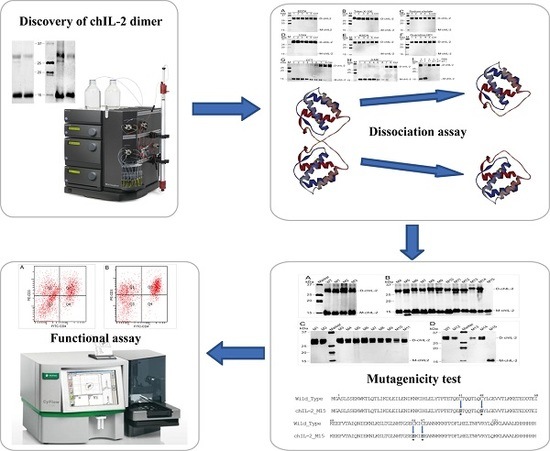
Four Cysteine Residues Contribute to Homodimerization of Chicken Interleukin-2
Abstract
1. Introduction
2. Results
2.1. Purified ChIL-2 Formed a Dimer
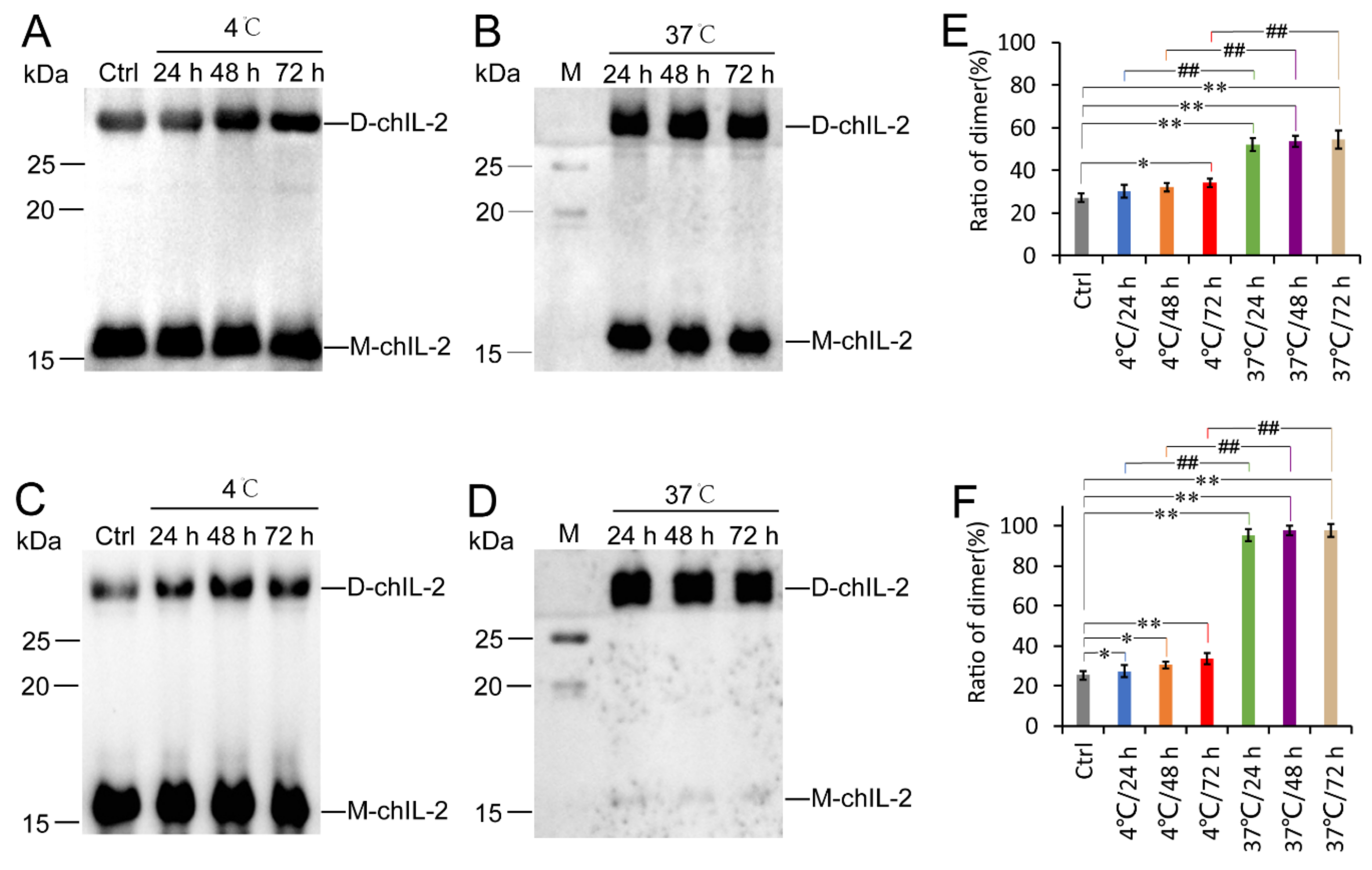
2.2. Increasing Temperature Promotes Dimerization of ChIL-2
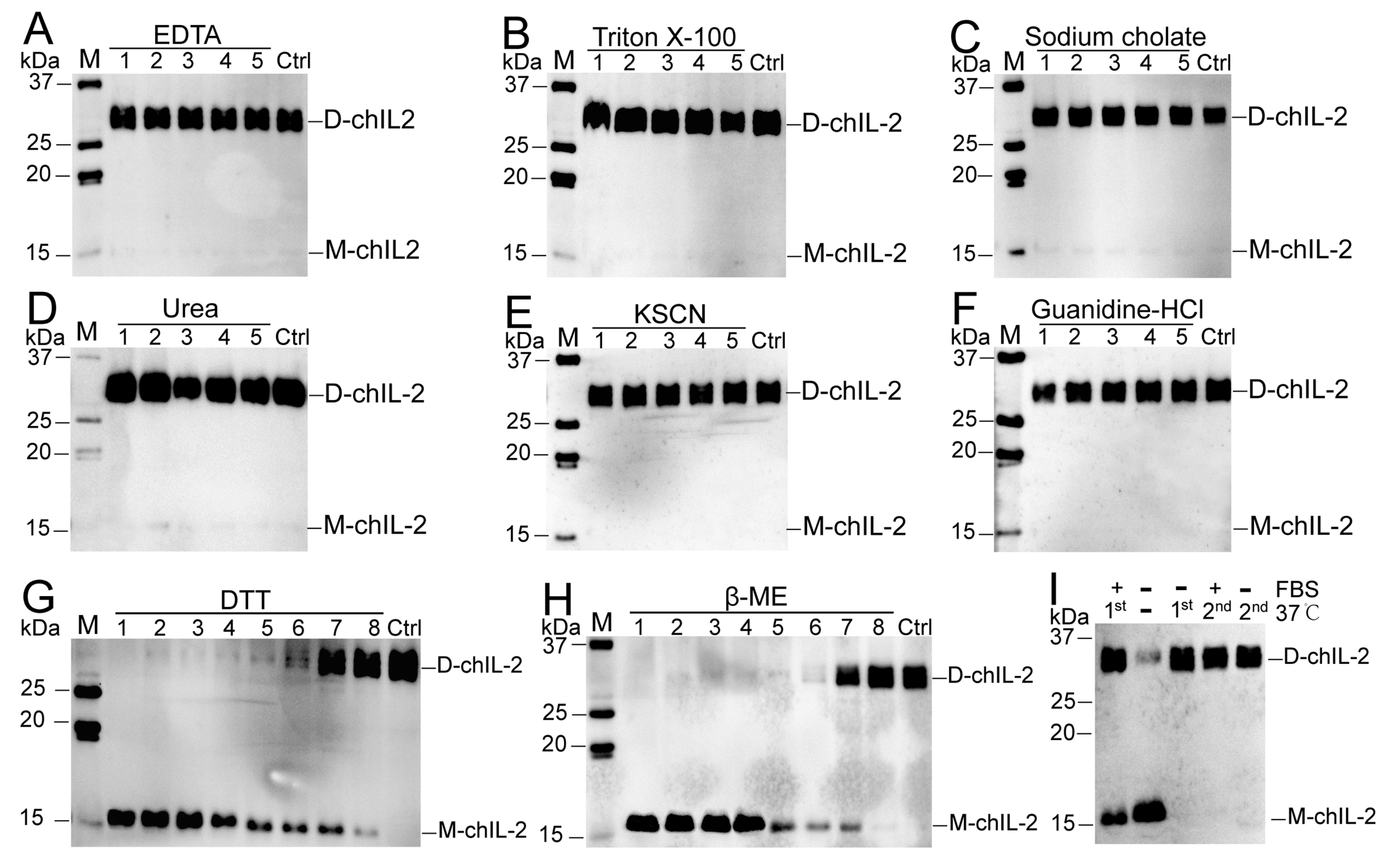
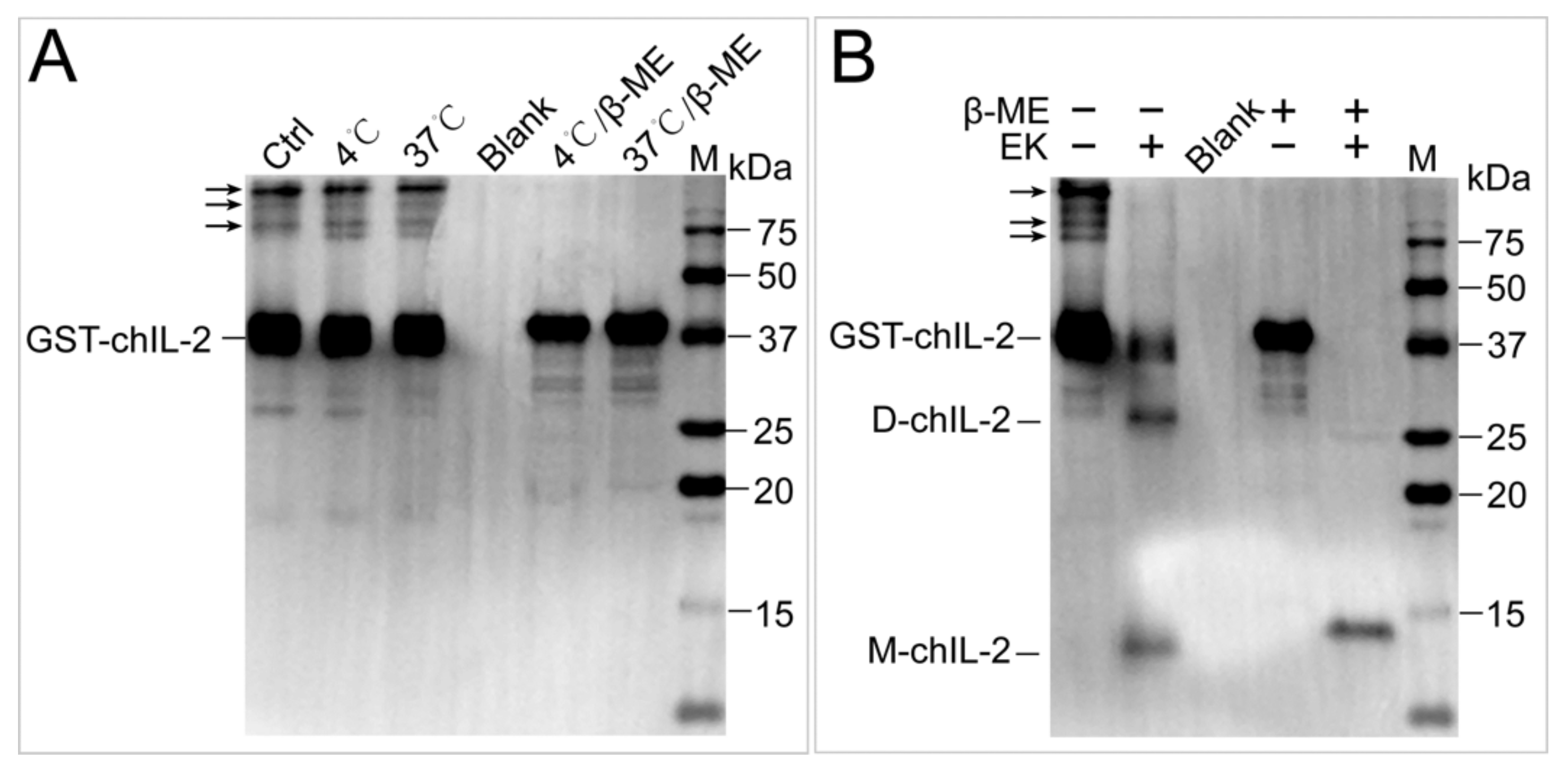
2.3. Reducing Agents Dissociated ChIL-2 Homodimer
2.4. The ChIL-2 Mutant with Four Cys Residue Mutations Fails to Form Dimeric ChIL-2
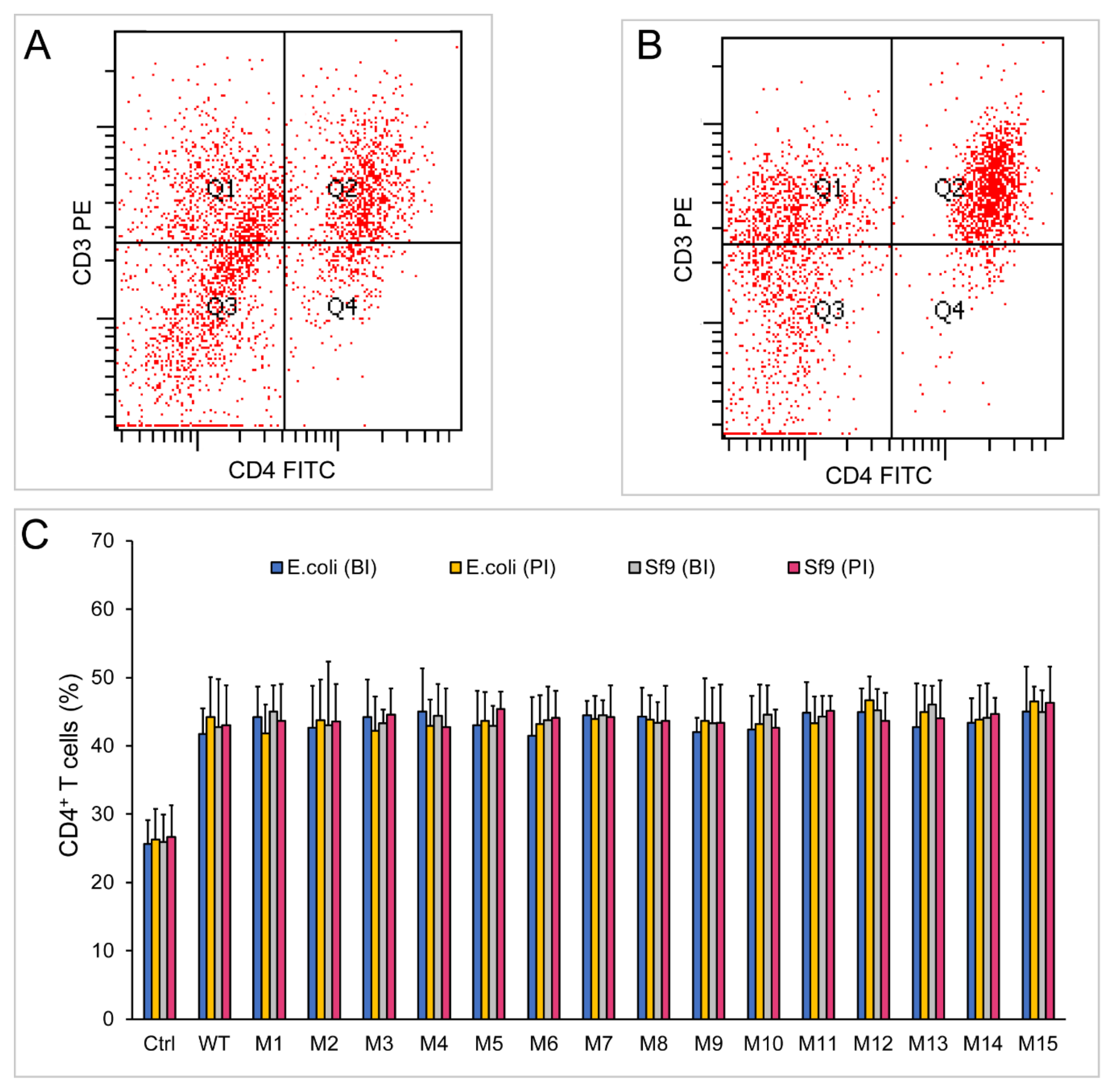
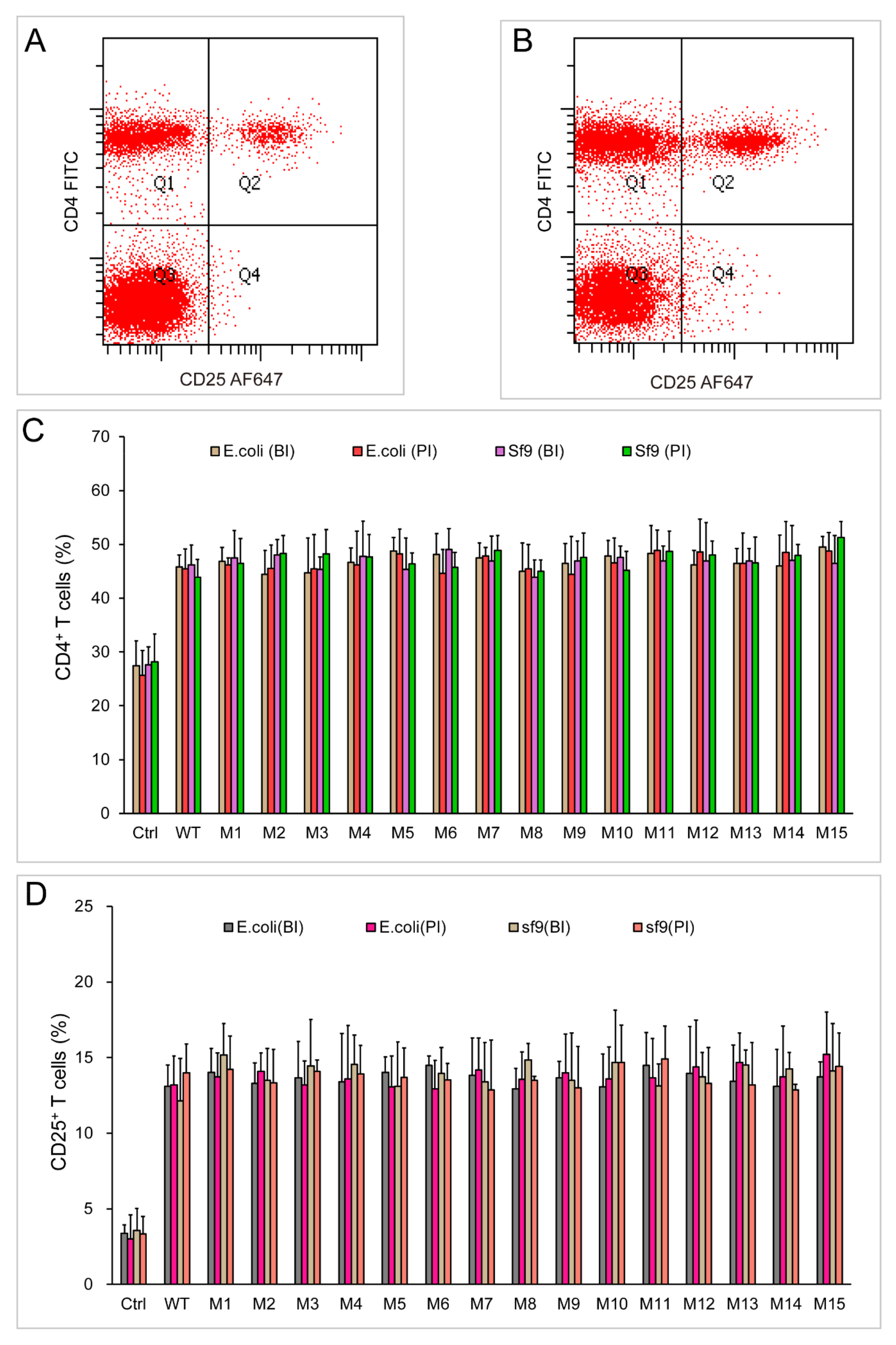
2.5. Both Dimeric and Monomeric ChIL-2 Stimulated T Cells Expansion
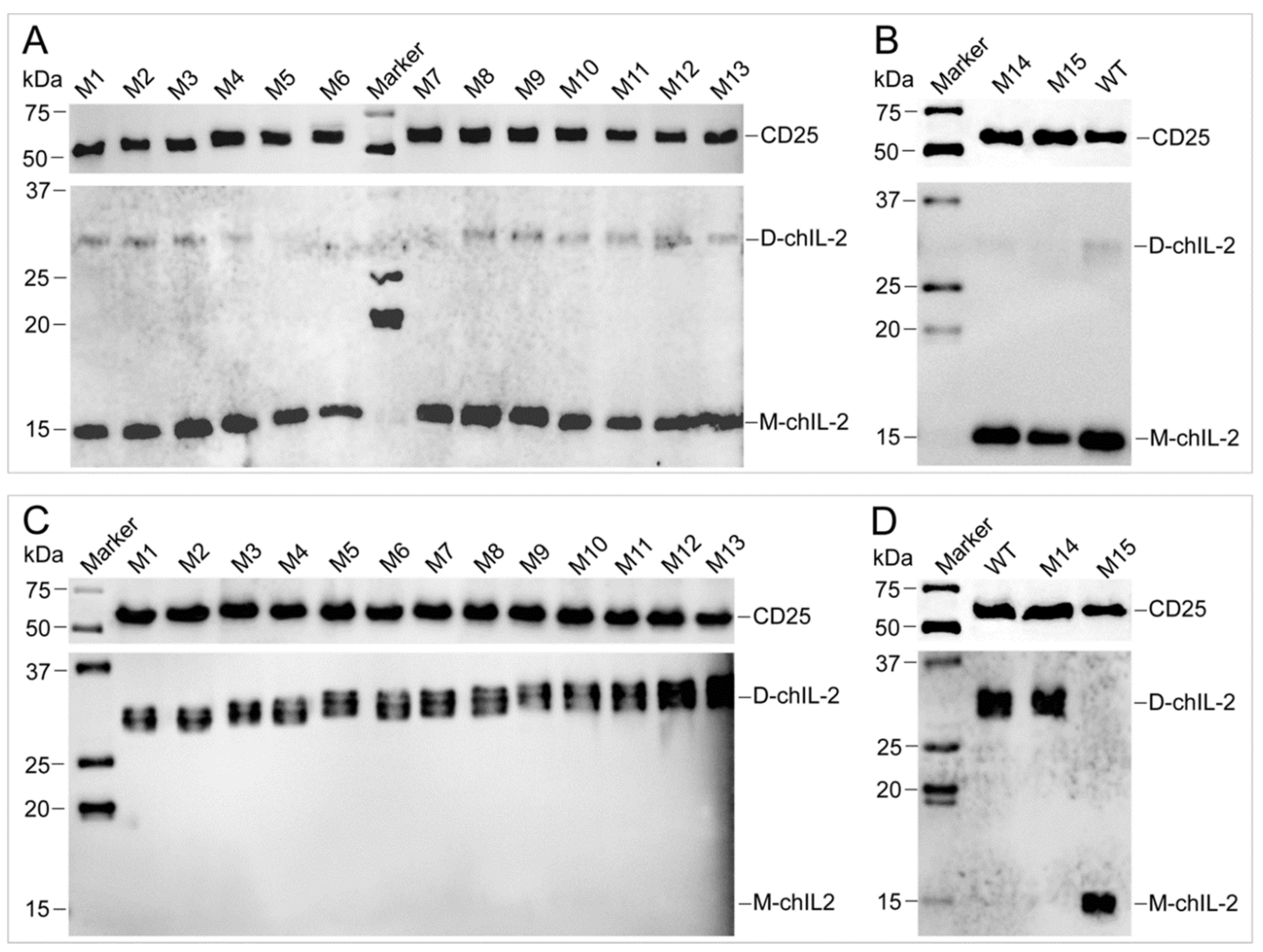
2.6. Different Forms of ChIL-2 can Effectively Bind to ChIL-2 Receptor
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of ChIL-2
4.2. Homodimerization of ChIL-2 Protein
4.3. The Effect of Temperature on ChIL-2 Dimerization
4.4. Dissociation of the ChIL-2 Dimer
4.5. Determination of Cysteine Sites Involved in Dimerization
4.6. Identification of Differences in Stimulatory Activity between Monomeric and Dimeric ChIL2
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IL-2 | Interleukin-2 |
| KSCN | Potassium thiocyanate |
| EDTA | Ethylene Diamine Tetraacetic Acid |
| β-ME | β-Methylphenethylamine |
| DTT | Dithiothreitol |
| Cys | Cysteine |
| Sf9 | Spodoptera frugiperda |
| E. coli | Escherichia coli |
| ConA | Concanavalin A |
| PHA | Phytohemagglutinin |
| FBS | Fetal bovine serum |
| EK | Enterokinase |
| MS | Mass spectrometry |
| HMS | Honeybee melittin signalling sequence |
| i.v. | Intravenous injection |
| i.p. | Intraperitoneal injection |
References
