Abstract
Plant-growth-promoting bacteria (PGPB) are beneficial microorganisms that can also protect against disease and environmental stress. Silicon (Si) is the second most abundant element in soil, and is known to increase plant growth, grain yield, resistance to biotic stress, and tolerance to abiotic stress. Combined treatment of PGPB and Si has been shown to further enhance plant growth and crop yield. To determine the global effects of the PGPB and Si on rice growth, we compared rice plants treated with Paenibacillus yonginensis DCY84T (DCY84T) and Si with untreated rice. To identify the genes that respond to DCY84T+Si treatment in rice, we performed an RNA-Seq transcriptome analysis by sampling treated and untreated roots on a weekly basis for three weeks. Overall, 576 genes were upregulated, and 394 genes were downregulated in treated roots, using threshold fold-changes of at least 2 (log2) and p-values < 0.05. Gene ontology analysis showed that phenylpropanoids and the L-phenylalanine metabolic process were prominent in the upregulated genes. In a metabolic overview analysis using the MapMan toolkit, pathways involving phenylpropanoids and ethylene were strongly associated with upregulated genes. The functions of seven upregulated genes were identified as being associated with drought stress through a literature search, and a stress experiment confirmed that plants treated with DCY84T+Si exhibited greater drought tolerance than the untreated control plants. Furthermore, the predicted protein–protein interaction network analysis associated with DCY84T+ Si suggests mechanisms underlying growth promotion and stress tolerance.
1. Introduction
Modern intensive agriculture depends on synthetic chemical fertilizers containing essential plant nutrients such as nitrogen, phosphorus, and potassium [1,2]. However, excessive use of such fertilizers leads to the accumulation of insoluble phosphates in the soil, and also results in ecosystem disturbances and environmental pollution [3,4]. Recently, the inoculation of plant-growth-promoting bacteria (PGPB) into crops has been shown to improve crop growth and increase resistance from various environmental stresses [5]. In addition, PGPB have been reported to promote plant growth through mechanisms such as biological nitrogen fixation, phosphate solubilization, plant hormone regulation, and siderophore production [5,6,7]. PGPB, which are beneficial microorganisms found primarily on the root surface (“rhizobacteria”) that can also protect against disease and environmental stresses [8,9].
The genus Paenibacillus is a well-known member of the PGPB group, together with Acetobacter, Azotobacter, Burkholderia, and Pseudomonas [10]. To date, about 150 species of Paenibacillus have been identified (http://www.bacterio.net/paenibacillus.html); they are widespread, having been isolated from alkaline soil, ginseng field soil, rice field soil, and gamma-irradiated Antarctic soil [11,12,13,14]. Paenibacillus yonginensis DCY84T (DCY84T) was found in the humus soil of Yongin forest in Gyeonggi-do, South Korea. It is a Gram-positive, rod-shaped, aerobic, spore-forming bacterium that is motile by means of peritrichous flagella [15]. In addition, DCY84T has been reported to not only promote plant growth, but also to protect plants from biotic and abiotic stresses. For example, Arabidopsis treated with DCY84T reportedly exhibited increased tolerance to salinity, drought, and heavy metal stresses [16].
Silicon (Si), the second most abundant element in soil [17], is classified as a “quasi-essential element for plant growth”, and has been reported to increase growth, grain yield, resistance to pathogens, and tolerance to abiotic stresses [18,19,20]. For example, Si-treated rice exhibited increased resistance to diseases such as leaf blast, sheath blight, brown spot, and stem rot [21]. Rice is a Si-hyperaccumulating species that absorbs Si in the form of silicic acid (H4SiO4) from the soil through the roots, and can accumulate Si up to 10% of shoot dry weight [22]. This makes Si an important limiting factor for rice production, and a supply of exogenous Si is necessary for stable rice production systems [23,24].
In previous studies, the contribution of DCY84T-treated seeds, Si-coated seeds, and DCY84T+Si-treated seeds to rice growth was analyzed by phenotypic observations [25]. At 60 days after sowing (DAS), rice seedlings from seeds treated with DCY84T+Si had a higher shoot length and an increased total fresh and dry weight compared to those with DCY84T-treated seedlings, Si-coated seedlings, and mock-inoculated seedlings [25]. More interestingly, DCY84T-treated seeds or Si-coated seeds did not significantly affect fertility percentage, whereas DCY84T+Si had a significantly higher number of spikelets per panicle, and increased grain yield up to 70% than mock-inoculated seedlings [25]. These results support the fact that the increase of plant growth and grain yield was maximized when DCY84T and Si were used together. Although a transcriptome analysis was previously performed by treating Si in rice roots, finding an improvement of suberization and lignification by this treatment [26], further transcriptome analysis on the role of combined DCY84T and Si treatment in early plant growth would be very useful for future applications to enhance crop yield.
In the current study, we performed whole-transcriptome shotgun sequencing (RNA-Seq) analysis on total RNA extracted from roots from control and DCY84T+Si-treated seedlings to investigate the effect of the treatment on plant growth. Through this analysis, we identified 576 and 394 genes which were significantly up- and down-regulated, respectively, in response to the combined treatment. Gene ontology (GO) enrichment analysis, MapMan analysis, and analysis of rice genes with known functions were carried out for these candidate genes. Here, we describe and discuss the effect of combined DCY84T and Si treatment which have improved initial growth and increased resistance to environmental stresses.
2. Results and Discussion
2.1. Combined DCY84T and Si Treatment Stimulates Root Growth of Rice Plants
We compared various traits of rice seedlings grown from untreated seed and from seed subjected to DCY84T+Si treatment. Seedlings from treated rice seeds (Oryza sativa, japonica variety Chilbo), at 3 d after germination, produced plumules and radicles that were significantly longer than those from control seeds (Figure S1). Thereafter, the plants grown from the two types of seeds were monitored for four weeks at intervals of one week (Table 1; Figure S1). A statistical analysis showed that at one week after sowing, plants treated with combined DCY84T+Si increased both length and dry weight in root and leaf compared to control plants. However, at two weeks after sowing, all significant differences by the combined treatment disappeared except for root length, which was also lost at three weeks after sowing. On the other hand, the mean root dry weight of the DCY84T+Si-treated plants at three weeks after sowing was 20% heavier than that of the control (Table 1). These results are very similar to our previous measurements of root length and dry weight in paddy fields [25]. In addition, rice seedlings at three weeks after sowing consumed most of nutrients in the endosperm of germinating seeds, and more stably adapted to the environment. Therefore, we performed RNA-Seq analysis by sampling the control and DCY84T+Si-treated roots from seedlings at 21 DAS.

Table 1.
Comparison of the length and dry weight of the leaves and roots between normal conditions and both DCY84T and Si treatments.
