GABA-Alleviated Oxidative Injury Induced by Salinity, Osmotic Stress and their Combination by Regulating Cellular and Molecular Signals in Rice
Abstract
1. Introduction
2. Results
2.1. Effects of GABA Priming on the Morpho-Physiological Parameters under Salinity, Osmotic Stress and OS+S
2.2. Effects of GABA Priming on the Photosynthetic and Water Relation Parameters under Salinity, Osmotic Stress and OS+S
2.3. Effects of GABA Priming on the Sugar, Protein, Starch and GABA Contents under Salinity, Osmotic Stress and OS+S
2.4. Effects of GABA Priming on ROS, MDA, Proline and GR under Salinity, Osmotic Stress and OS+S
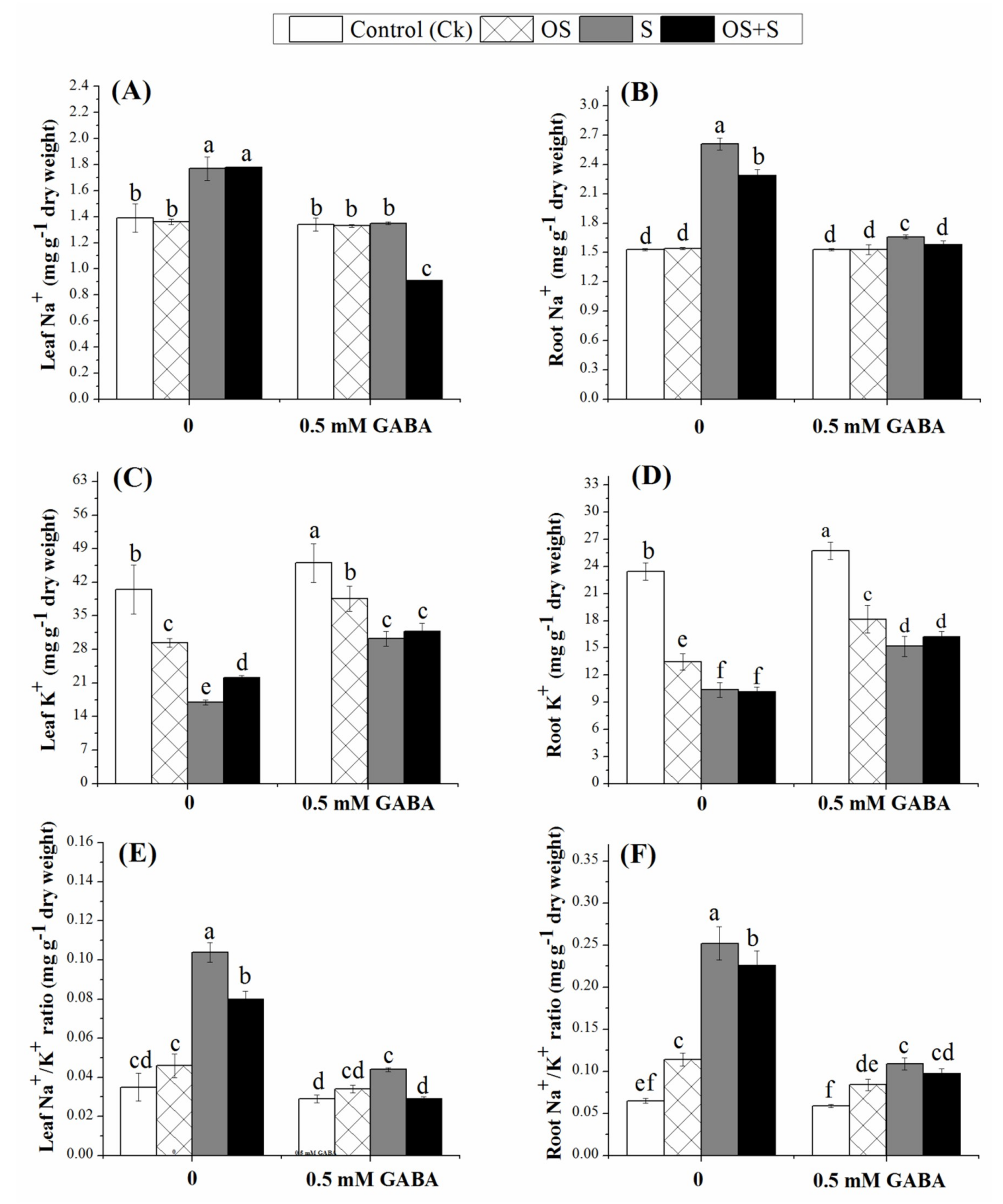
2.5. Effects of GABA Priming on Ion Accumulation under Salinity, Osmotic Stress and OS+S
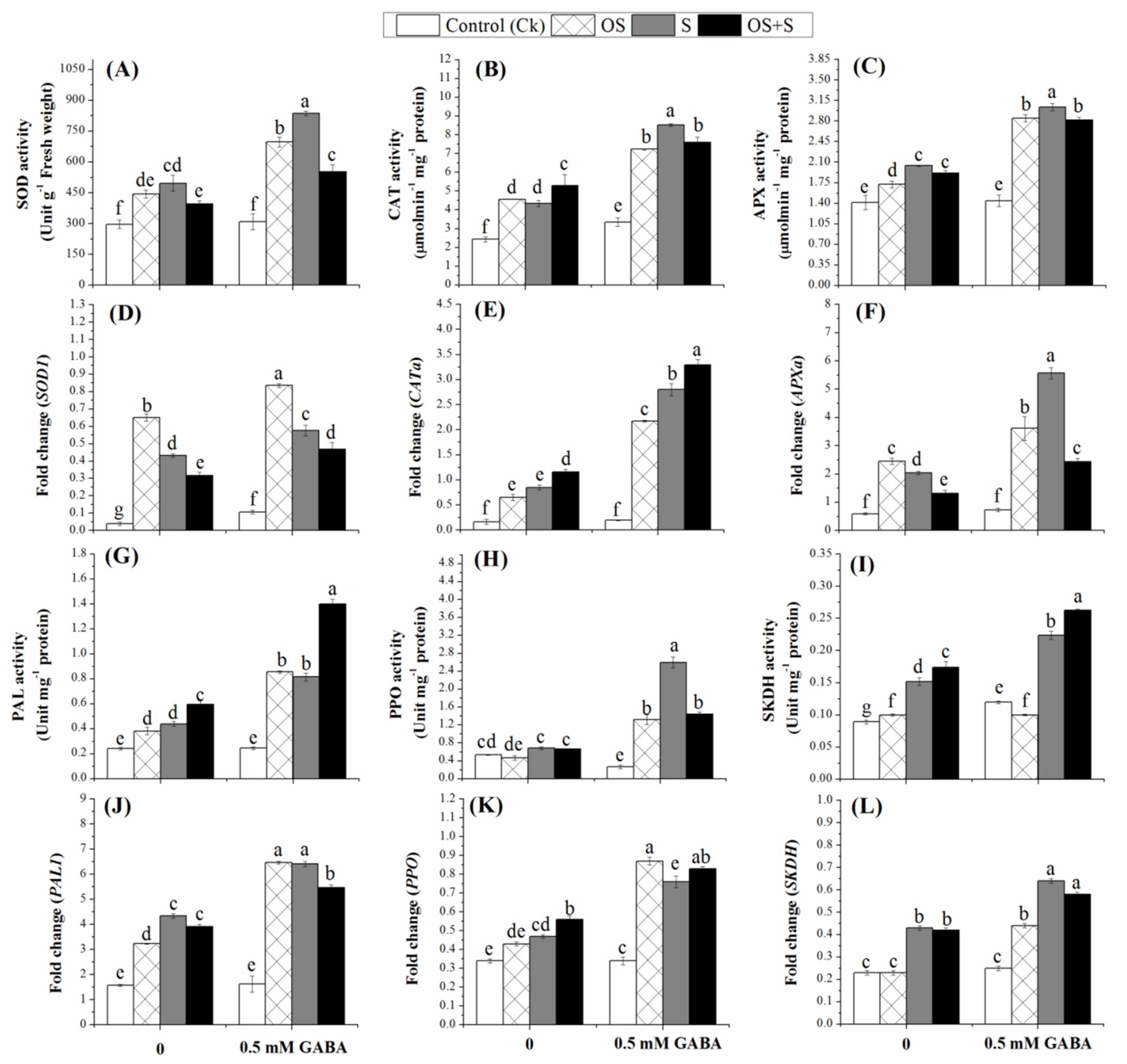
2.6. Effects of GABA Priming on Enzyme Activities under Salinity, Osmotic Stress and OS+S
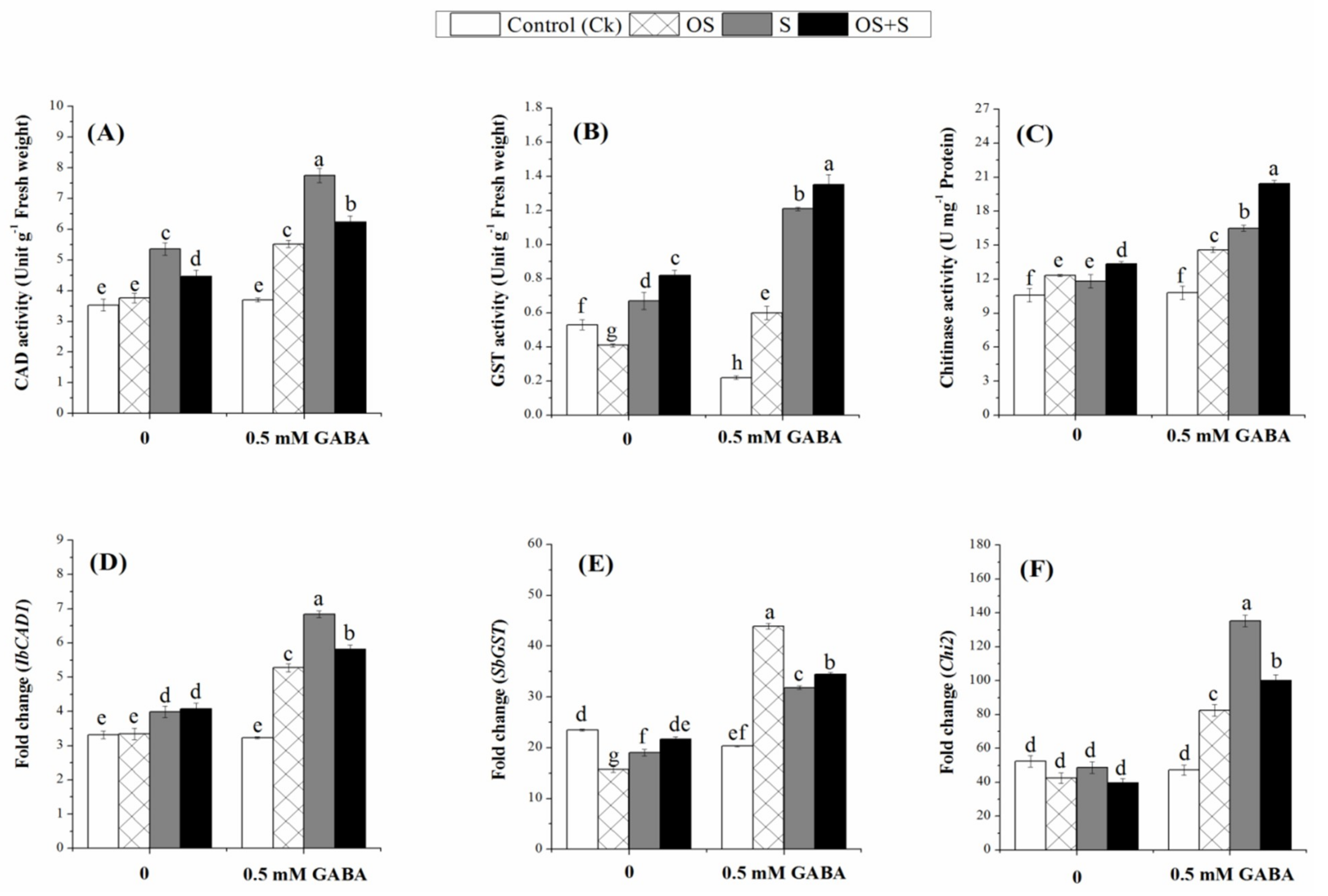
2.7. Effects of GABA Priming on Phenolic Metabolism under Salinity, Osmotic Stress and OS+S
2.8. Effects of GABA Priming on Detoxification-related Enzymes under Salinity, Osmotic Stress and OS+S
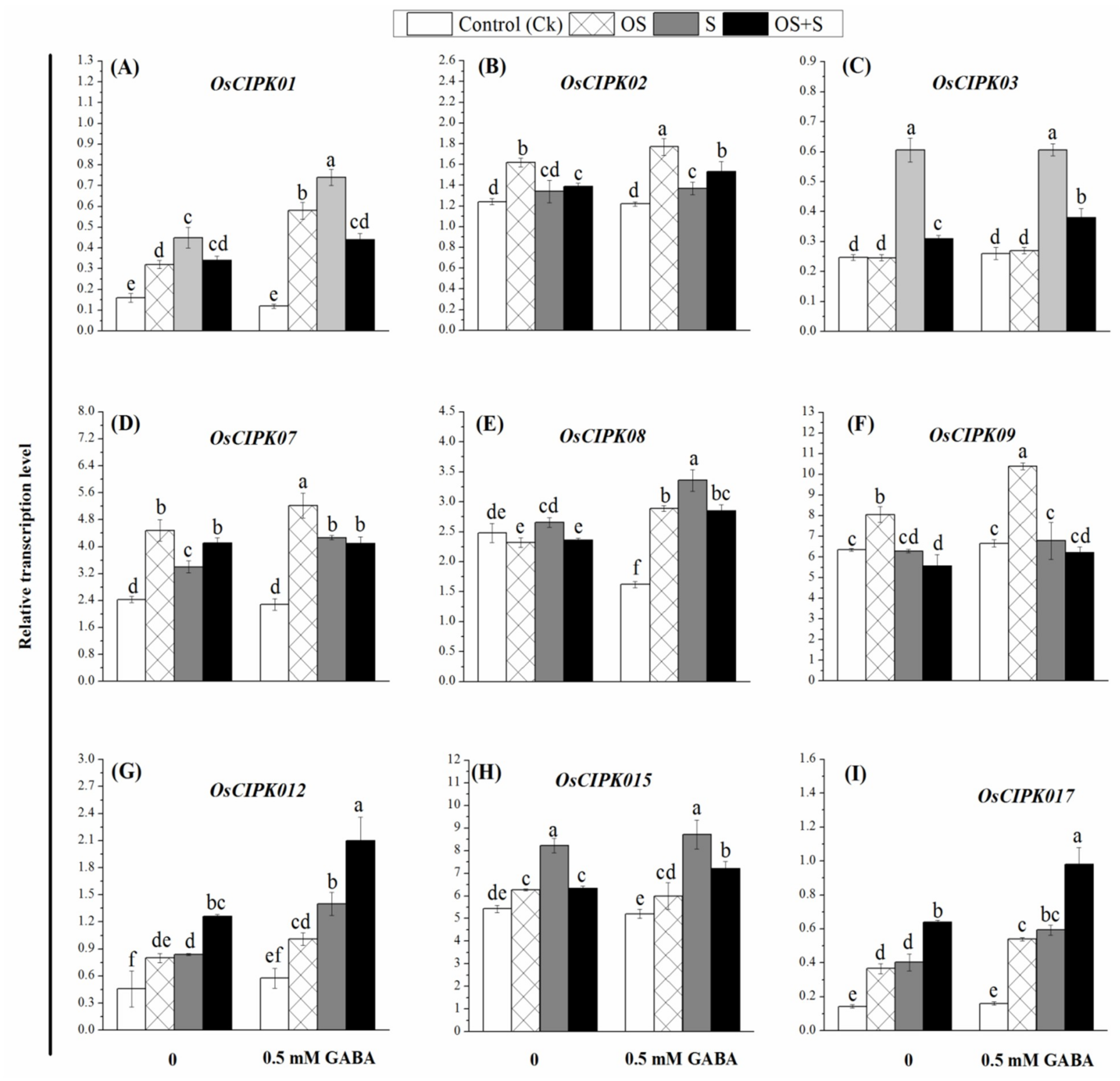
2.9. Effects of GABA Priming on Gene Expression under Salinity, Osmotic Stress and OS+S
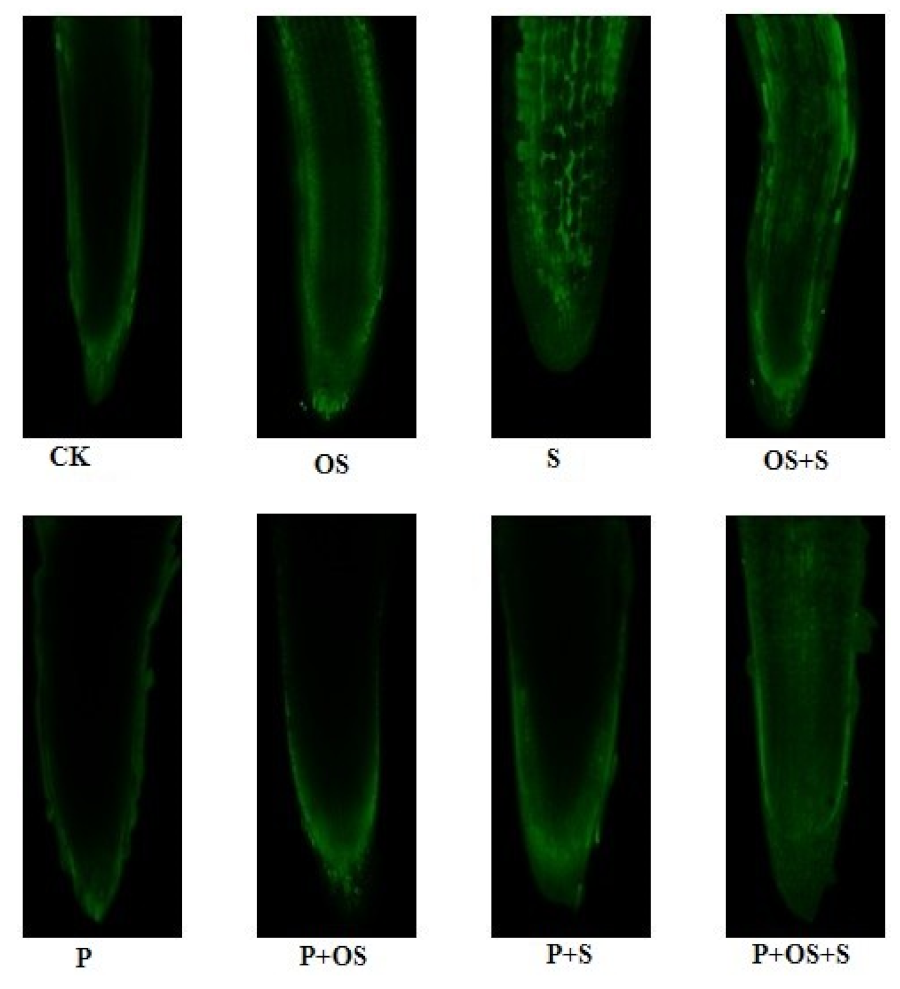
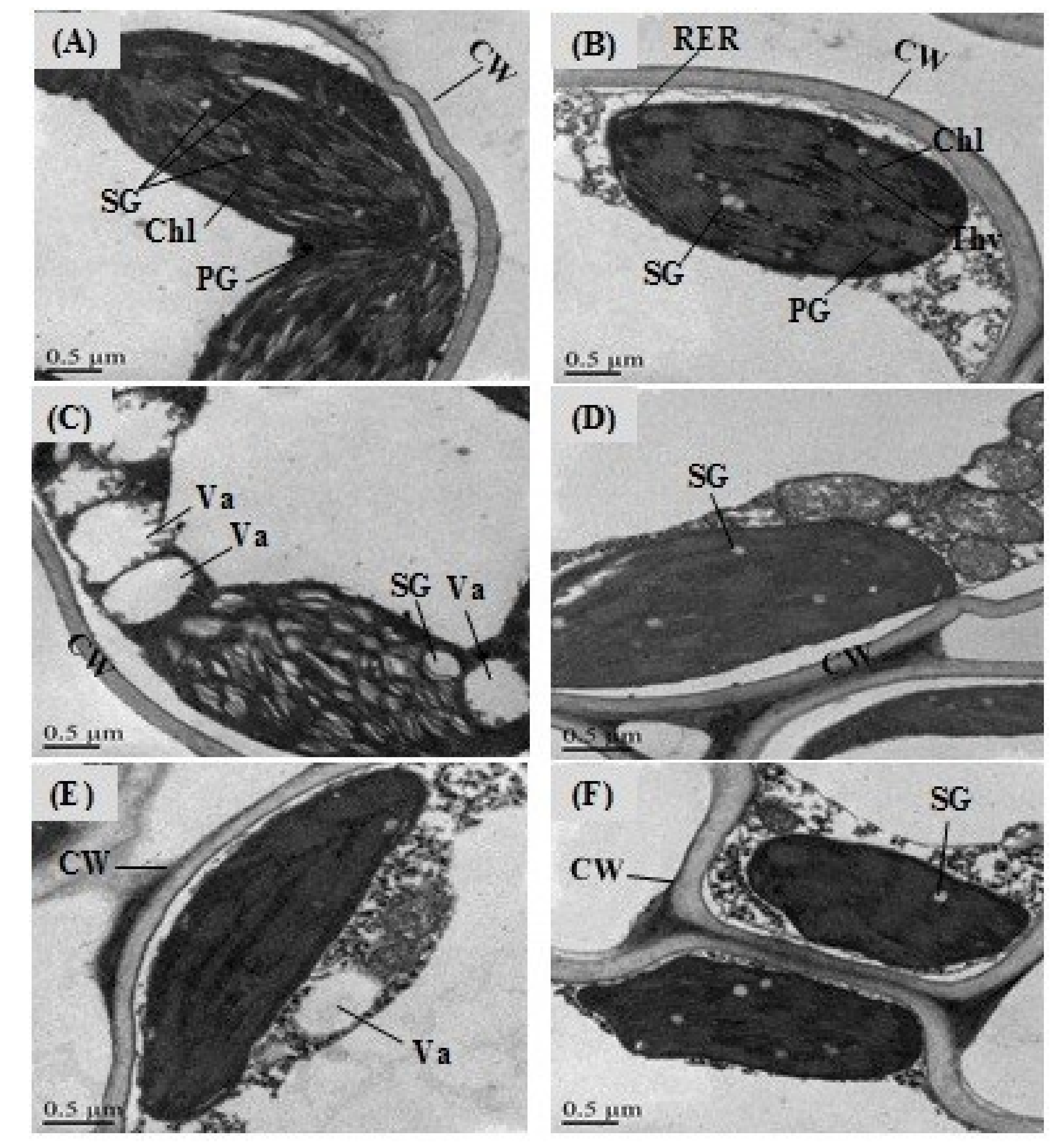
2.10. Effects of GABA Priming on the Nuclear DNA Content and Ultramorphology of the Cell under Salinity, Osmotic Stress and OS+S
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Determination of Photosynthesis and Leaf water Relation
4.3. Biochemical Analysis
4.4. Analysis of MDA, Proline and ROS Contents
4.5. Determination of GABA Content
4.6. Assay of Phenolic Metabolism-related Enzymes
4.7. Measurements of Detoxification-related Enzymes
4.8. Measurement of Na+ and K+ Ions in the Leaf and Root Tissues
4.9. Analysis of Gene Expression
4.10. Ultra-structure and Flow Cytometry Analysis
4.11. Experimental Design and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ. 2009, 32, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M. Breeding for salinity tolerance in rice. In Salt-affected Soils of Pakistan, India and Thailand; IRRI, Ed.; International Rice Research Institute: Manila, Philippines, 1986; pp. 39–63. [Google Scholar]
- Zhu, G.Y.; Kinet, M.; Lutts, S. Characterization of rice (Oryza sativa L.) F3 populations selected for salt resistance. I. Physiological behaviour during vegetative growth. Euphytica 2001, 121, 251–263. [Google Scholar] [CrossRef]
- Castillo, E.G.; Tuong, T.P.; Ismail, A.M.; Inubushi, K. Response to salinity in rice: Comparative effects of osmotic and ionic stresses. Plant Prod. Sci. 2007, 10, 159–170. [Google Scholar] [CrossRef]
- Kaiser, W.M. Effects of water deficit on photosynthetic capacity. Plant Physiol. 2009, 71, 142. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Dai, H.; Zheng, W.; Cao, F.; Zhang, G.; Sun, D.; Wu, F. Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol. Biochem. 2013, 63, 49–60. [Google Scholar] [CrossRef]
- Wang, R.; Chen, S.; Zhou, X.; Shen, X.; Deng, L.; Zhu, H.; Shao, J.; Shi, Y.; Dai, S.; Fritz, E.P.; et al. Ionic homeostasis and reactive oxygen species control in leaves and xylem sap of two poplars subjected to NaCl stress. Tree Physiol. 2008, 28, 947–957. [Google Scholar] [CrossRef]
