Connexin43 Is Required for the Effective Activation of Spleen Cells and Immunoglobulin Production
Abstract
1. Introduction
2. Results
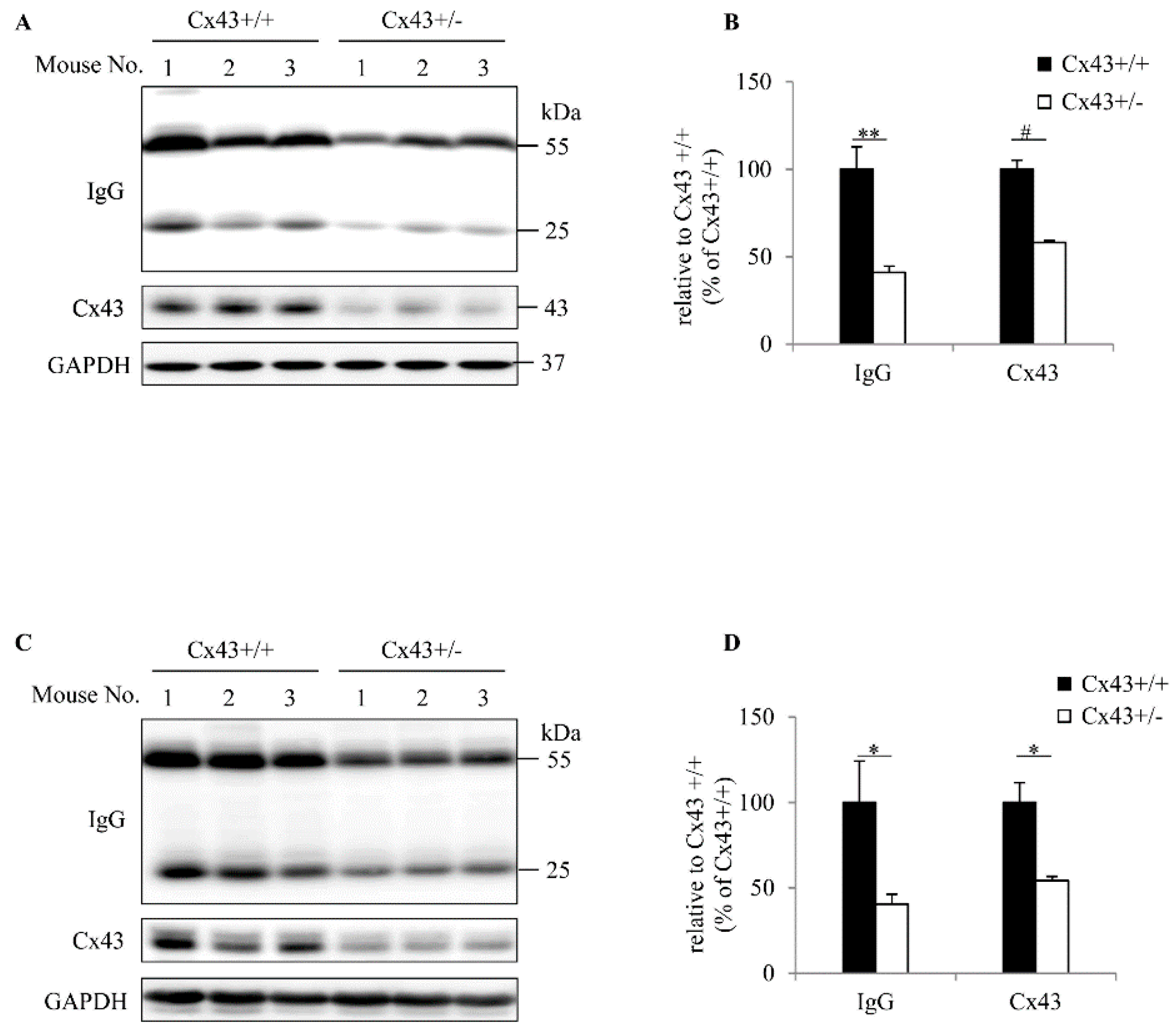
2.1. Cx43+/+ Has a Higher Level of Serum IgG Than Cx43+/− Mouse
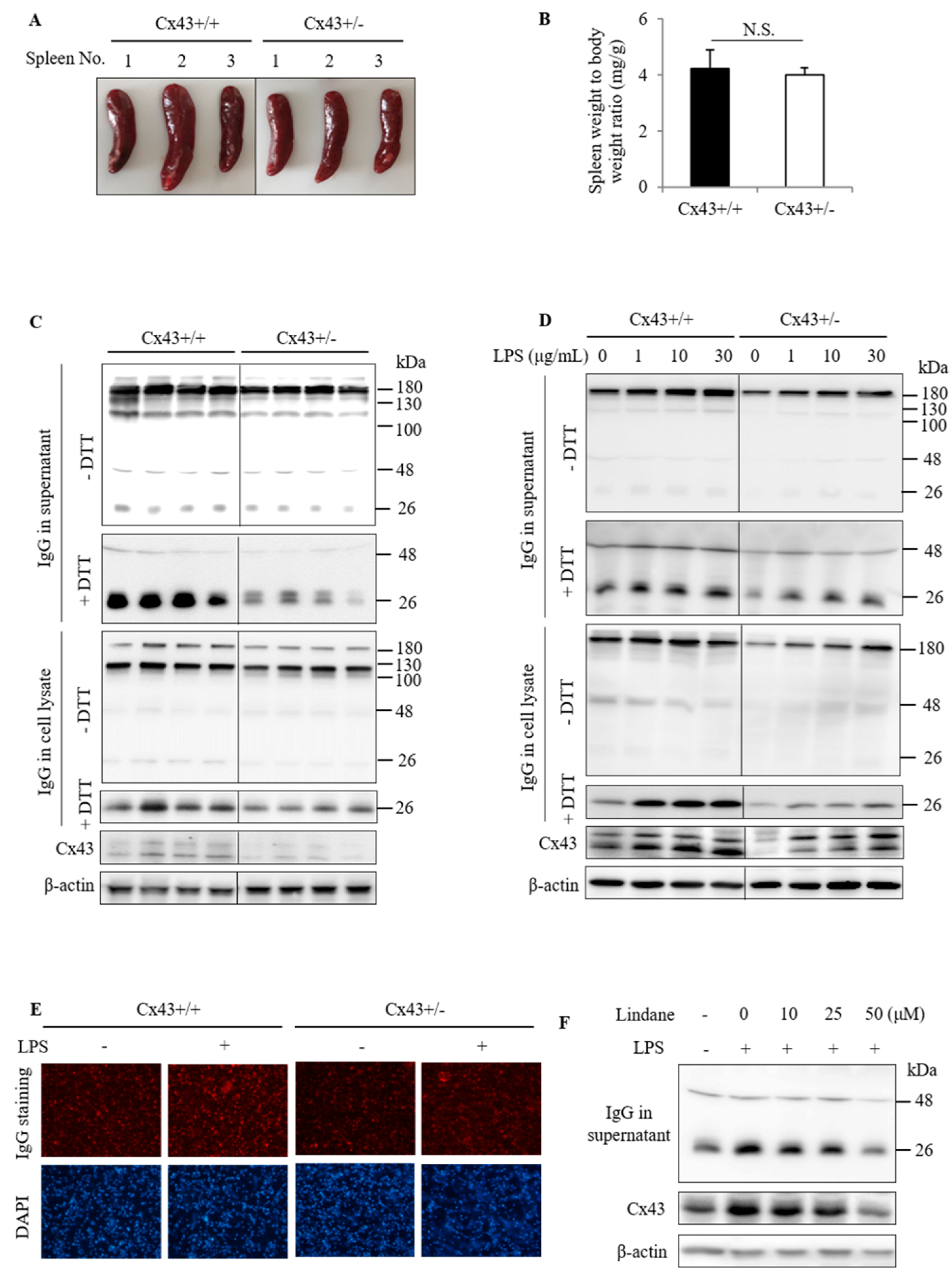
2.2. Cx43+/+ Spleen Cells Produce More IgG Than Cx43+/− Cells
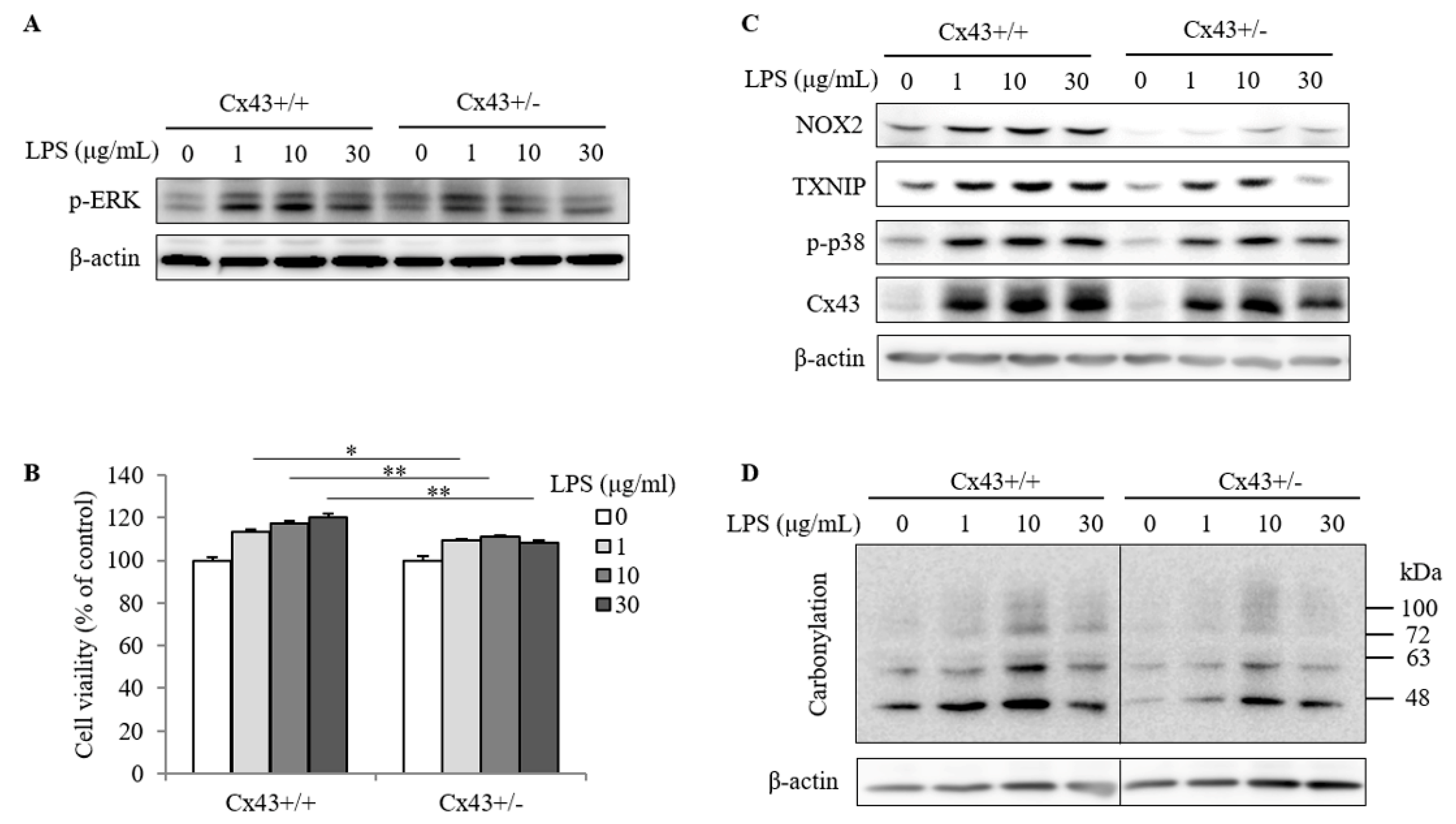
2.3. Cx43+/+ Spleen Cells Display a Stronger Response to LPS Than Cx43+/− Cells in Cell Proliferation and Redox Signaling
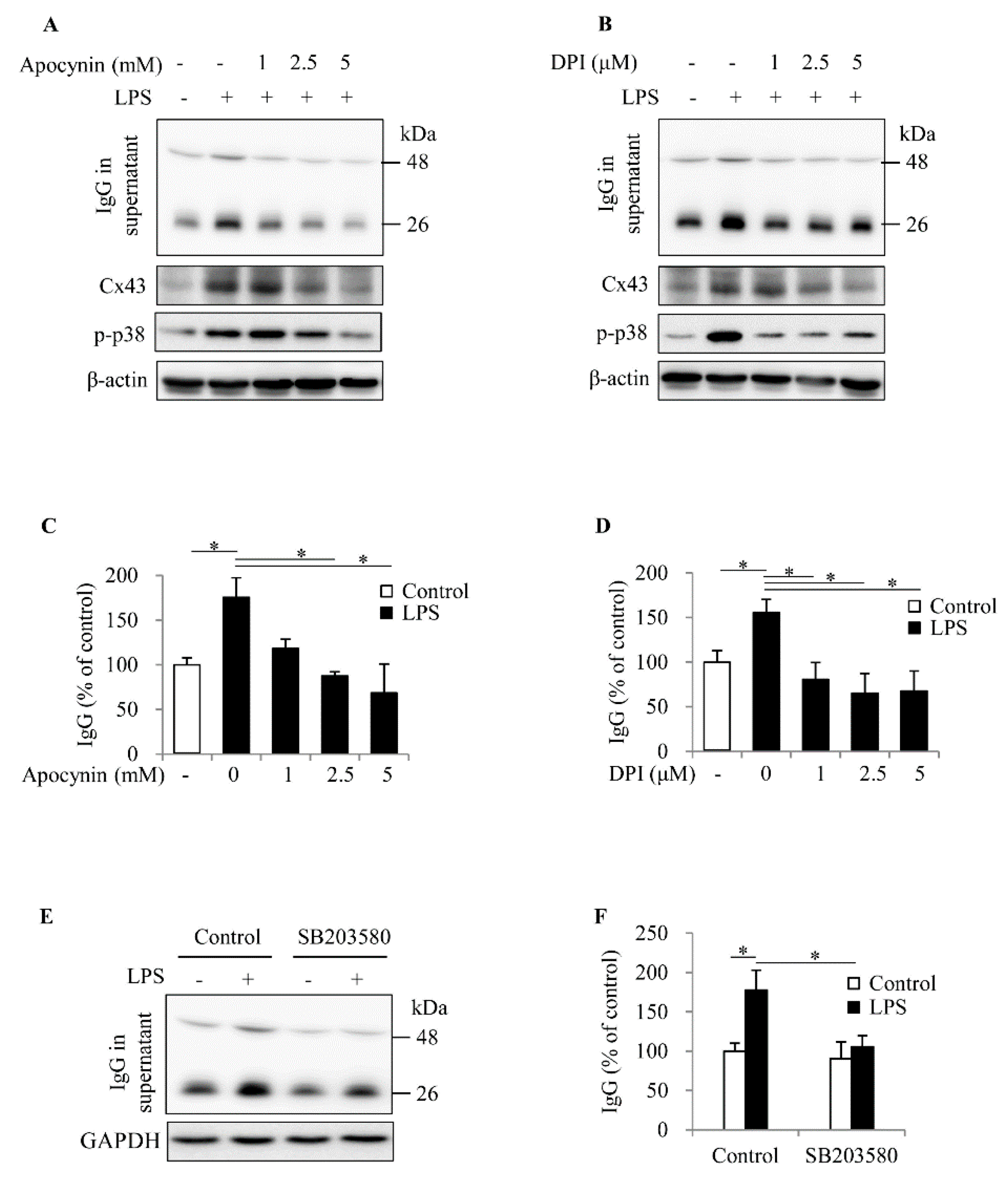
2.4. ROS Contributes to Spleen Cell Activation and IgG Production
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Cells
4.4. Western Blot Analysis
4.5. Easy-Titer IgG Assay
4.6. Polymerase Chain Reaction
4.7. Assessment of Cell Proliferation with WST Reagent
4.8. Immunofluorescent Staining of IgG in Spleen Cells
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASK | Apoptosis signal-regulating kinase |
| Cx | Connexin |
| Cx43 | Connexin43 |
| DMNQ | 2,3-Dimethoxy-1,4-naphthoquinone |
| DC | Dendritic cell |
| GJ | Gap junction |
| Ig | Immunoglobulin |
| IgG | Immunoglobulin G |
| LPS | Lipopolysaccharide |
| MW | Molecular weight |
| NADPH oxidase | NOX |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| ROS | Reactive oxygen species |
| Trx | Thioredoxin |
| TXNIP | Thioredoxin-interacting protein |
References
- Saez, J.C.; Berthoud, V.M.; Branes, M.C.; Martinez, A.D.; Beyer, E.C. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol. Rev. 2003, 83, 1359–1400. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Oite, T.; Kitamura, M. Gap junctional intercellular communication in the juxtaglomerular apparatus. Am. J. Physiol. Renal 2009, 296, F939–F946. [Google Scholar] [CrossRef] [PubMed]
