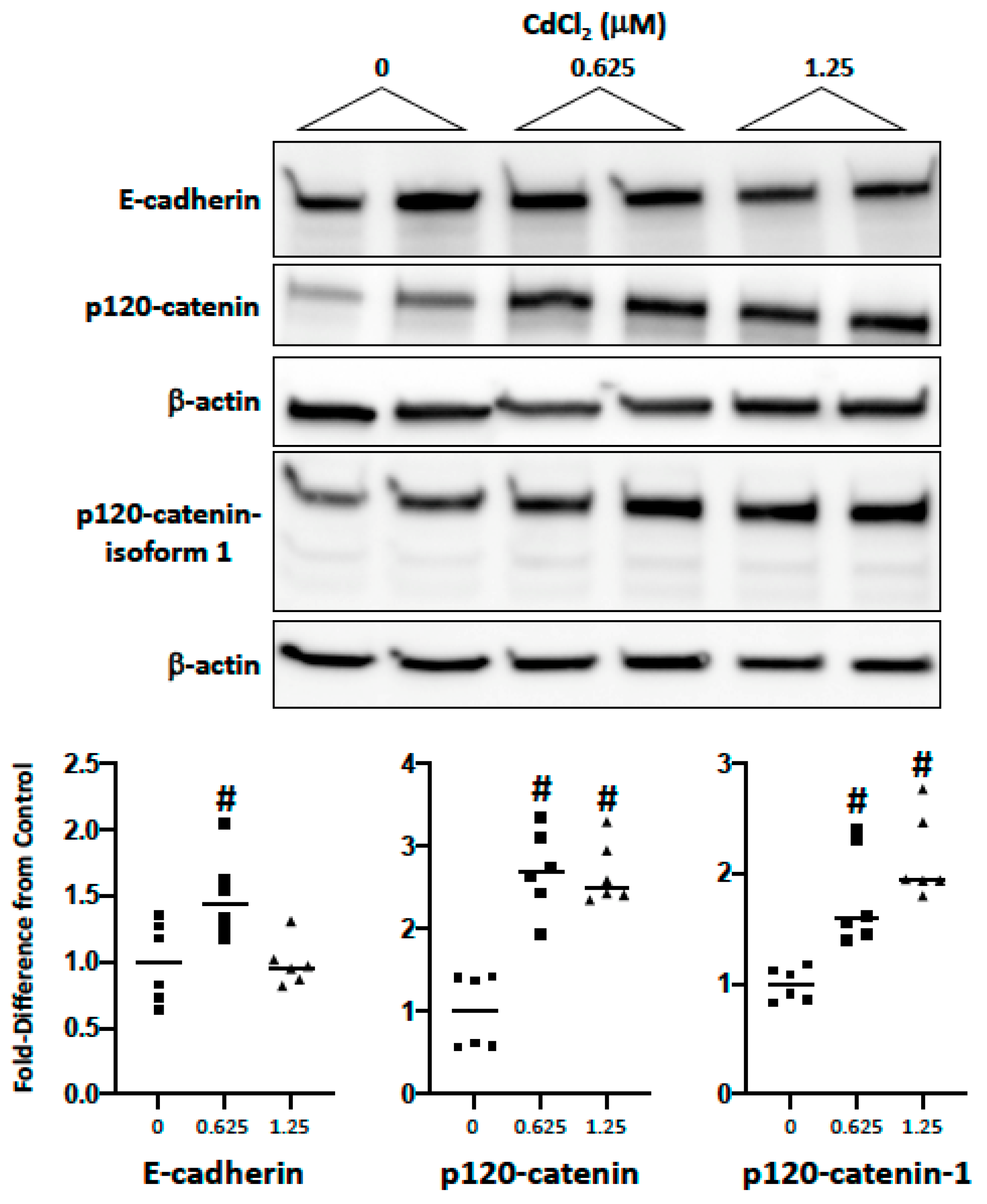
2.2. Impact of Cadmium and Lead on Cadherin and Catenin Expression
2.3. Impact of Cadmium and Lead on MMP-9 Expression
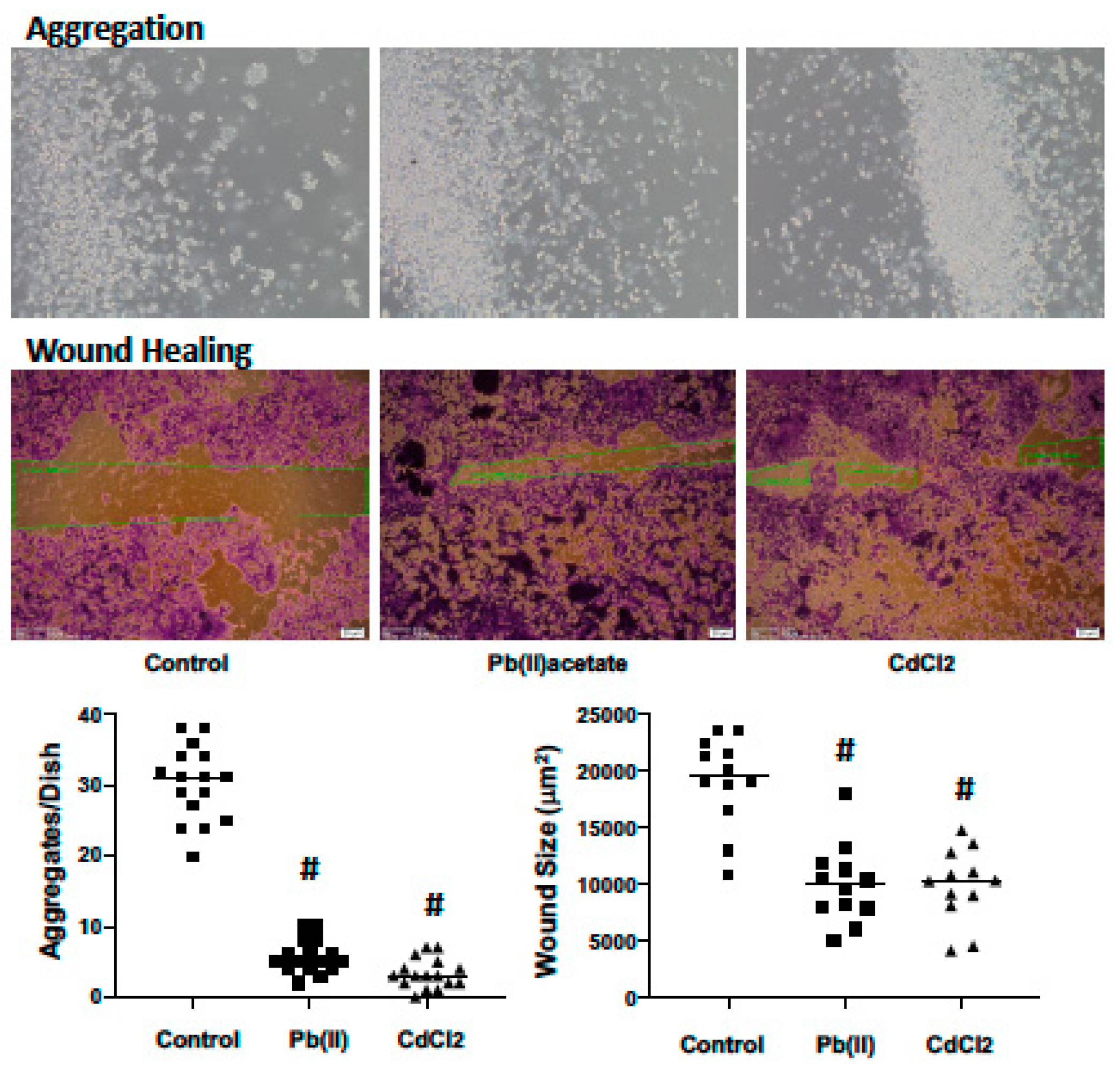
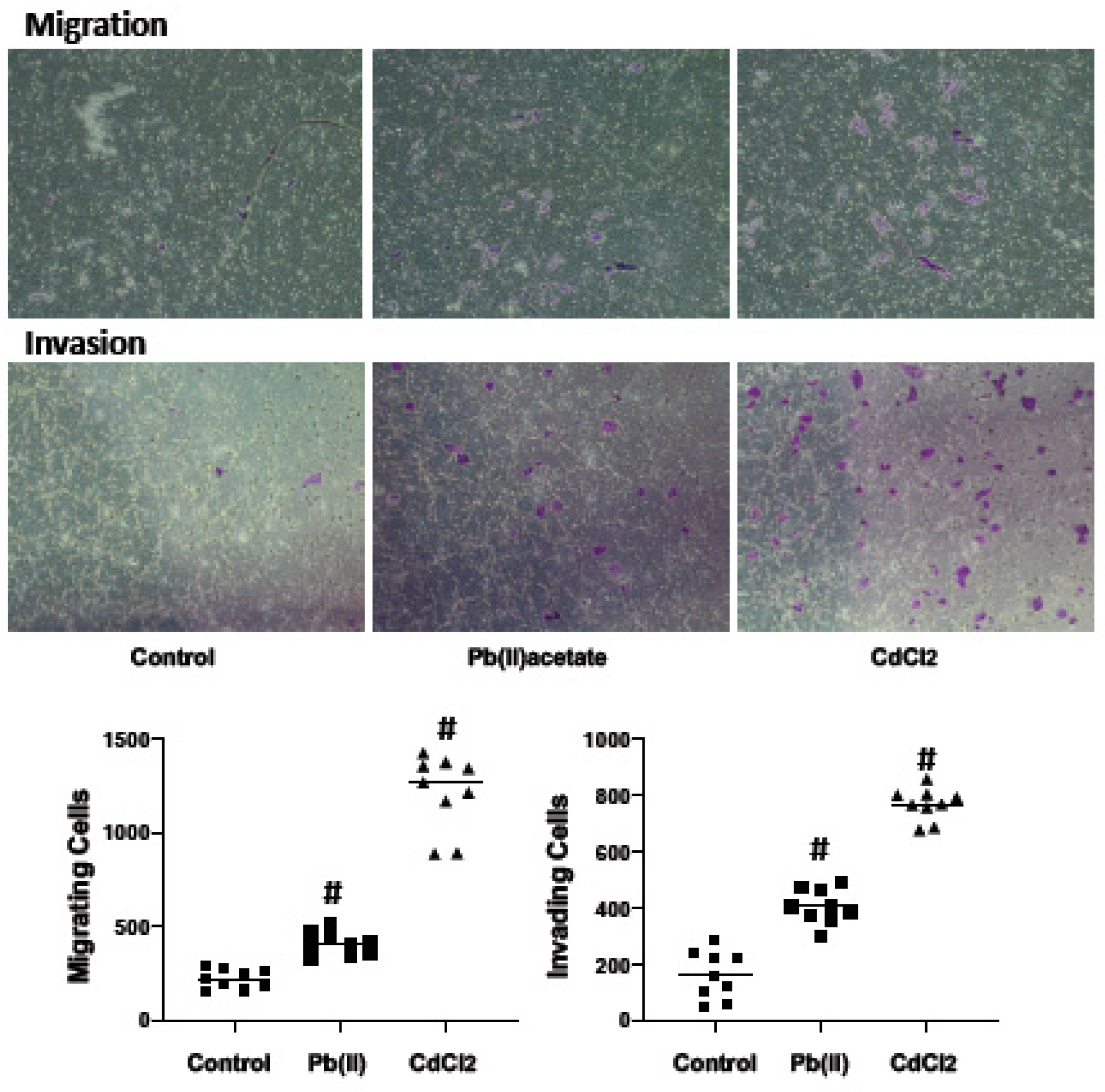
2.4. Impact of Cadmium and Lead on Renca cell Adhesion, Migration and Invasion
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Cell Viability
4.3. Western Blot
4.4. Cell Aggregation
4.5. Wound Healing
4.6. Cell Migration and Invasion
4.7. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Surveillance Epidemiology and End Results. SEER Stat Fact Sheets. National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/kidrp.html (accessed on 10 December 2019).
