From Local to Global Modeling for Characterizing Calcium Dynamics and Their Effects on Electrical Activity and Exocytosis in Excitable Cells
Abstract
1. Introduction
2. Results
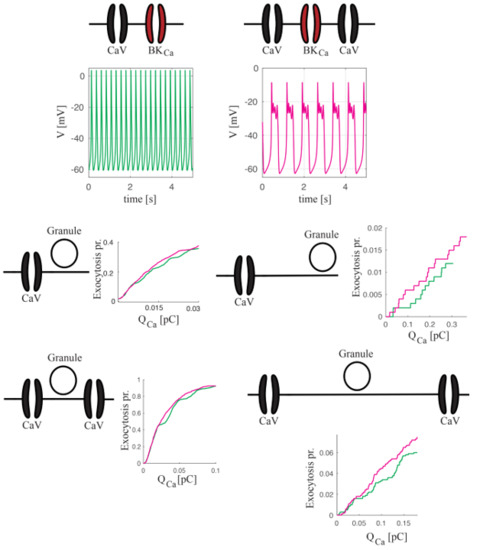
2.1. Whole-Cell Electrical Activity Modeling Respecting Local Control
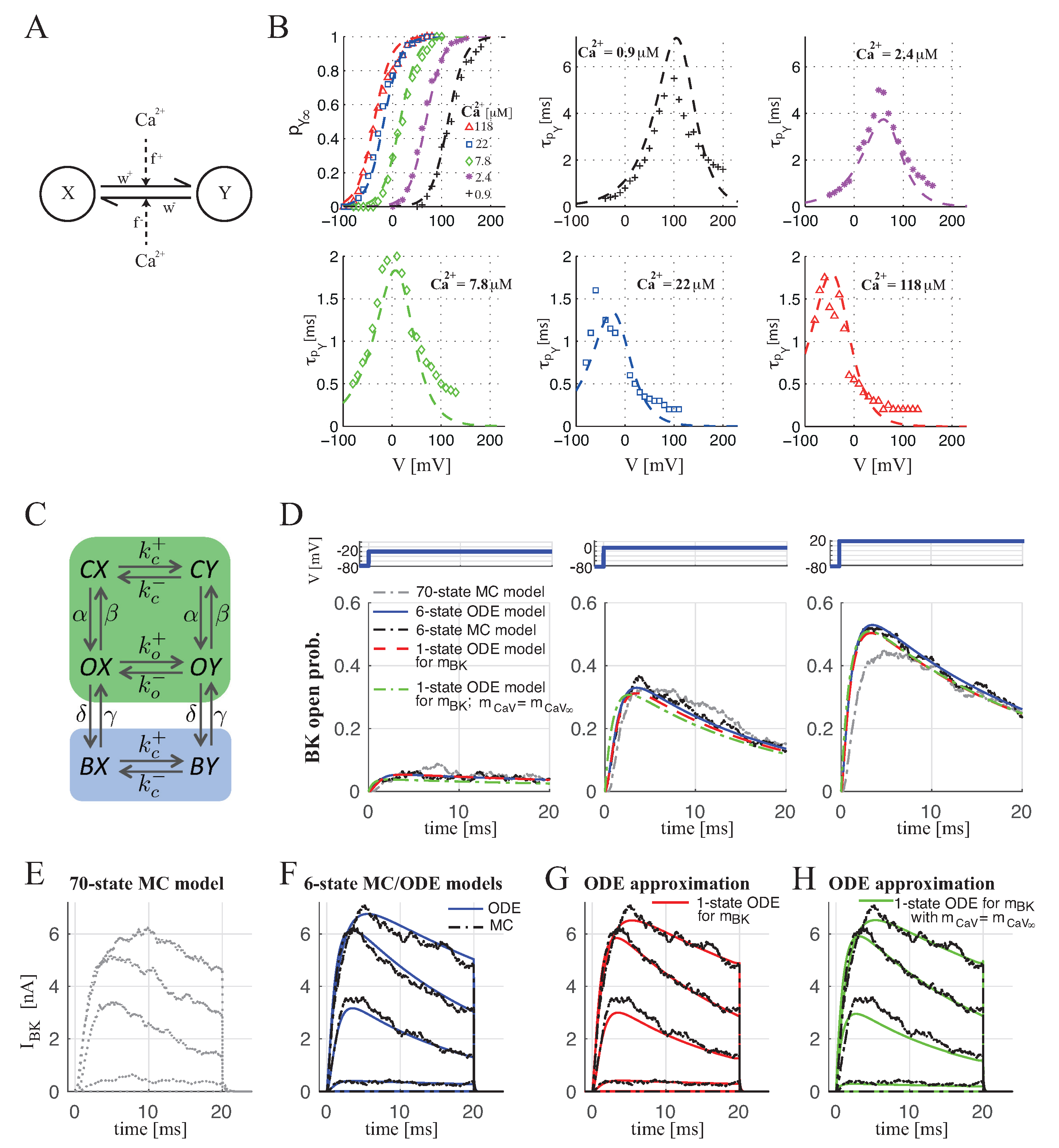
2.1.1. BKCa–CaV Complex with 1:1 Stoichiometry
2.1.2. BKCa–CaV Complex with 1:n Stoichiometry
2.1.3. Concise Whole-Cell Modeling Respecting Local Control
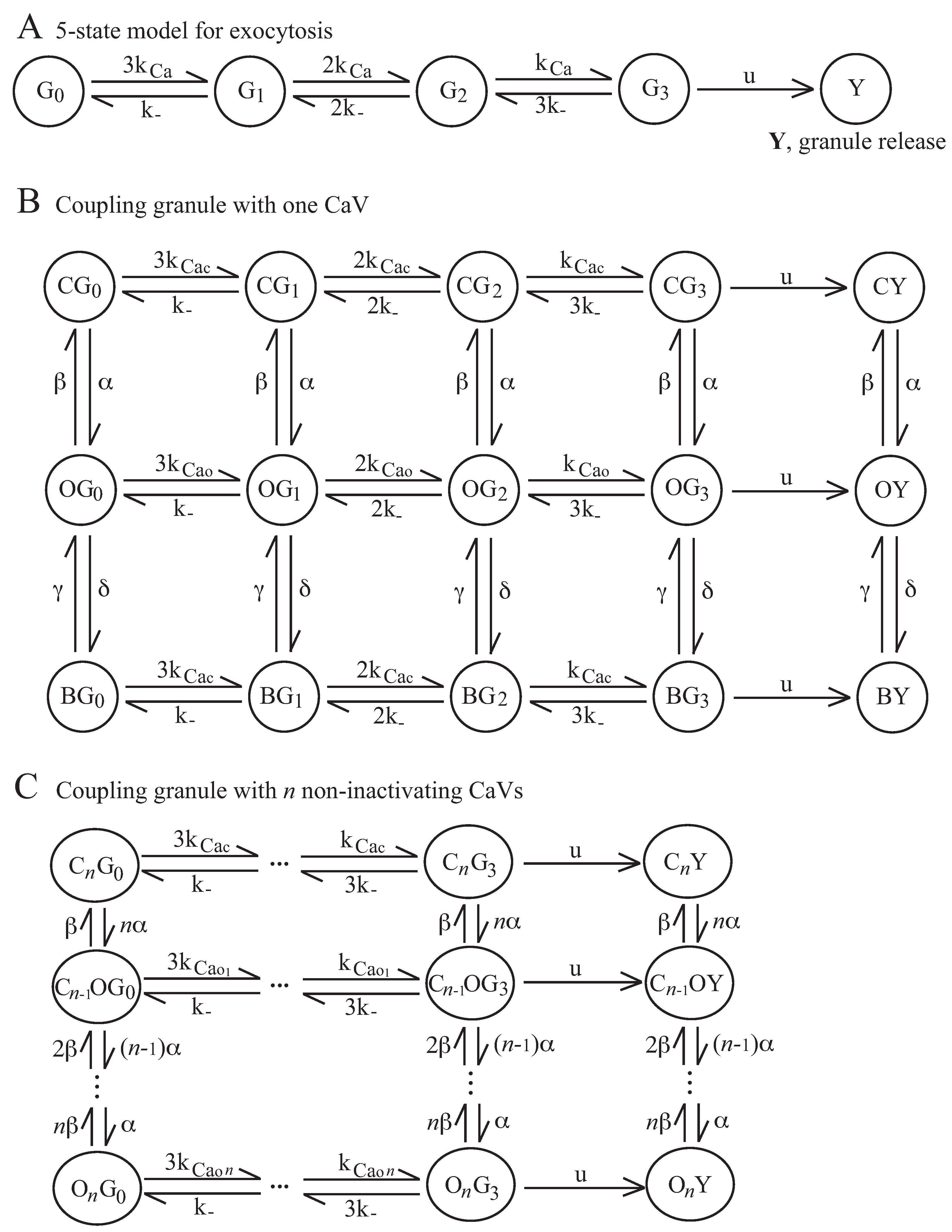
2.2. Coupling Electrical Activity with Exocytosis
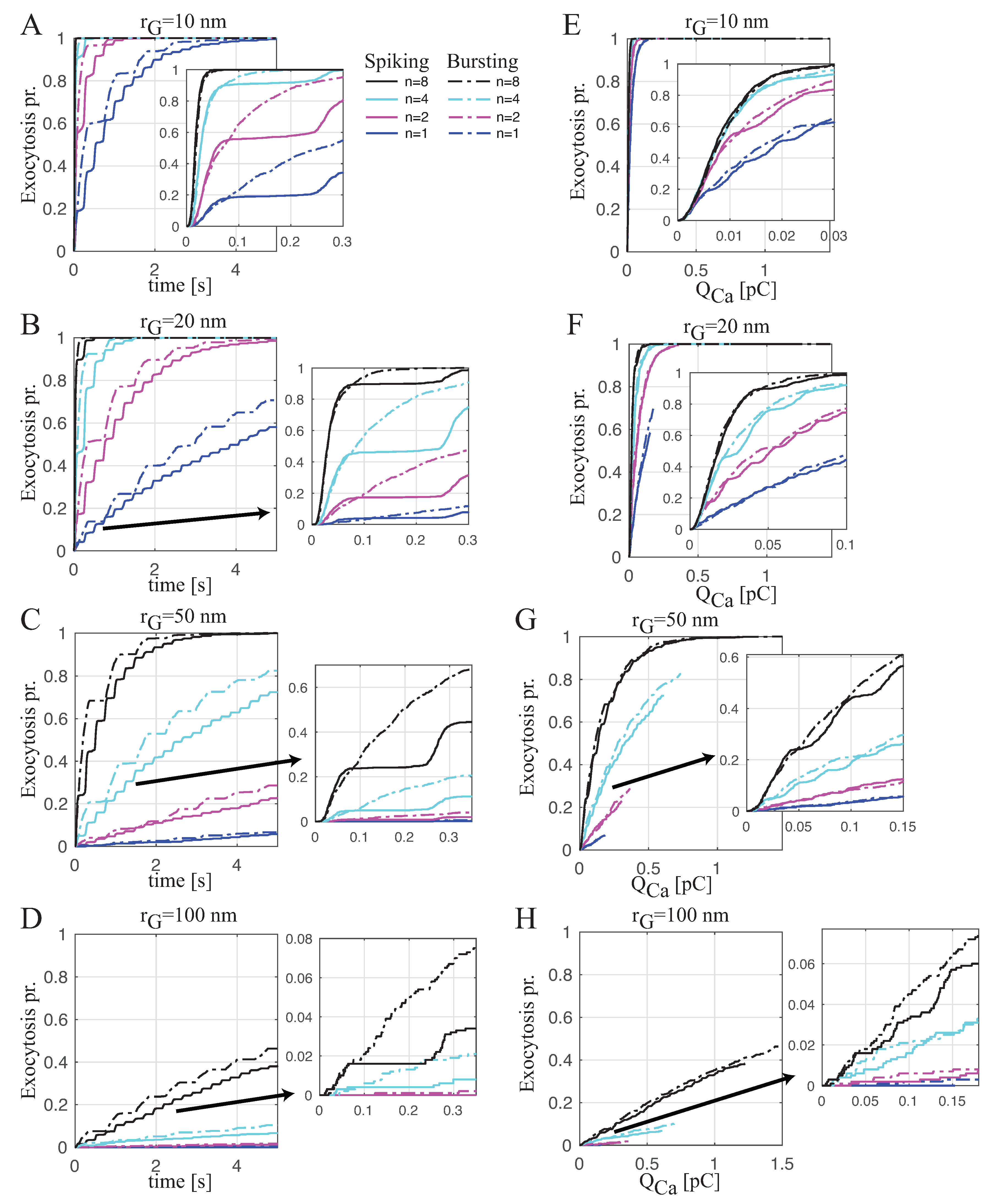
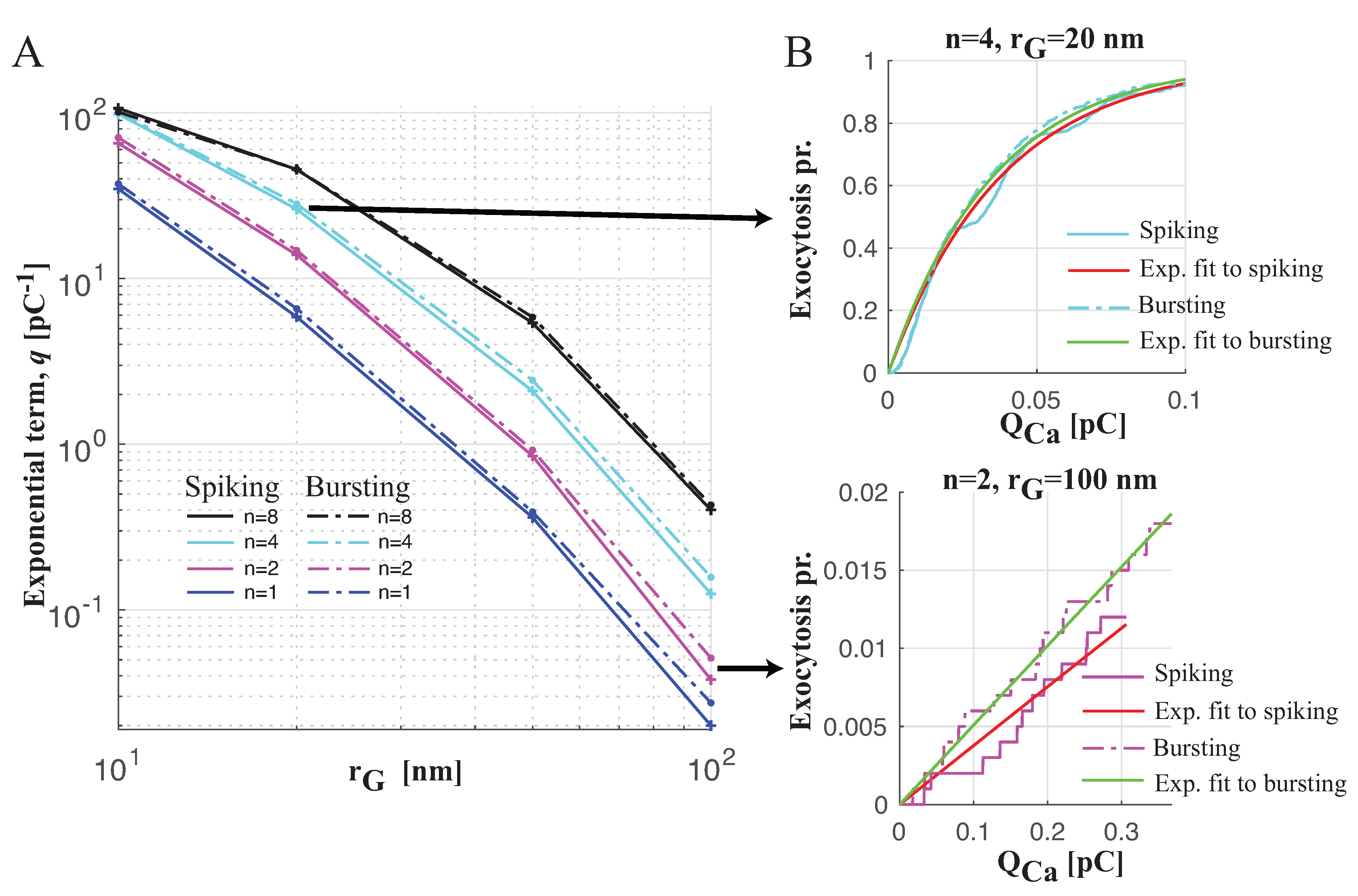
Spiking versus Bursting on Evoking Exocytosis
3. Discussion
4. Methods
4.1. Electrophysiological Model of Pituitary Cells
4.1.1. BKCa Channel Modeling
4.1.2. CaV Modeling
4.1.3. BKCa Channel Open Probability for BKCa–CaV Complex with 1:1 Stoichiometry
4.1.4. BKCa Channel Open Probability for BKCa–CaV Complex with 1:n Stoichiometry
4.2. Exocytosis Model
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]
- Anderson, D.; Mehaffey, W.H.; Iftinca, M.; Rehak, R.; Engbers, J.D.T.; Hameed, S.; Zamponi, G.W.; Turner, R.W. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat. Neurosci. 2010, 13, 333–337. [Google Scholar] [CrossRef]
- Berkefeld, H.; Fakler, B.; Schulte, U. Ca2+-Activated K+ Channels: From Protein Complexes to Function. Physiol. Rev. 2010, 90, 1437–1459. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yamamura, H.; Ohya, S.; Imaizumi, Y. Caveolin-1 Facilitates the Direct Coupling between Large Conductance Ca2+-activated K+(BKCa) and Cav1.2 Ca2+ Channels and Their Clustering to Regulate Membrane Excitability in Vascular Myocytes. J. Biol. Chem. 2013, 288, 36750–36761. [Google Scholar] [CrossRef] [PubMed]