- Singh, N.P.; Pal, P.K.; Vaishali, S.K. Morpho-physiological characterization of Indian wheat genotypes and their evaluation under drought condition. Afr. J. Biotechnol. 2014, 13, 2022–2027. [Google Scholar]
- Turkan, I.; Bor, M.; Ozdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, C. Physiological responses and expression profile of NADPH oxidase in rice (Oryza sativa) seedlings under different levels of submergence. Rice 2016, 9, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletov, S.; Mittler, R. When defense pathways collide: The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.; Thomashow, M.F. Arabidopsis Transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 2002, 14, 1675–1690. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Mekonnen, D.W.; Flüggea, U.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef]
- Jordan, B.R.; Givan, C.V. Effects of light and inhibitors on glutamate metabolism in leaf discs of Vicia faba L. Plant Physiol. 1979, 64, 1043–1047. [Google Scholar] [CrossRef]
- Bown, A.W.; MacGregor, K.B.; Shelp, B.J. Gamma-aminobutyrate: Defense against invertebrate pests. Trends Plant Sci. 2006, 11, 424–427. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite. Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Wang, M.; Sun, M.; Gu, Z.; Yang, R. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 2019, 270, 593–601. [Google Scholar] [CrossRef]
- Renault, H.; Roussel, V.; Amrani, A.E.; Arzel, M.; Renault, D.; Bouchereau, A. The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol. 2010, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.M.; Singh, N.K.; Cherry, J.H.; Locy, R.D. Nitrate uptake and utilization is modulated by exogenous gamma-aminobutyric acid in Arabidopsis thaliana seedlings. Plant Physiol. Biochem. 2010, 48, 443. [Google Scholar] [CrossRef] [PubMed]
- Beuve, N.; Rispail, N.; Laine, P.; Cliquet, J.B.; Ourry, A.; Le Deuneff, E. Putative role of gamma-aminobutyric acid (GABA) as a long-distance signal in up-regulation of nitrate uptake in Brassica napus L. Plant Cell Environ. 2004, 27, 1035–1046. [Google Scholar] [CrossRef]
- Palanivelu, R.; Brass, L.; Edlund, A.F.; Preuss, D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 2003, 114, 47–59. [Google Scholar] [CrossRef]
- Deleu, C.; Faes, P.; Niogret, M.F.; Bouchereau, A. Effects of the inhibitor of the g-aminobutyrate-transaminase, vinyl-gaminobutyrate, on development and nitrogen metabolism in Brassica napus seedlings. Plant Physiol. Biochem. 2013, 64, 60–69. [Google Scholar] [CrossRef]
- Niu, L.; Dong, B.; Song, Z.; Meng, D.; Fu, Y. Genome-wide identification and characterization of CIPK family and analysis responses to various stresses in apple (Malus domestica). Int. J. Mol. Sci. 2018, 19, 2131. [Google Scholar] [CrossRef]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-shinozaki, K.; Shinozaki, K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999, 17, 287–291. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W.; et al. The γ-Aminobutyric Acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. In. J. Mol. Sci. 2018, 19, 2520–2528. [Google Scholar] [CrossRef]
