Lack of Delta-Sarcoglycan (Sgcd) Results in Retinal Degeneration
Abstract
1. Introduction
2. Results
2.1. Changes in Retinal Structure among Three-Month-Old Mice Knocked-Out (KO) and Wild-Type (WT)
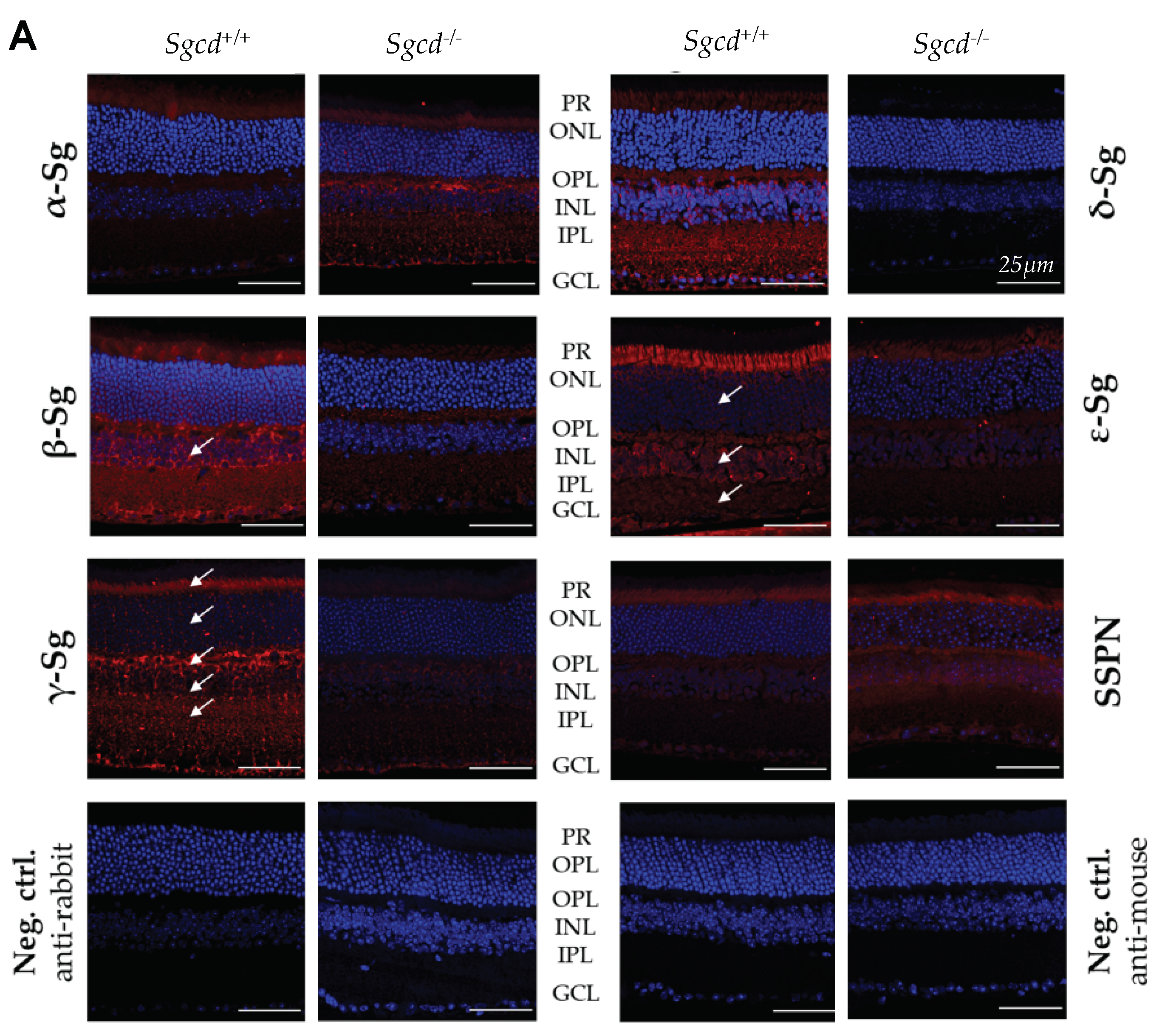
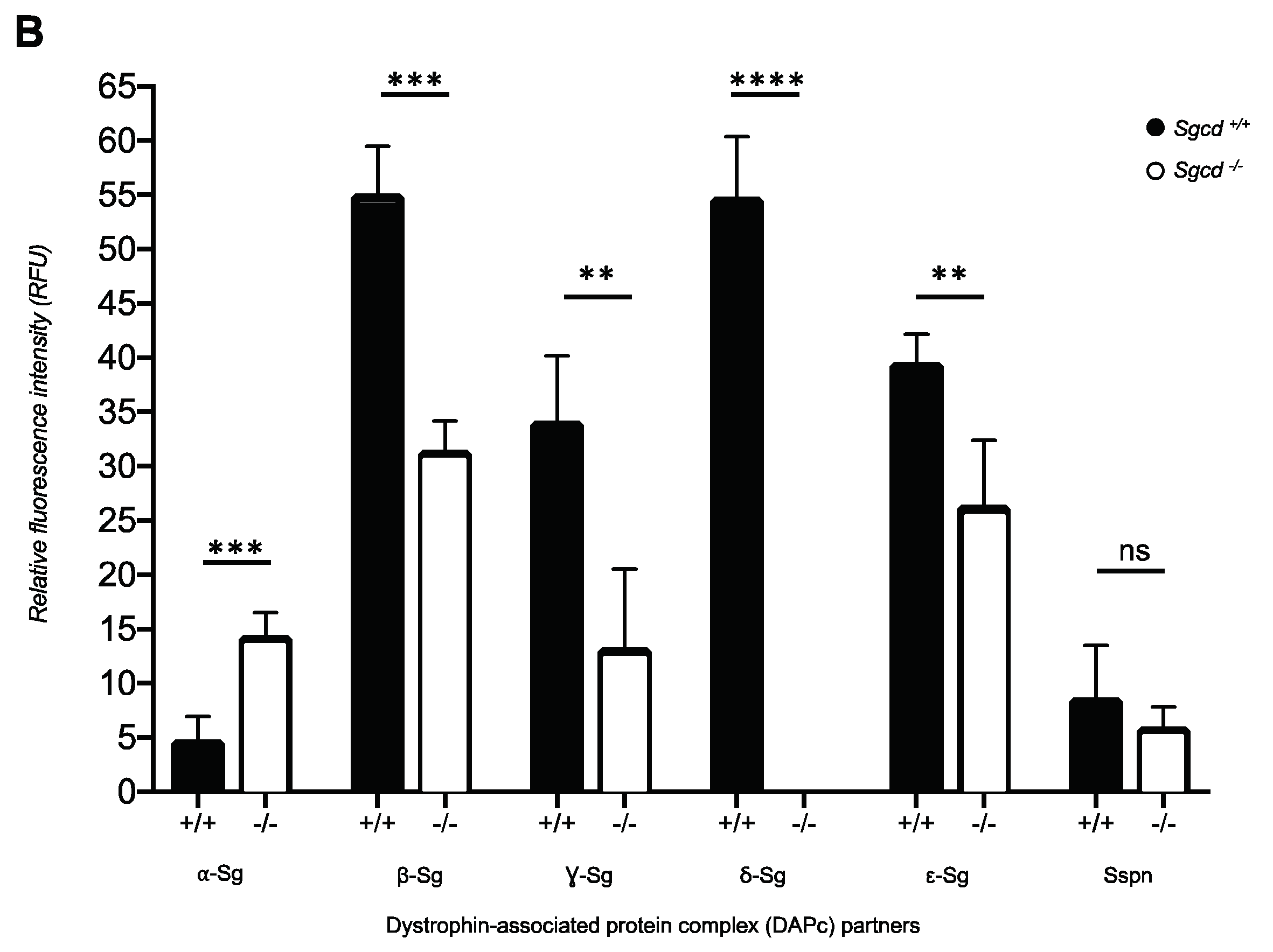
2.2. Sarcoglycan Complex Expression and Localization by Immunofluorescence among Three-Month-Old Mice
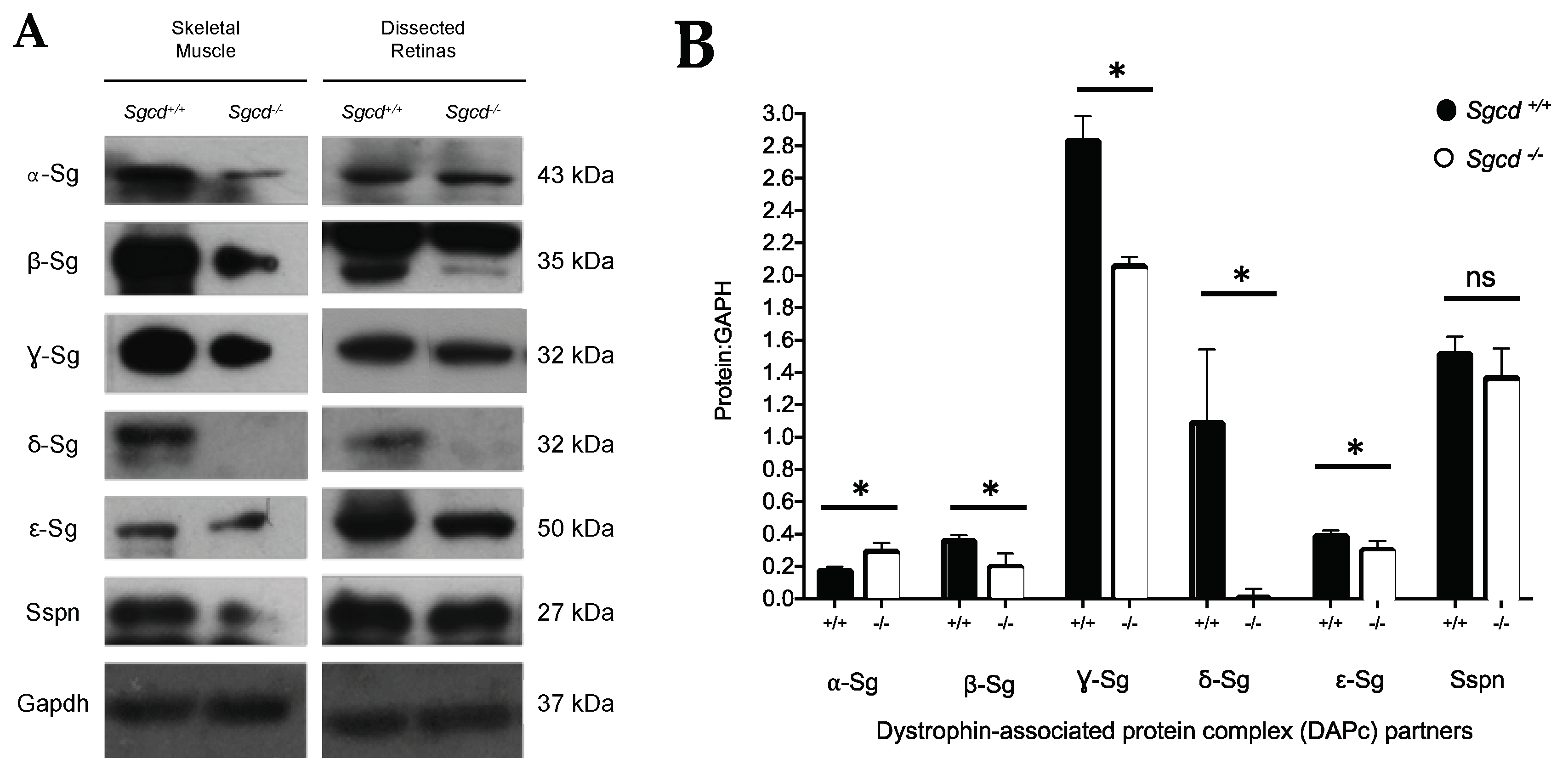
2.3. Changes in Sarcoglycan Complex Protein Expression in Dissected Retinas of Three-Month-Old Mice
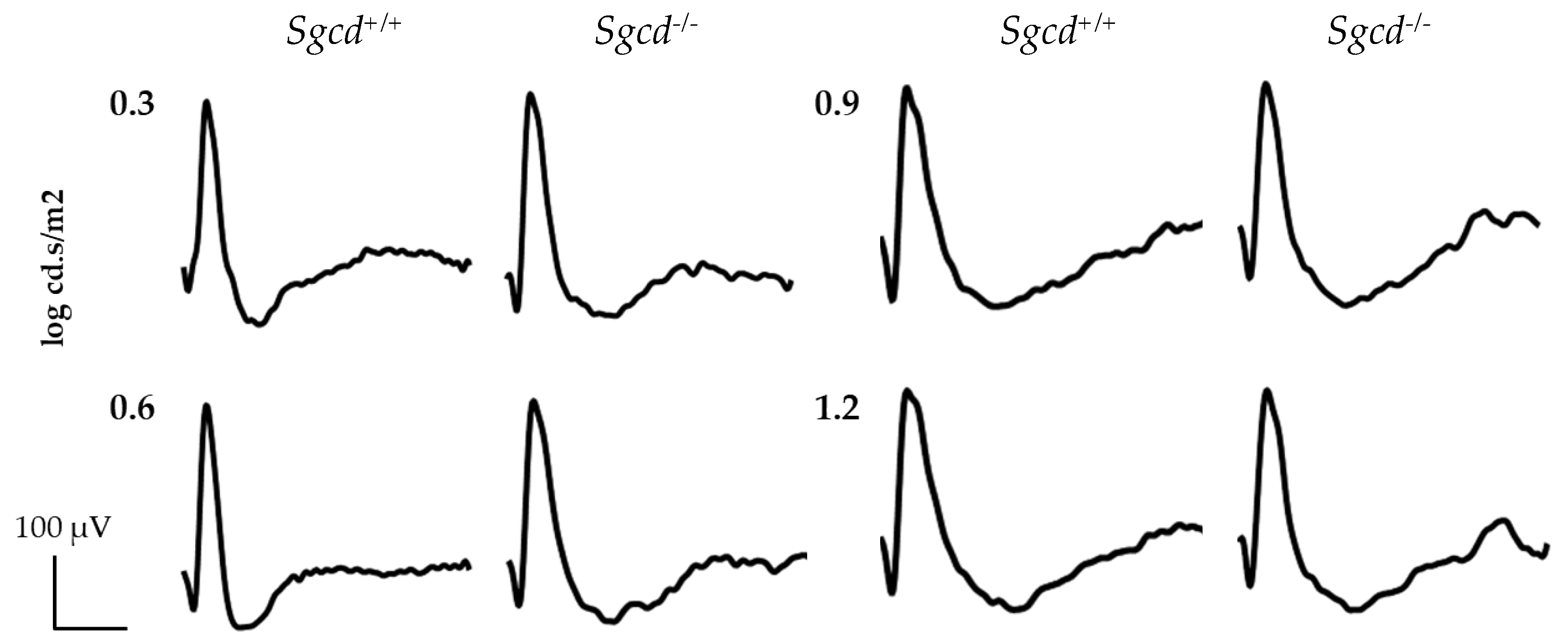
2.4. Effect of Sgcd Knock-Out in Retinal Function by Electroretinography among Three-Month-Old Mice
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethical Approval
4.2. Mice Genotyping
4.2.1. DNA Extraction
4.2.2. PCR Reactions
4.3. Morphological Analysis
4.3.1. Retinal Thickness
4.3.2. Frailty Index
4.3.3. Nuclei Density
4.4. Immunofluorescence of the Sarcoglycan-Sarcospan Complex
4.5. Western Blot of the Sarcoglycan-Sarcospan Complex
4.6. Electroretinographic (ERG) Assessments
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| ANOVA | Analysis of variance |
| ARVO | The Association for Research in Vision and Ophthalmology |
| DAPc | Dystrophin-associated protein complex |
| Dp71 | Dystrophin isoform of 71 kilodaltons |
