Mycobacterium abscessus, an Emerging and Worrisome Pathogen among Cystic Fibrosis Patients
Abstract
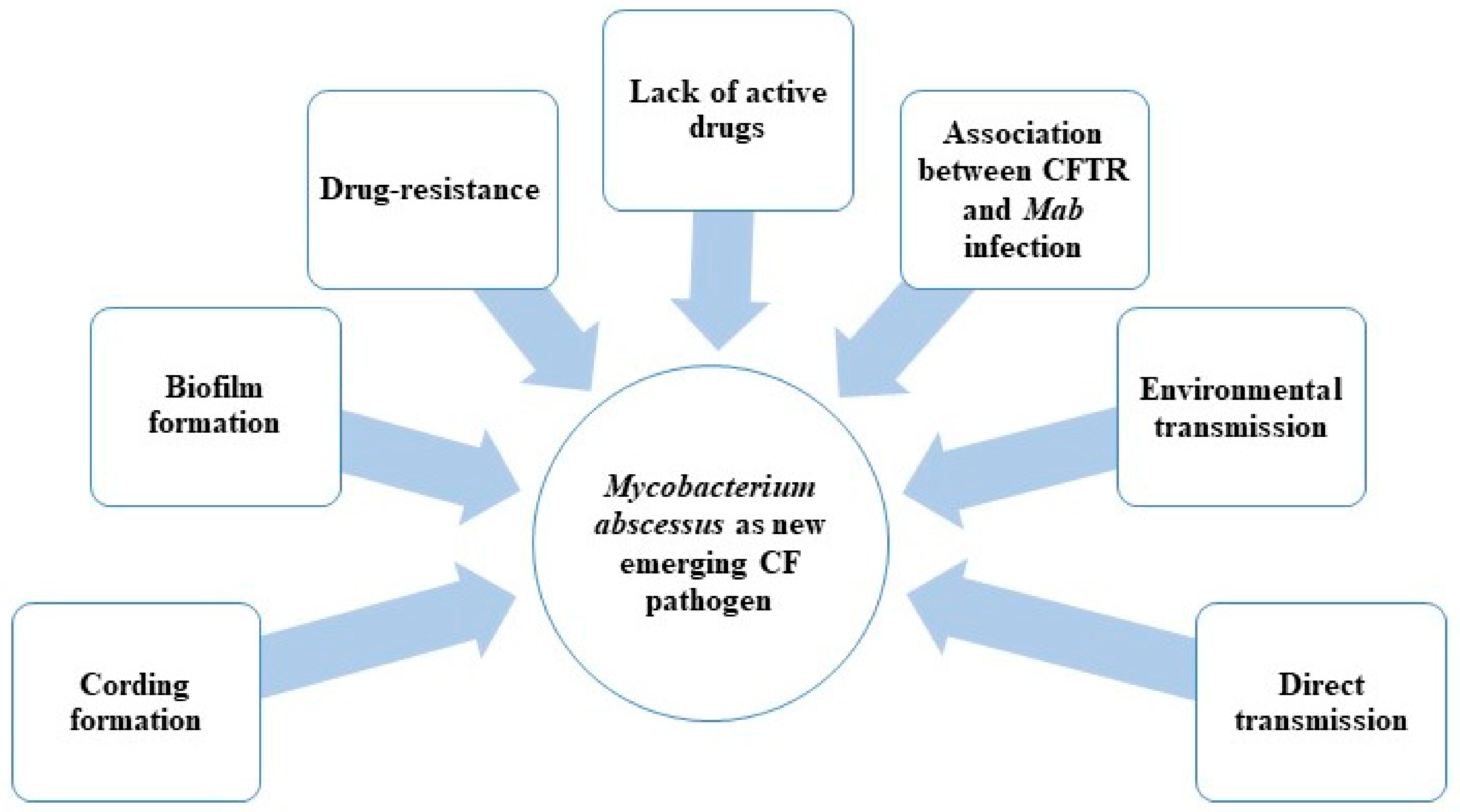
1. Introduction
- Possible direct person-to-person transmission;
- Biofilm and drug resistance;
- Association between CFTR (cystic fibrosis transmembrane conductance regulator) mutations and formation of granuloma in the presence of Mab infection;
- Lack of active drugs (in particular with bactericidal activity) (Figure 1).
2. Possible M. abscessus Direct Transmission among CF Patients
3. Pathogenesis of M. abscessus
- -
- Arrest of lipid transport;
- -
- Production of serpentine cords;
- -
- Growth as extracellular cords, allowing escape from the innate immune defenses;
- -
CFTR Mutations Specialize M. abscessus as CF Pathogen
4. Current Therapy against M. abscessus Infections
Mechanisms of Resistance to Drugs Used in Therapy
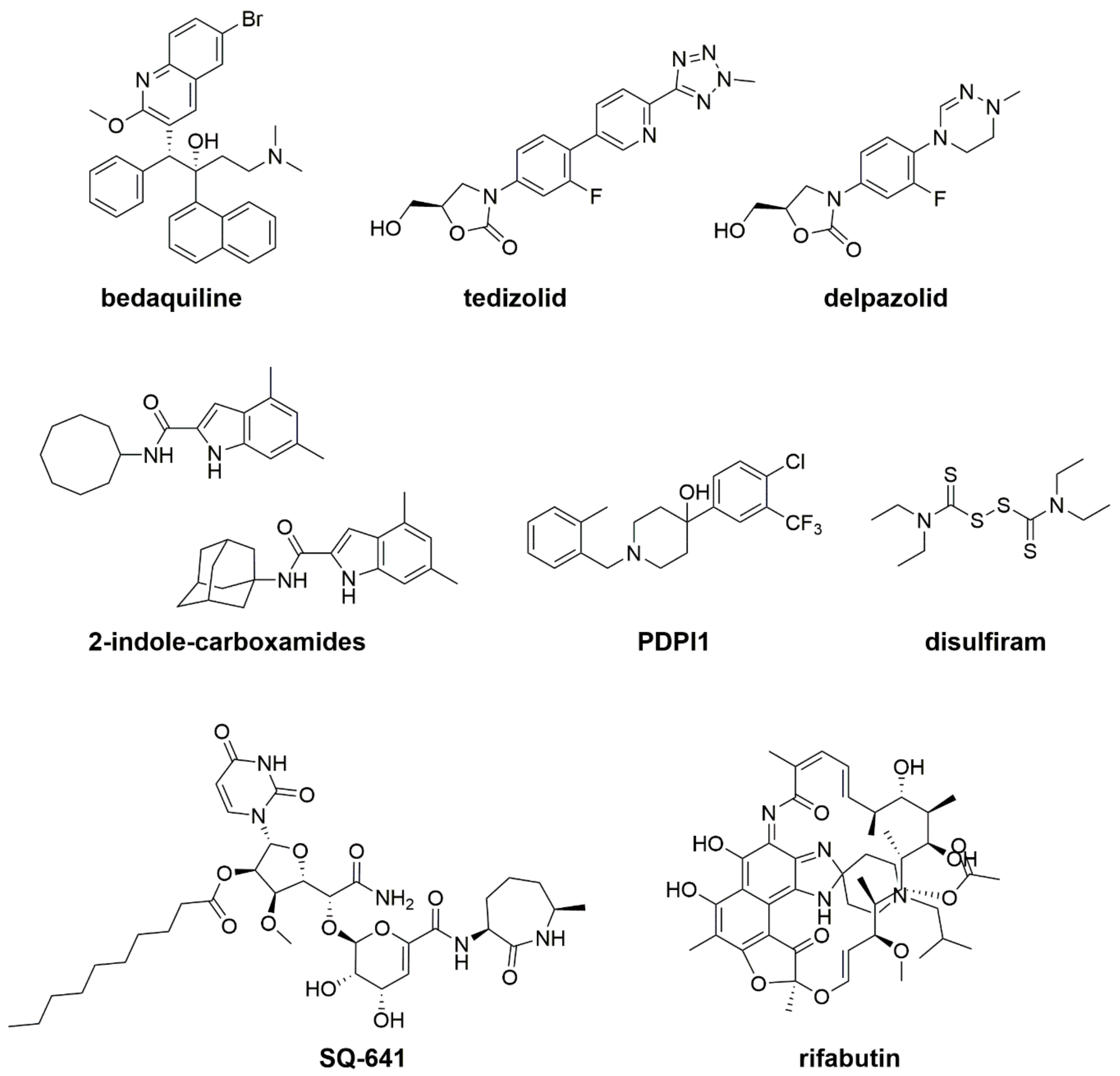
5. New Drugs and New Treatments in Preclinical and Clinical Trials
5.1. Bedaquiline
5.2. New Oxazolidinone Derivatives: Tedizolid and Delpazolid
5.3. MmpL3 Inhibitors: Indole-2-Carboxamides and PIPD1
5.4. Capuramycin SQ641
5.5. Repurposing and Repositioning Drugs: Rifabutin, Disulfiram, and β-Lactams
5.6. Tigecycline
5.7. Inhaled Formulation Nitric Oxide
5.8. Liposomal Amikacin for Inhalation
5.9. Inhaled Molgramostim
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pranke, I.; Golec, A.; Hinzpeter, A.; Edelman, A.; Sermet-Gaudelus, I. Emerging Therapeutic Approaches for Cystic Fibrosis. From Gene Editing to Personalized Medicine. Front. Pharmacol. 2019, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Skolnik, K.; Kirkpatrick, G.; Quon, B.S. Nontuberculous Mycobacteria in Cystic Fibrosis. Curr. Treat. Options Infect. Dis. 2016, 8, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Viviani, L.; Harrison, M.J.; Zolin, A.; Haworth, C.S.; Floto, R.A. Epidemiology of nontuberculous mycobacteria (NTM) amongst individuals with cystic fibrosis (CF). J. Cyst. Fibros. 2016, 15, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Martiniano, S.L.; Nick, J.A.; Daley, C.L. Nontuberculous Mycobacterial Infections in Cystic Fibrosis. Thorac. Surg. Clin. 2019, 29, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Daniel-Wayman, S.; Abate, G.; Barber, D.L.; Bermudez, L.E.; Coler, R.N.; Cynamon, M.H.; Daley, C.L.; Davidson, R.M.; Dick, T.; Floto, R.A.; et al. Advancing Translational Science for Pulmonary Nontuberculous Mycobacterial Infections. A Road Map for Research. Am. J. Respir. Crit. Care Med. 2019, 199, 947–951. [Google Scholar] [CrossRef]
- Salsgiver, E.L.; Fink, A.K.; Knapp, E.A.; LiPuma, J.J.; Olivier, K.N.; Marshall, B.C.; Saiman, L. Changing Epidemiology of the Respiratory Bacteriology of Patients with Cystic Fibrosis. Chest 2016, 149, 390–400. [Google Scholar] [CrossRef]
- Mougari, F.; Guglielmetti, L.; Raskine, L.; Sermet-Gaudelus, I.; Veziris, N.; Cambau, E. Infections caused by Mycobacterium abscessus: Epidemiology, diagnostic tools and treatment. Expert Rev. Anti-Infect. Ther. 2016, 14, 1139–1154. [Google Scholar] [CrossRef]
- Wu, M.L.; Aziz, D.B.; Dartois, V.; Dick, T. NTM drug discovery: Status, gaps and the way forward. Drug Discov. Today 2018, 23, 1502–1519. [Google Scholar] [CrossRef]
- Qvist, T.; Pressler, T.; Høiby, N.; Katzenstein, T.L. Shifting paradigms of nontuberculous mycobacteria in cystic fibrosis. Respir. Res. 2014, 15, 41. [Google Scholar] [CrossRef]
- Andrew, E.C.; Connell, T.; Robinson, P.; Curtis, N.; Massie, J.; Robertson, C.; Harrison, J.; Shanthikumar, S.; Bryant, P.A.; Starr, M.; et al. Pulmonary Mycobacterium abscessus complex in children with cystic fibrosis: A practical management guideline. J. Paediatr. Child Health 2019, 55, 502–511. [Google Scholar] [CrossRef]
- van Dorn, A. Multidrug-resistant Mycobacterium abscessus threatens patients with cystic fibrosis. Lancet Respir. Med. 2017, 5, 15. [Google Scholar] [CrossRef]