- Chad, J.; Eckert, R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys. J. 1984, 45, 993–999. [Google Scholar] [CrossRef]
- Simon, S.; Llinás, R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys. J. 1985, 48, 485–498. [Google Scholar] [CrossRef]
- Neher, E. Vesicle Pools and Ca2+ Microdomains: New Tools for Understanding Their Roles in Neurotransmitter Release. Neuron 1998, 20, 389–399. [Google Scholar] [CrossRef]
- Fakler, B.; Adelman, J.P. Control of KCa Channels by Calcium Nano/Microdomains. Neuron 2008, 59, 873–881. [Google Scholar] [CrossRef]
- Berkefeld, H.; Fakler, B. Ligand-Gating by Ca2+ Is Rate Limiting for Physiological Operation of BKCa Channels. J. Neurosci. 2013, 33, 7358–7367. [Google Scholar] [CrossRef]
- Cox, D.H. Modeling a Ca2+ Channel/BKCa Channel Complex at the Single-Complex Level. Biophys. J. 2014, 107, 2797–2814. [Google Scholar] [CrossRef][Green Version]
- Buchholz, P.; Kriege, J.; Felko, I. Phase-Type Distributions. In Input Modeling with Phase-Type Distributions and Markov Models; Springer International Publishing: Cham, Switzerland, 2014; pp. 5–28. [Google Scholar] [CrossRef]
- Segel, L.A.; Slemrod, M. The Quasi-Steady-State Assumption: A Case Study in Perturbation. SIAM Rev. 1989, 31, 446–477. [Google Scholar] [CrossRef]
- Montefusco, F.; Tagliavini, A.; Ferrante, M.; Pedersen, M.G. Concise Whole-Cell Modeling of BKCa-CaV Activity Controlled by Local Coupling and Stoichiometry. Biophys. J. 2017, 112, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.S.; Huertas, M.A.; Sobie, E.A.; Jafri, M.S.; Smith, G.D. A Probability Density Approach to Modeling Local Control of Calcium-Induced Calcium Release in Cardiac Myocytes. Biophys. J. 2007, 92, 2311–2328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burgoyne, R.D.; Morgan, A. Secretory Granule Exocytosis. Physiol. Rev. 2003, 83, 581–632. [Google Scholar] [CrossRef]
