Analysis of the Molecular Signaling Signatures of Muscle Protein Wasting Between the Intercostal Muscles and the Gastrocnemius Muscles in db/db Mice
Abstract
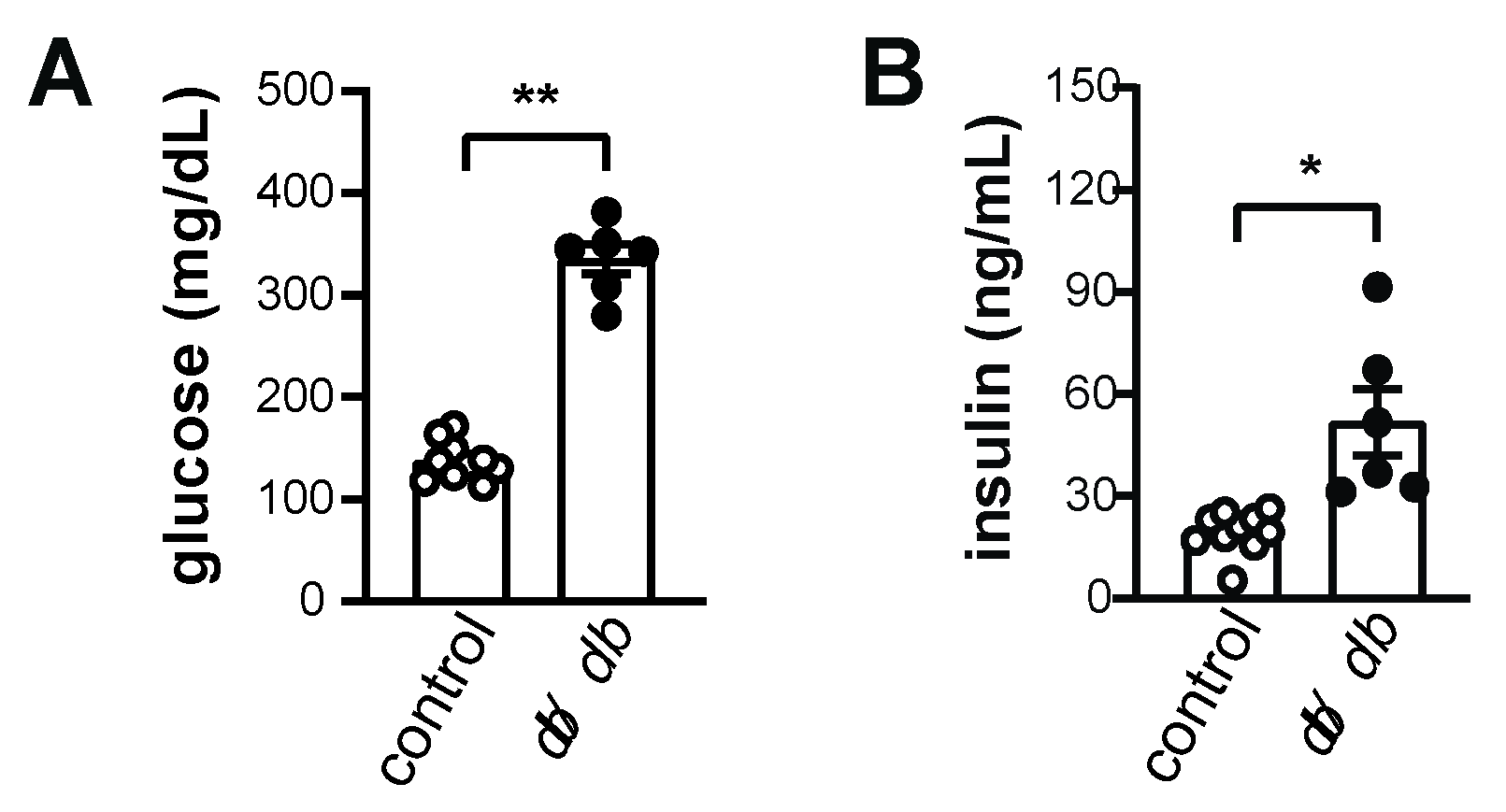
1. Introduction
2. Results
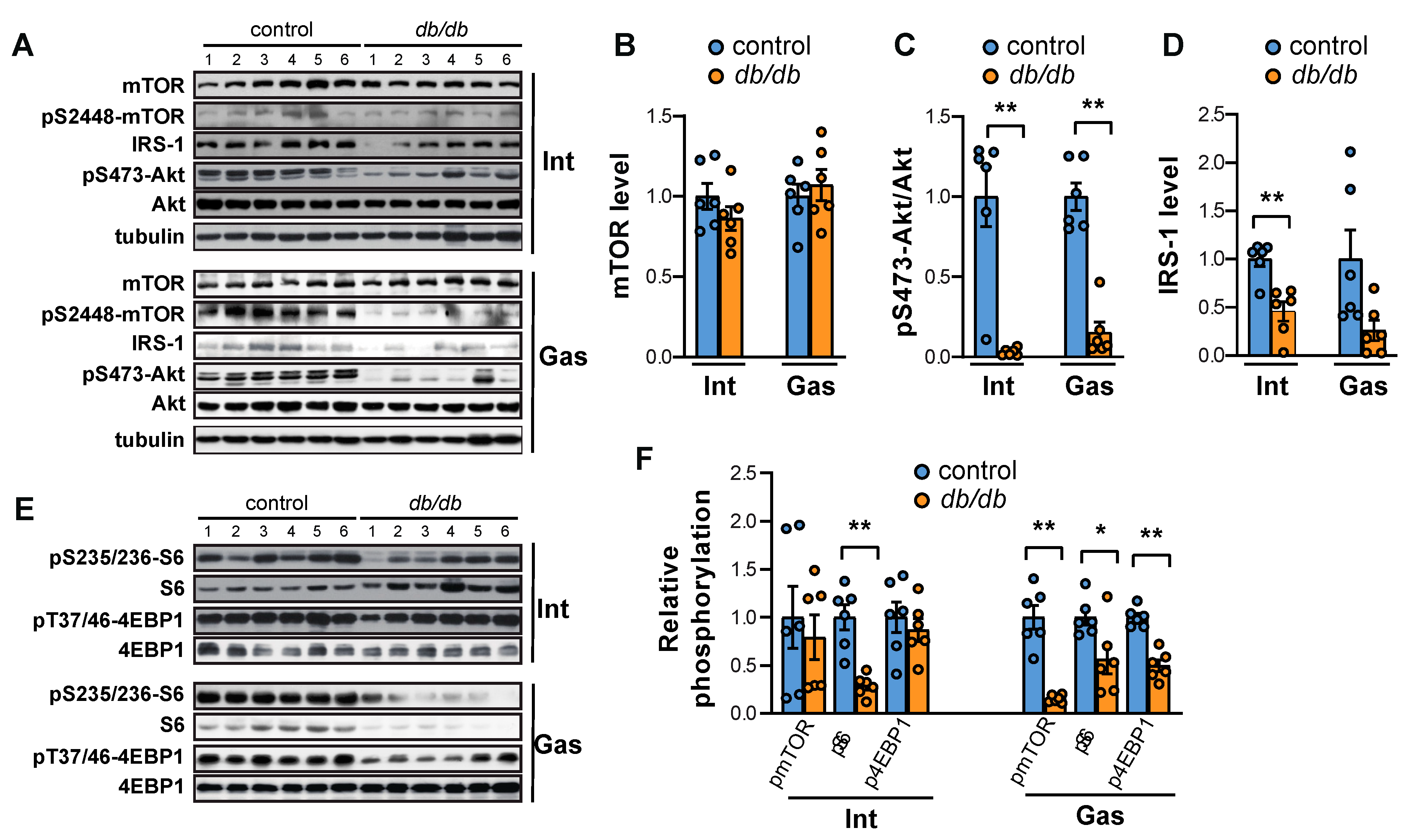
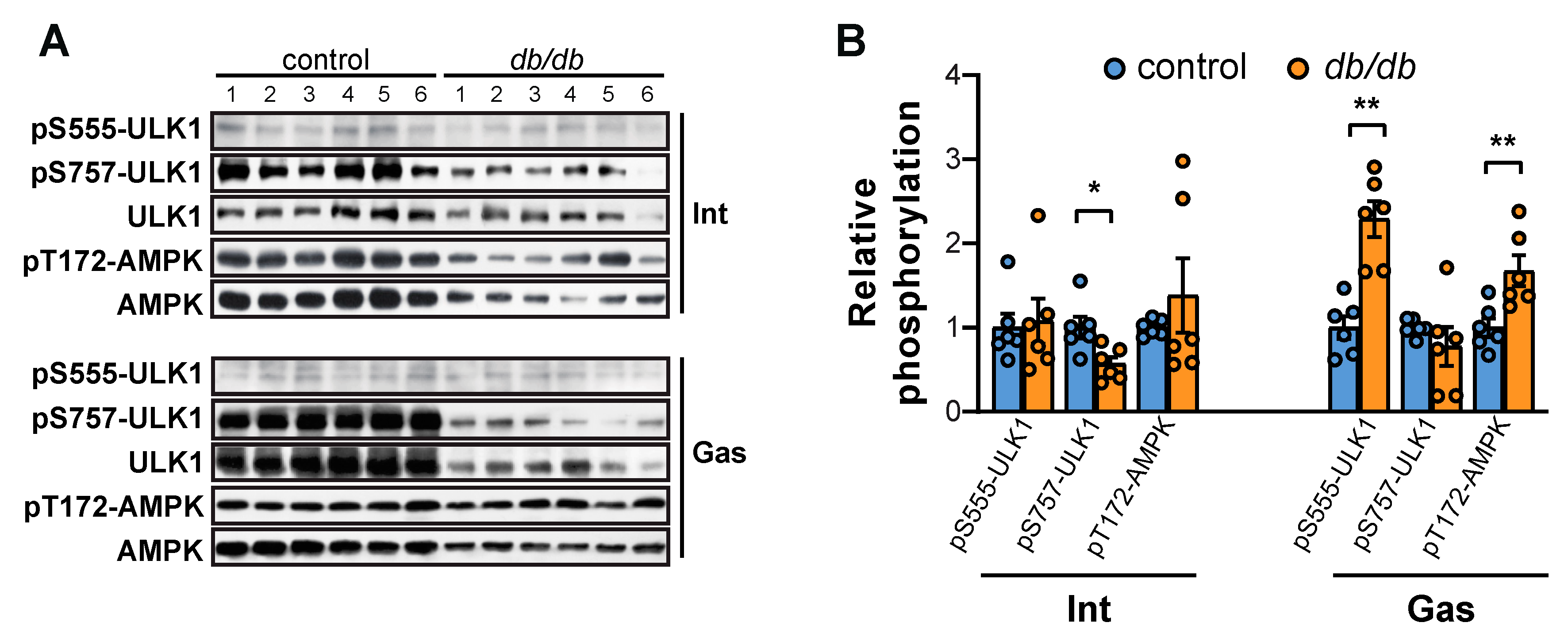
2.1. Both mTORC1 and Akt Are Suppressed in the Intercostal and Gastrocnemius Muscles in db/db Mice
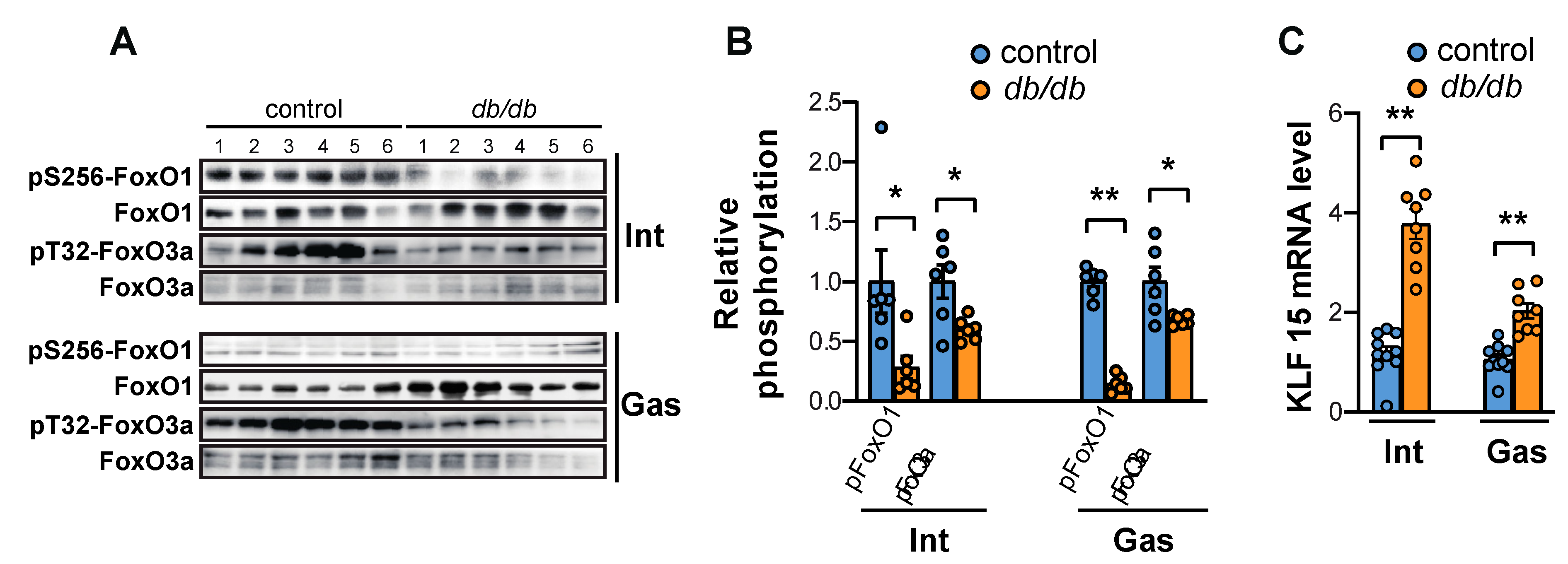
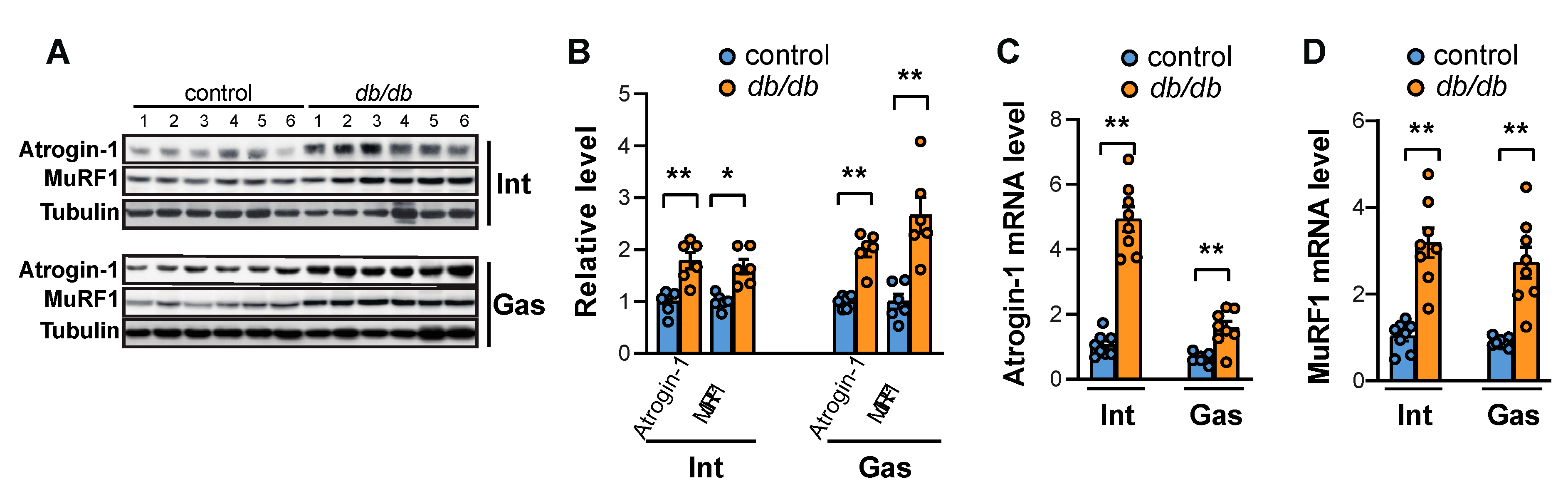
2.2. FoxO Activation and the Expression of Atrogin-1 and MuRF1 Are Induced in the Muscles of db/db Mice
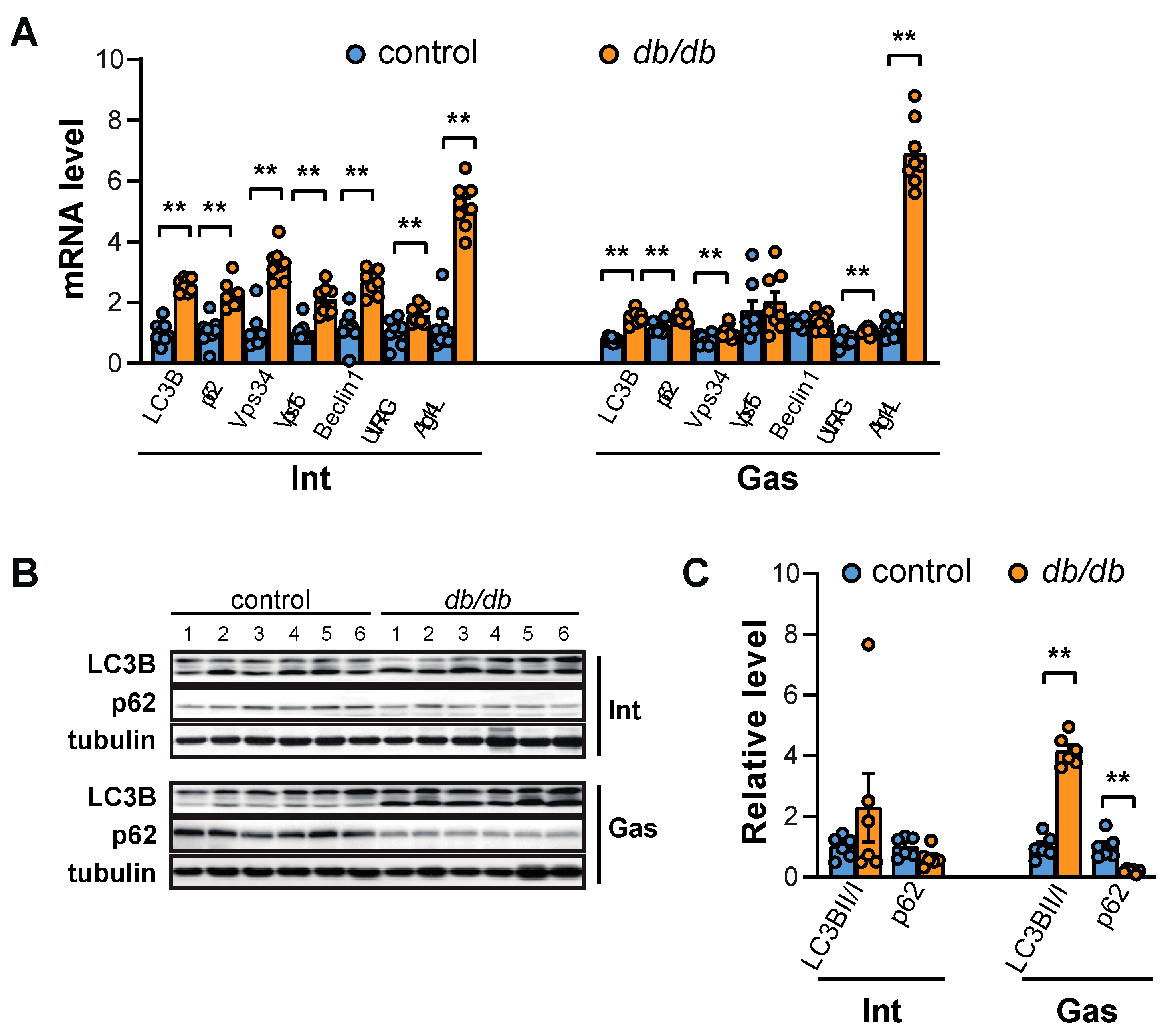
2.3. Autophagy is Elicited in the Gastrocnemius Muscle but not the Intercostal Muscles of db/db Mice
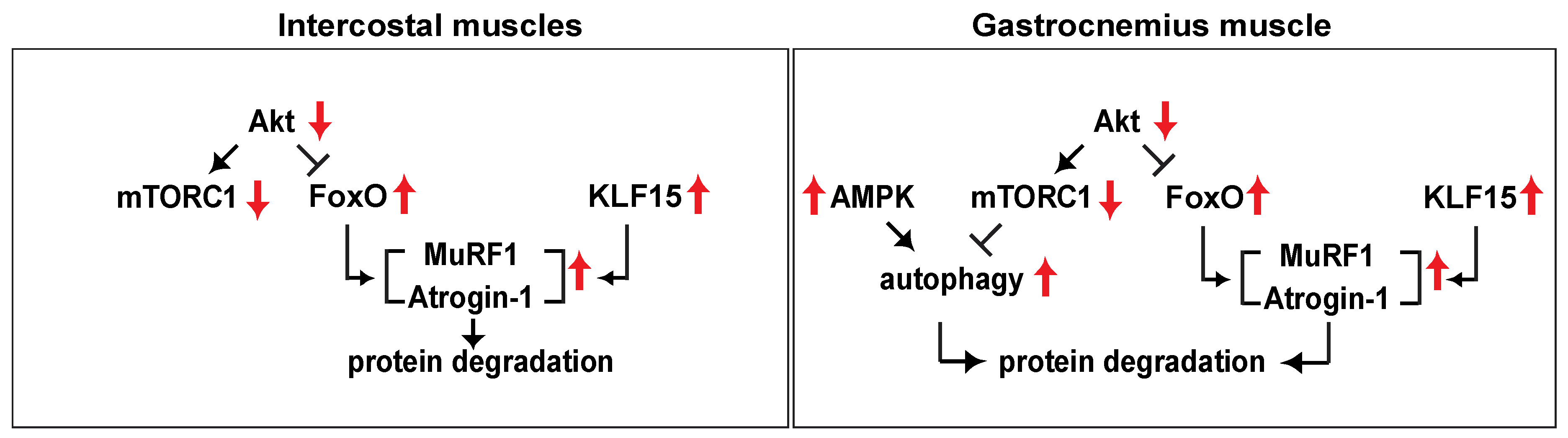
3. Discussion
4. Materials and Methods
4.1. Antibodies and Other Reagents
4.2. Animals
4.3. Tissue Lysis, Immunoprecipitation, and Western Blot Analysis
4.4. RNA Isolation and Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| mTOR | Mammalian target of rapamycin |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| mTORC1 | mTOR complex1 |
| mTORC2 | mTOR complex2 |
| db/db mice | Diabetic mice |
| DIO mice | Diet-induced obese mice |
| HFD | High fat diet |
| RC | Regular Chow |
References
- Martin-Timon, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Canizo-Gomez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, M.J.; Hachamovitch, R.; Kang, X.; Hayes, S.W.; Friedman, J.D.; Germano, G.; Pfisterer, M.E.; Berman, D.S. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur. Heart J. 2004, 25, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Naya, M.; Foster, C.R.; Gaber, M.; Hainer, J.; Klein, J.; Dorbala, S.; Blankstein, R.; Carli, M.F.D. Association Between Coronary Vascular Dysfunction and Cardiac Mortality in Patients With and Without Diabetes Mellitus. Circulation 2012, 126, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Han, T.S.; Al-Gindan, Y.Y.; Govan, L.; Hankey, C.R.; Lean, M.E.J. Associations of BMI, waist circumference, body fat, and skeletal muscle with type 2 diabetes in adults. Acta Diabetol. 2019, 56, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Van Eetvelde, B.L.M.; Cambier, D.; Vanden Wyngaert, K.; Celie, B.; Calders, P. The Influence of Clinically Diagnosed Neuropathy on Respiratory Muscle Strength in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 8065938. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Hamalainen, N.; Pette, D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J. Histochem. Cytochem. 1993, 41, 733–743. [Google Scholar] [CrossRef]
- Mizuno, M. Human respiratory muscles: Fibre morphology and capillary supply. Eur. Respir. J. 1991, 4, 587–601. [Google Scholar]
- Gaster, M.; Staehr, P.; Beck-Nielsen, H.; Schroder, H.D.; Handberg, A. GLUT4 Is Reduced in Slow Muscle Fibers of Type 2 Diabetic Patients. Is Insulin Resistance in Type 2 Diabetes a Slow, Type 1 Fiber Disease? Diabetes 2001, 50, 1324–1329. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Romanino, K.; Cloetta, D.; Lin, S.; Mascarenhas, J.B.; Oliveri, F.; Xia, J.; Casanova, E.; Costa, C.F.; Brink, M.; et al. Skeletal muscle-Specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008, 8, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Pallafacchina, G.; Calabria, E.; Serrano, A.L.; Kalhovde, J.M.; Schiaffino, S. A protein kinase B-Dependent and rapamycin-Sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc. Natl. Acad. Sci. USA 2002, 99, 9213–9218. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, A.L.; Warnes, C.; Liu, J.; Zhang, C.; Kawasome, H.; Terada, N.; Boppart, M.D.; Schoenherr, C.J.; Chen, J. mTOR regulates skeletal muscle regeneration in vivo through kinase-dependent and kinase-Independent mechanisms. Am. J. Physiol. Cell Physiol. 2009, 297, C1434–C1444. [Google Scholar] [CrossRef]
- Castets, P.; Lin, S.; Rion, N.; Di Fulvio, S.; Romanino, K.; Guridi, M.; Frank, S.; Tintignac, L.A.; Sinnreich, M.; Ruegg, M.A. Sustained activation of mTORC1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-Onset myopathy. Cell Metab. 2013, 17, 731–744. [Google Scholar] [CrossRef]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumi, J.; Ramos, D.; Ruberte, J.; Simo, R.; Hernandez, C. The db/db mouse: A useful model for the study of diabetic retinal neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef]
- Tang, H.; Inoki, K.; Brooks, S.V.; Okazawa, H.; Lee, M.; Wang, J.; Kim, M.; Kennedy, C.L.; Macpherson, P.C.D.; Ji, X.; et al. mTORC1 underlies age-Related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell 2019, 18, e12943. [Google Scholar] [CrossRef]