- Moon, B.; Kim, T.; Seoh, J. Functional modulation of regulatory T cells by IL-2. PLoS ONE 2015, 10, e0141864. [Google Scholar] [CrossRef]
- Sojka, D.K.; Bruniquel, D.; Schwartz, R.H.; Singh, N.J. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J. Immunol. 2004, 172, 6136–6143. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Liu, K.D. Overview of interleukin-2 function, production and clinical applications. Cytokine 2004, 28, 109–123. [Google Scholar] [CrossRef]
- Mortara, L.; Balza, E.; Bruno, A.; Poggi, A.; Orecchia, P.; Carnemolla, B. Anti-cancer therapies employing IL-2 cytokine tumor targeting: Contribution of innate, adaptive and immunosuppressive cells in the anti-tumor efficacy. Front. Immunol. 2018, 9, 2905. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lin, J.; Leonard, W.J. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011, 23, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Oxenius, A. Interleukin 2: From immunostimulation to immunoregulation and back again. EMBO Rep. 2007, 8, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Caggiari, L.; Zanussi, S.; Crepaldi, C.; Bortolin, M.T.; Caffau, C.; D’Andrea, M.; De Paoli, P. Different rates of CD4+ and CD8+ T-cell proliferation in interleukin-2–treated human immunodeficiency virus-positive subjects. Cytometry 2001, 46, 233–237. [Google Scholar] [CrossRef]
- Nguyen, T.; Russell, J. The regulation of FasL expression during activation-induced cell death (AICD). Immunology 2001, 103, 426–434. [Google Scholar] [CrossRef]
- Vámosi, G.; Bodnár, A.; Vereb, G.; Jenei, A.; Goldman, C.K.; Langowski, J.; Tóth, K.; Mátyus, L.; Szöllösi, J.; Waldmann, T.A.; et al. IL-2 and IL-15 receptor alpha-subunits are coexpressed in a supramolecular receptor cluster in lipid rafts of T cells. Proc. Natl. Acad. Sci. USA 2004, 101, 11082–11087. [Google Scholar] [CrossRef]
