Amoxicillin Modulates ApoA-I Transcription and Secretion, Predominantly via PPARα Transactivation Inhibition
Abstract
:1. Introduction
2. Results
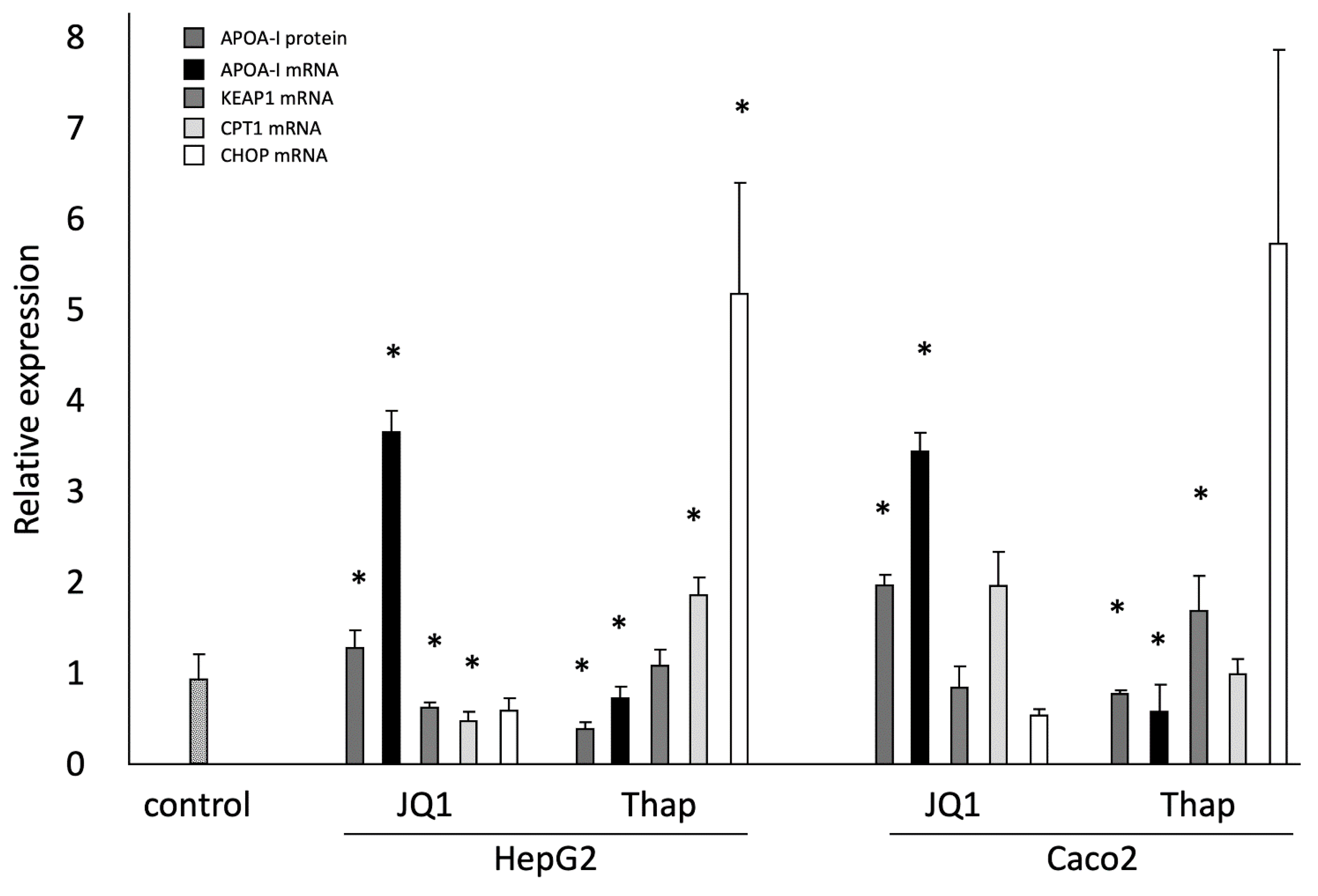
2.1. Effects of JQ1(+) and Thap as Controls on ApoA-I Gene Expression; ApoA-I Protein Secretion; and KEAP1, CPT1, and CHOP Gene Expressions in HepG2 and Caco-2 Cells
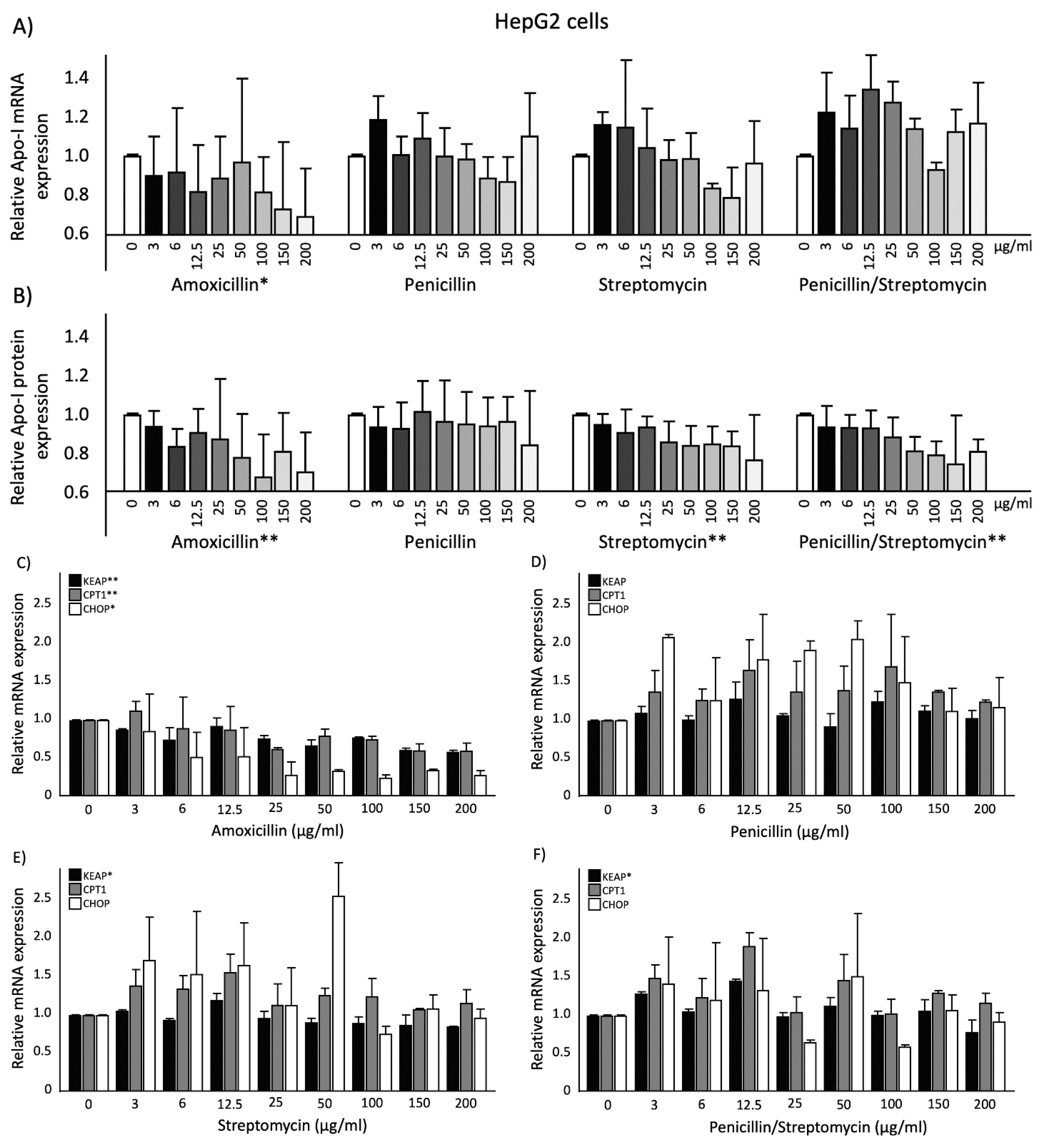
2.2. Effects of Antibiotics on ApoA-I Gene Expression and Protein Secretion in HepG2 Cells
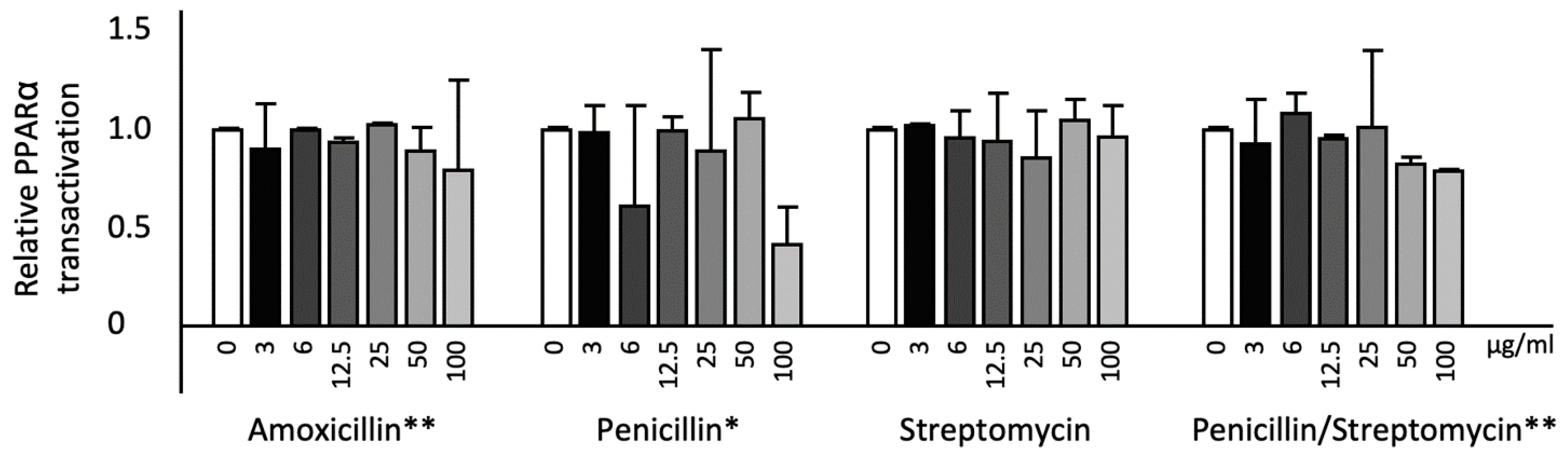
2.3. Effects of Antibiotics on KEAP1, CPT1, and CHOP Gene Expression and PPARα Transactivation in HepG2cells
2.4. Effects of Antibiotics on ApoA-I Gene Expression and Protein Secretion in Caco-2 Cells
2.5. Effects of Antibiotics on KEAP1, CPT1, and CHOP Gene Expression in Caco-2 Cells
3. Discussion
4. Material and methods
4.1. Materials
4.2. Cell Culture and Antibiotics Treatment
4.3. ApoA-I Protein Concentration in Cell Culture Medium
4.4. mRNA Expression Quantification
4.5. Luciferase Assay
4.6. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- WHO. 19th WHO Model List of Essential Medicine. 2015. Available online: https://www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf (accessed on 8 October 2019).
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Danese, E.; Mattiuzzi, C.; Favaloro, E.J. The Intriguing link between the Intestinal microbiota and cardiovascular disease. Semin. Thromb. Hemost. 2017, 43, 609–613. [Google Scholar] [PubMed]
- Battson, M.L.; Lee, D.M.; Weir, T.L.; Gentile, C.L. The gut microbiota as a novel regulator of cardiovascular function and disease. J. Nutr. Biochem. 2018, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, H. The role of gut microbiota in atherosclerosis and hypertension. Front. Pharmacol. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-Oxide: A link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef]
- De Smet, E. Plant Stanol Esters: Focus on Intestinal Lipoprotein Metabolism. Ph.D. Thesis, Maastricht University, Maastricht, The Netherlands, 2014. Available from BOXPress. [Google Scholar]