| ERG | Electroretinography or electroretinogram, as applicable |
| GCL | Ganglion cell layer |
| H&E | Hematoxylin and eosin |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| KO | Knocked-out for Sgcd−/− gene |
| LGMD2 | Limb-girdle muscular dystrophy |
| M ± IQR | Median ± interquartile range |
| ms | Millisecond |
| ONL | Outer nuclear layer |
| OPL | Outer plexiform layer |
| OR | Odds ratio |
| PBS | Phosphate-buffered saline |
| PR | Photoreceptor |
| RR | Risk ratio |
| Sg(s) | Sarcoglycan(s) |
| Sspn | Sarcospan(s) |
| Sgcd/SGCD | Delta-sarcoglycan murine protein (Lowercase) or human (Uppercase) |
| Sgcd/SGCD | Delta-sarcoglycan murine gene (Lowercase) or human (Uppercase) |
| µV | Microvolt |
| WT | Wild type for Sgcd+/+. |
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Heal. 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Edwards, A.O.; Ritter, R.; Abel, K.J.; Manning, A.; Panhuysen, C.; Farrer, L.A. Complement factor H polymorphism and age-related macular degeneration. Science 2005, 308, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Camp, N.J.; Sun, H.; Tong, Z.; Gibbs, D.; Cameron, D.J.; Chen, H.; Zhao, Y.; Pearson, E.; Li, X.; et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 2006, 314, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.V.; Zenteno, J.C.; Fernández-López, J.C.; Rodríguez-Corona, U.; Falfán-Valencia, R.; Sebastian, L.; Morales, F.; Ochoa-Contreras, D.; Carnevale, A.; Silva-Zolezzi, I. CFH