- Machtaler, S.; Choi, K.; Dang-Lawson, M.; Falk, L.; Pournia, F.; Naus, C.C.; Matsuuchi, L. The role of the gap junction protein connexin43 in B lymphocyte motility and migration. FEBS Lett. 2014, 588, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mao, Z.; Zhang, Z.; Obata, F.; Yang, X.; Zhang, X.; Huang, Y.; Mitsui, T.; Fan, J.; Takeda, M.; et al. Connexin43 Contributes to Inflammasome Activation and Lipopolysaccharide-Initiated Acute Renal Injury via Modulation of Intracellular Oxidative Status. Antioxid. Redox Signal. 2019, in press. [Google Scholar] [CrossRef]
- Krenacs, T.; Rosendaal, M. Immunohistological detection of gap junctions in human lymphoid tissue: connexin43 in follicular dendritic and lymphoendothelial cells. J. Histochem. Cytochem. 1995, 43, 1125–1137. [Google Scholar] [CrossRef]
- Krenacs, T.; van Dartel, M.; Lindhout, E.; Rosendaal, M. Direct cell/cell communication in the lymphoid germinal center: Connexin43 gap junctions functionally couple follicular dendritic cells to each other and to B lymphocytes. Eur. J. Immunol. 1997, 27, 1489–1497. [Google Scholar] [CrossRef]
- Matsue, H.; Yao, J.; Matsue, K.; Nagasaka, A.; Sugiyama, H.; Aoki, R.; Kitamura, M.; Shimada, S. Gap junction-mediated intercellular communication between dendritic cells (DCs) is required for effective activation of DCs. J. Immunol. 2006, 176, 181–190. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Taffet, S.M. A model system to study Connexin 43 in the immune system. Mol. Immunol. 2009, 46, 2938–2946. [Google Scholar] [CrossRef]