- Stephenson, D.; Perry, A.; Appleby, M.R.; Lee, D.; Davison, J.; Johnston, A.; Jones, A.L.; Nelson, A.; Bourke, S.J.; Thomas, M.F.; et al. An evaluation of methods for the isolation of nontuberculous mycobacteria from patients with cystic fibrosis, bronchiectasis and patients assessed for lung transplantation. BMC Pulm. Med. 2019, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.M. A Closer Look at the Genomic Variation of Geographically Diverse Mycobacterium abscessus Clones That Cause Human Infection and Disease. Front. Microbiol. 2018, 9, 2988. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.M.; Grogono, D.M.; Rodriguez-Rincon, D.; Everall, I.; Brown, K.P.; Moreno, P.; Verma, D.; Hill, E.; Drijkoningen, J.; Gilligan, P.; et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 2016, 354, 751–757. [Google Scholar] [CrossRef]
- Lopeman, R.C.; Harrison, J.; Desai, M.; Cox, J.A.G. Mycobacterium abscessus: Environmental Bacterium Turned Clinical Nightmare. Microorganisms 2019, 7, 90. [Google Scholar] [CrossRef]
- Thomson, R.; Tolson, C.; Sidjabat, H.; Huygens, F.; Hargreaves, M. Mycobacterium abscessus isolated from municipal water—A potential source of human infection. BMC Infect. Dis. 2013, 13, 241. [Google Scholar] [CrossRef]
- Floto, R.A.; Olivier, K.N.; Saiman, L.; Daley, C.L.; Herrmann, J.L.; Nick, J.A.; Noone, P.G.; Bilton, D.; Corris, P.; Gibson, R.L.; et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016, 71 (Suppl. 1), i1–i22. [Google Scholar] [CrossRef]
- Sood, G.; Parrish, N. Outbreaks of nontuberculous mycobacteria. Curr. Opin. Infect. Dis. 2017, 30, 404–409. [Google Scholar] [CrossRef]
- King, D.N.; Donohue, M.J.; Vesper, S.J.; Villegas, E.N.; Ware, M.W.; Vogel, M.E.; Furlong, E.F.; Kolpin, D.W.; Glassmeyer, S.T.; Pfaller, S. Microbial pathogens in source and treated waters from drinking water treatment plants in the United States and implications for human health. Sci. Total Environ. 2016, 562, 987–995. [Google Scholar] [CrossRef]
- Thomson, R.; Tolson, C.; Carter, R.; Coulter, C.; Huygens, F.; Hargreaves, M. Isolation of nontuberculous mycobacteria (NTM) from household water and shower aerosols in patients with pulmonary disease caused by NTM. J. Clin. Microbiol. 2013, 51, 3006–3011. [Google Scholar] [CrossRef]
- Torvinen, E.; Suomalainen, S.; Paulin, L.; Kusnetsov, J. Mycobacteria in Finnish cooling tower waters. Apmis 2014, 122, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Alqumber, M.A. Prevalence of mycobacteria in water reservoirs of Albaha, Saudi Arabia. Saudi Med. J. 2014, 35, 466–471. [Google Scholar] [PubMed]
- Williams, M.M.; Chen, T.H.; Keane, T.; Toney, N.; Toney, S.; Armbruster, C.R.; Butler, W.R.; Arduino, M.J. Point-of-use membrane filtration and hyperchlorination to prevent patient exposure to rapidly growing mycobacteria in the potable water supply of a skilled nursing facility. Infect. Control Hosp. Epidemiol. 2011, 32, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Honda, J.R.; Hasan, N.A.; Davidson, R.M.; Williams, M.D.; Epperson, L.E.; Reynolds, P.R.; Smith, T.; Iakhiaeva, E.; Bankowski, M.J.; Wallace, R.J., Jr.; et al. Environmental Nontuberculous Mycobacteria in the Hawaiian Islands. PLoS Negl. Trop. Dis. 2016, 10, e0005068. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yu, Z.; Zhang, H.; Yang, M.; Shi, B.; Liu, X. Diversity of bacteria and mycobacteria in biofilms of two urban drinking water distribution systems. Can. J. Microbiol. 2012, 58, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Delafont, V.; Mougari, F.; Cambau, E.; Joyeux, M.; Bouchon, D.; Héchard, Y.; Moulin, L. First evidence of amoebae-mycobacteria association in drinking water network. Environ. Sci. Technol. 2014, 48, 11872–11882. [Google Scholar] [CrossRef]
- Donohue, M.J.; Mistry, J.H.; Donohue, J.M.; O’Connell, K.; King, D.; Byran, J.; Covert, T.; Pfaller, S. Increased Frequency of Nontuberculous Mycobacteria Detection at Potable Water Taps within the United States. Environ. Sci. Technol. 2015, 49, 6127–6133. [Google Scholar] [CrossRef]
- Puk, K.; Banach, T.; Wawrzyniak, A.; Adaszek, Ł.; Ziętek, J.; Winiarczyk, S.; Guz, L. Detection of Mycobacterium marinum, M. peregrinum, M. fortuitum and M. abscessus in aquarium fish. J. Fish Dis. 2018, 41, 153–156. [Google Scholar] [CrossRef]
- Schets, F.M.; van den Berg, H.H.; de Zwaan, R.; van Soolingen, D.; de Roda Husman, A.M. The microbiological quality of water in fish spas with Garra rufa fish, the Netherlands, October to November 2012. Euro Surveill. 2015, 20, 2–8. [Google Scholar] [CrossRef]