haplotypes and ARMS2, C2, C3, and CFB alleles show association with susceptibility to age-related macular degeneration in Mexicans. Mol. Vis. 2014, 20, 105–116. [Google Scholar] [PubMed]
- Ryu, E.; Fridley, B.L.; Tosakulwong, N.; Bailey, K.R.; Edwards, A.O. Genome-wide association analyses of genetic, phenotypic, and environmental risks in the age-related eye disease study. Mol. Vis. 2010, 16, 2811–2821. [Google Scholar]
- Tong, Y.; Liao, J.; Zhang, Y.; Zhou, J.; Zhang, H.; Mao, M. LOC387715/HTRA1 gene polymorphisms and susceptibility to age-related macular degeneration: A HuGE review and meta-analysis. Mol. Vis. 2010, 16, 1958–1981. [Google Scholar]
- Lu, F.; Liu, S.; Hao, Q.; Liu, L.; Zhang, J.; Chen, X.; Hu, W.; Huang, P. Association between Complement Factor C2/C3/CFB/CFH Polymorphisms and Age-Related Macular Degeneration: A Meta-Analysis. Genet. Test. Mol. Biomark. 2018, 22, 526–540. [Google Scholar] [CrossRef]
- Perez-Ortiz, A.C.; Luna-Angulo, A.; Zenteno, J.C.; Rendon, A.; Cortes-Ballinas, L.G.; Jimenez-Collado, D.; Antonio-Aguirre, B.; Peralta-Ildefonso, M.J.; Ramírez, I.; Jacob-Kuttothara, S.; et al. Significant Association Between Variant in SGCD and Age-Related Macular Degeneration. Genes (Basel) 2018, 9, 467. [Google Scholar] [CrossRef]