- Ring, S.; Karakhanova, S.; Johnson, T.; Enk, A.H.; Mahnke, K. Gap junctions between regulatory T cells and dendritic cells prevent sensitization of CD8(+) T cells. J. Allergy Clin. Immunol. 2010, 125, 237–246. [Google Scholar] [CrossRef]
- Machtaler, S.; Dang-Lawson, M.; Choi, K.; Jang, C.; Naus, C.C.; Matsuuchi, L. The gap junction protein Cx43 regulates B-lymphocyte spreading and adhesion. J. Cell Sci. 2011, 124, 2611–2621. [Google Scholar] [CrossRef]
- Zhang, H.C.; Zhang, Z.S.; Zhang, L.; Wang, A.; Zhu, H.; Li, L.; Si, J.Q.; Li, X.Z.; Ma, K.T. Connexin 43 in splenic lymphocytes is involved in the regulation of CD4(+)CD25(+) T lymphocyte proliferation and cytokine production in hypertensive inflammation. Int. J. Mol. Med. 2018, 41, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Chi, Y.; Zhang, X.; Zhang, H.; Li, G.; Sun, W.; Takeda, M.; Yao, J. A novel TXNIP-based mechanism for Cx43-mediated regulation of oxidative drug injury. J. Cell Mol. Med. 2015, 19, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, X.; Gao, K.; Zhang, Z.; Huang, Y.; Yoda, R.; Yao, J. The pivotal role of extracellular signal-regulated kinase in gap junction-mediated regulation of TXNIP. Cell Signal. 2017, 38, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Gao, K.; Chi, Y.; Li, K.; Zhu, Y.; Wan, Y.; Sun, W.; Matsue, H.; Kitamura, M.; Yao, J. NADPH oxidase-mediated upregulation of connexin43 contributes to podocyte injury. Free Radic. Biol. Med. 2012, 53, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Huang, T.; Zhu, Y.; Yan, Q.; Chi, Y.; Jiang, J.X.; Wang, P.; Matsue, H.; Kitamura, M.; Yao, J. Connexin43 hemichannels contribute to cadmium-induced oxidative stress and cell injury. Antioxid. Redox Signal. 