- Varello, K.; Prearo, M.; Serracca, L.; Meloni, D.; Rossini, I.; Righetti, M.; Pezzolato, M.; Fioravanti, M.L.; Ercolini, C.; Bozzetta, E. Granulomatous lesions in a wild mullet population from the eastern Ligurian Sea (Italy): Mycobacteriosis vs. pseudotuberculosis. J. Fish Dis. 2014, 37, 553–558. [Google Scholar] [CrossRef]
- Favaro, L.; Scanzio, T.; Varello, K.; Caffara, M.; Righetti, M.; Bozzetta, E.; Prearo, M. Mixed mycobacterial infection in an adult koi carp Cyprinus carpio L. J. Fish Dis. 2014, 37, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, R.G.; Florio, D.; Fioravanti, M.L.; Rossi, M.; Prearo, M. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J. Fish Dis. 2008, 31, 433–441. [Google Scholar] [PubMed]
- Chang, T.C.; Hsieh, C.Y.; Chang, C.D.; Shen, Y.L.; Huang, K.C.; Tu, C.; Chen, L.C.; Wu, Z.B.; Tsai, S.S. Pathological and molecular studies on mycobacteriosis of milkfish Chanos chanos in Taiwan. Dis. Aquat. Org. 2006, 72, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Reisfeld, L.; Ikuta, C.Y.; Ippolito, L.; Silvatti, B.; Ferreira Neto, J.S.; Catão-Dias, J.L.; Rosas, F.C.W.; Neto, J.A.; da Silva, V.M.F. Cutaneous mycobacteriosis in a captive Amazonian manatee Trichechus inunguis. Dis. Aquat. Org. 2018, 127, 231–236. [Google Scholar] [CrossRef]
- Katale, B.Z.; Mbugi, E.V.; Botha, L.; Keyyu, J.D.; Kendall, S.; Dockrell, H.M.; Michel, A.L.; Kazwala, R.R.; Rweyemamu, M.M.; van Helden, P.; et al. Species diversity of non-tuberculous mycobacteria isolated from humans, livestock and wildlife in the Serengeti ecosystem, Tanzania. BMC Infect. Dis. 2014, 14, 616. [Google Scholar] [CrossRef]
- Clayton, L.A.; Stamper, M.A.; Whitaker, B.R.; Hadfield, C.A.; Simons, B.; Mankowski, J.L. Mycobacterium abscessus pneumonia in an Atlantic bottlenose dolphin (Tursiops truncatus). J. Zoo Wildl. Med. 2012, 43, 961–965. [Google Scholar] [CrossRef]
- Jassies-van der Lee, A.; Houwers, D.J.; Meertens, N.; van der Zanden, A.G.; Willemse, T. Localised pyogranulomatous dermatitis due to Mycobacterium abscessus in a cat: A case report. Vet. J. 2009, 179, 304–306. [Google Scholar] [CrossRef]
- Lunn, J.A.; Martin, P.; Zaki, S.; Malik, R. Pneumonia due to Mycobacterium abscessus in two domestic ferrets (Mustelo putorius furo). Aust. Vet. J. 2005, 83, 542–546. [Google Scholar] [CrossRef]
- Karlson, A.G.; Seibold, H.R.; Wolf, R.H. Mycobacterium abscessus infection in an owl monkey (Aotus trivirgatua). Pathol. Vet. 1970, 7, 448–454. [Google Scholar]
- Jang, S.S.; Hirsh, D.C. Rapidly growing members of the genus Mycobacterium affecting dogs and cats. J. Am. Anim. Hosp. Assoc. 2002, 38, 217–220. [Google Scholar] [CrossRef]
- Falkinham, J.O., 3rd. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg. Infect. Dis. 2011, 17, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.M.; Grogono, D.M.; Greaves, D.; Foweraker, J.; Roddick, I.; Inns, T.; Reacher, M.; Haworth, C.S.; Curran, M.D.; Harris, S.R.; et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: A retrospective cohort study. Lancet 2013, 381, 1551–1560. [Google Scholar] [CrossRef]
- Yan, J.; Kevat, A.; Martinez, E.; Teese, N.; Johnson, K.; Ranganathan, S.; Harrison, J.; Massie, J.; Daley, A. Investigating transmission of Mycobacterium abscessus amongst children in an Australian cystic fibrosis centre. J. Cyst. Fibros. 2019, in press. [Google Scholar] [CrossRef]
- Tortoli, E.; Kohl, T.A.; Trovato, A.; Baldan, R.; Campana, S.; Cariani, L.; Colombo, C.; Costa, D.; Cristadoro, S.; Di Serio, M.C.; et al. Mycobacterium abscessus in patients with cystic fibrosis: Low impact of inter-human transmission in Italy. Eur. Respir. J. 2017, 50, 1602525. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; García-Coca, M. Mycobacterium Biofilms. Front. Microbiol. 2018, 8, 2651. [Google Scholar] [CrossRef] [PubMed]
- Benwill, J.L.; Wallace, R.J., Jr. Mycobacterium abscessus: Challenges in diagnosis and treatment. Curr. Opin. Infect. Dis. 2014, 27, 506–510. [Google Scholar] [CrossRef]
- Qvist, T.; Eickhardt, S.; Kragh, K.N.; Andersen, C.B.; Iversen, M.; Høiby, N.; Bjarnsholt, T. Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur. Respir. J. 2015, 46, 1823–1826. [Google Scholar] [CrossRef]