- Rahimov, F.; Kunkel, L.M. Cellular and molecular mechanisms underlying muscular dystrophy. J. Cell Biol. 2013, 201, 499–510. [Google Scholar] [CrossRef]
- Barboni, M.T.S.; Nagy, B.V.; de Araújo Moura, A.L.; Damico, F.M.; da Costa, M.F.; Kremers, J.; Ventura, D.F. ON and OFF electroretinography and contrast sensitivity in duchenne muscular dystrophy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3195–3204. [Google Scholar] [CrossRef]
- Costa, M.F.; Oliveira, A.G.F.; Feitosa-Santana, C.; Zatz, M.; Ventura, D.F. Red-green color vision impairment in Duchenne muscular dystrophy. Am. J. Hum. Genet. 2007, 80, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Fort, P.; Estrada, F.J.; Bordais, A.; Mornet, D.; Sahel, J.A.; Picaud, S.; Vargas, H.R.; Coral-Vázquez, R.M.; Rendon, A. The sarcoglycan-sarcospan complex localization in mouse retina is independent from dystrophins. Neurosci. Res. 2005, 53, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Claudepierre, T.; Mornet, D.; Pannicke, T.; Forster, V.; Dalloz, C.; Bolaños, F.; Sähel, J.; Reichenbach, A.; Rendon, A. Expression of Dp71 in Muller glial cells: A comparison with utrophin- and dystrophin-associated proteins. Investig. Ophthalmol. Vis. Sci. 2000, 41, 294–304. [Google Scholar]