- Anil, T.M.; Harish, C.; Lakshmi, M.N.; Harsha, K.; Onkaramurthy, M.; Sathish Kumar, V.; Shree, N.; Geetha, V.; Balamurali, G.V.; Gopala, A.S.; et al. CNX-012-570, a direct AMPK activator provides strong glycemic and lipid control along with significant reduction in body weight; studies from both diet-Induced obese mice and db/db mice models. Cardiovasc. Diabetol. 2014, 13, 27. [Google Scholar] [CrossRef]
- O’Neill, B.T.; Bhardwaj, G.; Penniman, C.M.; Krumpoch, M.T.; Suarez Beltran, P.A.; Klaus, K.; Poro, K.; Li, M.; Pan, H.; Dreyfuss, J.M.; et al. FoxO Transcription Factors are Critical Regulators of Diabetes-Related Muscle Atrophy. Diabetes 2018, 68, 556–570. [Google Scholar] [CrossRef]
- Shimizu, N.; Yoshikawa, N.; Ito, N.; Maruyama, T.; Suzuki, Y.; Takeda, S.; Nakae, J.; Tagata, Y.; Nishitani, S.; Takehana, K.; et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011, 13, 170–182. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-Related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, D.; Cao, P.; Lecker, S.; Hu, Z. Atrogin-1 Affects Muscle Protein Synthesis and Degradation When Energy Metabolism Is Impaired by the Antidiabetes Drug Berberine. Diabetes 2010, 59, 1879–1889. [Google Scholar] [CrossRef]
- Lutz, T.A.; Woods, S.C. Overview of animal models of obesity. Curr. Protoc. Pharmacol. 2012, 58, 5–61. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.X.; Qiu, Y.; Hansen, M.K.; Zhu, L.; Zhang, V.; Xie, M.; Okamoto, Y.; Mattie, M.D.; Higashiyama, H.; Asano, S.; et al. Adipose mitochondrial biogenesis is suppressed in db/db and high-Fat diet-Fed mice and improved by rosiglitazone. Diabetes 2007, 56, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010, 33, 1497–1499. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; De Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; De Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef]