- Alqarawi, A.A.; Hashem, A.; AbdAllah, E.F.; Al-Huqail, A.A.; Alshahrani, T.S.; Alshalawi, S.A.R.; Egamberdieva, D. Protective role of gamma amminobutyric acid on Cassia italica Mill under salt stress. Legume Res. 2016, 39, 396–404. [Google Scholar] [CrossRef]
- He, F.; Shen, H.; Lin, C.; Fu, H.; Sheteiwy, M.S.; Guan, Y.; Huang, Y.; Hu, J. Transcriptome analysis of chilling-imbibed embryo revealed membrane recovery related genes in maize. Front. Plant Sci. 2017, 7, 1978. [Google Scholar] [CrossRef]
- Grattan, S.; Zeng, L.; Shannon, M.; Robert, S.R. Rice is more sensitive to salinity than previously thought. Calif. Agric. 2002, 56, 189–195. [Google Scholar] [CrossRef]
- Centritto, M.; Lauteri, M.; Monteverdi, M.C.; Serraj, R. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J. Exp. Bot. 2009, 60, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.X.; Wang, Y.Y.; Sun, W.N.; Lou, Q.J.; Mei, H.W.; Shen, S.H.; Chen, H. Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J. Plant Physiol. 2012, 169, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, W.; Yao, M.; Xie, T.; Li, L.; Jing, L.; Shi, W. γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci. Rep. 2017, 7, 43609. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Y.; Zhang, X.Q.; Ma, X.; Hang, L.K.; Yan, Y.H. Exogenous spermidine improves seed germination of white clover under water stress via involvement in starch metabolism, antioxidant defenses and relevant gene expression. Molecules 2014, 19, 18003–18024. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Gu, Q.; Dong, Q.; Zhang, Z.; Lin, C.; Hu, W.; Pan, R.; Guan, Y.; Hu, J. Spermidine enhances heat tolerance of rice seeds by modulating endogenous starch and polyamine metabolism. Molecules 2019, 24, 1395. [Google Scholar] [CrossRef]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Hamoud, Y.A.; Wang, Z.; Guo, X.; Shaghaleh, H.; Sheteiwy, M.; Chen, S.; Qiu, R.; Elbashier, M. Effect of Irrigation regimes and soil texture on the potassium utilization efficiency of rice. Agronomy 2019, 9, 100. [Google Scholar] [CrossRef]
- Hamoud, Y.A.; Shaghaleh, H.; Sheteiwy, M.; Guo, X.; Elshaikh, N.A.; Khan, N.; Oumarou, A.; Rahim, S.F. Impact of alternative wetting and soil drying and soil clay content on the morphological and physiological traits of rice roots and their relationships to yield and nutrient use-efficiency. Agric. Water Manag. 2019, 223, 105706. [Google Scholar] [CrossRef]
- Trovato, M.; Mattioli, R.; Costantino, P. Multiple roles of proline in plant stress tolerance and development. Rend. Lincei 2008, 19, 325–346. [Google Scholar] [CrossRef]