- Khera, A.V.; Cuchel, M.; De La Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef]
- Du, Y.; Wang, L.; Si, S.; Yang, Y.; Hong, B. A novel compound 4010B-30 upregulates apolipoprotein A-I gene expression through activation of PPARgamma in HepG2 cells. Atherosclerosis 2015, 239, 589–598. [Google Scholar] [CrossRef]
- Smolders, L.; Plat, J.; Mensink, R.P. Dietary strategies and novel pharmaceutical approaches targeting serum ApoA-I metabolism. J. Nutr. Metab. 2017, 2017, 5415921. [Google Scholar] [CrossRef]
- Shi, J.; Vakoc, C.R. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol. Cell 2014, 54, 728–736. [Google Scholar] [CrossRef]
- Martin, G.; Duez, H.; Blanquart, C.; Berezowski, V.; Poulain, P.; Fruchart, J.C.; Najib-Fruchart, J.; Glineur, C.; Staels, B. Statin-induced inhibition of the Rho-signaling pathway activates PPARalpha and induces HDL apoA-I. J. Clin. Investig. 2001, 107, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Q.; Chen, Z.; Chen, L.X. Endoplasmic reticulum stress: A novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol. Sin. 2016, 37, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Van der Krieken, S.E.; Popeijus, H.E.; Mensink, R.P.; Plat, J. Link between ER-stress, PPAR-alpha activation, and BET inhibition in relation to apolipoprotein A-I transcription in HepG2 cells. J. Cell. Biochem. 2017, 118, 2161–2167. [Google Scholar] [CrossRef]
- Etebu, E.; Arikekpar, I. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. Int. J. Appl. Microbiol. Biotechnol. Res. 2016, 4, 90–101. [Google Scholar]
- Mandell, L.A.; Ball, P.; Tillotson, G. Antimicrobial safety and tolerability: Differences and dilemmas. Clin. Infect. Dis. 2001, 32, S72–S79. [Google Scholar] [CrossRef]
- Salvo, F.; De Sarro, A.; Caputi, A.P.; Polimeni, G. Amoxicillin and amoxicillin plus clavulanate: A safety review. Expert Opin. Drug Saf. 2009, 8, 111–118. [Google Scholar] [CrossRef]
- Bhattacharya, S. The facts about penicillin allergy. J. Adv. Pharm. Technol. Res. 2010, 1, 11–17. [Google Scholar]
- Crofton, J. The MRC randomized trial of streptomycin and its legacy: A view from the clinical front line. J. R. Soc. Med. 2006, 99, 531–534. [Google Scholar] [CrossRef]