- Aandahl, E.M.; Michaëlsson, J.; Moretto, W.J.; Hecht, F.M.; Nixon, D.F. Human CD4+CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 2004, 78, 2454–2459. [Google Scholar] [CrossRef]
- Cabrera, R.; Tu, Z.; Xu, Y.; Firpi, R.J.; Rosen, H.R.; Liu, C.; Nelson, D.R. An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology 2004, 40, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Wylezinski, L.S.; Hawiger, J. Interleukin 2 activates brain microvascular endothelial cells resulting in destabilization of adherens junctions. J. Biol. Chem. 2016, 291, 22913–22923. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lu, C. Interleukin-2 and its effects in the central nervous system. Biol. Signals Recept. 1998, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Leng, Q.; Mixson, A.J. Alteration in the IL-2 signal peptide affects secretion of proteins in vitro and in vivo. J. Gene Med. 2005, 7, 354–365. [Google Scholar] [CrossRef]
- McDermott, D.F.; Atkins, M.B. Interleukin-2 therapy of metastatic renal cell carcinoma-predictors of response. Semin. Oncol. 2006, 33, 583–587. [Google Scholar] [CrossRef]
- Sereti, I.; Anthony, K.B.; Martinez-Wilson, H.; Lempicki, R.; Adelsberger, J.; Metcalf, J.A.; Hallahan, C.W.; Follmann, D.; Davey, R.T.; Kovacs, J.A.; et al. IL-2–induced CD4+ T-cell expansion in HIV-infected patients is associated with long-term decreases in T-cell proliferation. Blood 2004, 104, 775. [Google Scholar] [CrossRef][Green Version]