2.2. RNA-Seq Analysis Identified Global Candidate Genes Associated with DCY84T+Si Treatment
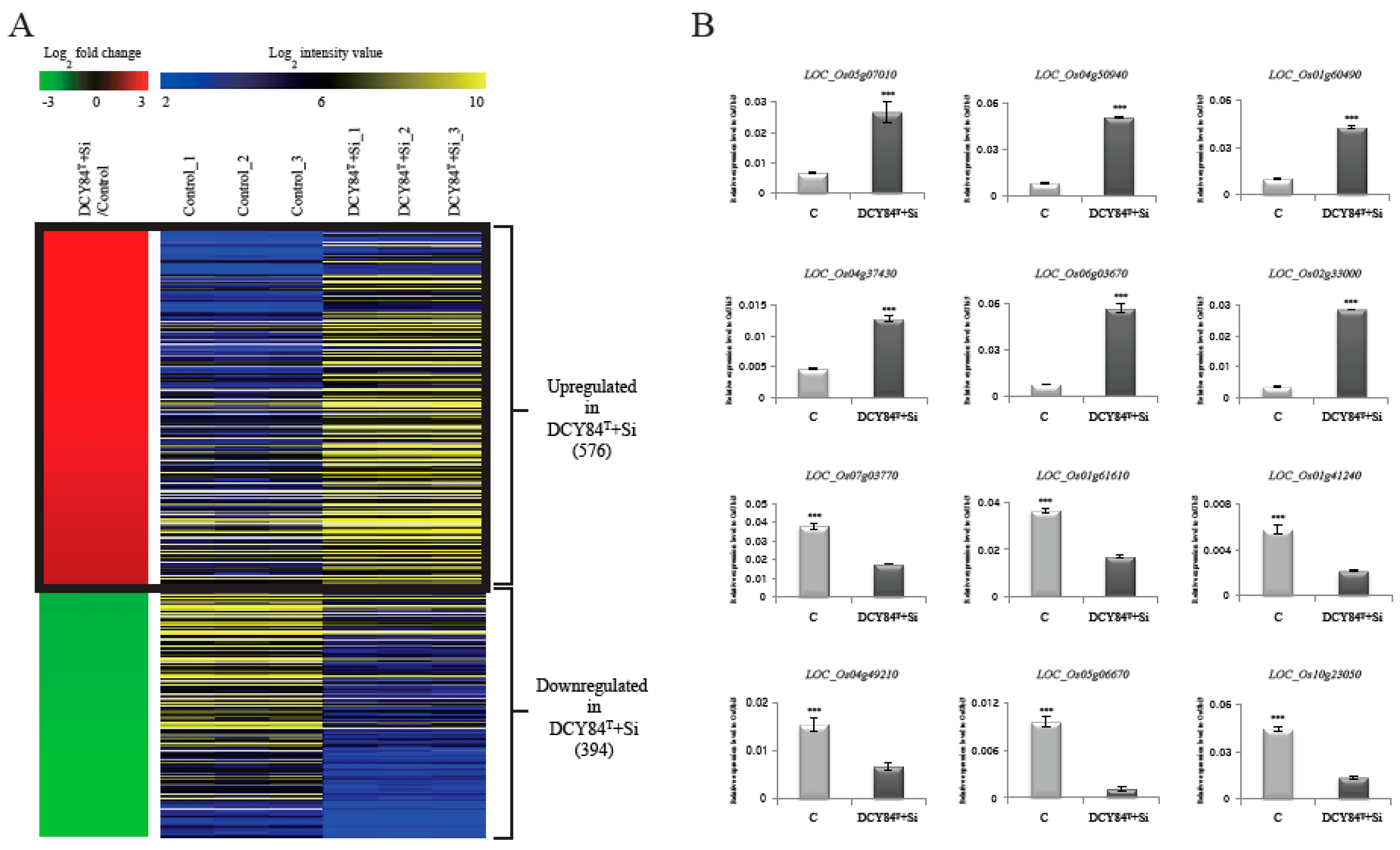
To identify the genes showing differential expression patterns in response to DCY84T+Si treatment, we compared three-week-old roots from treated seedlings with those from untreated control seedlings, with three biological replicates. RNA-Seq revealed that 576 genes were upregulated, and 394 genes were downregulated in the treated plants relative to the corresponding genes in the controls (p-values < 0.05 and log2 fold-changes > 2; Figure 1A). A heatmap was constructed with data for log2 fold-change values for both treated plants and control plants, along with log2 intensities in the two sample types of the 970 differentially-expressed genes (DEGs), including both the upregulated and downregulated genes (Figure 1A; Table S1). In addition, we compared RNA-seq data with the previous data (GSE23723) that performed a microarray with mock and Si-treated roots in rice. We identified 147 genes (log2 fold-changes > 2; Table S2) that were upregulated in Si treatment, and compared them with 576 genes that were upregulated in DCY84T+Si treatment. Interestingly, it was found that only three genes overlap between the two groups. These results indicate that the genes, in response to Si treatment and those in response to DCY84T+Si treatment, might be quite different.
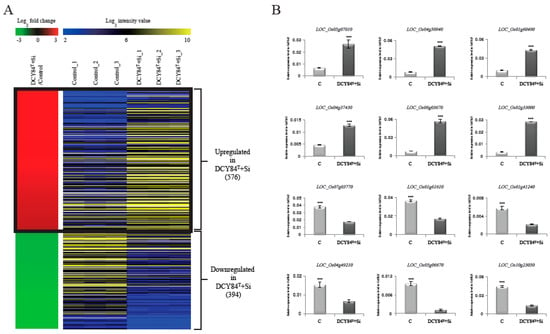
Figure 1.
Heat map of differentially-expressed genes during DCY84T+Si treatment. Using RNA-seq data analysis under criteria of FPKM > 4, p-values < 0.05, and log2 ratio of < −2 for > 2 of DCY84T+Si-treated plant roots versus normal plant roots (control), we identified 1455 differentially-expressed genes (A). In the left panel, red indicates upregulation in DCY84T+Si/control comparisons; green indicates downregulation in DCY84T+Si/control comparisons. The right panel shows average normalized log2 FPKM values from RNA-seq experiments; blue indicates the lowest expression level, and yellow the highest. The effects of DCY84T+Si were checked by monitoring expression patterns of 12 genes (B). The y axis indicates expression level relative to OsUBI5/Os01g22490 (internal control); the x axis indicates samples used for qRT-PCR. *** p < 0.001. Detailed data about RNA-seq analysis are presented in Table S1.
To verify the DEGs, we selected 6 genes that are upregulated and 6 that are downregulated when treated with DCY84T+Si (Table S3). Then, we checked the expression patterns of 12 genes by real-time quantitative PCR (qPCR). As a result, we confirmed that the RNA-Seq data showed significant positive correlation with qPCR data (Figure 1B). Notably, transcriptional factors or transporter with confirmed expression patterns by qRT-PCR might be primary targets to increase biomass mediated by DCY84T+Si treatment.
2.3. GO Enrichment Analysis Revealed the Significant Biological Processes Associated with DCY84T+Si Treatment
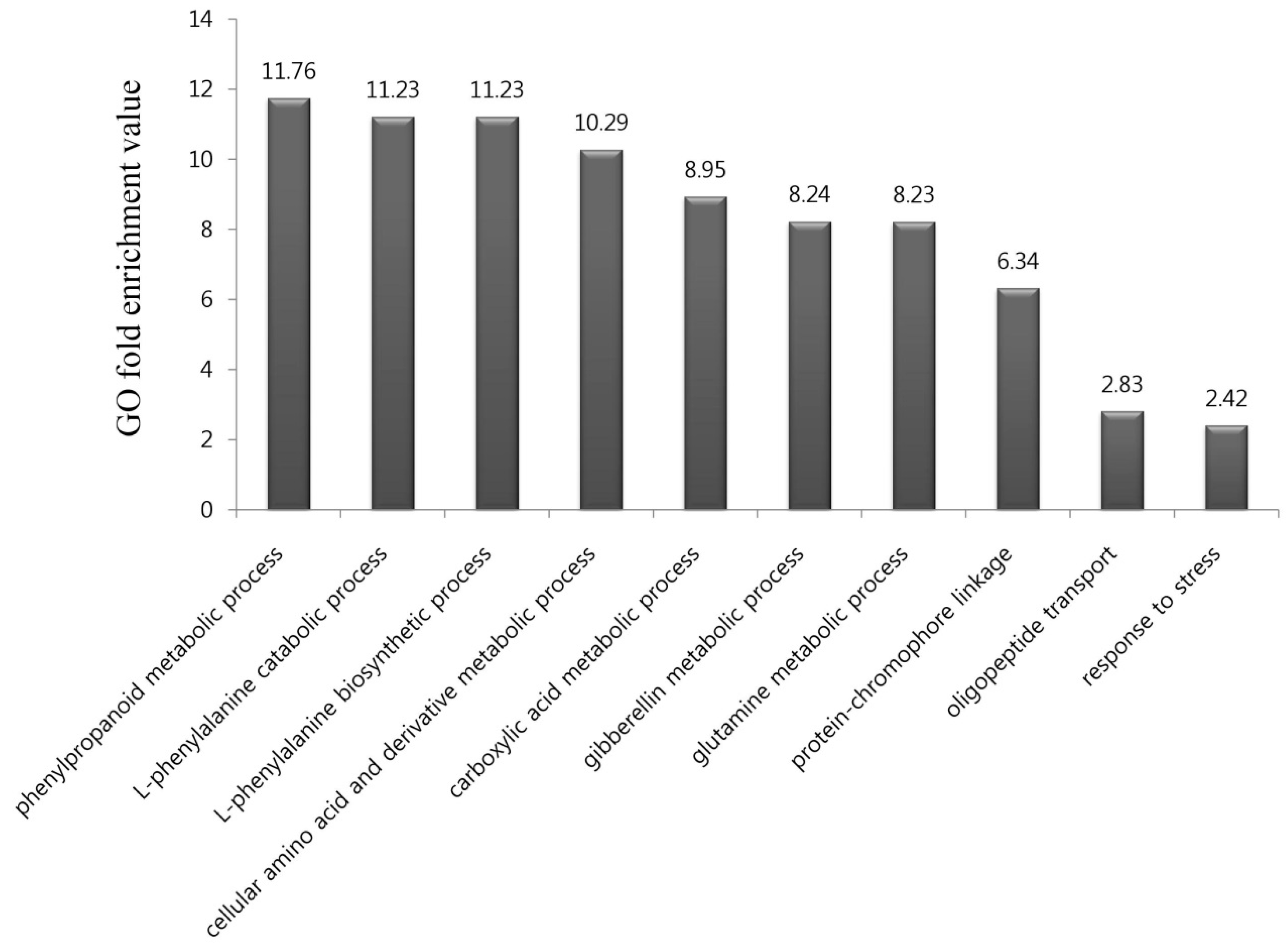
To identify the biological functions of the 576 genes upregulated by DCY84T+Si treatment, we performed a GO term enrichment analysis of those genes in the “biological process” category. In all, ten GO terms were highly over-represented in our gene list, with p-values < 0.01 and fold-enrichment values of >2-fold (Figure 2; Table S4), as previously reported [27]. They included biological processes relating to the phenylpropanoid metabolic process (11.76-fold enrichment), L-phenylalanine catabolic process (11.23), L-phenylalanine biosynthetic process (11.23), cellular amino acid and derivative metabolic process (10.29), carboxylic acid metabolic process (8.95), gibberellin metabolic process (8.24), glutamine metabolic process (8.23), protein-chromophore linkage (6.34), oligopeptide transport (2.83), and response to stress (2.42).