2011, 14, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Vene, R.; Delfino, L.; Castellani, P.; Balza, E.; Bertolotti, M.; Sitia, R.; Rubartelli, A. Redox remodeling allows and controls B-cell activation and differentiation. Antioxid. Redox Signal. 2010, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Tang, M.; Suzuki, M.; Gunasekara, C.; Anbe, Y.; Hiraoka, Y.; Liu, J.; Grasberger, H.; Ohkita, M.; Matsumura, Y.; et al. Essential Role of NOX-Dependent Production of Reactive Oxygen Species in Maintenance of Sustained B Cell Receptor Signaling and B Cell Proliferation. J. Immunol. 2019, 202, 2546–2557. [Google Scholar] [CrossRef]
- Shao, Y.; Kim, S.Y.; Shin, D.; Kim, M.S.; Suh, H.W.; Piao, Z.H.; Jeong, M.; Lee, S.H.; Yoon, S.R.; Lim, B.H.; et al. TXNIP regulates germinal center generation by suppressing BCL-6 expression. Immunol. Lett. 2010, 129, 78–84. [Google Scholar] [CrossRef]
- Elgueta, R.; Tobar, J.A.; Shoji, K.F.; De Calisto, J.; Kalergis, A.M.; Bono, M.R.; Rosemblatt, M.; Saez, J.C. Gap Junctions at the Dendritic Cell-T Cell Interface Are Key Elements for Antigen-Dependent T Cell Activation. J. Immunol. 2009, 183, 277–284. [Google Scholar] [CrossRef]
- Andersson, J.; Sjoberg, O.; Moller, G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur. J. Immunol. 1972, 2, 349–353. [Google Scholar] [CrossRef]