- Barg, S. Mechanisms of Exocytosis in Insulin-Secreting Beta-Cells and Glucagon-Secreting Alpha-Cells. Pharmacol. Toxicol. 2003, 92, 3–13. [Google Scholar] [CrossRef]
- Jahn, R.; Hanson, P.I. SNAREs line up in new environment. Nature 1998, 393, 14–15. [Google Scholar] [CrossRef]
- Thorn, P.; Zorec, R.; Rettig, J.; Keating, D.J. Exocytosis in non-neuronal cells. J. Neurochem. 2016, 137, 849–859. [Google Scholar] [CrossRef]
- Pedersen, M.G.; Tagliavini, A.; Cortese, G.; Riz, M.; Montefusco, F. Recent advances in mathematical modeling and statistical analysis of exocytosis in endocrine cells. Math. Biosci. 2017, 283, 60–70. [Google Scholar] [CrossRef]
- Bornschein, G.; Schmidt, H. Synaptotagmin Ca2+ Sensors and Their Spatial Coupling to Presynaptic Cav Channels in Central Cortical Synapses. Front. Mol. Neurosci. 2019, 11. [Google Scholar] [CrossRef]
- Gandasi, N.R.; Yin, P.; Riz, M.; Chibalina, M.V.; Cortese, G.; Lund, P.E.; Matveev, V.; Rorsman, P.; Sherman, A.; Pedersen, M.G.; et al. Ca2+ channel clustering with insulin-containing granules is disturbed in type 2 diabetes. J. Clin. Investig. 2017, 127, 2353–2364. [Google Scholar] [CrossRef]
- Montefusco, F.; Pedersen, M.G. Explicit Theoretical Analysis of How the Rate of Exocytosis Depends on Local Control by Ca2+ Channels. Comput. Math. Methods Med. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vivas, O.; Moreno, C.M.; Santana, L.F.; Hille, B. Proximal clustering between BK and CaV1.3 channels promotes functional coupling and BK channel activation at low voltage. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Stojilkovic, S.S. Ca2+-regulated exocytosis and SNARE function. Trends Endocrinol. Metab. TEM 2005, 16, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Tagliavini, A.; Tabak, J.; Bertram, R.; Pedersen, M.G. Is bursting more effective than spiking in evoking pituitary hormone secretion? A spatiotemporal simulation study of calcium and granule dynamics. Am. J. Physiol.-Endocrinol. Metab. 2016, 310, E515–E525. [Google Scholar] [CrossRef]
