Assessment of Bones Deficient in Fibrillin-1 Microfibrils Reveals Pronounced Sex Differences
Abstract
1. Introduction
2. Results
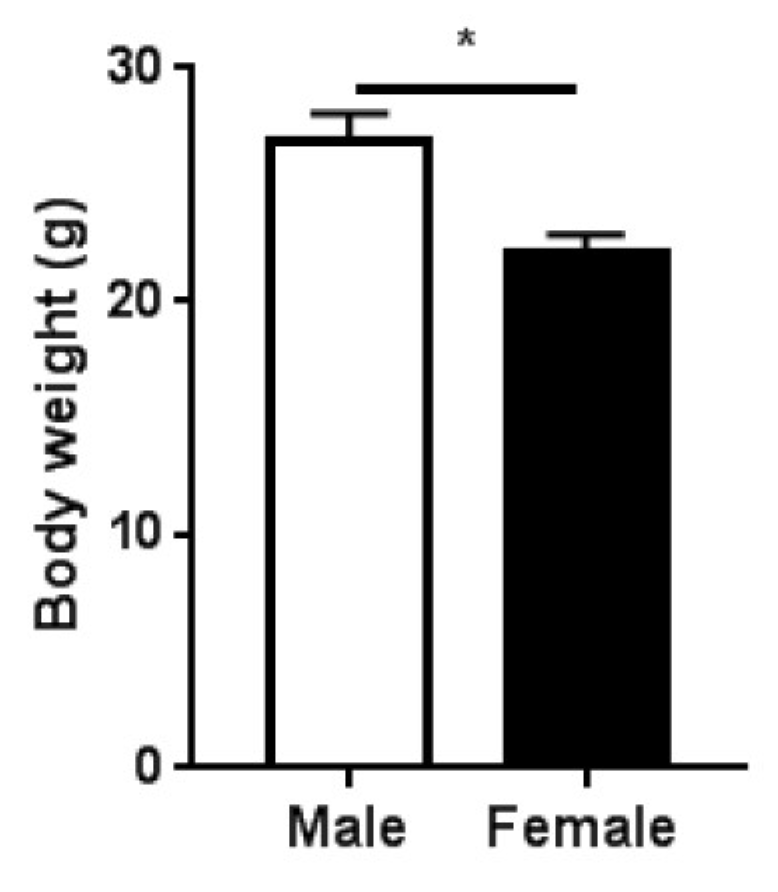
2.1. Study Design and Body Mass
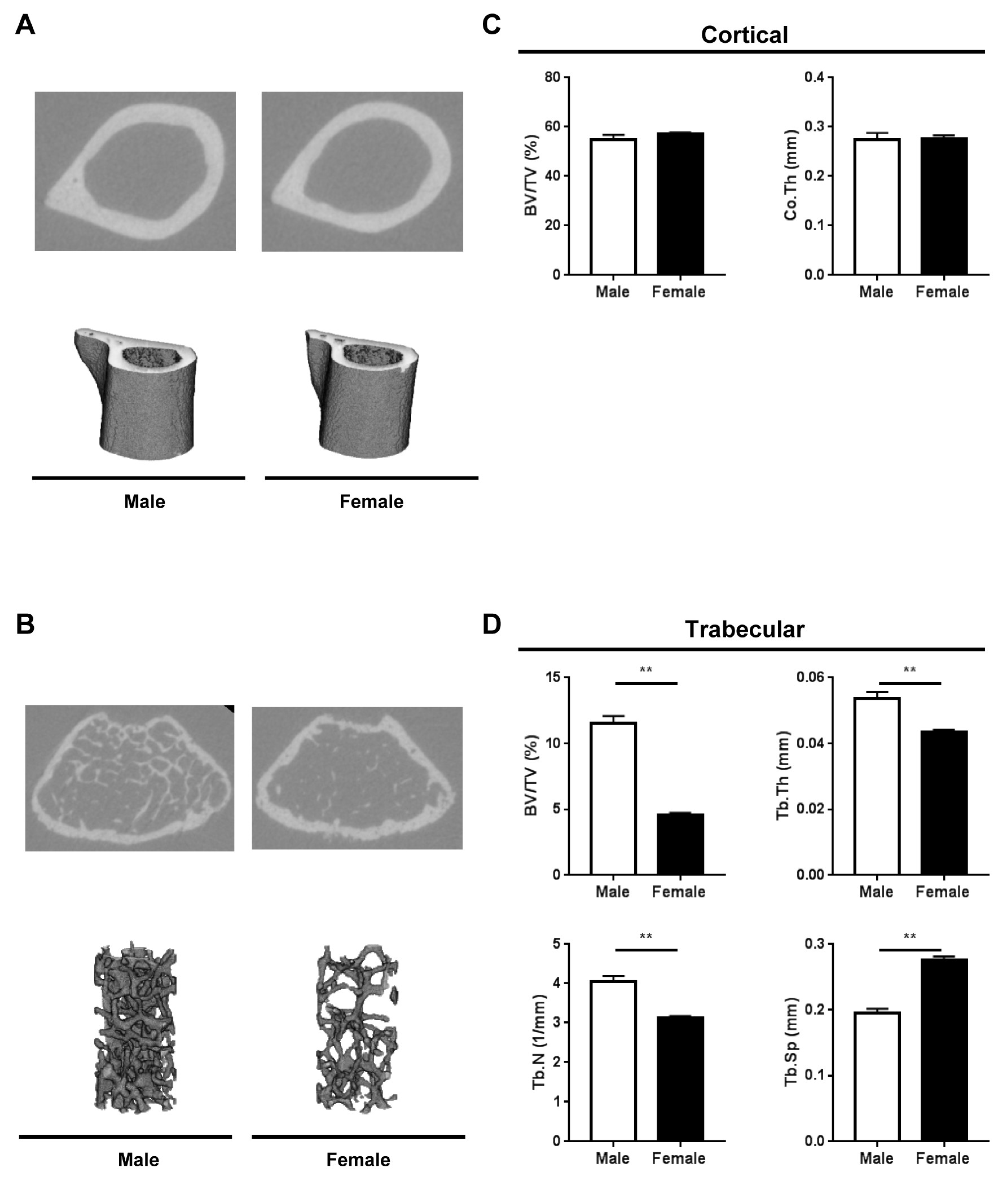
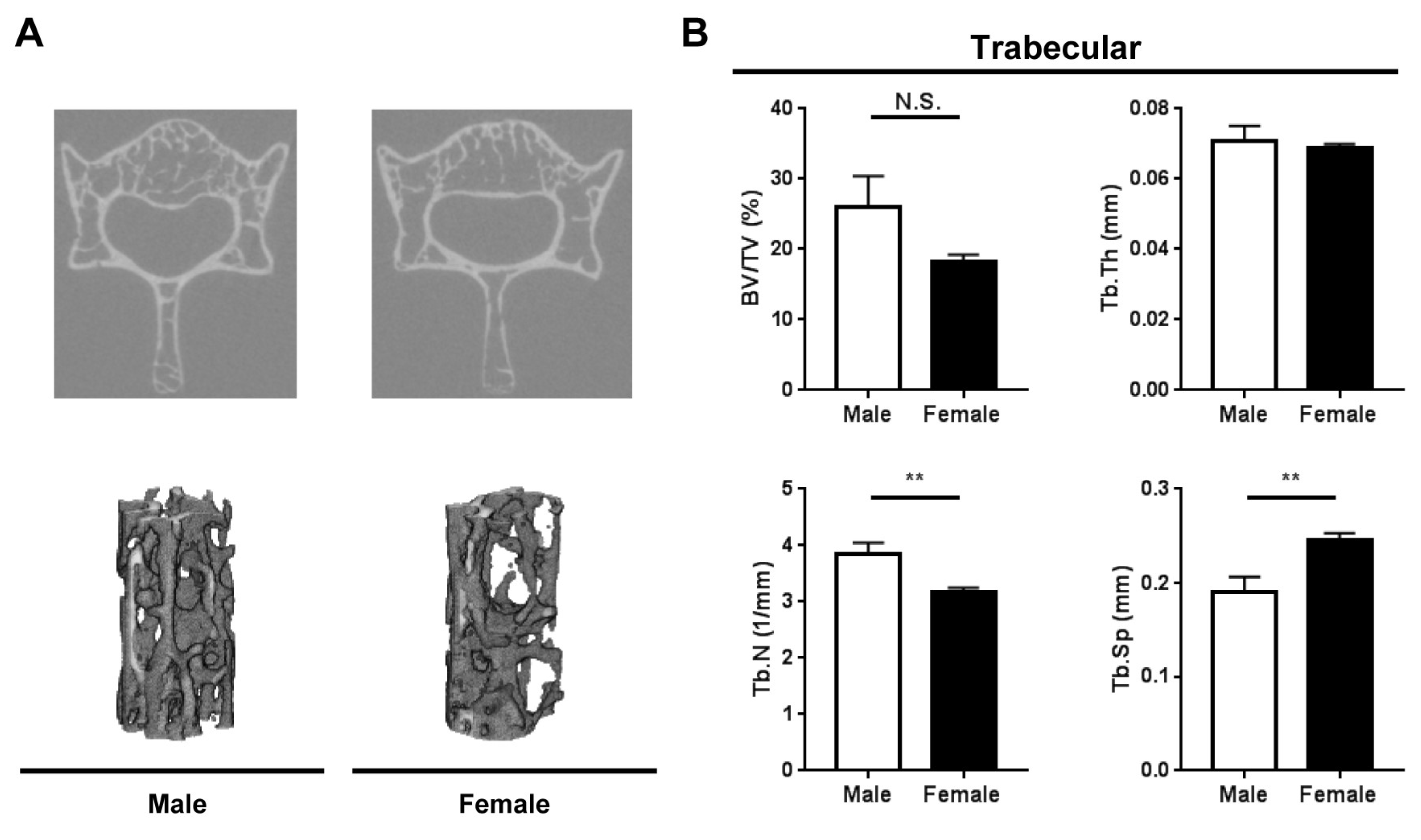
2.2. Bone Microarchitecture
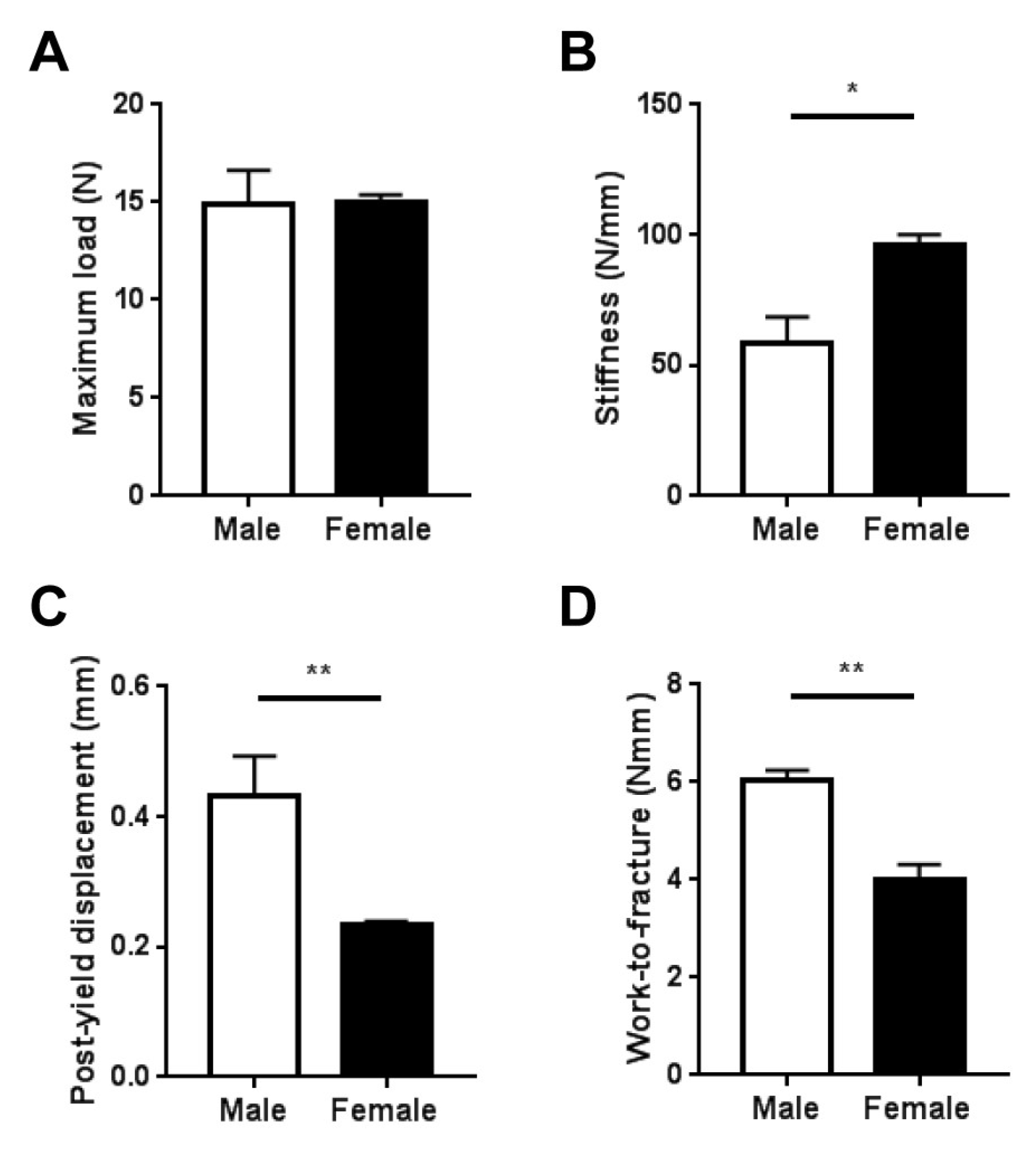
2.3. Whole-Bone Mechanical Properties
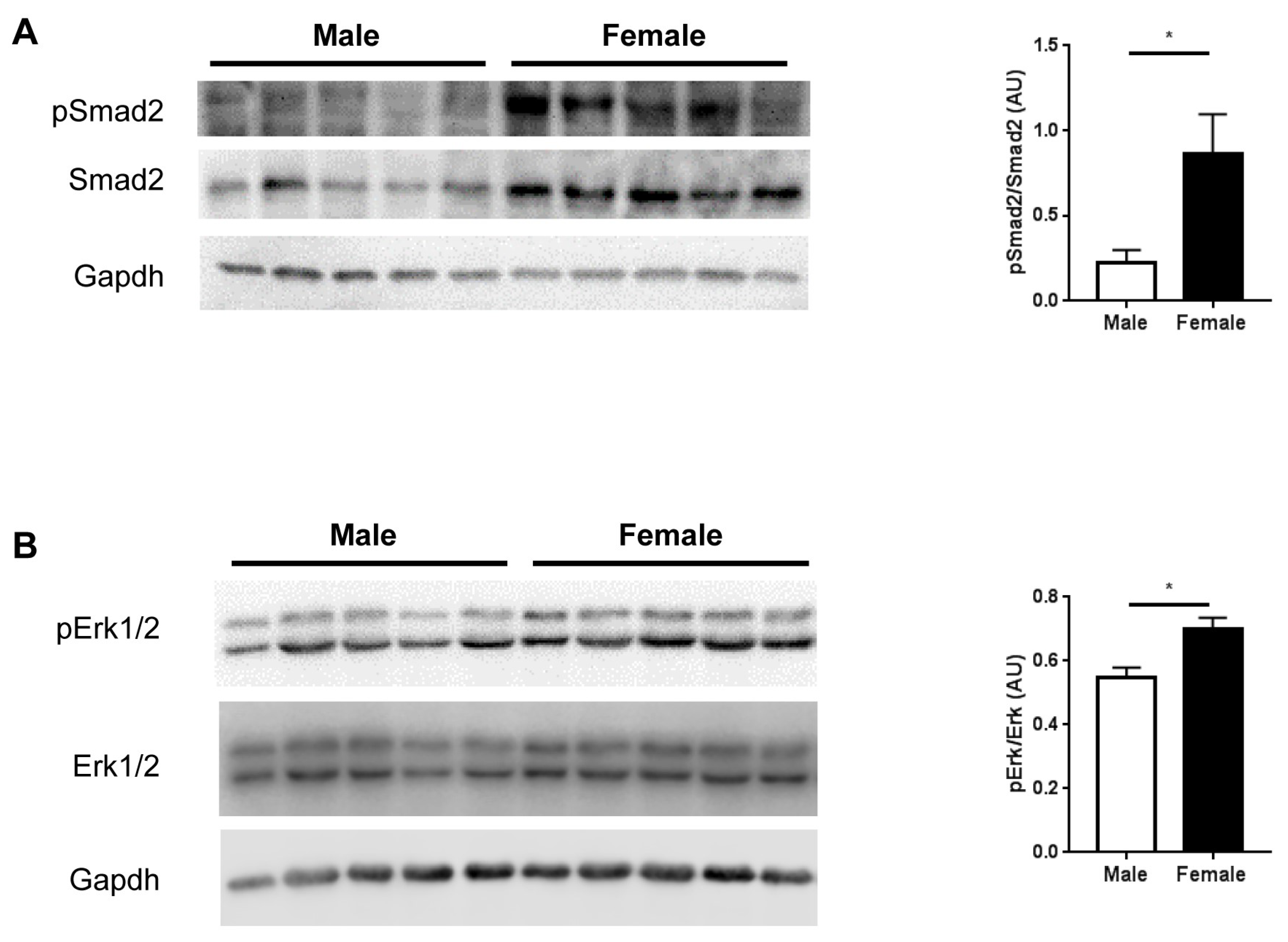
2.4. TGFβ Signaling
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Micro-Computed Tomography
4.3. Biomechanical Measurement
4.4. Immunoblotting
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BMD | bone mineral density |
| MFS | Marfan syndrome |
| μCT | micro-computed tomography |
| TGFβ | transforming growth factor beta |
References
- Ramirez, F.; Sakai, L.Y. Biogenesis and function of fibrillin assemblies. Cell Tissue Res. 2010, 339, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, F.; Caescu, C.; Wondimu, E.; Galatioto, J. Marfan syndrome; A connective tissue disease at the crossroads of mechanotransduction, TGFbeta signaling and cell stemness. Matrix Biol. 2018, 71, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Judge, D.P.; Dietz, H.C. Marfan’s syndrome. Lancet 2005, 366, 1965–1976. [Google Scholar] [CrossRef]
- Ramirez, F.; Carta, L.; Lee-Arteaga, S.; Liu, C.; Nistala, H.; Smaldone, S. Fibrillin-rich microfibrils-structural and instructive determinants of mammalian development and physiology. Connect. Tissue Res. 2008, 49, 1–6. [Google Scholar] [CrossRef]