- Hack, A.A.; Lam, M.Y.; Cordier, L.; Shoturma, D.I.; Ly, C.T.; Hadhazy, M.A.; Hadhazy, M.R.; Sweeney, H.L.; McNally, E.M. Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex. J. Cell Sci. 2000, 113, 2535–2544. [Google Scholar]
- Tarakci, H.; Berger, J. The sarcoglycan complex in skeletal muscle. Front. Biosci. 2016, 21, 744–756. [Google Scholar]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; LIMBURG, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Heal. 2017, 5, e888–e897. [Google Scholar] [CrossRef]
- Shahidi, M.; Blair, N.P.; Mori, M.; Gieser, J.; Pulido, J.S. Retinal topography and thickness mapping in atrophic age related macular degeneration. Br. J. Ophthalmol. 2002, 86, 623–626. [Google Scholar] [CrossRef]
- Brandl, C.; Brücklmayer, C.; Günther, F.; Zimmermann, M.E.; Küchenhoff, H.; Helbig, H.; Weber, B.H.F.; Heid, I.M.; Stark, K.J. Retinal Layer Thicknesses in Early Age-Related Macular Degeneration: Results From the German AugUR Study. Investig. Opthalmology Vis. Sci. 2019, 60, 1581. [Google Scholar] [CrossRef]
- Blain, A.M.; Straub, V.W. δ-Sarcoglycan-deficient muscular dystrophy: From discovery to therapeutic approaches. Skelet. Muscle 2011, 1, 1–12. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Wang, P.; Liu, J.; Li, B.; Yu, Y.; Wu, H.; Kang, R.; Zhang, X.; Wang, Z. Deciphering the scalene association among type-2 diabetes mellitus, prostate cancer, and chronic myeloid leukemia via enrichment analysis of disease-gene network. Cancer Med. 2019, 8, 2268–2277. [Google Scholar] [CrossRef]