- Al-Jowari, S. Comparative investigation of amoxicillin and cephalexin administration on some biochemical parameters in male rabbits. IJSR 2018, 7, 1481–1484. [Google Scholar]
- Sato, H.; Zhang, L.S.; Martinez, K.; Chang, E.B.; Yang, Q.; Wang, F.; Howles, P.N.; Hokari, R.; Miura, S.; Tso, P. Antibiotics suppress activation of intestinal mucosal mast cells and reduce dietary lipid absorption in Sprague-Dawley rats. Gastroenterology 2016, 5, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Jahagirdar, R.; Zhang, H.; Azhar, S.; Tobin, J.; Attwell, S.; Yu, R.; Wu, J.; McLure, K.G.; Hansen, H.C.; Wagner, G.S. A novel BET bromodomain inhibitor, RVX-208, shows reduction of atherosclerosis in hyperlipidemic ApoE deficient mice. Atherosclerosis 2014, 236, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kempen, H.J.; Bellus, D.; Fedorov, O.; Nicklisch, S.; Filippakopoulos, P.; Picaud, S.; Knapp, S. Stimulation of hepatic apolipoprotein A-I production by novel thieno-triazolodiazepines: Roles of the classical benzodiazepine receptor, PAF receptor, and bromodomain binding. Lipid Insights 2013, 6, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hussong, M.; Börno, S.; Kerick, M.; Wunderlich, A.; Franz, A.; Sültmann, H.; Timmermann, B.; Lehrach, H.; Hirsch-Kauffmann, M.; Schweiger, M. The bromodomain protein BRD4 regulates the KEAP1/NRF2-dependent oxidative stress response. Cell Death Dis. 2014, 5, e1195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Lawson, B.; Brewer, J.W.; Zinszner, H.; Sanjay, A.; Mi, L.J.; Boorstein, R.; Kreibich, G.; Hendershot, L.M.; Ron, D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol. Cell. Biol. 1996, 16, 4273–4280. [Google Scholar] [CrossRef]
- Naem, E.; Haas, M.J.; Wong, N.C.; Mooradian, A.D. Endoplasmic reticulum stress in HepG2 cells inhibits apolipoprotein A-I secretion. Life Sci. 2013, 92, 72–80. [Google Scholar] [CrossRef]
- Guillemot, D.; Carbon, C.; Balkau, B.; Geslin, P.; Lecoeur, H.; Vauzelle-Kervroëdan, F.; Bouvenot, G.; Eschwége, E. Low dosage and long treatment duration of β-lactam: Risk factors for carriage of penicillin-resistant streptococcus pneumoniae. JAMA 1998, 279, 365–370. [Google Scholar] [CrossRef]