- Tarpey, I.; Davis, P.J.; Sondermeijer, P.; van Geffen, C.; Verstegen, I.; Schijns, V.E.; Kolodsick, J.; Sundick, R. Expression of chicken interleukin-2 by turkey herpesvirus increases the immune response against Marek’s disease virus but fails to increase protection against virulent challenge. Avian Pathol. 2007, 36, 69–74. [Google Scholar] [CrossRef]
- Vainio, O.; Ratcliffe, M.J.; Leanderson, T. Chicken T-cell growth factor: Use in the generation of a long-term cultured T-cell line and biochemical characterization. Scand. J. Immunol. 1986, 23, 135–142. [Google Scholar] [CrossRef]
- Hilton, L.S.; Bean, A.G.D.; Kimpton, W.G.; Lowenthal, J.W. Interleukin-2 directly induces activation and proliferation of chicken T cells in vivo. J. Interferon. Cytokine Res. 2002, 22, 755–763. [Google Scholar] [CrossRef]
- Li, Z.; Tang, X.; Suo, J.; Qin, M.; Yin, G.; Liu, X.; Suo, X. Transgenic Eimeria mitis expressing chicken interleukin 2 stimulated higher cellular immune response in chickens compared with the wild-type parasites. Front. Microbiol. 2015, 6, 533. [Google Scholar] [CrossRef]
- Tarpey, I.; van Loon, A.A.; de Haas, N.; Davis, P.J.; Orbell, S.; Cavanagh, D.; Britton, P.; Casais, R.; Sondermeijer, P.; Sundick, R. A recombinant turkey herpesvirus expressing chicken interleukin-2 increases the protection provided by in ovo vaccination with infectious bursal disease and infectious bronchitis virus. Vaccine 2007, 25, 8529–8535. [Google Scholar] [CrossRef] [PubMed]
- Millet, S.; Maertens, L. The European ban on antibiotic growth promoters in animal feed: From challenges to opportunities. Vet. J. 2011, 187, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Hilton, L.S.; Bean, A.G.D.; Lowenthal, J.W. The emerging role of avian cytokines as immunotherapeutics and vaccine adjuvants. Vet. Immunol. Immunopathol. 2002, 85, 119–128. [Google Scholar] [CrossRef]