- Durbeej, M.; Campbell, K.P. Muscular dystrophies involving the dystrophin-glycoprotein complex: An overview of current mouse models. Curr. Opin. Genet. Dev. 2002, 12, 349–361. [Google Scholar] [CrossRef]
- Imamura, M.; Mochizuki, Y.; Engvall, E.; Takeda, S. E-Sarcoglycan compensates for lack of α-sarcoglycan in a mouse model of limb-girdle muscular dystrophy. Hum. Mol. Genet. 2005, 14, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Lancioni, A.; Rotundo, I.L.; Kobayashi, Y.M.; D’Orsi, L.; Aurino, S.; Nigro, G.; Piluso, G.; Acampora, D.; Cacciottolo, M.; Campbell, K.P.; et al. Combined deficiency of alpha and epsilon sarcoglycan disrupts the cardiac dystrophin complex. Hum. Mol. Genet. 2011, 20, 4644–4654. [Google Scholar] [CrossRef] [PubMed]
- Lebakken, C.S.; Venzke, D.P.; Hrstka, R.F.; Consolino, C.M.; Faulkner, J.A.; Williamson, R.A.; Campbell, K.P. Sarcospan-Deficient Mice Maintain Normal Muscle Function. Mol. Cell. Biol. 2000, 20, 1669–1677. [Google Scholar] [CrossRef]
- Pappuru, R.R.; Ouyang, Y.; Nittala, M.G.; Hemmati, H.D.; Keane, P.A.; Walsh, A.C.; Sadda, S.R. Relationship between outer retinal thickness substructures and visual acuity in eyes with dry age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6743–6748. [Google Scholar] [CrossRef]