- Tabak, J.; Toporikova, N.; Freeman, M.E.; Bertram, R. Low dose of dopamine may stimulate prolactin secretion by increasing fast potassium currents. J. Comput. Neurosci. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Berkefeld, H.; Sailer, C.A.; Bildl, W.; Rohde, V.; Thumfart, J.O.; Eble, S.; Klugbauer, N.; Reisinger, E.; Bischofberger, J.; Oliver, D.; et al. BKCa-Cav Channel Complexes Mediate Rapid and Localized Ca2-Activated K+ Signaling. Science 2006, 314, 615–620. [Google Scholar] [CrossRef]
- Roper, P.; Callaway, J.; Shevchenko, T.; Teruyama, R.; Armstrong, W. AHP’s, HAP’s and DAP’s: How Potassium Currents Regulate the Excitability of Rat Supraoptic Neurones. J. Comput. Neurosci. 2003, 15, 367–389. [Google Scholar] [CrossRef]
- Pedersen, M.G. A Biophysical Model of Electrical Activity in Human Beta-Cells. Biophys. J. 2010, 99, 3200–3207. [Google Scholar] [CrossRef]
- Riz, M.; Braun, M.; Pedersen, M.G. Mathematical Modeling of Heterogeneous Electrophysiological Responses in Human Beta-Cells. PLoS Comput. Biol. 2014, 10, e1003389. [Google Scholar] [CrossRef]
- Pallotta, B.S.; Magleby, K.L.; Barrett, J.N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature 1981, 293, 471–474. [Google Scholar] [CrossRef]
- Benton, M.D.; Lewis, A.H.; Bant, J.S.; Raman, I.M. Iberiotoxin-sensitive and -insensitive BK currents in Purkinje neuron somata. J. Neurophysiol. 2013, 109, 2528–2541. [Google Scholar] [CrossRef] [PubMed]