- Okamoto, T.; Yoshiyama, H.; Nakazawa, T.; Park, I.D.; Chang, M.W.; Yanai, H.; Okita, K.; Shirai, M. A change in PBP1 is involved in amoxicillin resistance of clinical isolates of Helicobacter pylori. J. Antimicrob. Chemother. 2002, 50, 849–856. [Google Scholar] [CrossRef]
- Adesanoye, O.; Ifezue, A.; Farombi, E. Influence of chloramphenicol and amoxicillin on rat liver microsomal enzymes and lipid peroxidation. Afr. J. Biomed. Res. 2014, 17, 135–142. [Google Scholar]
- Neal, M.J. Medical Pharmacology at a Glance, 7th ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 82–83. [Google Scholar]
- Amigó, N.; Mallol, R.; Heras, M.; Martínez-Hervás, S.; Blanco-Vaca, F.; Escolà-Gil, J.C.; Plana, N.; Yanes, Ó.; Masana, L.; Correig, X. Lipoprotein hydrophobic core lipids are partially extruded to surface in smaller HDL: “Herniated” HDL, a common feature in diabetes. Sci. Rep. 2016, 6, 19249. [Google Scholar] [CrossRef]
- Pan, C.; Kumar, C.; Bohl, S.; Klingmueller, U.; Mann, M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol. Cell. Proteom. 2009, 8, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Mohammadi, M.H.; Rafatian, N.; Toroghi, M.K.; Wang, E.Y.; Zhao, Y.; Korolj, A.; Ahadian, S.; Radisic, M. Cardiovascular disease models: A game changing paradigm in drug discovery and screening. Biomaterials 2019, 198, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. 2005, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- DrugBank. Amoxicillin. 2005. Available online: https://www.drugbank.ca/drugs/DB01060 (accessed on 8 October 2019).
- DrugBank. Benzylpenicillin. 2005. Available online: https://www.drugbank.ca/drugs/DB01053 (accessed on 8 October 2019).
- DrugBank. Streptomycin. 2005. Available online: https://www.drugbank.ca/drugs/DB01082 (accessed on 8 October 2019).
- Sinicina, I.; Pankratz, H.; Büttner, A.; Mall, G. Death due to neurogenic shock following gastric rupture in an anorexia nervosa patient. Forensic Sci. Int. 2005, 155, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, A.; Guttmann, J.; Gonzalez, G.; Gonzalez, C. Absorption and disposition kinetics of amoxicillin in normal human subjects. Antimicrob. Agents Chemother. 1980, 17, 199–202. [Google Scholar] [CrossRef]