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.K.; Lipworth, L.; Tarone, R.E.; Blot, W.J. Cancer Epidemiology and Prevention; Schottenfeld, D., Fraumeni, J.F., Eds.; Oxford University Press: New York, NY, USA, 2006; Volume 57, pp. 1087–1100. [Google Scholar]
- Thompson, R.H.; Ordonez, M.A.; Iasonos, A.; Secin, F.P.; Guillonneau, B.; Russo, P.; Touijer, K. Renal cell carcinoma in young and old patients—Is there a diference? J. Urol. 2008, 180, 1262–1266. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Magi-Galluzzi, C. Epithelial-to-mesenchymal transition in renal neoplasms. Adv. Anat. Pathol. 2014, 21, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Piva, F.; Giuletti, M.; Santoni, M.; Occhipinti, G.; Scarpelli, M.; Lopez-Beltran, A.; Cheng, L.; Principato, G.; Montironi, R. Epithelial to mesenchymal transition in renal cell carcinoma: Implications for cancer therapy. Mol. Diagn. 2016, 20, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals 2010, 23, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Env. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Talio, M.C.; Luconi, M.O.; Masi, A.N.; Fernández, L.P. Cadmium monitoring in saliva and urine as indicator of smoking addiction. Sci. Total Env. 2010, 408, 3125–3132. [Google Scholar] [CrossRef]
- Kolonel, L.N. Association of cadmium with renal cancer. Cancer 1976, 37, 1782–1787. [Google Scholar] [CrossRef]
- Song, J.K.; Lou, H.; Yin, X.H.; Huang, G.L.; Luo, S.Y.; Lin, D.R.; Yuan, B.; Zhang, W.; Zhu, J.G. Association between cadmium exposure and renal cancer risk: A meta-analysis of observational studies. Sci. Rep. 2015, 5, 17976. [Google Scholar] [CrossRef]
- Boffetta, P.; Fontana, L.; Stewart, P.; Zaridze, D.; Szeszenia-Dabrowska, N.; Janout, V.; Bencko, V.; Foretova, L.; Jinga, V.; Matveev, V.; et al. Occupational exposure to arsenic, cadmium, chromium, lead and nickel, and renal cell carcinoma: A case-control study from Central and Eastern Europe. Occup. Env. Med. 2011, 68, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Southard, E.B.; Roff, A.; Fortugno, T.; Richie, J.P.; Kaag, M.; Chinchilli1, V.M.; Virtamo, J.; Albanes, D.; Weinstein, S.; Wilson, R.T. Lead, calcium uptake, and related genetic variants in association with renal cell carcinoma in a cohort of male Finnish smokers. Cancer Epidemiol. Biomark. Prev. 2012, 21, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Calvo, B.F.; Junior, S.D.; Rodrigues, C.J.; Krug, F.J.; Marumo, J.T.; Schor, N.; Bellini, M.H. Variation in the distribution of trace elements in renal cell carcinoma. Biol. Trace Elem. Res. 2009, 130, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Pirincci, N.; Gecit, I.; Gunes, M.; Kaba, M.; Tanik, S.; Yuksel, M.B.; Arsian, H.; Demir, H. Levels of serum trace elements in renal cell carcinoma cases. Asian Pac. J. Cancer Prev. 2013, 14, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Inamura, H.; Miyayama, T.; Matsuoka, M. Involvement of Notch1 signaling in malignant progression of A549 cells subjected to prolonged cadmium exposure. J. Biol. Chem. 2017, 292, 7942–7953. [Google Scholar] [CrossRef]
- Shan, Z.; Wei, Z.; Shaikh, Z.A. Suppression of ferroportin expression by cadmium stimulates proliferation, EMT, and migration in triple-negative breast cancer cells. Toxicol. Appl. Pharm. 2018, 356, 36–43. [Google Scholar] [CrossRef]