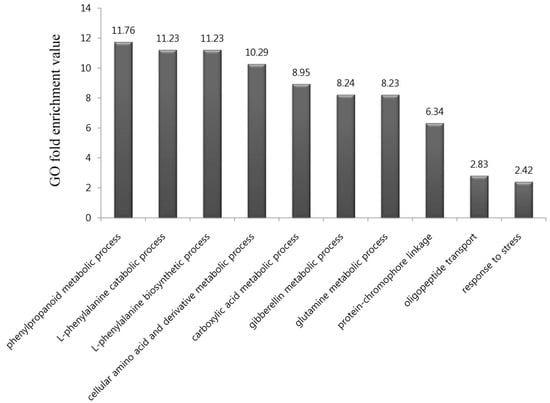
Figure 2.
Gene ontology (GO) enrichment analysis in the “biological process” category for genes upregulated and downregulated in response to DCY84T+Si treatment. In all, 10 GO terms were over-represented under > two-fold enrichment value, with p-values < 0.01. Details of GO assignments are presented in Table S4.
Of these, the phenylpropanoid metabolic process was significantly enriched by DCY84T+Si treatment (Figure 2). Phenylpropanoids are well known as defense chemicals that protect plants against pathogen attacks through induced systemic resistance (ISR) mediated by PGPB. For example, when Burkholderia sp., a member of the PGPB group, was treated with Vitis vinifera, phenolic compounds accumulated and the cell walls of the exodermis and cortical cell layers were strengthened, increasing disease resistance [28]. In addition, when root-inducing T-DNA peas are treated with the endophytic bacterium B. pumilus, the pea root-rotting fungus Fusarium oxysporum forms wall appositions containing phenolic compounds to limit pathogen growth [29]. Finally, treatment of B. pumilus with tomato plants inoculated with the vascular fungus F. oxysporum reduces the growth of pathogens through the formation of wall appositions composed of phenolic compounds [30]. Interestingly, rice treated with DCY84T is more resistant to Xanthomonas oryzae pv. oryzae (Xoo) compared with controls [16]. These results suggest that the synthesis of phenylpropanoid may also occur in rice roots treated with DCY84T+Si.
Then, the L-phenylalanine catabolic and L-phenylalanine biosynthetic processes were significantly enriched. As an aromatic amino acid, phenylalanine is a central molecule in plant metabolism, and its biosynthetic pathways and regulation have been extensively studied [31]. Phenylalanine acts as a precursor to a range of phenolic secondary metabolites, including phenylpropanoids, flavonoids, and lignin [32]. There have been several reports that salt stress stimulates the phenylpropanoid biosynthetic pathway to produce various phenolic compounds with antioxidant ability. For example, plants of Thymus vulgaris and Thymus daenensis grown under salt stress exhibited increased antioxidant activity as a result of the increased production of phenolic compounds [33]. In addition, the transformation of Arabidopsis thaliana with the grape bHLH transcription factor gene, VvbHLH1, which regulates flavonoid biosynthesis, increased the accumulation of flavonoids and enhanced salt tolerance in the transgenic plant [34]. In a previous study, DCY84T-treated rice improved plant growth in saline coastal soils [16]. These results suggest that treatment with DCY84T+Si may increase the production of phenylpropanoids or other phenolic compounds through stimulation of the phenylpropanoid biosynthetic pathway.
2.4. MapMan Analysis Revealed Involvement of DCY84T+Si Treatment in Phenylpropanoid Metabolism and Ethylene Regulation
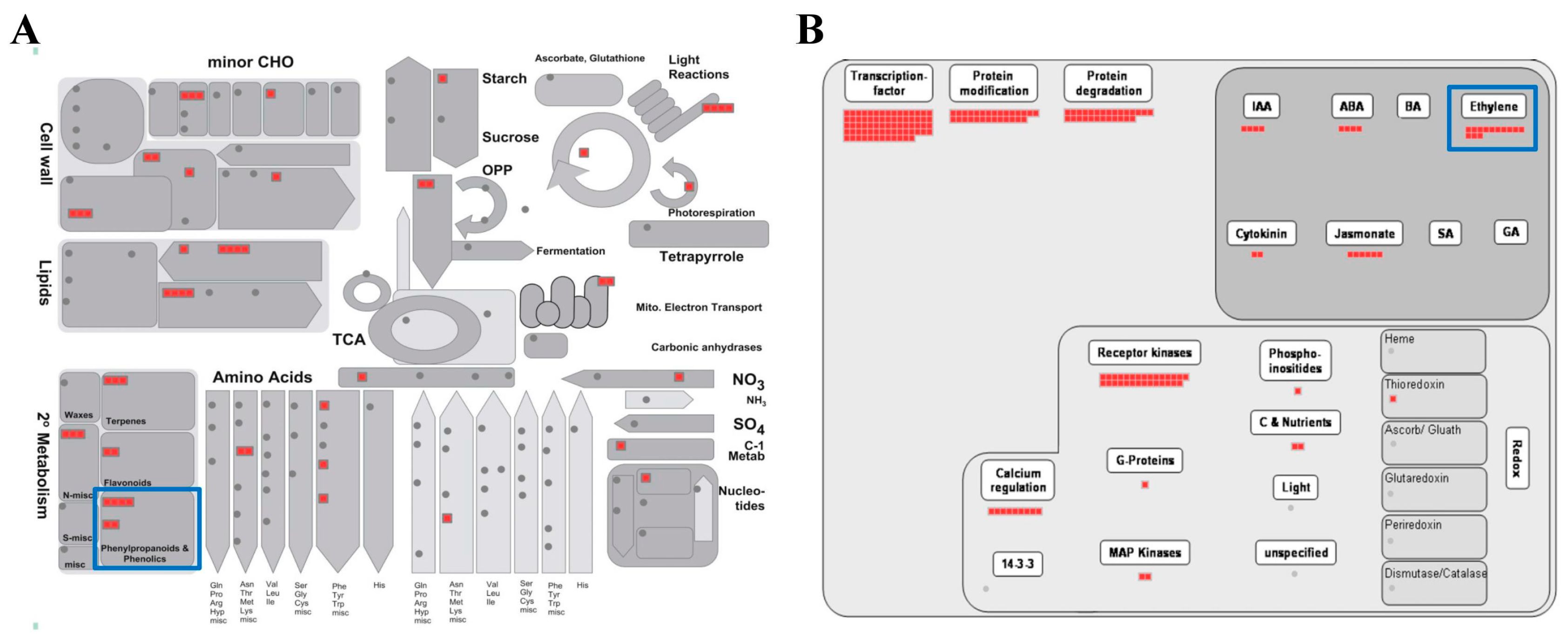
The MapMan program is an effective tool for visualizing diverse overviews associated with high-throughput transcriptomics data [35]. We uploaded the fold-change data and locus IDs of the 576 upregulated genes to various overviews installed in the MapMan program (Figure 3; Tables S5 and S6). In the metabolism overview, we identified six genes upregulated in “Phenylpropanoids & Phenolics” metabolism belonging to secondary metabolism (blue box in Figure 3A). As we found that the phenylpropanoid metabolic process was the biological process which is most enriched by GO enrichment analysis in Section 2.3, it was predictable that many phenylpropanoid-associated genes would be identified in the metabolism overview.
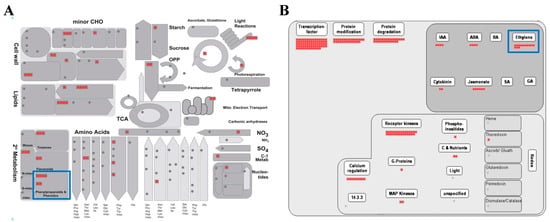
Figure 3.