- Ali, A.; Bagchi, A. An overview of protein-protein interaction. Curr. Opin. Chem. Biol. 2015, 9, 53–65. [Google Scholar] [CrossRef]
- Marianayagam, N.J.; Sunde, M.; Matthews, J.M. The power of two: Protein dimerization in biology. Trends Biochem. Sci. 2004, 29, 618–625. [Google Scholar] [CrossRef]
- Herscovitch, M.; Comb, W.; Ennis, T.; Coleman, K.; Yong, S.; Armstead, B.; Kalaitzidis, D.; Chandani, S.; Gilmore, T.D. Intermolecular disulfide bond formation in the NEMO dimer requires Cys54 and Cys347. Biochem. Biophys. Res. Commun. 2008, 367, 103–108. [Google Scholar] [CrossRef]
- Mi Hwang, E.; Kim, E.; Yarishkin, O.; Ho Woo, D.; Han, K.-S.; Park, N.; Bae, Y.; Woo, J.; Kim, D.; Park, M.; et al. A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nat. Commun. 2014, 5, 3227. [Google Scholar] [CrossRef]
- Lother, H.; Muther, H.; Gessner, A.; Abdallah, S.; Kuhlcke, K. Intermolecular cystine-bonding of murine interleukin 2 indicates that ligand dimerization is important for the formation of the high-affinity receptor complex. Growth Factors 1992, 7, 117–129. [Google Scholar] [CrossRef]
- Tsuji, T.; Nakagawa, R.; Sugimoto, N.; Fukuhara, K. Characterization of disulfide bonds in recombinant proteins: Reduced human interleukin 2 in inclusion bodies and its oxidative refolding. Biochemistry 1987, 26, 3129–3134. [Google Scholar] [CrossRef]
- Gorman, J.J.; Wallis, T.P.; Pitt, J.J. Protein disulfide bond determination by mass spectrometry. Mass Spectrom. Rev. 2002, 21, 183–216. [Google Scholar] [CrossRef]