- Singh, N.K.; Bracker, C.A.; Hasegawa, P.M.; Handa, A.K.; Buckel, S.; Hermodson, M.A. Characterization of osmotin: A thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987, 85, 529. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Wang, C.; Fan, L.; Gao, H.; Wu, X.; Li, J.; Lv, G.; Gong, B. Polyamine biosynthesis and degradation are modulated by exogenous gamma-aminobutyric acid in root-zone hypoxia-stressed melon roots. Plant Physiol. Biochem. 2014, 82, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.M.; Nadira, U.A.; Bibi, N.; Cao, F.; He, X.; Zhang, G.; Wu, F. Secondary metabolism and antioxidants are involved in the tolerance to drought and salinity, separately and combined, in Tibetan wild barley. Environ. Exp. Bot. 2015, 111, 1–12. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Kao, C.H. Differential effect of sorbitol and polyethylene glycol on antioxidant enzymes in rice leaves. Plant Growth Regul. 2003, 39, 83–90. [Google Scholar] [CrossRef]
- Shivakrishna, M.P.; Reddy, K.A.; Rao, D.M. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar] [CrossRef]
- Zgallaï, H.; Steppe, K.; Lemeur, R. Photosynthetic, physiological and biochemical responses of tomato plants to polyethylene glycol-induced water deficit. J. Integr. Plant Biol. 2005, 47, 1470–1478. [Google Scholar] [CrossRef]
- Seif, S.N.; Tafazzoli, E.; Talaii, A.; Aboutalebi, A.; Abdosi, V. Evaluation of two grape cultivars (Vitis vinifera L.) against salinity stress and surveying the effect of methyl jasmonate and epibrassinolide on alleviation the salinity stress. In. J. Biosci. 2014, 5, 116–125. [Google Scholar]
- Heber, U.; Tyankova, L.; Santarius, K.A. Stabilization and inactivation of biological membranes during freezing in the presence of amino acids. BBA Biomembr. 1971, 241, 578–592. [Google Scholar] [CrossRef]
- Das, I.; Krzyzosiak, A.; Schneider, K.; Wrabetz, L.; Antonio, M.; Barry, N.; igurdardottir, A.; Bertolotti, A. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Res. Rep. 2015, 348, 1–5. [Google Scholar] [CrossRef]
- Juan, M.; Rivero, R.M.; Romero, L.; Ruiz, J.M. Evaluation of some nutritional and biochemical indicators in selecting salt-resistant tomato cultivars. Environ. Exp. Bot. 2005, 54, 193–201. [Google Scholar] [CrossRef]
- Tejera, N.A.; Soussi, M.; Lluch, C. Physiological and nutritional indicators of tolerance to salinity in chickpea plants growing under symbiotic conditions. Environ. Exp. Bot. 2006, 58, 17–24. [Google Scholar] [CrossRef]
- Turkyilmaz, B.; Aktas, Y.; Guven, A. Salinity induced differences in growth and nutrient accumulation in five barley cultivars. Turk. J. Field Crops 2011, 16, 84–92. [Google Scholar]
- Assaha, D.V.M.; Uedam, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Qui, L.; Wu, D.Z.; Ali, S.; Cai, S.; Dai, F.; Jin, X.; Wu, F.B.; Zhang, G.P. Evaluation of salinity tolerance and analysis of allelic function of HvHKT2 in Tibetan wild barley. Theor. Appl. Genet. 2011, 122, 695–703. [Google Scholar]
- Yeo, A.R. Molecular biology of salt tolerance in the context of whole-plant physiology. J. Exp. Bot. 1998, 49, 915–929. [Google Scholar] [CrossRef]
- Davenport, R.J.; Munoz-Mayor, A.; Jha, D.; Essah, P.A.; Rus, A.; Tester, M. The Na+ transporter AtHKT1; 1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ. 2007, 30, 497–507. [Google Scholar] [CrossRef]
- Berthomieu, P.; Conejero, G.; Nublat, A.; Brackenbury, W.J.; Lambert, C.; Savio, C.; Uozumi, N.; Oiki, S.; Yamada, K.; Cellier, F. Functional analysis of AtHKT1 in Arabidopsis shows that Na 1 recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003, 22, 2004–2014. [Google Scholar] [CrossRef]