MapMan analysis of genes associated with a response to DCY84T+Si treatment. Overviews: (A) metabolism-response overview, and (B) regulation overview. Red boxes indicate genes upregulated by DCY84T+Si. Detailed information is presented in Tables S5 and S6.
We also identified 13 upregulated genes in the ethylene regulation pathway using a regulation overview (blue box in Figure 3B). Rhizobacteria, which include most PGPBs, colonize the roots of growing plants and react, using host-derived chemicals in root exudates, and using tryptophan to synthesize the natural auxin indole-3-acetic acid (IAA) [36]. Both bacterial IAA and endogenous plant IAA can stimulate plant growth and induce the synthesis of aminocyclopropane-1-carboxylate (ACC) synthase, one of the ethylene biosynthesis pathway enzymes in plants, where it plays a role in converting S-adenosyl methionine to ACC, the immediate precursor of ethylene. Some of the ACC molecules synthesized are converted to ammonia and α-ketobutyrate by ACC deaminase in bacteria [9,37,38,39,40]. Thus, the activity of bacterial ACC deaminase decreases the concentration of ethylene produced in plants, which results in increased plant growth [38]. We observed that the root length and dry weight of plants treated with DCY84T+Si increased significantly compared to the control (Table 1; Figure S1). These results suggest that DCY84T with Si treatment might be a PGPB capable of synthesizing ACC deaminase.
2.5. Functions of Candidate Genes Associated with DCY84T+Si Were Evaluated through Literature Searches
To further evaluate the functional significance of our candidate DEGs, we identified the known functions for these DEGs from previous studies. Of the DEGs, we found 30 genes upregulated by DCY84T+Si treatment with known functions (Table 2). Twelve of the genes were related to responses to various abiotic stresses, namely, drought-responsive ERF 1 (OsDERF1; [39]) and basic helix–loop–helix domain148 (OsbHLH148; [40]) for drought; ERF protein associated with tillering and panicle branching (OsEATB; [41]) and SALT-RESPONSIVE ERF1 (SERF1; [42]) for salinity; OsWRKY76 [43] for cold; zinc finger protein252 (ZFP252; [44]) and Ca2+-dependent protein kinase 4 (OsCPK4; [45]) for drought and salinity; trehalose-6-phosphate phosphatase1 (OsTPP1; [46]) for salinity and cold; mitogen-activated protein kinase5 (OsMAPK5; [47]), dehydration-responsive element-binding transcription factor 1F (OsDREB1F; [48]), and OsDREB1C [49] for drought, salinity, and cold; and basic helix–loop–helix 133 (OsbHLH133; [50]) for tolerance to other soil stresses. With respect to biotic stresses, the functions of seven genes were identified: fatty acid desaturase7 (OsFAD7; [51]), 1-aminocyclopropane-1-carboxylic acid synthase 2 (OsACS2; [52,53]), OsMAPK5 [47], and BROAD-SPECTRUM RESISTANCE 1 (BSR1; [54]) for rice blast resistance; OsWRKY71 [55] for bacterial blight resistance; and OsWRKY28 [56,57] and OsWRKY76 [43,56] for both blast and bacterial blight resistance. Nine genes were related to morphological traits, namely, Elicitor 5 (EL5; [58]), tryptophan deficient dwarf 1 (tdd1; [59]), and cZ-O-glucosyltransferase 2 (cZOGT2; [60]) for root traits; response to exogenous JA 1 (RERJ1; [61]), cytochrome P450 monooxygenase 734A4 (CYP734A4; [62]), and OsCPK4 [45] for dwarf habit; BRASSINOSTEROID UPREGULATED1 (bu1; [63]) for leaf and seed change (grain size); and dense and erect panicle 3 (dep3; [64]) and ERF protein associated with tillering and panicle branching (OsEATB; [41]) for panicle development. Two of the genes were related to physiological traits, including DEFECT IN EARLY EMBRYO SAC1 (OsDEES1; [65]) for sterility and OsACS2 [66] for spikelet fertility.

Table 2.
Summary of functionally-characterized genes through literature searches associated with DCY84T and Si.
As expected from earlier results from the current study (Table 1; Figure S1), several DEGs were found among the known genes associated with root biomass traits (Table 2). In addition, most of the known DEG genes are more likely associated with resistance- or tolerance-related traits (Table 2). These findings indicate that our candidate genes are potentially involved in plant responses to abiotic stresses, including drought, salinity, and cold, as well as growth promotion. Previous literature had reported that A. thaliana treated with DCY84T was more tolerant of drought, salinity, and aluminum treatments [16]. Our findings and the previous study support the hypothesis that rice plants treated with DCY84T+Si can better withstand various abiotic stresses such as drought or cold. Furthermore, uncharacterized DEGs might be useful targets for further study to enhance abiotic stress tolerance or growth promotion.
2.6. Treatment with DCY84T+Si Resulted in Increased Rice Drought Tolerance
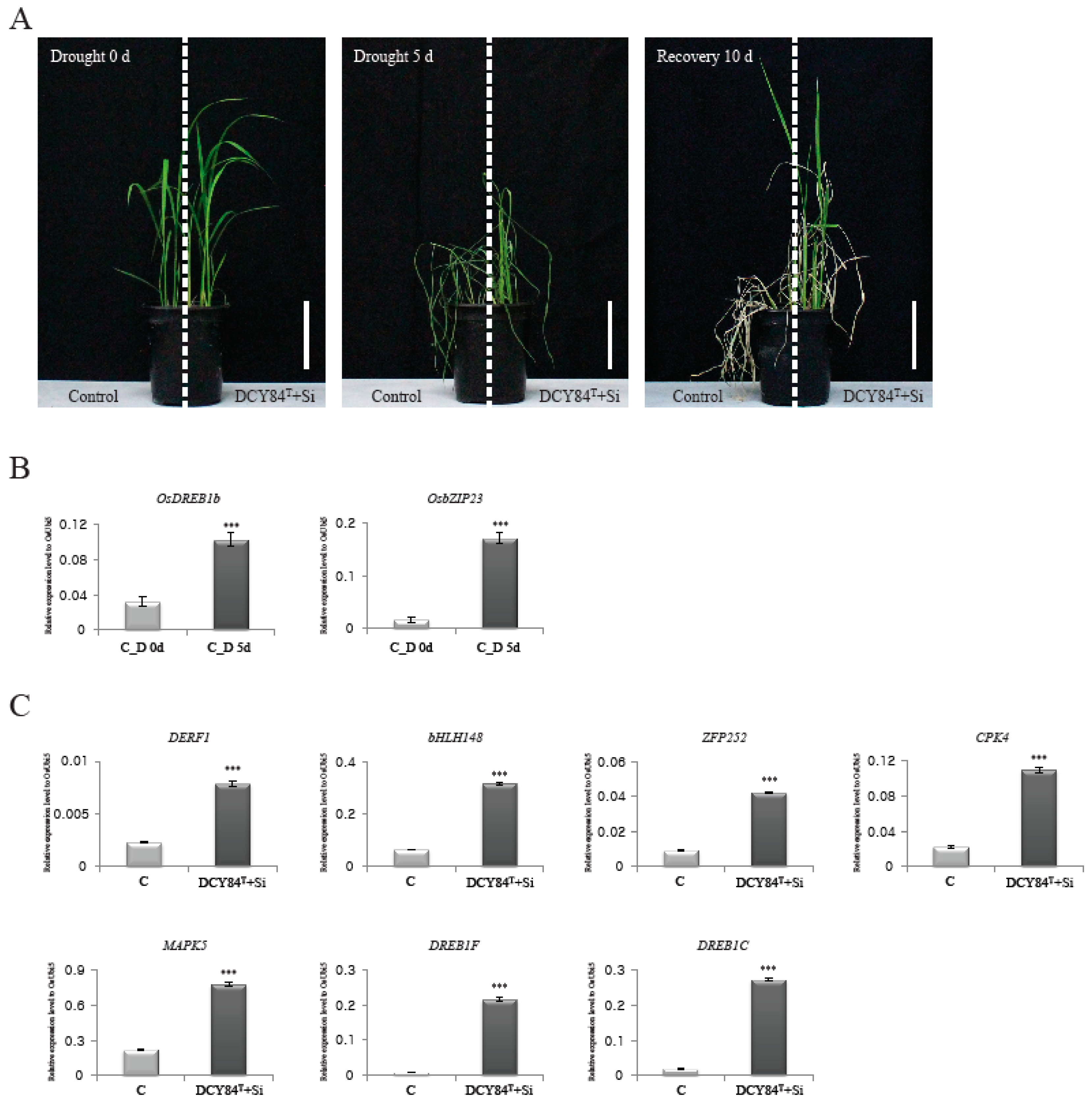
We had predicted the positive effects of DCY84T+Si treatment on root biomass, biotic stress resistance, and abiotic stress tolerance through GO enrichment analysis, MapMan analysis, and analysis of rice DEGs with known functions. Of these, tolerance of abiotic stresses, including drought stress, was hypothesized to be significantly affected by DCY84T+Si. To test this, we conducted the following experiment to determine whether rice plants treated with DCY84T+Si showed greater tolerance to drought stress. Control and DCY84T+Si-treated plants were grown for four weeks and then subjected to drought stress for five days, after which the plants were allowed to recover for 10 days (Figure 4A). Plants treated with DCY84T+Si showed significantly greater tolerance to drought stress than the control plants. The expression patterns were then examined for two genes that had been identified as molecular markers of the drought-stress response, i.e., OsDREB2b (LOC_Os05g27930) and OsbZIP23 (LOC_Os02g52780) [67]. As expected, stressed roots (from Day 5) showed increased expression of those genes, supporting the hypothesis that samples collected under drought stress were well qualified for further analysis (Figure 4B). Of the known genes, seven upregulated genes related to drought stress were selected (Table 2), and their expression patterns in treated and control plants were compared. All seven genes were found to be expressed at a significantly higher level in plants treated with DCY84T+Si than in control plants (Figure 4C). These results suggest that treatment with DCY84T+Si increased the expression of several drought-related genes, resulting in increased drought tolerance being conferred upon the treated rice plants.