- Fennelly, K.P.; Ojano-Dirain, C.; Yang, Q.; Liu, L.; Lu, L.; Progulske-Fox, A.; Wang, G.P.; Antonelli, P.; Schultz, G. Biofilm Formation by Mycobacterium abscessus in a Lung Cavity. Am. J. Respir. Crit. Care Med. 2016, 193, 692–693. [Google Scholar] [CrossRef]
- Hunt-Serracin, A.C.; Parks, B.J.; Boll, J.; Boutte, C. Biofilm-associated Mycobacterium abscessus cells have altered antibiotic tolerance and surface glycolipids in Artificial Cystic Fibrosis Sputum Media. Antimicrob. Agents Chemother. 2019, 63, e02488-18. [Google Scholar] [CrossRef]
- Gutiérrez, A.V.; Viljoen, A.; Ghigo, E.; Herrmann, J.L.; Kremer, L. Glycopeptidolipids, a Double-Edged Sword of the Mycobacterium abscessus Complex. Front. Microbiol. 2018, 9, 1145. [Google Scholar] [CrossRef]
- Davidson, L.B.; Nessar, R.; Kempaiah, P.; Perkins, D.J.; Byrd, T.F. Mycobacterium abscessus glycopeptidolipid prevents respiratory epithelial TLR2 signaling as measured by HβD2 gene expression and IL-8 release. PLoS ONE 2011, 6, e29148. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Sweet, L. The mycobacterial glycopeptidolipids: Structure, function, and their role in pathogenesis. Glycobiology 2008, 18, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.T.; Rhoades, E.; Recht, J.; Pang, X.; Alsup, A.; Kolter, R.; Lyons, C.R.; Byrd, T.F. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 2006, 152, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Rüger, K.; Hampel, A.; Billig, S.; Rücker, N.; Suerbaum, S.; Bange, F.C. Characterization of rough and smooth morphotypes of Mycobacterium abscessus isolates from clinical specimens. J. Clin. Microbiol. 2014, 52, 244–250. [Google Scholar] [CrossRef]
- Bernut, A.; Herrmann, J.L.; Kissa, K.; Dubremetz, J.F.; Gaillard, J.L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA 2014, 111, E943–E952. [Google Scholar] [CrossRef]
- Bernut, A.; Viljoen, A.; Dupont, C.; Sapriel, G.; Blaise, M.; Bouchier, C.; Brosch, R.; de Chastellier, C.; Herrmann, J.L.; Kremer, L. Insights into the smooth-to-rough transitioning in Mycobacterium bolletii unravels a functional Tyr residue conserved in all mycobacterial MmpL family members. Mol. Microbiol. 2016, 99, 866–883. [Google Scholar] [CrossRef]
- Medjahed, H.; Reyrat, J.M. Construction of Mycobacterium abscessus defined glycopeptidolipid mutants: Comparison of genetic tools. Appl. Environ. Microbiol. 2009, 75, 1331–1338. [Google Scholar] [CrossRef]
- Nessar, R.; Reyrat, J.M.; Davidson, L.B.; Byrd, T.F. Deletion of the mmpL4b gene in the Mycobacterium abscessus glycopeptidolipid biosynthetic pathway results in loss of surface colonization capability, but enhanced ability to replicate in human macrophages and stimulate their innate immune response. Microbiology 2011, 157, 1187–1195. [Google Scholar] [CrossRef]
- Bernut, A.; Herrmann, J.L.; Ordway, D.; Kremer, L. The diverse cellular and animal models to decipher the physiopathological traits of Mycobacterium abscessus infection. Front. Cell Infect. Microbiol. 2017, 7, 100. [Google Scholar] [CrossRef]
- Halloum, I.; Carrère-Kremer, S.; Blaise, M.; Viljoen, A.; Bernut, A.; Le Moigne, V.; Vilchèze, C.; Guérardel, Y.; Lutfalla, G.; Herrmann, J.L.; et al. Deletion of a dehydratase important for intracellular growth and cording renders rough Mycobacterium abscessus avirulent. Proc. Natl. Acad. Sci. USA 2016, 113, E4228–E4237. [Google Scholar] [CrossRef]
- Koh, W.J.; Jeong, B.H.; Kim, S.Y.; Jeon, K.; Park, K.U.; Jhun, B.W.; Lee, H.; Park, H.Y.; Kim, D.H.; Huh, H.J.; et al. Mycobacterial Characteristics and Treatment Outcomes in Mycobacterium abscessus Lung Disease. Clin. Infect. Dis. 2017, 64, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Esther, C.R., Jr.; Esserman, D.A.; Gilligan, P.; Kerr, A.; Noone, P.G. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. 2010, 9, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Ardito, F.; Fiscarelli, E.; La Sorda, M.; D’Argenio, P.; Ricciotti, G.; Fadda, G. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J. Clin. Microbiol. 2001, 39, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, B.E.; Gilljam, M.; Lindblad, A.; Ridell, M.; Wold, A.E.; Welinder Olsson, C. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J. Clin. Microbiol. 2007, 45, 1497–1504. [Google Scholar] [CrossRef]