- Bourgois, A.; Kitajima, K.; Hunter, I.R.; Askonas, B.A. Surface Immunoglobulins of Lipopolysaccharide-Stimulated Spleen-Cells-Behavior of Igm, Igd and Igg. Eur. J. Immunol. 1977, 7, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.K.; Galanos, C.; Koenig, S.; Oettgen, H.F. B-cell activation by lipopolysaccharide. Distinct pathways for induction of mitosis and antibody production. J. Exp. Med. 1977, 146, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.C.; Fang, S.H.; Lee, M.T.; Sheu, S.Y.; Lai, S.Y.; Chen, Y.J.; Huang, F.L.; Wang, P.S.; Stocco, D.M.; Hwang, J.J. Lindane, a gap junction blocker, suppresses FSH and transforming growth factor beta1-induced connexin43 gap junction formation and steroidogenesis in rat granulosa cells. J. Endocrinol. 2005, 184, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Leavy, O. Inflammation: Regulating ROS. Nat. Rev. Immunol. 2014, 14, 357. [Google Scholar] [CrossRef] [PubMed]
- Khiem, D.; Cyster, J.G.; Schwarz, J.J.; Black, B.L. A p38 MAPK-MEF2C pathway regulates B-cell proliferation. Proc. Natl. Acad. Sci. USA 2008, 105, 17067–17072. [Google Scholar] [CrossRef]
- Medgyesi, D.; Hobeika, E.; Biesen, R.; Kollert, F.; Taddeo, A.; Voll, R.E.; Hiepe, F.; Reth, M. The protein tyrosine phosphatase PTP1B is a negative regulator of CD40 and BAFF-R signaling and controls B cell autoimmunity. J. Exp. Med. 2014, 211, 427–440. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, H.; Xu, Y.; Lee, P.; Li, Y.; Wilson, J.C.; Vidal, O.; Choi, H.S.; Sun, Y.; Yang, L.J.; et al. Maintenance of autoantibody production in pristane-induced murine lupus. Arthritis Res. Ther. 2015, 17, 384. [Google Scholar] [CrossRef]