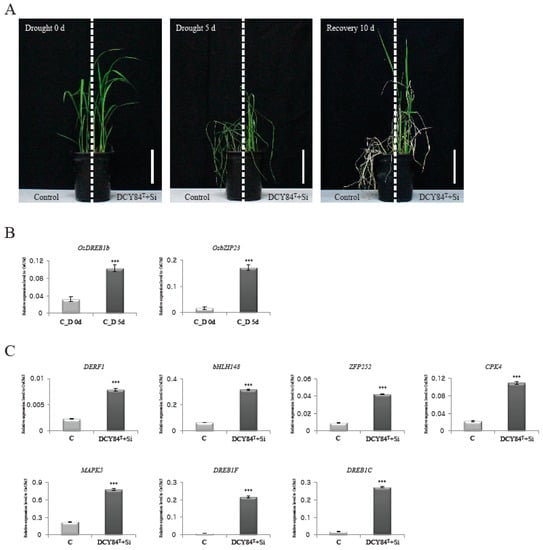
Figure 4.
Drought-stress response mediated by DCY84T+Si. Control and DCY84T+Si treated plants grown in plastic pots for 4 weeks was exposed to drought stress for 5 d. Photo were taken 10 d after re-watering (A). Effects of water deficiency were checked by monitoring expression patterns of drought-stress marker genes, OsDREB1b and OsZIP23 (B). Analyses of transcripts of DERF1, bHLH148, ZFP252, CPK4, MAPK5, DREB1F, and DREB1C for control and DCY84T+Si (C). The expression levels were normalized to that of OsUBI5/Os01g22490 (internal control) using real-time PCR analysis. C, control without drought treatment; D 0d, Drought stress treatment for 0 day; D 5d, Drought stress treatment for 5 days. Scale bar = 10 cm. N = 3 (A). *** p < 0.001 (B,C).
2.7. Analyses of Predicted Protein–Protein Interactions Associated with DCY84T+Si Treatment Suggest a Regulatory Model
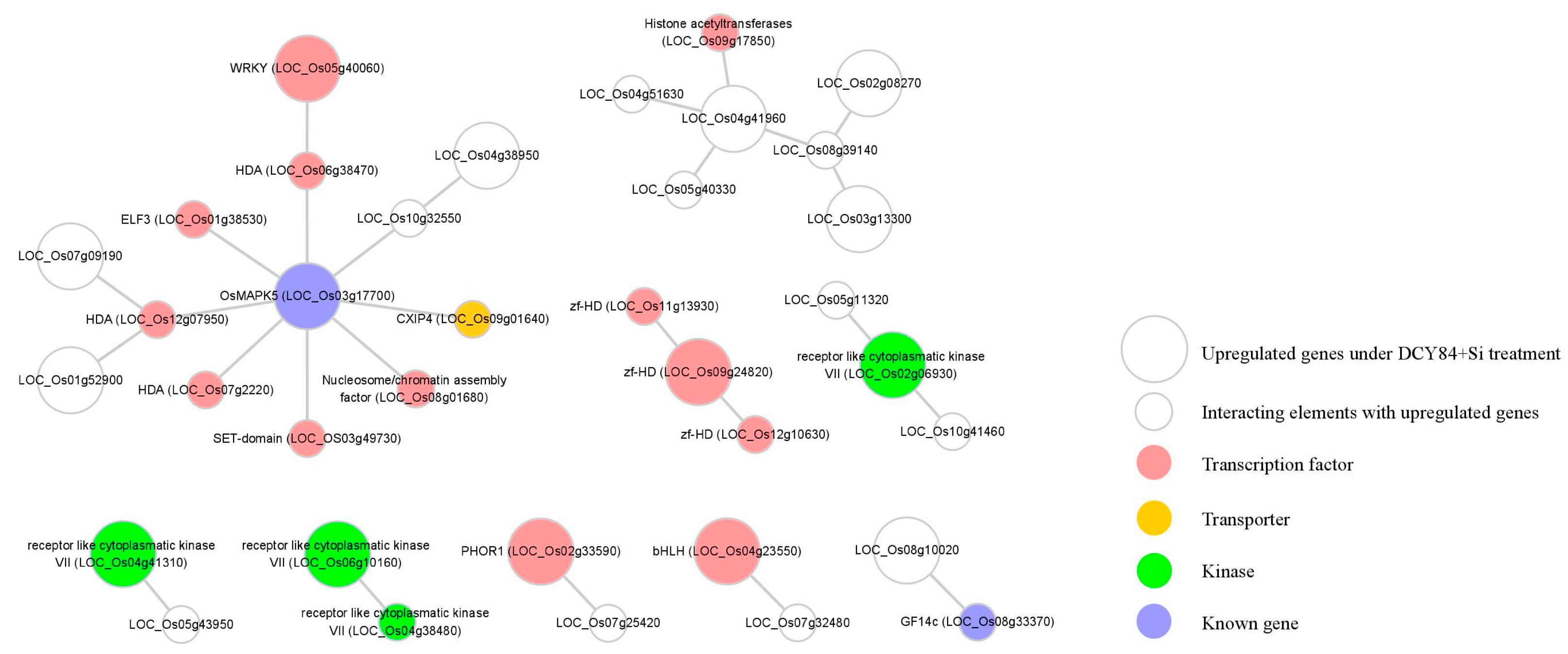
Regulatory genes, such as those encoding transcription factors, are primary targets when investigating diverse abiotic stress responses and developmental processes. Understanding the regulatory relationship among upregulated genes can lead to a new strategy for increasing tolerance to environmental stress as a result of DCY84T+Si treatment. We utilized the Rice Interactions Viewer to generate a hypothetical protein–protein interaction network associated with the 576 genes upregulated in response to DCY84T+Si treatment [68]. We then refined the network by using genes in the following four categories as the query: 13 transcription factors (pink circles, Figure 5), one transporter (yellow circle), four kinases (green circles), and two genes functionally characterized to be associated with abiotic stress tolerance (blue circles, Figure 5).
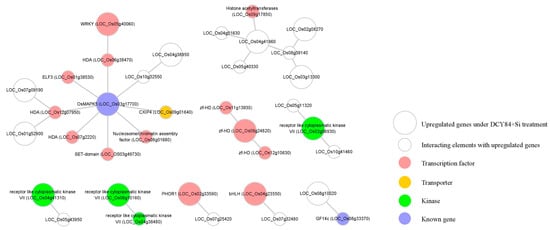
Figure 5.
Construction of regulatory network associated with genes upregulated under DCY84T+Si treatment. Using Rice Interaction Viewer and Cytoscape tools, we queried the predicted protein–protein interaction network associated with 13 transcription factors (pink circles), one transporter (yellow circle), four kinase (green circles), and two functionally-characterized genes in environmental stress (blue circles). The large circle represents the upregulated gene under DCY84T+Si treatment. The small circle represents an interacting element with upregulated genes.