- Qvist, T.; Taylor-Robinson, D.; Waldmann, E.; Olesen, H.V.; Hansen, C.R.; Mathiesen, I.H.; Høiby, N.; Katzenstein, T.L.; Smyth, R.L.; Diggle, P.J.; et al. Comparing the harmful effects of nontuberculous mycobacteria and Gram negative bacteria on lung function in patients with cystic fibrosis. J. Cyst. Fibros. 2016, 15, 380–385. [Google Scholar] [CrossRef]
- Dubois, V.; Viljoen, A.; Laencina, L.; Le Moigne, V.; Bernut, A.; Dubar, F.; Blaise, M.; Gaillard, J.L.; Guérardel, Y.; Kremer, L.; et al. MmpL8(MAB) controls Mycobacterium abscessus virulence and production of a previously unknown glycolipid family. Proc. Natl. Acad. Sci. USA 2018, 115, E10147–E10156. [Google Scholar] [CrossRef]
- Laencina, L.; Dubois, V.; Le Moigne, V.; Viljoen, A.; Majlessi, L.; Pritchard, J.; Bernut, A.; Piel, L.; Roux, A.L.; Gaillard, J.L.; et al. Identification of genes required for Mycobacterium abscessus growth in vivo with a prominent role of the ESX-4 locus. Proc. Natl. Acad. Sci. USA 2018, 115, E1002–E1011. [Google Scholar] [CrossRef]
- Bernut, A.; Nguyen-Chi, M.; Halloum, I.; Herrmann, J.L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus-Induced Granuloma Formation Is Strictly Dependent on TNF Signaling and Neutrophil Trafficking. PLoS Pathog. 2016, 12, e1005986. [Google Scholar] [CrossRef]
- Bernut, A.; Dupont, C.; Ogryzko, N.V.; Neyret, A.; Herrmann, J.L.; Floto, R.A.; Renshaw, S.A.; Kremer, L. CFTR Protects against Mycobacterium abscessus Infection by Fine-Tuning Host Oxidative Defenses. Cell Rep. 2019, 26, 1828–1840.e4. [Google Scholar] [CrossRef]
- Leung, J.M.; Olivier, K.N. Nontuberculous mycobacteria: The changing epidemiology and treatment challenges in cystic fibrosis. Curr. Opin. Pulm. Med. 2013, 19, 662–669. [Google Scholar] [CrossRef]
- Floto, R.A.; Olivier, K.N.; Saiman, L.; Daley, C.L.; Herrmann, J.L.; Nick, J.A.; Noone, P.G.; Bilton, D.; Corris, P.; Gibson, R.L.; et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: Executive summary. Thorax 2016, 71, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Lobo, L.J.; Chang, L.C.; Esther, C.R., Jr.; Gilligan, P.H.; Tulu, Z.; Noone, P.G. Lung transplant outcomes in cystic fibrosis patients with pre-operative Mycobacterium abscessus respiratory infections. Clin. Transplant. 2013, 27, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, in press. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Luthra, S.; Rominski, A.; Sander, P. The Role of Antibiotic-Target-Modifying and Antibiotic-Modifying Enzymes in Mycobacterium abscessus Drug Resistance. Front. Microbiol. 2018, 9, 2179. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef]
- Nash, K.A.; Brown-Elliott, B.A.; Wallace, R.J., Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob. Agents Chemother. 2009, 53, 1367–1376. [Google Scholar] [CrossRef]
- Pfister, P.; Jenni, S.; Poehlsgaard, J.; Thomas, A.; Douthwaite, S.; Ban, N.; Böttger, E.C. The structural basis of macrolide-ribosome binding assessed using mutagenesis of 23S rRNA positions 2058 and 2059. J. Mol. Biol. 2004, 342, 1569–1581. [Google Scholar] [CrossRef]
- Hurst-Hess, K.; Rudra, P.; Ghosh, P. Mycobacterium abscessus WhiB7 Regulates a Species-Specific Repertoire of Genes to Confer Extreme Antibiotic Resistance. Antimicrob. Agents Chemother. 2017, 61, e01347-17. [Google Scholar] [CrossRef]
- Prammananan, T.; Sander, P.; Brown, B.A.; Frischkorn, K.; Onyi, G.O.; Zhang, Y.; Böttger, E.C.; Wallace, R.J., Jr. A single 16S ribosomal RNA substitution is responsible for resistance to amikacin and other 2-deoxystreptamine aminoglycosides in Mycobacterium abscessus and Mycobacterium chelonae. J. Infect. Dis. 1998, 177, 1573–1581. [Google Scholar] [CrossRef]