- Storm, J.F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J. Physiol. 1987, 385, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Ramracheya, R.; Bengtsson, M.; Zhang, Q.; Karanauskaite, J.; Partridge, C.; Johnson, P.R.; Rorsman, P. Voltage-Gated Ion Channels in Human Pancreatic Beta-Cells: Electrophysiological Characterization and Role in Insulin Secretion. Diabetes 2008, 57, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Houamed, K.M.; Sweet, I.R.; Satin, L.S. BK channels mediate a novel ionic mechanism that regulates glucose-dependent electrical activity and insulin secretion in mouse pancreatic beta-cells. J. Physiol. 2010, 588, 3511–3523. [Google Scholar] [CrossRef]
- Barg, S.; Ma, X.; Eliasson, L.; Galvanovskis, J.; Göpel, S.O.; Obermüller, S.; Platzer, J.; Renström, E.; Trus, M.; Atlas, D.; et al. Fast exocytosis with few Ca2+ channels in insulin-secreting mouse pancreatic beta-cells. Biophys. J. 2001, 81, 3308–3323. [Google Scholar] [CrossRef]
- Barg, S.; Galvanovskis, J.; Gopel, S.O.; Rorsman, P.; Eliasson, L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes 2000, 49, 1500–1510. [Google Scholar] [CrossRef]
- Voets, T.; Neher, E.; Moser, T. Mechanisms Underlying Phasic and Sustained Secretion in Chromaffin Cells from Mouse Adrenal Slices. Neuron 1999, 23, 607–615. [Google Scholar] [CrossRef]
- Milescu, L.S.; Yamanishi, T.; Ptak, K.; Mogri, M.Z.; Smith, J.C. Real-Time Kinetic Modeling of Voltage-Gated Ion Channels Using Dynamic Clamp. Biophys. J. 2008, 95, 66–87. [Google Scholar] [CrossRef][Green Version]
- Montefusco, F.; Pedersen, M.G. Mathematical modelling of local calcium and regulated exocytosis during inhibition and stimulation of glucagon secretion from pancreatic alpha-cells. J. Physiol. 2015, 593, 4519–4530. [Google Scholar] [CrossRef]
- Latorre, R.; Brauchi, S. Large conductance Ca2+-activated K+ (BK) channel: Activation by Ca2+ and voltage. Biol. Res. 2006, 39, 385–401. [Google Scholar] [CrossRef]