We predicted that OsMAPK5 interacts with six TFs and one transporter in the network (Figure 5). According to previous studies, the OsMAPK5 gene, its protein, and its kinase activity were all reported to be induced by abscisic acid and various biotic and abiotic stresses [47]. Interestingly, the expression of OsMAPK5 was also shown to be altered by some PGPBs. For example, greenhouse-grown rice treated with Bacillus amyloliquefaciens NBRISN13 showed decreased expression of OsMAPK5 in the leaves [69]. In contrast to this result, we found that the expression of OsMAPK5 was increased in the rice root when treated with DCY84T+Si (Figure 4B). Xiong et al. [47] reported that OsMAPK5-overexpressed lines not only increased kinase activity, but also increased tolerance to drought, salt, and cold stresses. These results do not provide information on the pathway by which DCY84T+Si induces OsMAPK5 expression, but our network model suggests the possible regulatory pathways associated with OsMAPK5 which was stimulated in response to DCY84T+Si treatment. Further functional analysis using our transcriptional regulation model might shed light on the OsMAPK5-mediated signaling and transcriptional regulation pathway.
3. Materials and Methods
3.1. Plant Materials and Phenotypic Observation
The Paenibacillus yonginensis strain DCY84T was grown at 30 °C on trypticase soy broth for 16 h. The culture broth was centrifuged, and the pelleted cells were resuspended in a dilute saline solution (0.85% NaCl). Surface-sterilized seeds of O. sativa, japonica cv. Chilbo (Rural Development Administration, Jeonju, Korea) were treated by soaking them in the bacterial suspension (108 CFU ml−1) or saline solution (control) for 30 min. The binder solution was composed of 10% Na2SiO3, 5% humic acid, 3% sodium alginate, 0.05% molybdenum, and 0.01% carboxymethyl chitosan. 500 g of Zeolite was mixed with 2 kg of inoculated rice seeds, and 300 mL of binder solution was sprayed [25,70]. For the control seeds, only a binder was added. Treated and control seeds were allowed to germinate by imbibing them in water for three days and then placing the seedlings in a growth incubator (Younghwa Science, Daegu, Korea) for four weeks (14-h light/10-h dark, 28 °C/22 °C). To observe the effects of DCY84T+Si on the growth of rice, the length and dry weight of the roots of control and treated plants were measured at seven-day intervals for four weeks (Table 1; Figure S1).
3.2. Statistical Analyses
Thirty plants were used at each stage to determine the lengths and dry weight of roots and leaves. All data were presented as mean ± standard deviation. Statistical analyses were performed using the Student’s t test (* p < 0.05; ** p < 0.01; and *** p < 0.001). Then, we sampled the roots of the control and DCY84T+Si-treated plants grown for three weeks for RNA-Seq analysis.
3.3. RNA-Seq Analysis
We used the Illumina platform to generate sequences (approximately 26 GB) of three independent total RNA samples from the roots of each of the DCY84T+Si-treated and control seedlings. In each transcriptome sample, 100-bp paired-end sequences were assessed with a FastQC toolkit [71]. Any adapter contaminations or low-quality sequences (pPhred +33 and -q 20) were removed using both Cutadapt [72] and its wrapper tool, Trimgalore [73]. The resultant high-quality sequences were used for our TopHat2 pipeline [74]. On average, 94% of the filtered sequences were mapped to the International Rice Genome Sequencing Project (IRGSP) 1.0 reference genome [75], and the gene features were estimated based on the gff3 annotation file from the Rice Genome Annotation Project (RGAP) database (http://rice.plantbiology.msu.edu) [76]. DEGs were evaluated using Cuffdiff to compare treatment conditions. Genes with p-values < 0.05 and log2 fold-changes > 2 (i.e., fold-change > 4) were considered to be differentially expressed. Further screening among the initial DEGs was based on fragments per kilobase per million fragments mapped (FPKM) values [77]. The selected DEGs created a heatmap using the Multi Experiment Viewer (MeV_4-9-0) software tool [78,79].
3.4. GO Enrichment Analysis
We employed the GO enrichment tool [80] to determine the biological roles of selected genes listed in the Rice Oligonucleotide Array Database (http://ricephylogenomics-khu.org/road/go_analysis.php). This included any genes that were upregulated during DCY84T+Si treatment. An enrichment value higher than standard (1) meant that the selected GO term was over-represented. Terms with >3-fold enrichment values were also considered.
3.5. MapMan Analysis
The rice MapMan classification system covers 36 BINs, each of which could be extended in a hierarchical manner into subBINs [67,81,82]. Using diverse MapMan tools, a significant gene list selected from high-throughput data analysis can be integrated to produce diverse overviews. Here, we generated a dataset carrying locus IDs from the RGAP annotation version 7.0 in addition to average log2 fold-change data for controls versus DCY84T+Si conditions. To describe the functional classification of genes upregulated in response to DCY84T+Si, we used two overviews: metabolism and regulation.
3.6. Analysis of Rice Genes with Known Functions
To evaluate the functional significance of our candidate genes, we compared our gene list with the Overview of Functionally-Characterized Genes in Rice Online database (http://qtaro.abr.affrc.go.jp/ogro), which summarizes rice genes with known functions [83], and then grouped them into three major categories: resistance- or tolerance-related trait, morphological trait, and physiological trait.
3.7. Drought Stress Treatment
Plants of japonica rice (Oryza sativa) cv. Chilbo from either control seeds or seeds dressed with DCY84T+Si were allowed to germinate by imbibing them in water for three days and then placing the seedlings in a growth incubator (Younghwa Science, Daegu, Korea) for four weeks (14-h light/10-h dark, 28 °C (day)/22 °C (night), humidity 80%). On average, 800 g of dried soil was used to grow ten plants (five control plants vs. five DCY84T+Si plants) in each pot. Ten replicate pots were used for phenotypic observation. Drought stress was applied to four-week-old seedlings by withholding irrigation for 5 d (14-h light/10-h dark, 28 °C (day)/22 °C (night), humidity 40%), after which the seedlings were rewatered for 10 d (14-h light/10-h dark, 28 °C (day)/22 °C (night), humidity 80%). Plant phenotypes were photographed with a camera (Canon EOS 550D; Canon, Tokyo, Japan) before and after drought stress treatment and during the recovery phase.
3.8. RNA Extraction and Quantitative RT-PCR (qRT-PCR) Anayslis
Three independent replicates (three plants per replicate) of each of the three-week-old control roots and DCY84T+Si-treated roots were sampled and immediately frozen in liquid nitrogen. Total RNA was extracted using RNAiso Plus according to the manufacturer’s protocol (Takara Bio, Kyoto, Japan). The first-strand cDNA was synthesized with MMLV Reverse Transcriptase (Promega, Fitchburg, WI, USA) and the oligo(dT)-15 primer. The qPCR was performed by Qiagen Rotor-Gene Q real-time PCR cycler using the following thermal cycling procedure: 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 1 min. To normalize the amplified transcripts, we used a primer pair for rice ubiquitin 5 (OsUbi5/Os01g22490) [84]. Finally, a Student’s t-test was used for statistical analysis. All primers for the genes used in these analyses are presented in Table S7.
4. Conclusions
We demonstrated that a combination DCY84T+Si treatment increases root growth in the seedling stage, and increases tolerance of drought stress. GO analysis, MapMan, and literature searches indicated that the tolerance response might be due to the upregulation of genes involved in the phenylpropanoid and ethylene metabolic pathways. Gene indexed mutants are available for more than half of the rice genome with a well-established gene editing system [84]. With the benefit for further research as a model crop plant, understanding and exploiting the interactions between bacteria and plants can be a powerful tool by which to enhance agronomic traits such as nutrient use efficiency, crop yield, and stress tolerance.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/23/5883/s1. Table S1. Collection of differentially-expressed genes between DCY84T+Si-treated samples and untreated controls. Table S2. List of differentially-expressed genes between control and Si treatment (GSE23723). Table S3. 12 genes information selected for qRT-PCR analysis among differentially-expressed genes. Table S4. Gene ontology (GO) assignments in the “biological process” category for genes upregulated by DCY84T+Si. Table S5. Metabolism overview of MapMan terms assigned to genes upregulated by DCY84T+Si. Table S6. Detailed information of MapMan terms assigned to genes upregulated by DCY84T+Si. Table S7. Primer sequences for real-time quantitative PCR (qPCR) analyses. Figure S1. Physiological responses during the DCY84T+Si treatment of rice seedlings grown in soil.
Author Contributions
Formal analysis, Y.-H.Y.; Investigation, M.K. and H.R.A.; Resources, G.T.L., S.K., D.S., J.-O.K. and Y.-J.K.; Software, A.K.N.C. and W.-J.H.; Supervision, K.-H.J.; Writing—original draft, Y.-H.Y.; Writing—review & editing, K.-H.J.
Funding
This work was supported by grants from the National Research Foundation of Korea (NRF-2016R1D1A1A09919568), Next-Generation BioGreen 21 Program (PJ01366401 and PJ01325901 to JKH), the Rural Development Administration, Republic of Korea and the Collaborative Genome Program of the Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (MOF) (No. 20180430 to JKH). The funding agencies did not participate in the design of the study, the collection, analysis, and interpretation of data, nor in writing the manuscript.