- Pepato, M.T.; Migliorini, R.H.; Goldberg, A.L.; Kettelhut, I.C. Role of different proteolytic pathways in degradation of muscle protein from streptozotocin-Diabetic rats. Am. J. Physiol. 1996, 271, E340–E347. [Google Scholar] [CrossRef] [PubMed]
- Sitnick, M.; Bodine, S.C.; Rutledge, J.C. Chronic high fat feeding attenuates load-Induced hypertrophy in mice. J. Physiol. 2009, 587, 5753–5765. [Google Scholar] [CrossRef] [PubMed]
- Turpin, S.M.; Ryall, J.G.; Southgate, R.; Darby, I.; Hevener, A.L.; Febbraio, M.A.; Kemp, B.E.; Lynch, G.S.; Watt, M.J. Examination of ‘lipotoxicity’ in skeletal muscle of high-Fat fed and ob/ob mice. J. Physiol. 2009, 587, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Kinugawa, S.; Hirabayashi, K.; Matsushima, S.; Inoue, N.; Ohta, Y.; Hamaguchi, S.; Sobirin, M.A.; Ono, T.; Suga, T.; et al. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1069–H1077. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Hu, J.; Du, J.; Mitch, W.E. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006, 147, 4160–4168. [Google Scholar] [CrossRef]
- Ostler, J.E.; Maurya, S.K.; Dials, J.; Roof, S.R.; Devor, S.T.; Ziolo, M.T.; Periasamy, M. Effects of insulin resistance on skeletal muscle growth and exercise capacity in type 2 diabetic mouse models. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E592–E605. [Google Scholar] [CrossRef]