- Cox, D.; Cui, J.; Aldrich, R. Allosteric Gating of a Large Conductance Ca-activated K+ Channel. J. Gen. Physiol. 1997, 110, 257–281. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Rapin, A.M.; Holmstrand, E.C.; Cox, D.H. Elimination of the BKCa Channel’s High-Affinity Ca2+ Sensitivity. J. Gen. Physiol. 2002, 120, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.M.; Zeng, X.; Lingle, C.J. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature 2002, 418, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Yusifov, T.; Savalli, N.; Gandhi, C.S.; Ottolia, M.; Olcese, R. The RCK2 domain of the human BKCa channel is a calcium sensor. Proc. Natl. Acad. Sci. USA 2007, 105, 376–381. [Google Scholar] [CrossRef]
- Horrigan, F.T.; Aldrich, R.W. Allosteric Voltage Gating of Potassium Channels II. J. Gen. Physiol. 1999, 114, 305–336. [Google Scholar] [CrossRef]
- Sherman, A.; Keizer, J.; Rinzel, J. Domain model for Ca2+-inactivation of Ca2+ channels at low channel density. Biophys. J. 1990, 58, 985–995. [Google Scholar] [CrossRef]
- Neher, E. Concentration profiles of intracellular Ca2+ in the presence of diffusible chelator. In Calcium Electrogenesis and Neuronal Functioning, Exp. Brain Res. 14; Springer-Verlag: Berlin, Germany, 1986; pp. 80–96. [Google Scholar]
- Naraghi, M.; Neher, E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 6961–6973. [Google Scholar] [CrossRef]
- Sherman, A.; Smith, G.D.; Dai, L.; Miura, R.M. Asymptotic Analysis of Buffered Calcium Diffusion near a Point Source. SIAM J. Appl. Math. 2001, 61, 1816–1838. [Google Scholar] [CrossRef]
- Matveev, V.; Zucker, R.S.; Sherman, A. Facilitation through Buffer Saturation: Constraints on Endogenous Buffering Properties. Biophys. J. 2004, 86, 2691–2709. [Google Scholar] [CrossRef]