- Nessar, R.; Reyrat, J.M.; Murray, A.; Gicquel, B. Genetic analysis of new 16S rRNA mutations conferring aminoglycoside resistance in Mycobacterium abscessus. J. Antimicrob. Chemother. 2011, 66, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Soroka, D.; Dubée, V.; Soulier-Escrihuela, O.; Cuinet, G.; Hugonnet, J.E.; Gutmann, L.; Mainardi, J.L.; Arthur, M. Characterization of broad-spectrum Mycobacterium abscessus class A β-lactamase. J. Antimicrob. Chemother. 2014, 69, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Rudra, P.; Hurst-Hess, K.; Lappierre, P.; Ghosh, P. High Levels of Intrinsic Tetracycline Resistance in Mycobacterium abscessus Are Conferred by a Tetracycline-Modifying Monooxygenase. Antimicrob. Agents Chemother. 2018, 62, e00119-18. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.; Gutiérrez, A.V.; Viljoen, A.; Rodriguez-Rincon, D.; Roquet-Baneres, F.; Blaise, M.; Everall, I.; Parkhill, J.; Floto, R.A.; Kremer, L. Mutations in the MAB_2299c TetR Regulator Confer Cross-Resistance to Clofazimine and Bedaquiline in Mycobacterium abscessus. Antimicrob. Agents Chemother. 2018, 63, e01316-18. [Google Scholar] [CrossRef]
- Guillemin, I.; Jarlier, V.; Cambau, E. Correlation between quinolone susceptibility patterns and sequences in the A and B subunits of DNA gyrase in mycobacteria. Antimicrob. Agents Chemother. 1998, 42, 2084–2088. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jhun, B.W.; Moon, S.M.; Shin, S.H.; Jeon, K.; Kwon, O.J.; Yoo, I.Y.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; et al. Mutations in gyrA and gyrB in Moxifloxacin-Resistant Mycobacterium avium Complex and Mycobacterium abscessus Complex Clinical Isolates. Antimicrob. Agents Chemother. 2018, 62, e00527-18. [Google Scholar] [CrossRef]
- Ye, M.; Xu, L.; Zou, Y.; Li, B.; Guo, Q.; Zhang, Y.; Zhan, M.; Xu, B.; Yu, F.; Zhang, Z.; et al. Molecular Analysis of Linezolid-Resistant Clinical Isolates of Mycobacterium abscessus. Antimicrob. Agents Chemother. 2019, 63, e01842-18. [Google Scholar] [CrossRef]
- Hansen, J.L.; Ippolito, J.A.; Ban, N.; Nissen, P.; Moore, P.B.; Steitz, T.A. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Mol. Cell 2002, 10, 117–128. [Google Scholar] [CrossRef]
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef]
- Hartkoorn, R.C.; Uplekar, S.; Cole, S.T. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 2979–2981. [Google Scholar] [CrossRef]
- Choo, S.W.; Wee, W.Y.; Ngeow, Y.F.; Mitchell, W.; Tan, J.L.; Wong, G.J.; Zhao, Y.; Xiao, J. Genomic reconnaissance of clinical isolates of emerging human pathogen Mycobacterium abscessus reveals high evolutionary potential. Sci. Rep. 2014, 4, 4061. [Google Scholar] [CrossRef] [PubMed]
- Maurer, F.P.; Bruderer, V.L.; Ritter, C.; Castelberg, C.; Bloemberg, G.V.; Böttger, E.C. Lack of antimicrobial bactericidal activity in Mycobacterium abscessus. Antimicrob. Agents Chemother. 2014, 58, 3828–3836. [Google Scholar] [CrossRef] [PubMed]
- Philley, J.V.; Wallace, R.J., Jr.; Benwill, J.L.; Taskar, V.; Brown-Elliott, B.A.; Thakkar, F.; Aksamit, T.R.; Griffith, D.E. Preliminary Results of Bedaquiline as Salvage Therapy for Patients with Nontuberculous Mycobacterial Lung Disease. Chest 2015, 148, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ye, M.; Guo, Q.; Zhang, Z.; Yang, S.; Ma, W.; Yu, F.; Chu, H. Determination of MIC Distribution and Mechanisms of Decreased Susceptibility to Bedaquiline among Clinical Isolates of Mycobacterium abscessus. Antimicrob. Agents Chemother. 2018, 62, e00175-18. [Google Scholar] [CrossRef]
- Viljoen, A.; Raynaud, C.; Johansen, M.D.; Roquet-Banères, F.; Herrmann, J.L.; Daher, W.; Kremer, L. Improved activity of bedaquiline by verapamil against Mycobacterium abscessus in vitro and in macrophages. Antimicrob. Agents Chemother. 2019, 63, e00705-19. [Google Scholar] [CrossRef]
- Obregón-Henao, A.; Arnett, K.A.; Henao-Tamayo, M.; Massoudi, L.; Creissen, E.; Andries, K.; Lenaerts, A.J.; Ordway, D.J. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob. Agents Chemother. 2015, 59, 6904–6912. [Google Scholar] [CrossRef]
- Lerat, I.; Cambau, E.; Roth Dit Bettoni, R.; Gaillard, J.L.; Jarlier, V.; Truffot, C.; Veziris, N. In Vivo evaluation of antibiotic activity against Mycobacterium abscessus. J. Infect. Dis. 2014, 209, 905–912. [Google Scholar] [CrossRef]
- Dupont, C.; Viljoen, A.; Thomas, S.; Roquet-Banères, F.; Herrmann, J.L.; Pethe, K.; Kremer, L. Bedaquiline Inhibits the ATP Synthase in Mycobacterium abscessus and Is Effective in Infected Zebrafish. Antimicrob. Agents Chemother. 2017, 61, e01225-17. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Wallace, R.J., Jr. In Vitro Susceptibility Testing of Tedizolid against Nontuberculous Mycobacteria. J. Clin. Microbiol. 2017, 55, 1747–1754. [Google Scholar] [CrossRef]