- Sim, G.C.; Radvanyi, L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Wrenshall, L.E.; Clabaugh, S.E.; Cool, D.R.; Arumugam, P.; Grunwald, W.C.; Smith, D.R.; Liu, G.C.; Miller, J.D. Identification of a cytotoxic form of dimeric interleukin-2 in murine tissues. PLoS ONE 2014, 9, e102191. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Arumugam, P.; Clabaugh, S.; Kozak, A.; Wrenshall, L. Dimeric IL-2 induces necrosis of interleukin-2 receptor positive cells. J. Immunol. 2015, 194, 187.4. [Google Scholar]
- Kaplan, D.; Smith, D.; Huang, R.; Yildirim, Z. Self-association of interleukin 2 bound to its receptor. FASEB J. 1995, 9, 1096–1102. [Google Scholar] [CrossRef]
- Eizenberg, O.; Kaplitt, M.G.; Eitan, S.; Pfaff, D.W.; Hirschberg, D.L.; Schwartz, M. Linear dimeric interleukin-2 obtained by the use of a defective herpes simplex viral vector: Conformation-activity relationship. Mol. Brain Res. 1994, 26, 156–162. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; Li, J.; Zheng, J.; Cai, N.; Gong, P.; Li, S.; Li, H.; Zhang, X. A novel recombinant BCG vaccine encoding Eimeria tenella rhomboid and chicken IL-2 induces protective immunity against coccidiosis. Korean J. Parasitol. 2014, 52, 251–256. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.; Cho, M. Rotational dynamics of thiocyanate ions in highly concentrated aqueous solutions. Phys. Chem. Chem. Phys. 2012, 14, 6233–6240. [Google Scholar] [CrossRef]
- Maestre, A.; Guardado, P.; Moyá, M.L. Thermodynamic study of bile salts micellization. J. Chem. Eng. Data 2014, 59, 433–438. [Google Scholar] [CrossRef]
- Robinson, N.C.; Talbert, L. Triton X-100 induced dissociation of beef heart cytochrome c oxidase into monomers. Biochemistry 1986, 25, 2328–2335. [Google Scholar] [CrossRef]