- Smaldone, S.; Ramirez, F. Fibrillin microfibrils in bone physiology. Matrix Biol. 2016, 52, 191–197. [Google Scholar] [CrossRef]
- Kohlmeier, L.; Gasner, C.; Marcus, R. Bone mineral status of women with Marfan syndrome. Am. J. Med. 1993, 95, 568–572. [Google Scholar] [CrossRef]
- Moura, B.; Tubach, F.; Sulpice, M.; Boileau, C.; Jondeau, G.; Muti, C.; Chevallier, B.; Ounnoughene, Y.; Le Parc, J.M. Bone mineral density in Marfan syndrome. A large case-control study. Jt. Bone Spine 2006, 73, 733–735. [Google Scholar] [CrossRef]
- Carter, N.; Duncan, E.; Wordsworth, P. Bone mineral density in adults with Marfan syndrome. Rheumatology (Oxf.) 2000, 39, 307–309. [Google Scholar] [CrossRef]
- Kohlmeier, L.; Gasner, C.; Bachrach, L.K.; Marcus, R. The bone mineral status of patients with Marfan syndrome. J. Bone Min. Res. 1995, 10, 1550–1555. [Google Scholar] [CrossRef]
- Haine, E.; Salles, J.P.; Khau Van Kien, P.; Conte-Auriol, F.; Gennero, I.; Plancke, A.; Julia, S.; Dulac, Y.; Tauber, M.; Edouard, T. Muscle and Bone Impairment in Children With Marfan Syndrome: Correlation With Age and FBN1 Genotype. J. Bone Min. Res. 2015, 30, 1369–1376. [Google Scholar] [CrossRef]
- Grover, M.; Brunetti-Pierri, N.; Belmont, J.; Phan, K.; Tran, A.; Shypailo, R.J.; Ellis, K.J.; Lee, B.H. Assessment of bone mineral status in children with Marfan syndrome. Am. J. Med. Genet. A 2012, 158, 2221–2224. [Google Scholar] [CrossRef] [PubMed]