- Wei, Z.; Shaikh, Z.A. Cadmium stimulates metastasis-associated phenotype in triple-negative breast cancer cells through integrin and catenin signaling. Toxicol. Appl. Pharm. 2017, 328, 70–80. [Google Scholar] [CrossRef]
- Wei, Z.; Shan, Z.; Shaikh, Z.A. Epithelial-mesenchymal transition in breast epithelial cells treated with cadmium and the role of Snail. Toxicol. Appl. Pharm. 2018, 344, 46–55. [Google Scholar] [CrossRef]
- Du, L.; Lei, Y.; Chen, J.; Song, H.; Wu, X. Potential ameliorative effects of qin ye dan against cadmium induced prostatic deficits via regulating Nrf-2/HO-1 and TGF-1/Smad pathways. Cell. Physiol. Biochem. 2017, 43, 1359–1368. [Google Scholar] [CrossRef]
- Thijssen, S.; Lambrichts, I.; Maringwa, J.; Kerkhove, E.V. Changes in expression of fibrotic markers and histopathological alterations in kidneys of mice chronically exposed to low and high Cd doses. Toxicology 2007, 238, 200–210. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Scharner, B.; Jurasovic, J.; Messner, B.; Bernhard, D.; Thévenod, F. Chronic cadmium exposure induces transcriptional activation of the Wnt pathway and upregulation of epithelial-to-mesenchymal transition markers in mouse kidney. Toxicol. Lett. 2010, 198, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Niewenhuis, R.J. Cadmium (Cd2+) disrupts Ca2+-dependent cell-cell junctions and alters the pattern of E-cadherin immunofluorescence in LLC-PK1 cells. Biochem. Biophys. Res. Commun. 1991, 181, 1118–1124. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Lamar, P.C.; Lynch, S.M. Cadmium alters the localization of N-cadherin, E-cadherin and beta-catenin in the proximal tubule epithelium. Toxicol. Appl. Pharm. 2003, 189, 180–195. [Google Scholar] [CrossRef]
- Cookman, G.R.; King, W.; Regan, C.M. Chronic low-level lead exposure impairs embryonic to adult conversion of the neural cell adhesion molecule. J. Neurochem. 1987, 49, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Breen, K.C.; Regan, C.M. Lead stimulates Golgi sialyltransferase at times coincident with the embryonic to adult conversion of the neural cell adhesion molecule (N-CAM). Toxicology 1988, 49, 71–76. [Google Scholar] [CrossRef]
- Hu, Q.; Fu, H.; Song, H.; Ren, T.; Li, L.; Ye, L.; Liu, T.; Dong, S. Low-level lead exposure attenuates the expression of three major isoforms of neural cell adhesion molecule. Neurotoxicology 2011, 32, 255–260. [Google Scholar] [CrossRef]
- Mansel, C.; Fross, S.; Rose, J.; Dema, E.; Mann, A.; Hart, H.; Klawinski, P.; Vohra, B.P.S. Lead exposure reduces survival, neuronal determination, and differentiation of P19 stem cells. Neurotoxicol. Teratol. 2019, 72, 58–70. [Google Scholar] [CrossRef]
- Beier, E.E.; Sheu, T.J.; Buckley, T.; Yukata, K.; O’Keefe, R.; Zuscik, M.J.; Puzas, J.E. Inhbition of beta-catenin signaling by Pb leads to incomplete fracture healing. J. Orthop. Res. 2014, 32, 1397–1405. [Google Scholar] [CrossRef]
- Beier, E.D.; Sheu, T.J.; Dang, D.; Holz, J.D.; Ubayawardena, R.; Babij, P.; Puzas, J.E. Heavy metal ion regulation of gene expression: Mechanism by which lead inhibits osteoblastic bone-forming activity through modulation of the wnt/-catenin signaling pathway. J. Biol. Chem. 2015, 290, 18216–18226. [Google Scholar] [CrossRef]