- Kera, T.; Kawai, H.; Hirano, H.; Kojima, M.; Watanabe, Y.; Motokawa, K.; Fujiwara, Y.; Ihara, K.; Kim, H.; Obuchi, S. Definition of Respiratory Sarcopenia With Peak Expiratory Flow Rate. J. Am. Med. Dir. Assoc. 2019, 20, 1021–1025. [Google Scholar] [CrossRef]
- Nakamura, J.; Kamiya, H.; Haneda, M.; Inagaki, N.; Tanizawa, Y.; Araki, E.; Ueki, K.; Nakayama, T. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001–2010: Report of the Committee on Causes of Death in Diabetes Mellitus. J. Diabetes Investig. 2017, 8, 397–410. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Lagirand-Cantaloube, J.; Offner, N.; Csibi, A.; Leibovitch, M.P.; Batonnet-Pichon, S.; Tintignac, L.A.; Segura, C.T.; Leibovitch, S.A. The initiation factor eIF3-F is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. Embo. J. 2008, 27, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-Lysosome and ubiquitin-Proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Goldberg, A.L.; Mitch, W.E. Protein Degradation by the Ubiquitin–Proteasome Pathway in Normal and Disease States. J. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar] [CrossRef]
- Waddell, D.S.; Baehr, L.M.; Van Den Brandt, J.; Johnsen, S.A.; Reichardt, H.M.; Furlow, J.D.; Bodine, S.C. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-Associated MuRF1 gene. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E785–E797. [Google Scholar] [CrossRef]