- Senadheera, P.; Singh, R.; Maathuis, F.J. Differentially expressed membrane transporters in rice roots may contribute to cultivar dependent salt tolerance. J. Exp. Bot. 2009, 60, 2553–2563. [Google Scholar] [CrossRef]
- Nayyar, H.; Kaur, R.; Kaur, S.; Singh, R. γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J. Plant Growth Regul. 2014, 33, 408–419. [Google Scholar] [CrossRef]
- Vijayakumari, K.; Puthur, J.T. γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum linn. plants subjected to PEG-induced stress. Plant Growth Regul. 2015, 78, 1–11. [Google Scholar] [CrossRef]
- Krishnan, S.; Laskowski, K.; Shukla, V.; Merewitz, E.B. Mitigation of drought stress damage by exogenous application of a non-protein amino acid gamma aminobutyric acid on perennial ryegrass. J. Am. Soc. Hortic. Sci. 2013, 138, 358–366. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 26, 217037. [Google Scholar] [CrossRef]
- Swigonska, S.; Amarowicz, R.; Król, A.; Mostek, A.; Badowiec, A.; Weidner, S. Influence of abiotic stress during soybean germination followed by recovery on the phenolic compounds of radicles and their antioxidant capacity. Acta Soc. Bot. Pol. 2014, 83, 209–218. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress. J. Plant Physiol. 2018, 231, 192–201. [Google Scholar] [CrossRef]
- Kovacik, J.; Klejdus, B.; Hedbavny, J.; Backor, M. Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 2009, 18, 544–554. [Google Scholar] [CrossRef]
- Kim, S.K.; Son, T.K.; Park, S.Y.; Lee, I.J.; Lee, B.H.; Kim, H.Y.; Lee, S.C. Influences of gibberellin and auxin on endogenous plant hormone and starch mobilization during rice seed germination under salt stress. J. Environ. Biol. 2007, 27, 181–186. [Google Scholar]
- Dai, H.X.; Cao, F.B.; Chen, X.; Zhang, M.; Ahmed, I.M.; Chen, Z.H.; Li, C.; Zhang, G.P.; Wu, F.B. Comparative proteomic analysis of aluminum tolerance in Tibetan wild and cultivated barleys. PLoS ONE 2013, 8, e63428. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y. (2013) Role of brassinosterods in alleviation of phenanthrene-cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef]
- Thipyapong, P.; Hunt, M.D.; Steffens, J.C. Antisense down regulation of polyphenol oxidase results in enhanced disease susceptibility. Planta 2004, 220, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.K.; Hwang, B.K. Induction by pathogen, salt and drought of a basic class II chitinase mRNA and its in situ localization in pepper (Capsicum annuum). Physiol. Plant. 2002, 114, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Moffatt, B.; Griffith, A.; Xiong, M.; Yang, F.; Wiseman, D.S.; Sarhan, S.B.; Danyluk, F.J.; Xue, Y.Q.; Hew, C.L. Chitinase genes responsive to cold Encode antifreeze proteins in winter cereals. Plant Physiol. 2000, 124, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, Y.; Xiong, L. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol. 2007, 144, 1416–1428. [Google Scholar] [CrossRef]
- Seki, M.; Narusaka, M.; Ishida, J.; Nanjo, T.; Fujita, M.; Oono, Y.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T.; et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 2002, 31, 279–292. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell 2003, 15, 745–759. [Google Scholar] [CrossRef]