- Trifiro, G.; Marelli, S.; Viecca, M.; Mora, S.; Pini, A. Areal bone mineral density in children and adolescents with Marfan syndrome: Evidence of an evolving problem. Bone 2015, 73, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Seeland, U.; Barcena de Arellano, M.L.; Dworatzek, E.; Regitz-Zagrosek, V. Why the study of the effects of biological sex is important. Commentary. Ann. Ist. Super Sanita 2016, 52, 149–150. [Google Scholar] [PubMed]
- Gray, J.R.; Bridges, A.B.; Mole, P.A.; Pringle, T.; Boxer, M.; Paterson, C.R. Osteoporosis and the Marfan syndrome. Postgrad. Med. J. 1993, 69, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Lee, S.Y.; Gayraud, B.; Andrikopoulos, K.; Shapiro, S.D.; Bunton, T.; Biery, N.J.; Dietz, H.C.; Sakai, L.Y.; Ramirez, F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. USA 1999, 96, 3819–3823. [Google Scholar] [CrossRef] [PubMed]
- Nistala, H.; Lee-Arteaga, S.; Carta, L.; Cook, J.R.; Smaldone, S.; Siciliano, G.; Rifkin, A.N.; Dietz, H.C.; Rifkin, D.B.; Ramirez, F. Differential effects of alendronate and losartan therapy on osteopenia and aortic aneurysm in mice with severe Marfan syndrome. Hum. Mol. Genet. 2010, 19, 4790–4798. [Google Scholar] [CrossRef]
- Glatt, V.; Canalis, E.; Stadmeyer, L.; Bouxsein, M.L. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Min. Res. 2007, 22, 1197–1207. [Google Scholar] [CrossRef]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef]