- Engel, J.D.; Kundu, S.D.; Yang, T.; Lang, S.; Goodwin, S.; Janulis, L.; Cho, J.S.; Chang, J.; Kim, S.J.; Lee, C. Transforming growth factor-beta type II receptor confers tumor suppressor activity in murine renal carcinoma (Renca) cells. Urology 1999, 54, 164–170. [Google Scholar] [CrossRef]
- Borgna, V.; Villegas, J.; Burzio, V.A.; Belmar, S.; Araya, M.; Jeldes, E.; Lobos-González, L.; Silva, V.; Villota, C.; Oliveira-Cruz, L.; et al. Mitochondrial ASncmtRNA-1 and ASncmtRNA-2 as potent targets to inhibit tumor growth and metastasis in the RenCa murine renal adenocarcinoma model. Oncotarget 2017, 8, 43692–43708. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.A.; James, B.R.; Wilber, A.; Griffith, T.S. A syngeneic mouse model of metastatic renal cell carcinoma for quantitative and longitudinal assessment of preclinical therapies. J. Vis. Expt. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wehrendt, D.P.; Carmona, F.; Wusener, A.E.G.; González, Á.; Martínez, J.M.L.; Arregui, C.O. P120-Catenin regulates early trafficking stages of the N-cadherin precursor complex. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Chuang, M.J.; Sun, K.H.; Tang, S.J.; Deng, M.W.; Wu, Y.H.; Sung, J.S.; Cha, T.L.; Sun, G.H. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci. 2008, 99, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Kallakury, B.V.; Karikehalli, S.; Haholu, A.; Sheehan, C.E.; Azumi, N.; Ross, J.S. Increased expression of matrix metalloproteinase 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin. Cancer Res. 2001, 7, 3113–3119. [Google Scholar] [PubMed]
- Cho, N.H.; Shim, H.S.; Rha, S.Y.; Kang, S.H.; Hong, S.H.; Choi, Y.D.; Sung, J.H.; Cho, S.H. Increased expression of matrix metalloproteinase 9 correlates with poor prognostic variables in renal cell carcinoma. Eur. Urol. 2003, 44, 560–566. [Google Scholar] [CrossRef]
- Delahunt, B. Histopathologic prognostic indicators for renal cell carcinoma. Semin. Diagn. Pathol. 1998, 15, 68–76. [Google Scholar]
- Shuch, B.; Bratslavsky, G.; Linehan, W.M.; Srinivasan, R. Sarcomatoid renal cell carcinoma: Comprehensive review of the biology and current treatment strategies. Oncologist 2012, 17, 46–54. [Google Scholar] [CrossRef]
- Conant, J.L.; Peng, Z.; Evans, M.F.; Naud, S.; Cooper, K. Sarcomatoid renal cell carcinoma is an examples of epithelial-mesenchymal transition. J. Clin. Pathol. 2011, 64, 1088–1092. [Google Scholar] [CrossRef]
- Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: Pathological significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 2004, 15, 1–12. [Google Scholar] [CrossRef]
- Reynolds, A.B.; Roesel, D.J.; Kanner, S.B.; Parsons, J.T. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol. Cell Biol. 1989, 9, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Kourtidis, A.; Yanagisawa, M.; Huveldt, D.; Copland, J.A.; Anastasiadis, P.Z. Pro-tumorigenic phosphorylation of p120 catenin in renal and breast cancer. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thoreson, M.A.; Reynolds, A.B. Altered expression of the catenin p120 in human cancer: Implications for tumor progression. Differentiation 2002, 70, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Schackmann, R.C.J.; Tenhagen, M.; van de Ven, R.A.H.; Derksen, P.W.B. P120-catenin in cancer-mechanisms, models and opportunities for intervention. J. Cell Sci. 2013, 126, 3615–3625. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.B. P120-catenin: Past and present. Biochim. Biophys. Acta 2007, 1773, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Allison, D.F.; Buckley, K.M.; Kottke, M.D.; Vincent, P.A.; Faundez, V.; Kowalczyk, A.P. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 2003, 163, 535–545. [Google Scholar] [CrossRef]