- Patra, M.; Mukhopadhyay, C.; Chakrabarti, A. Probing conformational stability and dynamics of erythroid and nonerythroid spectrin: Effects of urea and guanidine hydrochloride. PLoS ONE 2015, 10, e0116991. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paréj, K.; Hermann, Á.; Donáth, N.; Závodszky, P.; Gál, P.; Dobó, J. Dissociation and re-association studies on the interaction domains of Mannan-Binding Lectin (MBL)-associated serine proteases, MASP-1 and MASP-2, provide evidence for heterodimer formation. Mol. Immunol. 2014, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Gao, H.; Cui, X.; Zhao, Y.; Shi, X.; Li, Q.; Yan, S.; Gao, M.; Wang, M.; Liu, C.; et al. Avirulent Marek’s disease virus type 1 strain 814 vectored vaccine expressing avian influenza (AI) virus H5 haemagglutinin induced better protection than Turkey herpesvirus vectored AI vaccine. PLoS ONE 2013, 8, e53340. [Google Scholar] [CrossRef] [PubMed]
- Hathcock, K.S. T cell enrichment by nonadherence to nylon. Curr. Protoc. Immunol. 2001, 30, 2–3. [Google Scholar]

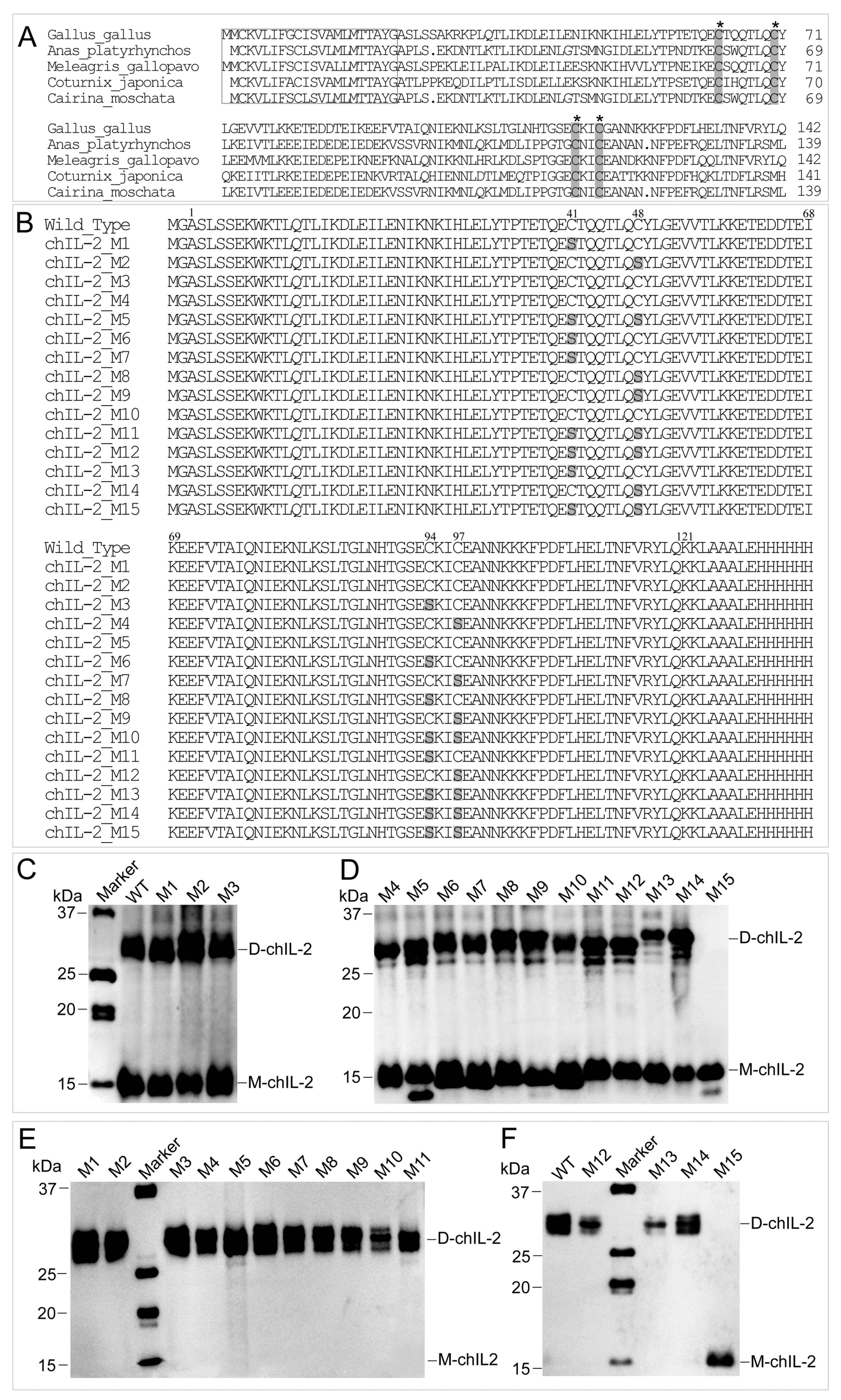

| Single Mutation | Two Mutations | Three Mutations | Four Mutations | |||
|---|---|---|---|---|---|---|
| M1 (C41S) | M5 (C41,48S) | M11 (C41,48,94S) | M15 (C41,48,94,97S) | |||
| M2 (C48S) | M6 (C41,94S) | M12 (C41,48,97S) | ||||
| M3 (C94S) | M7 (C41,97S) | M13 (C41,94,97S) | ||||
| M4 (C97S) | M8 (C48,94S) | M14 (C48,94,97S) | ||||
| M9 (C48,97S) | ||||||
| M10 (C94,97S) | ||||||
| Primer Name | Primer Sequence |
|---|---|
| P1 | 5′-GTCGACGTCCATGGGAGCATCTCTATCATCAGAA-3′ |
| P2 | 5′-GCGGCTGCGAAGCTTTTTTTGCAGATATCTCACA-3′ |
| P3 | 5′-CTCCACAGGTCGACATGTCGTACTACCATCAC-3′ |
| P4 | 5′-CGCGGTGCGGCCGCTTATCAGTGGTGGTGGTGGTGG-3′ |
| P5 | 5′-CCGCCGCCGGAATTCATGTCCCCTATACTAGGTTA-3′ |
| P6 | 5′-GGCCAAGACGTCGACGGATCCACGCGGAACCAGATCCGATTTTGGAGGATGGTC-3′ |