- Tuano, S.B.; Johnson, L.D.; Brodie, J.L.; Kirby, W.M. Comparative blood levels of hetacillin, ampicillin and penicillin G. N. Engl. J. Med. 1966, 275, 635–639. [Google Scholar] [CrossRef]
- Dailymed. Streptomycin Injection, Powder, lyophilized, for Solution. 2009. Available online: https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=2439 (accessed on 8 October 2019).
- Popeijus, H.E.; van Otterdijk, S.D.; van der Krieken, S.E.; Konings, M.; Serbonij, K.; Plat, J.; Mensink, R.P. Fatty acid chain length and saturation influences PPARα transcriptional activation and repression in HepG2 cells. Mol. Nutr. Food Res. 2014, 58, 2342–2349. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayyeb, J.Z.; Popeijus, H.E.; Mensink, R.P.; Konings, M.C.J.M.; Mulders, K.H.R.; Plat, J. Amoxicillin Modulates ApoA-I Transcription and Secretion, Predominantly via PPARα Transactivation Inhibition. Int. J. Mol. Sci. 2019, 20, 5967. https://doi.org/10.3390/ijms20235967
Tayyeb JZ, Popeijus HE, Mensink RP, Konings MCJM, Mulders KHR, Plat J. Amoxicillin Modulates ApoA-I Transcription and Secretion, Predominantly via PPARα Transactivation Inhibition. International Journal of Molecular Sciences. 2019; 20(23):5967. https://doi.org/10.3390/ijms20235967
Chicago/Turabian StyleTayyeb, Jehad Z., Herman E. Popeijus, Ronald P. Mensink, Maurice C.J.M. Konings, Kim H.R. Mulders, and Jogchum Plat. 2019. "Amoxicillin Modulates ApoA-I Transcription and Secretion, Predominantly via PPARα Transactivation Inhibition" International Journal of Molecular Sciences 20, no. 23: 5967. https://doi.org/10.3390/ijms20235967
APA StyleTayyeb, J. Z., Popeijus, H. E., Mensink, R. P., Konings, M. C. J. M., Mulders, K. H. R., & Plat, J. (2019). Amoxicillin Modulates ApoA-I Transcription and Secretion, Predominantly via PPARα Transactivation Inhibition. International Journal of Molecular Sciences, 20(23), 5967. https://doi.org/10.3390/ijms20235967