- Chaudhry, S.S.; Cain, S.A.; Morgan, A.; Dallas, S.L.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin-1 regulates the bioavailability of TGFbeta1. J. Cell Biol. 2007, 176, 355–367. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Holm, T.M.; Habashi, J.P.; Doyle, J.J.; Bedja, D.; Chen, Y.; van Erp, C.; Lindsay, M.E.; Kim, D.; Schoenhoff, F.; Cohn, R.D.; et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 2011, 332, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Becher, E.; Mahmoodzadeh, S.; Knosalla, C.; Hetzer, R.; Regitz-Zagrosek, V. Sex-specific modification of progesterone receptor expression by 17beta-oestradiol in human cardiac tissues. Biol. Sex Differ. 2010, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Bito, V.; Tinel, H.; Becher, E.; Baczko, I.; Knosalla, C.; Albrecht-Kupper, B.; Sipido, K.R.; Regitz-Zagrosek, V. Transcriptome characterization of estrogen-treated human myocardium identifies Myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J. Am. Coll. Cardiol. 2012, 59, 410–417. [Google Scholar] [CrossRef]
- Kararigas, G.; Nguyen, B.T.; Zelarayan, L.C.; Hassenpflug, M.; Toischer, K.; Sanchez-Ruderisch, H.; Hasenfuss, G.; Bergmann, M.W.; Jarry, H.; Regitz-Zagrosek, V. Genetic background defines the regulation of postnatal cardiac growth by 17beta-estradiol through a beta-catenin mechanism. Endocrinology 2014, 155, 2667–2676. [Google Scholar] [CrossRef]
- Esslinger, U.; Garnier, S.; Korniat, A.; Proust, C.; Kararigas, G.; Muller-Nurasyid, M.; Empana, J.P.; Morley, M.P.; Perret, C.; Stark, K.; et al. Exome-wide association study reveals novel susceptibility genes to sporadic dilated cardiomyopathy. PLoS ONE 2017, 12, e0172995. [Google Scholar] [CrossRef]