| P7 | 5′-CACGCACGCGTCGACGATGATGACGATAAAGCATCTCTATCATCAGAAAAATGG-3′ |
| P8 | 5′-CAGTCTCCCAAGCTTTTATTTTTGCAGATATCTCACAAAGTTGGTCAGTTCATGGAG-3′ |
| Primer Name | Primer Sequence |
|---|---|
| P1(C41S) | 5′-GAGACCCAGGAGaGCACCCAGCAAACT-3′ |
| P2(C41S) | 5′-AGTTTGCTGGGTGCtCTCCTGGGTCTC-3′ |
| P1(C48S) | 5′-GCAAACTCTGCAGTcTTACCTGGGAG-3′ |
| P2(C48S) | 5′-CTCCCAGGTAAgACTGCAGAGTTTGC-3′ |
| P1(C94S) | 5′-CACCGGAAGTGAAaGCAAGATCTGTG-3′ |
| P2(C94S) | 5′-CACAGATCTTGCtTTCACTTCCGGTG-3′ |
| P1(C97S) | 5′-GAATGCAAGATCTcTGAAGCTAACAAC-3′ |
| P2(C97S) | 5′-GTTGTTAGCTTCAgAGATCTTGCATTC-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.; Tan, H.; Zhou, H.; Wang, M.; Lü, Y.; Xu, J.; Zhang, H.; Han, L.; Ai, Y. Four Cysteine Residues Contribute to Homodimerization of Chicken Interleukin-2. Int. J. Mol. Sci. 2019, 20, 5744. https://doi.org/10.3390/ijms20225744
Deng C, Tan H, Zhou H, Wang M, Lü Y, Xu J, Zhang H, Han L, Ai Y. Four Cysteine Residues Contribute to Homodimerization of Chicken Interleukin-2. International Journal of Molecular Sciences. 2019; 20(22):5744. https://doi.org/10.3390/ijms20225744
Chicago/Turabian StyleDeng, Chen, Hailiang Tan, Hongda Zhou, Mengyun Wang, Yan Lü, Jiacui Xu, Huanmin Zhang, Limei Han, and Yongxing Ai. 2019. "Four Cysteine Residues Contribute to Homodimerization of Chicken Interleukin-2" International Journal of Molecular Sciences 20, no. 22: 5744. https://doi.org/10.3390/ijms20225744
APA StyleDeng, C., Tan, H., Zhou, H., Wang, M., Lü, Y., Xu, J., Zhang, H., Han, L., & Ai, Y. (2019). Four Cysteine Residues Contribute to Homodimerization of Chicken Interleukin-2. International Journal of Molecular Sciences, 20(22), 5744. https://doi.org/10.3390/ijms20225744