- Anderson, J.P.; Badruzsaufari, E.; Schank, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef]
- Bowler, C.; Fluhr, R. The role of calcium and activated oxygen as signals for controlling cross-tolerance. Trends Plant Sci. 2000, 5, 241–246. [Google Scholar] [CrossRef]
- West, G.; Inze, D.; Beemster, G.T. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, P.; Hou, H.; Zhang, H.; Wang, Y.; Yan, S.; Huang, Y.; Li, H.; Tan, J.; Hu, A.; et al. Transcriptional regulation of cell cycle genes in response to abiotic stresses correlates with dynamic changes in histone modifications in maize. PLoS ONE 2014, 9, e106070. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; An, J.; Yin, M.; Jia, X.; Guan, Y.; He, F.; Hu, J. Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings. Protoplasma 2018, 256, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Shen, H.; Xu, J.; Guan, Y.; Song, W.; Hu, J. Seed polyamines metabolism induced by seed priming with Spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ. Exp Bot. 2017, 137, 58–72. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y.; et al. The Synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Fu, Y.; Guan, Y.; Lin, C.; Cao, D.; Hu, W.; Sheteiwy, M.S.; Hu, J. Inhibitory effect of chemical combinations on seed germination and pre-harvest sprouting in hybrid rice. Plant Growth Regul. 2016, 80, 281–289. [Google Scholar] [CrossRef]
- Hu, Q.; Lin, C.; Guan, Y.; Sheteiwy, M.S.; Hu, W.; Hu, J. Inhibitory effect of eugenol on seed germination and pre-harvest sprouting of hybrid rice (Oryza sativa L.). Sci. Rep. 2017, 7, 5295. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Gong, D.; Gao, Y.; Pan, R.; Hu, J.; Guan, Y. Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ. Exp. Bot. 2018, 153, 236–248. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Guan, Y.; Cao, D.; Li, J.; Nawaz, A.; Hu, Q.; Hu, W.; Ning, M.; Hu, J. Seed priming with polyethylene glycol regulating the physiological an molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci. Rep. 2015, 5, 14278. [Google Scholar]
- Sheteiwy, M.S.; Fu, Y.; Hu, Q.; Nawaz, A.; Guan, Y.; Zhan, L.; Huang, Y.; Hu, J. Seed priming with polyethylene glycol induces antioxidative defense and metabolic performance of rice under nano-ZnO stress. Environ. Sci. Pollut. Res. 2016, 23, 19989–20002. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Dong, Q.; An, J.; Song, W.; Guan, Y.; He, F.; Huang, Y.; Hu, J. Regulation of ZnO nanoparticles-induced physiological and molecular changes by seed priming with humic acid in Oryza sativa seedlings. Plant Growth Regul. 2017, 83, 27–41. [Google Scholar] [CrossRef]