- Gaignebet, L.; Kararigas, G. En route to precision medicine through the integration of biological sex into pharmacogenomics. Clin. Sci. (Lond.) 2017, 131, 329–342. [Google Scholar] [CrossRef]
- Bhushan, R.; Altinbas, L.; Jager, M.; Zaradzki, M.; Lehmann, D.; Timmermann, B.; Clayton, N.P.; Zhu, Y.; Kallenbach, K.; Kararigas, G.; et al. An integrative systems approach identifies novel candidates in Marfan syndrome-related pathophysiology. J. Cell. Mol. Med. 2019, 23, 2526–2535. [Google Scholar] [CrossRef]
- Guo, G.; Booms, P.; Halushka, M.; Dietz, H.C.; Ney, A.; Stricker, S.; Hecht, J.; Mundlos, S.; Robinson, P.N. Induction of macrophage chemotaxis by aortic extracts of the mgR Marfan mouse model and a GxxPG-containing fibrillin-1 fragment. Circulation 2006, 114, 1855–1862. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Jepsen, K.J.; Silva, M.J.; Vashishth, D.; Guo, X.E.; van der Meulen, M.C. Establishing biomechanical mechanisms in mouse models: Practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J. Bone Min. Res. 2015, 30, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Dworatzek, E.; Baczko, I.; Kararigas, G. Effects of aging on cardiac extracellular matrix in men and women. Proteom. Clin. Appl. 2016, 10, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Octavia, Y.; Kararigas, G.; de Boer, M.; Chrifi, I.; Kietadisorn, R.; Swinnen, M.; Duimel, H.; Verheyen, F.K.; Brandt, M.M.; Fliegner, D.; et