- Montonen, O.; Aho, M.; Uitto, J.; Aho, S. Tissue distribution and cel type-specific expression of p120ctn isoforms. J. Histochem. Cytochem. 2001, 49, 1487–1496. [Google Scholar] [CrossRef]
- Wang, R.; Chen, Y.S.; Dashwood, W.M.; Li, Q.; Löhr, C.V.; Fischer, K.; Ho, E.; Williams, D.E.; Dashwood, R.H. Divergent roles of p120-catenin isoforms linked to altered cell viability, proliferation, and invasiveness in carcinogen-induced rat skin tumors. Mol. Carcinog. 2017, 56, 1733–1742. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Huveldt, D.; Kreinest, P.; Lohse, C.M.; Cheville, J.C.; Parker, A.S.; Copland, J.A.; Anastasiadis, P.Z. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion, and predicts metastatic disease. J. Biol. Chem. 2008, 283, 18344–18354. [Google Scholar] [CrossRef]
- Mo, Y.Y.; Reynolds, A.B. Identification of murine p120 isoforms and heterogenous expression of p120cas isoforms in human tumor cell lines. Cancer Res. 1996, 56, 2633–2640. [Google Scholar]
- Markham, N.O.; Doll, C.A.; Dohn, M.R.; Miller, R.K.; Yu, H.; Coffey, R.J.; McCrea, P.D.; Gamse, J.T.; Reynolds, A.B. DIPA-family coiled-coils bind conserved isoform-specific head domain of p120-catenin family: Potential roles in hydrocephalus and heterotopia. Mol. Biol. Cell 2014, 25, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Out, H.; Spentzos, D.; Kolia, S.; Blaheta, R.; Jonas, D.; Libermann, T.A. Gene signatures of progression and metastasis in renal cell cancer. Clin. Cancer Res. 2005, 11, 5730–5739. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: New insight with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Expt. 2014. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akin, R.; Hannibal, D.; Loida, M.; Stevens, E.M.; Grunz-Borgmann, E.A.; Parrish, A.R. Cadmium and Lead Decrease Cell–Cell Aggregation and Increase Migration and Invasion in Renca Mouse Renal Cell Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 6315. https://doi.org/10.3390/ijms20246315
Akin R, Hannibal D, Loida M, Stevens EM, Grunz-Borgmann EA, Parrish AR. Cadmium and Lead Decrease Cell–Cell Aggregation and Increase Migration and Invasion in Renca Mouse Renal Cell Carcinoma Cells. International Journal of Molecular Sciences. 2019; 20(24):6315. https://doi.org/10.3390/ijms20246315
Chicago/Turabian StyleAkin, Ryan, David Hannibal, Margaret Loida, Emily M. Stevens, Elizabeth A. Grunz-Borgmann, and Alan R. Parrish. 2019. "Cadmium and Lead Decrease Cell–Cell Aggregation and Increase Migration and Invasion in Renca Mouse Renal Cell Carcinoma Cells" International Journal of Molecular Sciences 20, no. 24: 6315. https://doi.org/10.3390/ijms20246315
APA StyleAkin, R., Hannibal, D., Loida, M., Stevens, E. M., Grunz-Borgmann, E. A., & Parrish, A. R. (2019). Cadmium and Lead Decrease Cell–Cell Aggregation and Increase Migration and Invasion in Renca Mouse Renal Cell Carcinoma Cells. International Journal of Molecular Sciences, 20(24), 6315. https://doi.org/10.3390/ijms20246315