- Zhou, W.J.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Li, H.S. Principle and Technology of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000; pp. 169–172. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of abscisic acid on reactive oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Z.; Cui, C.L.; Zhang, Y.T.; Wang, D.; Jing, Y.K.; Kim, Y. Active changes of lignification-related enzymes in pepper response to Glomus intraradices and/or Phytophthora capsici. J. Zhejiang Univ. Sci. B 2005, 6, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Wyrambik, D.; Grisebach, H. Purification and properties of isoenzymes of cinnamyl-alcohol dehydrogenase from soybean cell-suspension cultures. Eur. J. Biochem. 1975, 59, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Ying, G.E. Response of Glutathione and Glutathione S-transferase in Rice Seedlings Exposed to Cadmium Stress. Rice Sci. 2008, 15, 73–76. [Google Scholar]
- Zhao, X.; Wang, W.; Zhang, F.; Deng, J.; Li, Z.; Fu, B. Comparative metabolite profiling of two rice genotypes with contrasting salt stress tolerance at the seedling stage. PLoS ONE 2014, 9, e108020. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Zhao, L.; Li, J.; He, S.; Zhou, K.; Yang, F.; Huang, M.; Jiang, L.; Li, L. Trichostatin A selectively suppresses the cold-induced transcription of the ZmDREB1 gene in maize. PLoS ONE 2011, 6, e22132. [Google Scholar] [CrossRef]

| Treatments | GP | GE | RL | SL | SFW | SDW | SVI |
|---|---|---|---|---|---|---|---|
| Ck | 88.66 ± 0.57a | 80.66 ± 3.78ab | 12.40 ± 0.17b | 7.63 ± 5.41a | 0.160 ± 0.01b | 0.062 ± 0.002b | 1775 ± 459ab |
| Osmotic (OS) | 84.66 ± 0.41b | 79.33 ± 4.72bc | 9.53 ± 0.25d | 7.50 ± 0.30a | 0.126 ± 0.07b | 0.057 ± 0.002bc | 1443 ± 106bc |
| Salinity (S) | 76.66 ± 0.35de | 57.66 ± 0.57e | 7.60 ± 0.45f | 6.56 ± 0.37a | 0.096 ± 0.02b | 0.049 ± 0.003de | 1085 ± 53cd |
| OS+S | 73.66 ± 0.35e | 52.00 ± 2.00f | 6.56 ± 0.37g | 6.50 ± 0.50a | 0.061 ± 0.04c | 0.035 ± 0.001f | 962 ± 65d |
| Priming (P) | 89.00 ± 1.00a | 85.33 ± 0.57a | 14.30 ± 0.62a | 7.63 ± 5.40a | 0.192 ± 0.02a | 0.086 ± 0.002a | 1948 ± 518a |
| OS+P | 88.33 ± 1.15a | 81.66 ± 2.08ab | 10.78 ± 0.36c | 8.33 ± 0.11a | 0.131 ± 0.03b | 0.057 ± 0.006bc | 1688 ± 14ab |
| S+P | 80.33 ± 0.57c | 75.66 ± 0.57c | 8.50 ± 0.40e | 7.70 ± 0.26a | 0.114 ± 0.05b | 0.053 ± 0.003cd | 1301 ± 20b–d |
| OS+S+P | 79.00 ± 0.37cd | 69.66 ± 3.51d | 9.40 ± 0.36d | 9.00 ± 0.45a | 0.092 ± 0.01b | 0.044 ± 0.001e | 1453 ± 36bc |
| Treatments | Pn | Tr | Gs | Ci | SPAD | WP (Ψw) | OP (Ψs) | RWC | WUE |
|---|---|---|---|---|---|---|---|---|---|
| Ck | 6.67 ± 0.15b | 3.60 ± 0.20b | 0.613 ± 0.47b | 417.67 ± 35.30a | 11.70 ± 0.55b | −2.36 ± 0.15ab | −0.14 ± 0.01b | 33.04 ± 2.15b | 1.59 ± 0.19f |
| Osmotic (OS) | 3.46 ± 0.11d | 1.70 ± 0.10e | 0.453 ± 0.06c | 225.00 ± 5.00b | 6.33 ± 0.15e | −6.68 ± 0.17e | −0.17 ± 0.01b | 11.56 ± 1.48f | 3.38 ± 0.23d |
| Salinity (S) | 2.84 ± 0.09e | 1.23 ± 0.05f | 0.260 ± 0.03e | 115.00 ± 5.00d | 7.98 ± 0.45d | −9.43 ± 0.90f | −0.38 ± 0.04d | 16.81 ± 1.37e | 2.36 ± 0.07e |
| OS+S | 1.86 ± 0.13f | 0.88 ± 0.02g | 0.173 ± 0.02f | 136.67 ± 7.63cd | 8.20 ± 0.36d | −13.40 ± 0.95g | −0.26 ± 0.02c | 9.68 ± 0.50f | 2.17 ± 0.12e |
| Priming (P) | 11.10 ± 0.52a | 5.46 ± 0.30aa | 1.51 ± 0.05a | 410.00 ± 65.57a | 16.37 ± 0.12a | −1.60 ± 0.16a | −0.08 ± 0.01a | 63.61 ± 2.60a | 6.83 ± 0.10a |
| OS+P | 5.60 ± 0.19c | 3.07 ± 0.06c | 0.543 ± 0.05b | 248.33 ± 10.40b | 8.70 ± 0.85d | −3.69 ± 0.13c | −0.14 ± 0.01b | 25.86 ± 2.53c | 5.11 ± 0.41b |