- Egan Daniel, F.; Chun Matthew, G.H.; Vamos, M.; Zou, H.; Rong, J.; Miller Chad, J.; Lou Hua, J.; Raveendra-Panickar, D.; Yang, C.C.; Sheffler Douglas, J.; et al. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol. Cell 2015, 59, 285–297. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Csibi, A.; Raibon, A.; Cornille, K.; Gay, S.; Bernardi, H.; Candau, R. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J. Cell Biochem. 2012, 113, 695–710. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.C.; Fang, C.; Russell, R.C.; Kim, J.H.; Fan, W.; Liu, R.; Zhong, Q.; Guan, K.L. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 2013, 152, 290–303. [Google Scholar] [CrossRef]
- Gayle, A.; Dickinson, S.; Poole, C.; Pang, M.; Fauconnot, O.; Quint, J.K. Incidence of type II diabetes in chronic obstructive pulmonary disease: A nested case-Control study. NPJ Prim. Care Respir. Med. 2019, 29, 28. [Google Scholar] [CrossRef]
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 2008, 32, 962–969. [Google Scholar] [CrossRef]
| Target Gene | Sequence of Primer |
|---|---|
| mouse GAPDH F | TCCCACTCTTCCACCTTCGA |
| mouse GAPDH R | CAGGAAATGAGCTTGACAAAGTTG |
| mouse p62 F | GAAGCTGCCCTATACCCACA |
| mouse p62 R | GAGAAACCCATGGACAGCAT |
| mouse LC3B F | TTATAGAGCGATACAAGGGGGAG |
| mouse LC3B R | CGCCGTCTGATTATCTTGATGAG |
| mouse Vps34 F | CCTGGACATCAACGTGCAG |
| mouse Vps34 R | TGTCTCTTGGTATAGCCCAGAAA |
| mouse Vps15 F | GGTGGTCACGTTGCTAAGC |
| mouse Vps15 R | CGCAGGTGCCAATCATTCTTAT |
| mouse Beclin1 F | ATGGAGGGGTCTAAGGCGTC |
| mouse Beclin1 R | TCCTCTCCTGAGTTAGCCTCT |
| mouse UVRAG F | ACATCGCTGCTCGGAACATT |
| mouse UVRAG R | CTCCACGTCGGATTCAAGGAA |
| mouse Atg14L F | GAGGGCCTTTACGTGGCTG |
| mouse Atg14L R | AATAGACGAAATCACCGCTCTG |