- Boland, L.M.; Bean, B.P. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: Kinetics and voltage dependence. J. Neurosci. Off. J. Soc. Neurosci. 1993, 13, 516–533. [Google Scholar] [CrossRef]
- Weber, A.M.; Wong, F.K.; Tufford, A.R.; Schlichter, L.C.; Matveev, V.; Stanley, E.F. N-type Ca2+ channels carry the largest current: implications for nanodomains and transmitter release. Nat. Neurosci. 2010, 13, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Marcantoni, A.; Vandael, D.H.F.; Mahapatra, S.; Carabelli, V.; Sinnegger-Brauns, M.J.; Striessnig, J.; Carbone, E. Loss of Cav1.3 Channels Reveals the Critical Role of L-Type and BK Channel Coupling in Pacemaking Mouse Adrenal Chromaffin Cells. J. Neurosci. 2010, 30, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Berkefeld, H.; Fakler, B. Repolarizing Responses of BKCa-Cav Complexes Are Distinctly Shaped by Their Cav Subunits. J. Neurosci. 2008, 28, 8238–8245. [Google Scholar] [CrossRef] [PubMed]
- Rehak, R.; Bartoletti, T.M.; Engbers, J.D.T.; Berecki, G.; Turner, R.W.; Zamponi, G.W. Low Voltage Activation of KCa1.1 Current by Cav3-KCa1.1 Complexes. PLoS ONE 2013, 8, e61844. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Kukley, M.; Uebachs, M.; Beck, H.; Dietrich, D. Nanodomains of Single Ca2+ Channels Contribute to Action Potential Repolarization in Cortical Neurons. J. Neurosci. 2007, 27, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.S.; Houy, S.; Sørensen, J.B. C2-domain containing calcium sensors in neuroendocrine secretion. J. Neurochem. 2016, 139, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Voets, T. Dissection of Three Ca2+-Dependent Steps Leading to Secretion in Chromaffin Cells from Mouse Adrenal Slices. Neuron 2000, 28, 537–545. [Google Scholar] [CrossRef]
- Grabner, C.P.; Price, S.D.; Lysakowski, A.; Fox, A.P. Mouse Chromaffin Cells Have Two Populations of Dense Core Vesicles. J. Neurophysiol. 2005, 94, 2093–2104. [Google Scholar] [CrossRef]