| S+P | 3.73 ± 0.12d | 2.73 ± 0.07d | 0.426 ± 0.02c | 175.67 ± 4.04c | 9.60 ± 0.17c | −2.93 ± 0.06bc | −0.24 ± 0.01c | 30.51 ± 1.17b | 4.16 ± 0.12c |
| OS+S+P | 2.77 ± 0.19e | 1.78 ± 0.17e | 0.343 ± 0.03d | 154.33 ± 5.13cd | 9.80 ± 0.43c | −4.91 ± 0.61d | −0.26 ± 0.01c | 22.78 ± 0.67d | 3.52 ± 0.07d |
| Treatments | GABA Con. | Sugars | Protein | Starch | Proline | H2O2 | O2− | OH− | MDA | GR |
|---|---|---|---|---|---|---|---|---|---|---|
| Ck | 3.53 ± 0.10g | 12.50 ± 0.17a | 240.0 ± 8.6b | 66.37 ± 0.89b | 30.35 ± 2.07c | 1.64 ± 0.06de | 2.59 ± 0.15c | 0.103 ± 0.05d | 81.75 ± 4.8e | 6.72 ± 0.54f |
| Osmotic (OS) | 5.71 ± 0.10d | 6.30 ± 0.25d | 108.35 ± 6.2e | 33.25 ± 2.65de | 48.55 ± 1.89b | 3.52 ± 0.06b | 2.68 ± 0.18c | 0.153 ± 0.01b | 105.60 ± 1.5cd | 10.53 ± 0.07e |
| Salinity (S) | 4.76 ± 0.24e | 4.96 ± 0.20e | 102.81 ± 6.3e | 27.13 ± 1.47e | 73.19 ± 2.51a | 4.34 ± 0.18a | 3.95 ± 0.50a | 0.210 ± 0.04a | 159.90 ± 3.5a | 14.44 ± 0.42d |
| OS+S | 4.52 ± 0.24e | 4.17 ± 0.30f | 85.17 ± 5.84f | 28.84 ± 0.41e | 76.44 ± 1.7a | 4.15 ± 0.53a | 3.49 ± 0.05b | 0.156 ± 0.05b | 125.46 ± 2.0b | 17.31 ± 0.42c |
| Priming (P) | 4.07 ± 0.06f | 12.24 ± 0.30a | 361.01 ± 12.9a | 81.07 ± 7.62a | 30.40 ± 1.15c | 1.47 ± 0.21e | 2.69 ± 0.34c | 0.099 ± 0.05d | 83.24 ± 2.65e | 6.10 ± 0.18f |
| OS+P | 10.45 ± 0.05a | 9.90 ± 0.52b | 160.0 ± 5.60d | 63.90 ± 3.72b | 34.56 ± 1.04c | 1.98 ± 0.01d | 2.67 ± 0.27c | 0.126 ± 0.05b-d | 83.38 ± 10.4e | 18.61 ± 0.82bc |
| S+P | 8.42 ± 0.39b | 9.00 ± 0.46c | 215.37 ± 5.0c | 48.82 ± 3.26c | 41.68 ± 1.70bc | 2.69 ± 0.24c | 2.73 ± 0.07c | 0.136 ± 0.05bc | 112.02 ± 2.7c | 20.00 ± 0.21b |
| OS+S+P | 6.60 ± 0.16c | 10.19 ± 0.54b | 168.86 ± 16.3d | 39.47 ± 4.91d | 49.18 ± 6.30b | 2.95 ± 0.07c | 2.55 ± 0.09c | 0.116 ± 0.01cd | 102.67 ± 3.8d | 27.68 ± 2.00a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheteiwy, M.S.; Shao, H.; Qi, W.; Hamoud, Y.A.; Shaghaleh, H.; Khan, N.U.; Yang, R.; Tang, B. GABA-Alleviated Oxidative Injury Induced by Salinity, Osmotic Stress and their Combination by Regulating Cellular and Molecular Signals in Rice. Int. J. Mol. Sci. 2019, 20, 5709. https://doi.org/10.3390/ijms20225709
Sheteiwy MS, Shao H, Qi W, Hamoud YA, Shaghaleh H, Khan NU, Yang R, Tang B. GABA-Alleviated Oxidative Injury Induced by Salinity, Osmotic Stress and their Combination by Regulating Cellular and Molecular Signals in Rice. International Journal of Molecular Sciences. 2019; 20(22):5709. https://doi.org/10.3390/ijms20225709
Chicago/Turabian StyleSheteiwy, Mohamed S., Hongbo Shao, Weicong Qi, Yousef Alhaj Hamoud, Hiba Shaghaleh, Nasr Ullah Khan, Ruiping Yang, and Boping Tang. 2019. "GABA-Alleviated Oxidative Injury Induced by Salinity, Osmotic Stress and their Combination by Regulating Cellular and Molecular Signals in Rice" International Journal of Molecular Sciences 20, no. 22: 5709. https://doi.org/10.3390/ijms20225709
APA StyleSheteiwy, M. S., Shao, H., Qi, W., Hamoud, Y. A., Shaghaleh, H., Khan, N. U., Yang, R., & Tang, B. (2019). GABA-Alleviated Oxidative Injury Induced by Salinity, Osmotic Stress and their Combination by Regulating Cellular and Molecular Signals in Rice. International Journal of Molecular Sciences, 20(22), 5709. https://doi.org/10.3390/ijms20225709