- Kim, T.S.; Choe, J.H.; Kim, Y.J.; Yang, C.S.; Kwon, H.J.; Jeong, J.; Kim, G.; Park, D.E.; Jo, E.K.; Cho, Y.L.; et al. Activity of LCB01-0371, a Novel Oxazolidinone, against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2017, 61, e02752-16. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Onajole, O.K.; Stec, J.; Dupont, C.; Viljoen, A.; Richard, M.; Chaira, T.; Lun, S.; Bishai, W.; Raj, V.S.; et al. Targeting Mycolic Acid Transport by Indole-2-carboxamides for the Treatment of Mycobacterium abscessus Infections. J. Med. Chem. 2017, 60, 5876–5888. [Google Scholar] [CrossRef] [PubMed]
- Franz, N.D.; Belardinelli, J.M.; Kaminski, M.A.; Dunn, L.C.; Calado Nogueira de Moura, V.; Blaha, M.A.; Truong, D.D.; Li, W.; Jackson, M.; North, E.J. Design, synthesis and evaluation of indole-2-carboxamides with pan anti-mycobacterial activity. Bioorg. Med. Chem. 2017, 25, 3746–3755. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.N.; Prathipati, P.K.; Hegde, P.; Li, W.; Graham, K.F.; Mandal, S.; Drescher, K.M.; Destache, C.J.; Ordway, D.; Jackson, M.; et al. Indole-2-Carboxamides Are Active against Mycobacterium abscessus in a Mouse Model of Acute Infection. Antimicrob. Agents Chemother. 2019, 63, e02245-18. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Viljoen, A.; Dubar, F.; Blaise, M.; Bernut, A.; Pawlik, A.; Bouchier, C.; Brosch, R.; Guérardel, Y.; Lelièvre, J.; et al. A new piperidinol derivative targeting mycolic acid transport in Mycobacterium abscessus. Mol. Microbiol. 2016, 101, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, T.; Bogatcheva, E.; Krishnan, M.Y.; Collins, M.T.; Einck, L.; Nacy, C.A.; Reddy, V.M. In vitro antimicrobial activities of capuramycin analogues against non-tuberculous mycobacteria. J. Antimicrob. Chemother. 2010, 65, 2590–2597. [Google Scholar] [CrossRef]
- Aziz, D.B.; Low, J.L.; Wu, M.L.; Gengenbacher, M.; Teo, J.W.P.; Dartois, V.; Dick, T. Rifabutin Is Active against Mycobacterium abscessus Complex. Antimicrob. Agents Chemother. 2017, 61, e00155-17. [Google Scholar] [CrossRef]
- Ganapathy, U.S.; Dartois, V.; Dick, T. Repositioning rifamycins for Mycobacterium abscessus lung disease. Expert Opin. Drug Discov. 2019, 14, 867–878. [Google Scholar] [CrossRef]
- Das, S.; Garg, T.; Chopra, S.; Dasgupta, A. Repurposing disulfiram to target infections caused by non-tuberculous mycobacteria. J. Antimicrob. Chemother. 2019, 74, 1317–1322. [Google Scholar] [CrossRef]
- Story-Roller, E.; Maggioncalda, E.C.; Cohen, K.A.; Lamichhane, G. Mycobacterium abscessus and β-Lactams: Emerging Insights and Potential Opportunities. Front. Microbiol. 2018, 9, 2273. [Google Scholar] [CrossRef]
- Dubee, V.; Bernut, A.; Cortes, M.; Lesne, T.; Dorchene, D.; Lefebvre, A.-L.; Hugonnet, J.E.; Gutmann, L.; Mainardi, J.L.; Herrmann, J.L.; et al. β-Lactamase inhibition by avibactam in Mycobacterium abscessus. J. Antimicrob. Chemother. 2015, 70, 1051–1058. [Google Scholar] [CrossRef]
- Story-Roller, E.; Maggioncalda, E.C.; Lamichhane, G. Select β-Lactam Combinations Exhibit Synergy against Mycobacterium abscessus In Vitro. Antimicrob. Agents Chemother. 2019, 63, e02613-18. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Chen, L.; Manca, C.; Jenkins, S.; Glaser, L.; Vinnard, C.; Stone, G.; Lee, J.; Mathema, B.; Nuermberger, E.L.; et al. Dual β-Lactam Combinations Highly Active against Mycobacterium abscessus Complex In Vitro. MBio 2019, 10, e02895-18. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 1 October 2019).
- Wallace, R.J., Jr.; Dukart, G.; Brown-Elliott, B.A.; Griffith, D.E.; Scerpella, E.G.; Marshall, B. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J. Antimicrob. Chemother. 2014, 69, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Bentur, L.; Gur, M.; Ashkenazi, M.; Livnat-Levanon, G.; Mizrahi, M.; Tal, A.; Ghaffari, A.; Geffen, Y.; Aviram, M.; Efrati, O. Pilot study to test inhaled nitric oxide in cystic fibrosis patients with refractory Mycobacterium abscessus lung infection. J. Cyst. Fibros. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Drug Development Pipeline. Available online: https://www.cff.org/Trials/Pipeline/ (accessed on 1 October 2019).