Acknowledgments
We thank Gynheung An for providing valuable comments on this work and sharing the resources and facilities used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wartiainen, I.; Eriksson, T.; Zheng, W.; Rasmussen, U. Variation in the active diazotrophic community in rice paddy-nifH PCR-DGGE analysis of rhizosphere and bulk soil. Appl. Soil Ecol. 2008, 39, 65–75. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, V.V.; Kalagudi, G.M.; Gurudatta, B.V. Towards nitrogen autotrophic rice. Curr. Sci. 2001, 81, 451–457. [Google Scholar]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- De Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clement, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kang, S.J.; Yeo, S.H.; Oh, T.K. Paenibacillus alkaliterrae sp. nov., isolated from an alkaline soil in Korea. Int. J. Syst. Evol. Microbiol. 2005, 55, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Kim, H.B.; An, D.S.; Yang, H.C.; Oh, S.T.; Chung, H.J.; Yang, D.C. Paenibacillus soli sp. nov., a xylanolytic bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2007, 57, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.M.; Fritze, D.; Blanco, A.; Spröer, C.; Tindall, B.J.; Schumann, P.; Kroppenstedt, R.M.; Diaz, P.; Pastor, F.I.J. Paenibacillus barcinonensis sp. nov., a xylanase-producing bacterium isolated from a rice field in the Ebro River delta. Int. J. Syst. Evol. Microbiol. 2005, 55, 935–939. [Google Scholar]
- Dsouza, M.; Taylor, M.W.; Ryan, J.; MacKenzie, A.; Lagutin, K.; Anderson, R.F.; Turner, S.J.; Aislabie, J. Paenibacillus darwinianus sp. nov., isolated from gamma-irradiated Antarctic soil. Int. J. Syst. Evol. Microbiol. 2014, 64, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Sukweenadhi, J.; Kim, Y.J.; Lee, K.J.; Koh, S.C.; Hoang, V.A.; Nguyen, N.L.; Yang, D.C. Paenibacillus yonginensis sp. nov., a potential plant growth promoting bacterium isolated from humus soil of Yongin forest. Antonie van Leeuwenhoek 2014, 106, 935–945. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Kim, Y.J.; Choi, E.S.; Koh, S.C.; Lee, S.W.; Kim, Y.J.; Yang, D.C. Paenibacillus yonginensis DCY84T induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiol. Res. 2015, 172, 7–15. [Google Scholar] [CrossRef]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef]
- Epstein, E.; Bloom, A. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2005; p. 400. [Google Scholar]
- Liang, Y.; Sun, W.; Zhu, Y.G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Datnoff, L.E. Silicon and rice disease management. Fitopatol. Bras. 2005, 30, 457–469. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Savant, N.K.; Snyder, G.H.; Datnoff, L.E. Silicon management and sustainable rice production. Adv. Agron. 1996, 58, 151–199. [Google Scholar]
- Alvarez, J.; Datnoff, L.E. The economic potential of silicon for integrated management and sustainable rice production. Crop Prot. 2001, 20, 43–48. [Google Scholar] [CrossRef]
- Choi, E.S.; Sukweenadhi, J.; Kim, Y.J.; Jung, K.H.; Koh, S.C.; Hoang, V.A.; Yang, D.C. The effects of rice seed dressing with Paenibacillus yonginensis and silicon on crop development on South Korea’s reclaimed tidal land. Field Crop. Res. 2016, 188, 121–132. [Google Scholar] [CrossRef]
- Fleck, A.T.; Nye, T.; Repenning, C.; Stahl, F.; Zahn, M.; Schenk, M.K. Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J. Exp. Bot. 2011, 62, 2001–2011. [Google Scholar] [CrossRef]
- Jung, K.H.; Dardick, C.; Bartley, L.E.; Cao, P.; Phetsom, J.; Canlas, P.; Seo, Y.S.; Shultz, M.; Ouyang, S.; Yuan, Q.; et al. Refinement of light-responsive transcript lists using rice oligonucleotide arrays: Evaluation of gene-redundancy. PLoS ONE 2008, 3, e3337. [Google Scholar] [CrossRef]
- Compant, S.; Reiter, B.; Nowak, J.; Sessitsch, A.; Clément, C.; Barka, E.A. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685–1693. [Google Scholar] [CrossRef]
- Benhamou, N.; Kloepper, J.W.; Quadt-Hallman, A.; Tuzun, S. Induction of defense-related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol. 1996, 112, 919–929. [Google Scholar] [CrossRef]
- Benhamou, N.; Kloepper, J.W.; Tuzun, S. Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: Ultrastructure and cytochemistry of the host response. Planta 1998, 204, 153–168. [Google Scholar] [CrossRef]
- Galili, G.; Höfgen, R. Metabolic engineering of amino acids and storage proteins in plants. Metab. Eng. 2002, 4, 3–11. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crop. Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Chen, D.; Li, Z.; Peng, R.; Yao, Q. A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell. Tissue Organ Cult. 2016, 125, 387–398. [Google Scholar] [CrossRef]
- Jung, K.H.; An, G. Application of MapMan and RiceNet drives systematic analyses of the early heat stress transcriptome in rice seedlings. J. Plant Biol. 2012, 55, 436–449. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Glick, B.; Penrose, D.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, J.; Zhang, H.; Zhang, Z.; Quan, R.; Zhou, S.; Huang, R. Transcriptional activation of OsDERF1 in OsERF3 and OsAP2-39 negatively modulates ethylene synthesis and drought tolerance in rice. PLoS ONE 2011, 6, e25216. [Google Scholar] [CrossRef]
- Seo, J.S.; Joo, J.; Kim, M.J.; Kim, Y.K.; Nahm, B.H.; Song, S.I.; Cheong, J.J.; Lee, J.S.; Kim, J.K.; Choi, Y.D. OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J. 2011, 65, 907–921. [Google Scholar] [CrossRef]
- Qi, W.; Sun, F.; Wang, Q.; Chen, M.; Huang, Y.; Feng, Y.-Q.; Luo, X.; Yang, J. Rice ethylene-response AP2/ERF factor OsEATB restricts internode elongation by down-regulating a gibberellin biosynthetic gene. Plant Physiol. 2011, 157, 216–228. [Google Scholar] [CrossRef]
- Schmidt, R.; Mieulet, D.; Hubberten, H.-M.; Obata, T.; Hoefgen, R.; Fernie, A.R.; Fisahn, J.; San Segundo, B.; Guiderdoni, E.; Schippers, J.H.M.; et al. Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell 2013, 25, 2115–2131. [Google Scholar] [CrossRef] [PubMed]
- Yokotani, N.; Sato, Y.; Tanabe, S.; Chujo, T.; Shimizu, T.; Okada, K.; Yamane, H.; Shimono, M.; Sugano, S.; Takatsuji, H.; et al. WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J. Exp. Bot. 2013, 64, 5085–5097. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.Q.; Huang, J.; Guo, S.Q.; Yang, X.; Bao, Y.M.; Tang, H.J.; Zhang, H.S. Overexpression of a TFIIIA-type zinc finger protein gene ZFP252 enhances drought and salt tolerance in rice (Oryza sativa L.). FEBS Lett. 2008, 582, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Baldrich, P.; Messeguer, J.; Lalanne, E.; Coca, M.; San Segundo, B. Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiol. 2014, 165, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.F.; Chao, D.Y.; Shi, M.; Zhu, M.Z.; Gao, J.P.; Lin, H.X. Overexpression of the trehalose-6-phosphate phosphatase gene OsTPP1 confers stress tolerance in rice and results in the activation of stress responsive genes. Planta 2008, 228, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yang, Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid—Inducible mitogen-activated protein kinase. Plant Cell 2003, 15, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guan, Y.; Wu, Y.; Chen, H.; Chen, F.; Chu, C. Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol. Biol. 2008, 67, 589–602. [Google Scholar] [CrossRef]
- Ito, Y.; Katsura, K.; Maruyama, K.; Taji, T.; Kobayashi, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 2006, 47, 141–153. [Google Scholar] [CrossRef]