| mouse MuRF1 F | TCCTGGACGAGAAGAAGAGC |
| mouse MuRF1 R | TGCTCCCTGTACTGGAGGAT |
| mouse Atrogin-1 F | GCAACAAGGAGGTATACAGTAAGG |
| mouse Atrogin-1 R | TCCTTCGTACTTCCTTTGTGAAC |
| mouse KLF15 F | CTGCAGCAAGATGTACACCAA |
| mouse KLF15 R | TCATCTGAGCGTGAAAACCTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.W.; Baek, M.-O.; Choi, J.-Y.; Son, K.H.; Yoon, M.-S. Analysis of the Molecular Signaling Signatures of Muscle Protein Wasting Between the Intercostal Muscles and the Gastrocnemius Muscles in db/db Mice. Int. J. Mol. Sci. 2019, 20, 6062. https://doi.org/10.3390/ijms20236062
Kim KW, Baek M-O, Choi J-Y, Son KH, Yoon M-S. Analysis of the Molecular Signaling Signatures of Muscle Protein Wasting Between the Intercostal Muscles and the Gastrocnemius Muscles in db/db Mice. International Journal of Molecular Sciences. 2019; 20(23):6062. https://doi.org/10.3390/ijms20236062
Chicago/Turabian StyleKim, Kun Woo, Mi-Ock Baek, Ji-Young Choi, Kuk Hui Son, and Mee-Sup Yoon. 2019. "Analysis of the Molecular Signaling Signatures of Muscle Protein Wasting Between the Intercostal Muscles and the Gastrocnemius Muscles in db/db Mice" International Journal of Molecular Sciences 20, no. 23: 6062. https://doi.org/10.3390/ijms20236062
APA StyleKim, K. W., Baek, M.-O., Choi, J.-Y., Son, K. H., & Yoon, M.-S. (2019). Analysis of the Molecular Signaling Signatures of Muscle Protein Wasting Between the Intercostal Muscles and the Gastrocnemius Muscles in db/db Mice. International Journal of Molecular Sciences, 20(23), 6062. https://doi.org/10.3390/ijms20236062