- Andersson, S.A.; Pedersen, M.G.; Vikman, J.; Eliasson, L. Glucose-dependent docking and SNARE protein-mediated exocytosis in mouse pancreatic alpha-cell. Pflügers Arch. Eur. J. Physiol. 2011, 462, 443–454. [Google Scholar] [CrossRef]
- Olofsson, C.S.; Göpel, S.O.; Barg, S.; Galvanovskis, J.; Ma, X.; Salehi, A.; Rorsman, P.; Eliasson, L. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic beta-cells. Pflügers Arch. 2002, 444, 43–51. [Google Scholar] [CrossRef]
- Dean, P.M. Ultrastructural morphometry of the pancreatic beta-cell. Diabetologia 1973, 9, 115–119. [Google Scholar] [CrossRef] [PubMed][Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montefusco, F.; Pedersen, M.G. From Local to Global Modeling for Characterizing Calcium Dynamics and Their Effects on Electrical Activity and Exocytosis in Excitable Cells. Int. J. Mol. Sci. 2019, 20, 6057. https://doi.org/10.3390/ijms20236057
Montefusco F, Pedersen MG. From Local to Global Modeling for Characterizing Calcium Dynamics and Their Effects on Electrical Activity and Exocytosis in Excitable Cells. International Journal of Molecular Sciences. 2019; 20(23):6057. https://doi.org/10.3390/ijms20236057
Chicago/Turabian StyleMontefusco, Francesco, and Morten G. Pedersen. 2019. "From Local to Global Modeling for Characterizing Calcium Dynamics and Their Effects on Electrical Activity and Exocytosis in Excitable Cells" International Journal of Molecular Sciences 20, no. 23: 6057. https://doi.org/10.3390/ijms20236057
APA StyleMontefusco, F., & Pedersen, M. G. (2019). From Local to Global Modeling for Characterizing Calcium Dynamics and Their Effects on Electrical Activity and Exocytosis in Excitable Cells. International Journal of Molecular Sciences, 20(23), 6057. https://doi.org/10.3390/ijms20236057