- Wang, L.; Ying, Y.; Narsai, R.; Ye, L.; Zheng, L.; Tian, J.; Whelan, J.; Shou, H. Identification of OsbHLH133 as a regulator of iron distribution between roots and shoots in Oryza sativa. Plant Cell Environ. 2013, 36, 224–236. [Google Scholar] [CrossRef]
- Yara, A.; Yaeno, T.; Hasegawa, M.; Seto, H.; Montillet, J.L.; Kusumi, K.; Seo, S.; Iba, K. Disease resistance against Magnaporthe grisea is enhanced in transgenic rice with suppression of ω-3 fatty acid desaturases. Plant Cell Physiol. 2007, 48, 1263–1274. [Google Scholar] [CrossRef]
- Seo, S.; Mitsuhara, I.; Feng, J.; Iwai, T.; Hasegawa, M.; Ohashi, Y. Cyanide, a coproduct of plant hormone ethylene biosynthesis, contributes to the resistance of rice to blast fungus. Plant Physiol. 2011, 155, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, E.E.; Wang, Q.; Yang, Y. Transgenic rice with inducible ethylene production exhibits broad-spectrum disease resistance to the fungal pathogens Magnaporthe oryzae and Rhizoctonia solani. Plant Biotechnol. J. 2013, 11, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Maeda, S.; Sugano, S.; Ohtake, M.; Hayashi, N.; Ichikawa, T.; Kondou, Y.; Kuroda, H.; Horii, Y.; Matsui, M.; et al. Screening for resistance against Pseudomonas syringae in rice-FOX Arabidopsis lines identified a putative receptor-like cytoplasmic kinase gene that confers resistance to major bacterial and fungal pathogens in Arabidopsis and rice. Plant Biotechnol. J. 2011, 9, 466–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, X.; Wang, X.; Chu, C. OsWRKY71, a rice transcription factor, is involved in rice defense response. J. Plant Physiol. 2007, 164, 969–979. [Google Scholar] [CrossRef]
- Peng, Y.; Bartley, L.E.; Canlas, P.; Ronald, P.C. OsWRKY IIa transcription factors modulate rice innate immunity. Rice 2010, 3, 36–42. [Google Scholar] [CrossRef]
- Chujo, T.; Miyamoto, K.; Shimogawa, T.; Shimizu, T.; Otake, Y.; Yokotani, N.; Nishizawa, Y.; Shibuya, N.; Nojiri, H.; Yamane, H.; et al. OsWRKY28, a PAMP-responsive transrepressor, negatively regulates innate immune responses in rice against rice blast fungus. Plant Mol. Biol. 2013, 82, 23–37. [Google Scholar] [CrossRef]
- Koiwai, H.; Tagiri, A.; Katoh, S.; Katoh, E.; Ichikawa, H.; Minami, E.; Nishizawa, Y. RING-H2 type ubiquitin ligase EL5 is involved in root development through the maintenance of cell viability in rice. Plant J. 2007, 51, 92–104. [Google Scholar] [CrossRef]
- Sazuka, T.; Kamiya, N.; Nishimura, T.; Ohmae, K.; Sato, Y.; Imamura, K.; Nagato, Y.; Koshiba, T.; Nagamura, Y.; Ashikari, M.; et al. A rice tryptophan deficient dwarf mutant, tdd1, contains a reduced level of indole acetic acid and develops abnormal flowers and organless embryos. Plant J. 2009, 60, 227–241. [Google Scholar] [CrossRef]
- Kudo, T.; Makita, N.; Kojima, M.; Tokunaga, H.; Sakakibara, H. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin-O-glucosyltransferase in rice. Plant Physiol. 2012, 160, 319–331. [Google Scholar] [CrossRef]
- Kiribuchi, K.; Sugimori, M.; Takeda, M.; Otani, T.; Okada, K.; Onodera, H.; Ugaki, M.; Tanaka, Y.; Tomiyama-Akimoto, C.; Yamaguchi, T.; et al. RERJ1, a jasmonic acid-responsive gene from rice, encodes a basic helix-loop-helix protein. Biochem. Biophys. Res. Commun. 2004, 325, 857–863. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kawabe, A.; Tokida-Segawa, A.; Shimizu, B.I.; Takatsuto, S.; Shimada, Y.; Fujioka, S.; Mizutani, M. Rice CYP734As function as multisubstrate and multifunctional enzymes in brassinosteroid catabolism. Plant J. 2011, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nakagawa, H.; Tomita, C.; Shimatani, Z.; Ohtake, M.; Nomura, T.; Jiang, C.-J.; Dubouzet, J.G.; Kikuchi, S.; Sekimoto, H.; et al. BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol. 2009, 151, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Piao, R.; Shi, J.; Lee, S.I.; Jiang, W.; Kim, B.K.; Lee, J.; Han, L.; Ma, W.; Koh, H.J. Fine mapping and candidate gene analysis of dense and erect panicle 3, dep3, which confers high grain yield in rice (Oryza sativa L.). Appl. Genet. 2011, 122, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huang, H.-J.; Ren, S.-T.; Li, J.-J.; Sun, Y.; Sun, D.-Y.; Zhang, S.-Q. The rice wall-associated receptor-like kinase gene OsDEES1 plays a role in female gametophyte development. Plant Physiol. 2012, 160, 696–707. [Google Scholar] [CrossRef]
- Du, H.; Wu, N.; Cui, F.; You, L.; Li, X.; Xiong, L. A homolog of ETHYLENE OVERPRODUCER, OsETOL1, differentially modulates drought and submergence tolerance in rice. Plant J. 2014, 78, 834–849. [Google Scholar] [CrossRef]
- Yoo, Y.-H.; Nalini Chandran, A.K.; Park, J.-C.; Gho, Y.-S.; Lee, S.-W.; An, G.; Jung, K.-H. OsPhyB-mediating novel regulatory pathway for drought tolerance in rice root identified by a global RNA-Seq transcriptome analysis of rice genes in response to water deficiencies. Front. Plant Sci. 2017, 8, 580. [Google Scholar] [CrossRef]
- Ho, C.-L.; Wu, Y.; Shen, H.; Provart, N.J.; Geisler, M. A predicted protein interactome for rice. Rice 2012, 5, 15. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef]
- Singh, M.; Bhatia, P.; Sharma, P.; Khosla, B. Characterization for plant growth promoting rhizobacteria (PGPR) towards rice (Oryza sativa) seedling germination and growth. Ann. Biol. 2014, 30, 567–573. [Google Scholar]
- FastQC, version 0.11.7; Babraham Bioinformatics: Cambridge, UK. Available online: https://www.bioinformatics.babraham.ac.uk /projects/fastqc/ (accessed on 26 April 2010).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Trim Galore, version 0.5.0; Babraham Bioinformatics: Cambridge, UK. Available online: http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 14 March 2012).
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Zhu, W.; Hamilton, J.; Lin, H.; Campbell, M.; Childs, K.; Thibaud-Nissen, F.; Malek, R.L.; Lee, Y.; Zheng, L.; et al. The TIGR rice genome annotation resource: Improvements and new features. Nucleic Acids Res. 2007, 35, D883–D887. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef]
- Hoang, T.V.; Vo, K.T.X.; Hong, W.J.; Jung, K.H.; Jeon, J.S. Defense response to pathogens through Epigenetic regulation in rice. J. Plant Biol. 2018, 61, 1–10. [Google Scholar] [CrossRef]
- Xuan, Y.H.; Kumar, V.; Zhu, X.F.; Je, B.I.; Kim, C.M.; Huang, J.; Cho, J.H.; Yi, G.; Han, C.-d. IDD10 is involved in the interaction between NH4+ and auxin signaling in rice roots. J. Plant Biol. 2018, 61, 72–79. [Google Scholar] [CrossRef]
- Cao, P.; Jung, K.-H.; Choi, D.; Hwang, D.; Zhu, J.; Ronald, P.C. The rice oligonucleotide array database: An atlas of rice gene expression. Rice 2012, 5, 17. [Google Scholar] [CrossRef]
- Urbanczyk-Wochniak, E.; Usadel, B.; Thimm, O.; Nunes-Nesi, A.; Carrari, F.; Davy, M.; Bläsing, O.; Kowalczyk, M.; Weicht, D.; Polinceusz, A.; et al. Conversion of MapMan to allow the analysis of transcript data from Solanaceous species: Effects of genetic and environmental alterations in energy metabolism in the leaf. Plant Mol. Biol. 2006, 60, 773–792. [Google Scholar] [CrossRef]
- Usadel, B.; Nagel, A.; Thimm, O.; Redestig, H.; Blaesing, O.E.; Palacios-Rojas, N.; Selbig, J.; Hannemann, J.; Piques, M.C.; Steinhauser, D.; et al. Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiol. 2005, 138, 1195–1204. [Google Scholar] [CrossRef]
- Yamamoto, E.; Yonemaru, J.; Yamamoto, T.; Yano, M. OGRO: The overview of functionally characterized genes in rice online database. Rice 2012, 5, 26. [Google Scholar] [CrossRef]
- Jain, M.; Nijhawan, A.; Tyagi, A.K.; Khurana, J.P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006, 345, 646–651. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).