- Caimmi, D.; Martocq, N.; Trioleyre, D.; Guinet, C.; Godreuil, S.; Daniel, T.; Chiron, R. Positive Effect of Liposomal Amikacin for Inhalation on Mycobacterium abcessus in Cystic Fibrosis Patients. Open Forum Infect. Dis. 2018, 5, ofy034. [Google Scholar] [CrossRef]
- Diacon, A.H.; Pym, A.; Grobusch, M.P.; de los Rios, J.M.; Gotuzzo, E.; Vasilyeva, I.; Leimane, V.; Andries, K.; Bakare, N.; De Marez, T.; et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N. Engl. J. Med. 2014, 371, 723–732. [Google Scholar] [CrossRef]
- Guglielmetti, L.; Jaspard, M.; Le Dû, D.; Lachâtre, M.; Marigot-Outtandy, D.; Bernard, C.; Veziris, N.; Robert, J.; Yazdanpanah, Y.; Caumes, E.; et al. Long-term outcome and safety of prolonged bedaquiline treatment for multidrug-resistant tuberculosis. Eur. Respir. J. 2017, 49, 1601799. [Google Scholar] [CrossRef]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.; Neefs, J.M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef]
- Saxena, A.K.; Singh, A. Mycobacterial tuberculosis Enzyme Targets and their Inhibitors. Curr. Top. Med. Chem. 2019, 19, 337–355. [Google Scholar] [CrossRef]
- Fox, A. Engineered phages stymie drug-resistant infection. Science 2019, 364, 518–519. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Le Run, E.; Arthur, M.; Mainardi, J.L. In Vitro and Intracellular Activity of Imipenem Combined with Tedizolid, Rifabutin, and Avibactam against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2019, 63, e01915-18. [Google Scholar] [CrossRef]
- Lefebvre, A.L.; Le Moigne, V.; Bernut, A.; Veckerlé, C.; Compain, F.; Herrmann, J.L.; Kremer, L.; Arthur, M.; Mainardi, J.L. Inhibition of the β-Lactamase Bla(Mab) by Avibactam Improves the In Vitro and In Vivo Efficacy of Imipenem against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2017, 61, e02440-16. [Google Scholar] [CrossRef] [PubMed]

| Drugs | Targets | Mechanism of Resistance | Enzymes/Proteins Related to Mechanism of Resistance | References |
|---|---|---|---|---|
| Macrolides | 23S rRNA | Mutations in target gene | Rrl (MAB_r5052) | [77,78] |
| Modification of target | Erm(41) (MAB_2297) | [77] | ||
| Induction of WhiB7 activator | Activation of erm(41) (MAB_2297) | [8,79] | ||
| Amynoglicosides | 30S subunit of ribosome | Mutations in target genes | 16S rRNA (rrs, MAB_r5051) | [80,81] |
| RpsL (MAB_3851c) | ||||
| Enzymatic drug modification | AAC(2′) (MAB_4395) | [75] | ||
| Eis2 (MAB_4532c) | [75] | |||
| Induction of WhiB7 activator | Activation of eis2 (MAB_4532c) | [8,79] | ||
| β-lactams | Penicillin-binding protein | Enzymatic drug modification | Bla_Mab (MAB_2875) | [82] |
| Tetracyclines | 30S subunit of ribosome | Enzymatic drug modification | MabTetX (MAB_1496c) | [83] |
| Clofazimine | Mutations in the repressor → Over-expression of an efflux pump | MAB_2299c | [84] | |
| Fluoroquinolones | A subunit of DNA gyrase | Mutations in target gene | GyrA (MAB_0019) | [85,86] |
| Other mechanisms? | not detected | [86] | ||
| Linezolid | 23S rRNA | Mutations in target gene | Rrl (MAB_r5052) | [87] |
| Efflux pumps? | LmrS and MmpL9? |
| Drugs | Development Phase | Target | Mechanism of Resistance | References |
|---|---|---|---|---|
| Bedaquiline | Preclinical studies | ATP synthase | MmpS5-MmpL5 efflux pump | [84,93,94,95,96,97,98] |
| Tedizolid | Preclinical studies | 50S ribosome | - | [99] |
| Delpazolid | Preclinical studies | 50S ribosome | - | [100] |
| Indole-2-carboxamides | Preclinical studies | MmpL3 | - | [101,102,103] |
| PIPD1 | Preclinical studies | MmpL3 | - | [104] |
| SQ641 | Preclinical studies | Translocase-1 | - | [105] |
| Rifabutin | Preclinical studies | RNA polymerase | [106,107] | |
| Disulfiram | Preclinical studies | - | - | [108] |
| β-lactams (combinations) | Preclinical studies | Penicillin-binding protein | Bla_Mab | [82,109,110,111,112] |
| Tigecycline | Phase II | 30S subunit of ribosome | - | [83,113,114] |
| Nitric oxide | Phase II | - | - | [113,115,116] |
| Liposomal Amikacin for Inhalation | Phase II | 23S rRNA | - | [113,116,117] |
| Inhaled Molgramostim | Phase II | - | - | [113,116] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degiacomi, G.; Sammartino, J.C.; Chiarelli, L.R.; Riabova, O.; Makarov, V.; Pasca, M.R. Mycobacterium abscessus, an Emerging and Worrisome Pathogen among Cystic Fibrosis Patients. Int. J. Mol. Sci. 2019, 20, 5868. https://doi.org/10.3390/ijms20235868
Degiacomi G, Sammartino JC, Chiarelli LR, Riabova O, Makarov V, Pasca MR. Mycobacterium abscessus, an Emerging and Worrisome Pathogen among Cystic Fibrosis Patients. International Journal of Molecular Sciences. 2019; 20(23):5868. https://doi.org/10.3390/ijms20235868
Chicago/Turabian StyleDegiacomi, Giulia, José Camilla Sammartino, Laurent Roberto Chiarelli, Olga Riabova, Vadim Makarov, and Maria Rosalia Pasca. 2019. "Mycobacterium abscessus, an Emerging and Worrisome Pathogen among Cystic Fibrosis Patients" International Journal of Molecular Sciences 20, no. 23: 5868. https://doi.org/10.3390/ijms20235868
APA StyleDegiacomi, G., Sammartino, J. C., Chiarelli, L. R., Riabova, O., Makarov, V., & Pasca, M. R. (2019). Mycobacterium abscessus, an Emerging and Worrisome Pathogen among Cystic Fibrosis Patients. International Journal of Molecular Sciences, 20(23), 5868. https://doi.org/10.3390/ijms20235868