al. Folic acid reduces doxorubicin-induced cardiomyopathy by modulating endothelial nitric oxide synthase. J. Cell. Mol. Med. 2017, 21, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ruderisch, H.; Queiros, A.M.; Fliegner, D.; Eschen, C.; Kararigas, G.; Regitz-Zagrosek, V. Sex-specific regulation of cardiac microRNAs targeting mitochondrial proteins in pressure overload. Biol. Sex Differ. 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Thai, K.; Scholey, J.W. Angiotensin II type 1 receptor gene polymorphism predicts response to losartan and angiotensin II. Kidney Int. 1999, 56, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, J.; Lehoux, J.; Lanthier, L.; Cabana, J.; Guillemette, G.; Lavigne, P.; Leduc, R.; Escher, E. A single-nucleotide polymorphism of alanine to threonine at position 163 of the human angiotensin II type 1 receptor impairs Losartan affinity. Pharm. Genom. 2010, 20, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, P.F.; Raggio, C.; Davis, J.G. Marfan syndrome: Orthopedic and genetic review. Curr. Opin. Pediatr. 2002, 14, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Huang, C.; Liu, K.; Xu, G.; Yang, J.; Zhou, Y.; Feng, Y.; Kararigas, G.; Geng, B.; Cui, Q. Large-scale in silico identification of drugs exerting sex-specific effects in the heart. J. Transl. Med. 2018, 16, 236. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, W.; Wang, J.; Zhou, Y.; Geng, B.; Kararigas, G.; Yang, J.; Cui, Q. The DrugPattern tool for drug set enrichment analysis and its prediction for beneficial effects of oxLDL on type 2 diabetes. J. Genet. Genom. 2018, 45, 389–397. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altinbas, L.; Bormann, N.; Lehmann, D.; Jeuthe, S.; Wulsten, D.; Kornak, U.; Robinson, P.N.; Wildemann, B.; Kararigas, G. Assessment of Bones Deficient in Fibrillin-1 Microfibrils Reveals Pronounced Sex Differences. Int. J. Mol. Sci. 2019, 20, 6059. https://doi.org/10.3390/ijms20236059
Altinbas L, Bormann N, Lehmann D, Jeuthe S, Wulsten D, Kornak U, Robinson PN, Wildemann B, Kararigas G. Assessment of Bones Deficient in Fibrillin-1 Microfibrils Reveals Pronounced Sex Differences. International Journal of Molecular Sciences. 2019; 20(23):6059. https://doi.org/10.3390/ijms20236059
Chicago/Turabian StyleAltinbas, Lukas, Nicole Bormann, Daniel Lehmann, Sarah Jeuthe, Dag Wulsten, Uwe Kornak, Peter N. Robinson, Britt Wildemann, and Georgios Kararigas. 2019. "Assessment of Bones Deficient in Fibrillin-1 Microfibrils Reveals Pronounced Sex Differences" International Journal of Molecular Sciences 20, no. 23: 6059. https://doi.org/10.3390/ijms20236059
APA StyleAltinbas, L., Bormann, N., Lehmann, D., Jeuthe, S., Wulsten, D., Kornak, U., Robinson, P. N., Wildemann, B., & Kararigas, G. (2019). Assessment of Bones Deficient in Fibrillin-1 Microfibrils Reveals Pronounced Sex Differences. International Journal of Molecular Sciences, 20(23), 6059. https://doi.org/10.3390/ijms20236059