- Pongratz, G.; McAlees, J.W.; Conrad, D.H.; Erbe, R.S.; Haas, K.M.; Sanders, V.M. The level of IgE produced by a B cell is regulated by norepinephrine in a p38 MAPK- and CD23-dependent manner. J. Immunol. 2006, 177, 2926–2938. [Google Scholar] [CrossRef]
- Xie, X.; Lan, T.; Chang, X.; Huang, K.; Huang, J.; Wang, S.; Chen, C.; Shen, X.; Liu, P.; Huang, H. Connexin43 mediates NF-kappaB signalling activation induced by high glucose in GMCs: Involvement of c-Src. Cell Commun. Signal. 2013, 11, 38. [Google Scholar] [CrossRef]
- Li, W.; Bao, G.; Chen, W.; Qiang, X.; Zhu, S.; Wang, S.; He, M.; Ma, G.; Ochani, M.; Al-Abed, Y.; et al. Connexin 43 Hemichannel as a Novel Mediator of Sterile and Infectious Inflammatory Diseases. Sci. Rep. 2018, 8, 166. [Google Scholar] [CrossRef]
- Dosch, M.; Zindel, J.; Jebbawi, F.; Melin, N.; Sanchez-Taltavull, D.; Stroka, D.; Candinas, D.; Beldi, G. Connexin-43-dependent ATP release mediates macrophage activation during sepsis. Elife 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Tan, T.; Chan, C.; Laxton, V.; Chan, Y.W.; Liu, T.; Wong, W.T.; Tse, G. The Role of Connexins in Wound Healing and Repair: Novel Therapeutic Approaches. Front. Physiol. 2016, 7, 596. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Orta, E.; Evans, W.H. Gap junctions and connexins: Potential contributors to the immunological synapse. J. Leukoc. Biol. 2002, 72, 636–642. [Google Scholar] [PubMed]
- Oviedo-Orta, E.; Gasque, P.; Evans, W.H. Immunoglobulin and cytokine expression in mixed lymphocyte cultures is reduced by disruption of gap junction intercellular communication. FASEB J. 2001, 15, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Capasso, M.; Bhamrah, M.K.; Henley, T.; Boyd, R.S.; Langlais, C.; Cain, K.; Dinsdale, D.; Pulford, K.; Khan, M.; Musset, B.; et al. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat. Immunol. 2010, 11, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Czyz, J.; Piwowarczyk, K.; Paw, M.; Luty, M.; Wrobel, T.; Catapano, J.; Madeja, Z.; Ryszawy, D. Connexin-dependent intercellular stress signaling in tissue homeostasis and tumor development. Acta Biochim. Pol. 2017, 64, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Guida, L.; Zocchi, E.; Franco, L.; De Flora, A. Connexin 43 hemi channels mediate Ca2+/− regulated transmembrane NAD(+) fluxes in intact cells. FASEB J. 2001, 15, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Gao, K.; Li, K.; Nakajima, S.; Kira, S.; Takeda, M.; Yao, J. Purinergic control of AMPK activation by ATP released through connexin 43 hemichannels—Pivotal roles in hemichannel-mediated cell injury. J. Cell Sci. 2014, 127, 1487–1499. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Jiang, J.X. Gap junction and hemichannel-independent actions of connexins on cell and tissue functions—An update. FEBS Lett. 2014, 588, 1186–1192. [Google Scholar] [CrossRef]
- Shah, A.; Xia, L.; Goldberg, H.; Lee, K.W.; Quaggin, S.E.; Fantus, I.G. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NOX, Nox4, in mesangial cells. J. Biol. Chem. 2013, 288, 6835–6848. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; He, F.F.; Tang, H.; Lei, C.T.; Chen, S.; Meng, X.F.; Su, H.; Zhang, C. NOX-induced NALP3 inflammasome activation is driven by thioredoxin-interacting protein which contributes to podocyte injury in hyperglycemia. J. Diabetes Res. 2015, 2015, 504761. [Google Scholar] [CrossRef] [PubMed]
- Glass, A.M.; Snyder, E.G.; Taffet, S.M. Connexins and pannexins in the immune system and lymphatic organs. Cell Mol. Life Sci. 2015, 72, 2899–2910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Fang, X.; Niimi, M.; Huang, Y.; Piao, H.; Gao, S.; Fan, J.; Yao, J. Glutathione inhibits antibody and complement-mediated immunologic cell injury via multiple mechanisms. Redox Biol. 2017, 12, 571–581. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Mao, Z.; Zhang, X.; Yang, X.; Sawada, N.; Takeda, M.; Yao, J. Connexin43 Is Required for the Effective Activation of Spleen Cells and Immunoglobulin Production. Int. J. Mol. Sci. 2019, 20, 5789. https://doi.org/10.3390/ijms20225789
Huang Y, Mao Z, Zhang X, Yang X, Sawada N, Takeda M, Yao J. Connexin43 Is Required for the Effective Activation of Spleen Cells and Immunoglobulin Production. International Journal of Molecular Sciences. 2019; 20(22):5789. https://doi.org/10.3390/ijms20225789
Chicago/Turabian StyleHuang, Yanru, Zhimin Mao, Xiling Zhang, Xiawen Yang, Norifumi Sawada, Masayuki Takeda, and Jian Yao. 2019. "Connexin43 Is Required for the Effective Activation of Spleen Cells and Immunoglobulin Production" International Journal of Molecular Sciences 20, no. 22: 5789. https://doi.org/10.3390/ijms20225789
APA StyleHuang, Y., Mao, Z., Zhang, X., Yang, X., Sawada, N., Takeda, M., & Yao, J. (2019). Connexin43 Is Required for the Effective Activation of Spleen Cells and Immunoglobulin Production. International Journal of Molecular Sciences, 20(22), 5789. https://doi.org/10.3390/ijms20225789