- The Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Available online: https://www.arvo.org/About/policies/arvo-publications-ethics-statement/ (accessed on 24 September 2019).
- Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes. 2014. Available online: https://www.gov.uk/government/publications/code-of-practice-for-the-housing-and-care-of-animals-bred-supplied-or-used-for-scientific-purposes (accessed on 24 September 2019).
- Wilding, L.A.; Uchihashi, M.; Bergin, I.L.; Nowland, M.H. Enucleation for Treating Rodent Ocular Disease. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 328–332. [Google Scholar]
- Perez-Ortiz, A.C.; Solano-García, G.; Luna-Angulo, A.; Coral-Vazquez, R.M.; Garfias, Y.; Garcia-Perez, V.; de los Santos-Enriquez, S.; Rendon, A.; Ramirez-Sanchez, I.; Estrada-Mena, F.J. Changes in the sarcoglycan complex and effects of (−)-epicatechin in SGCD-null mice as a potential animal model for retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2016, 57, 1732. [Google Scholar]
- Smith, A. Color Gamut Transform Pairs. Siggraph Comput. 1978, 12, 12–19. [Google Scholar] [CrossRef]
- Frei, W. Digital image processing. IEEE Commun. Mag. 1981, 19, 53–54. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, H.; Bi, S. Segmentation of the clustered cells with optimized boundary detection in negative phase contrast images. PLoS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef]
- Díaz-Lezama, N.; Wu, Z.; Adán-Castro, E.; Arnold, E.; Vázquez-Membrillo, M.; Arredondo-Zamarripa, D.; Ledesma-Colunga, M.G.; Moreno-Carranza, B.; Martinez de la Escalera, G.; Colosi, P.; et al. Diabetes enhances the efficacy of AAV2 vectors in the retina: Therapeutic effect of AAV2 encoding vasoinhibin and soluble VEGF receptor 1. Lab. Investig. 2016, 96, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Margrain, T.; Binns, A.M. Detection of Early Age-Related Macular Degeneration Using Novel Functional Parameters of the Focal Cone Electroretinogram. PLoS ONE 2014, 9, e96742. [Google Scholar] [CrossRef] [PubMed]
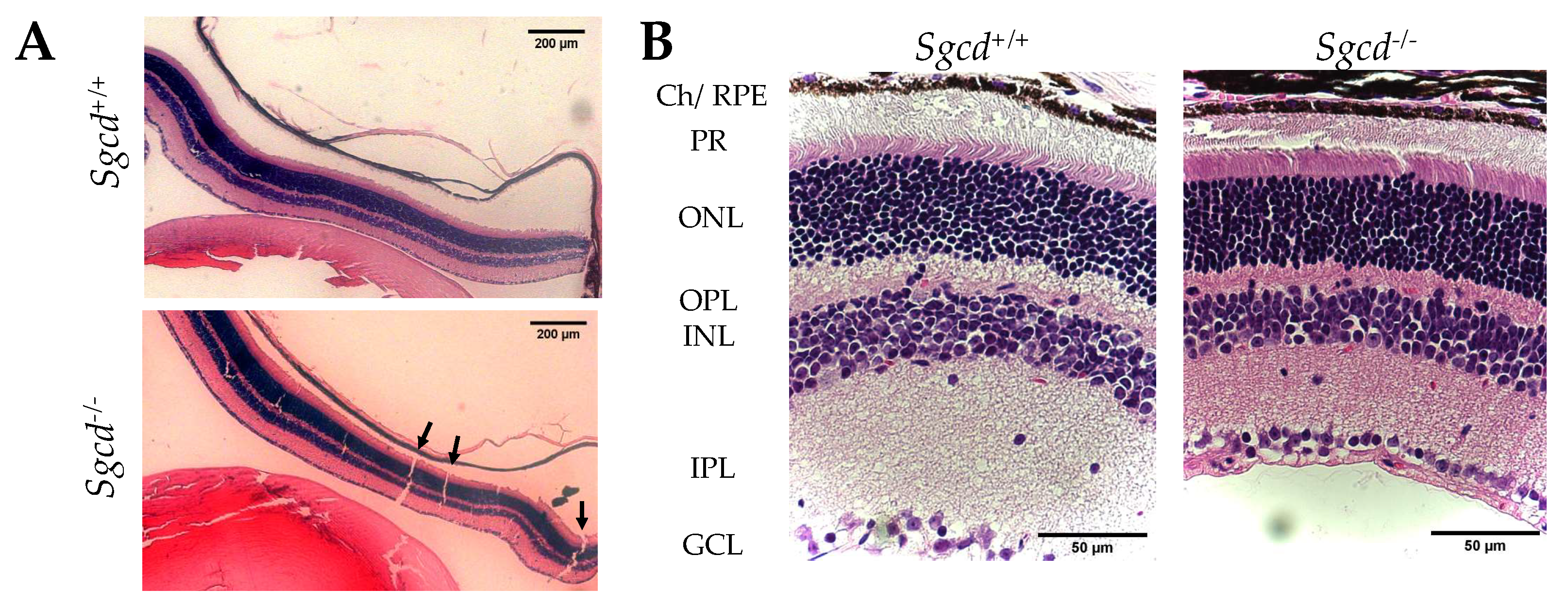
| Characteristic | Sgcd+/+ | Sgcd–/– | p-Value 1,2 |
|---|---|---|---|
| Retinal thickness (µm) 1 | 252.4 ± 51.5 | 149.0 ± 51.0 | <0.0001 |
| Differences any layer 2 | <0.0001 a | ||
| Photoreceptor | 42.6 ± 15.6 | 24.7 ± 8.3 | <0.0001 b |
| Outer segment | 109.6 ± 20.2 | 75.5 ± 15.0 | <0.0001 b |
| Inner segment | 135.8 ± 22.4 | 83.1 ± 24.3 | <0.0001 b |
| Outer nuclear | 67.0 ± 10.2 | 50.8 ± 11.8 | <0.0001 b |
| Outer plexiform | 19.9 ± 5.3 | 11.2 ± 2.7 | <0.0001 b |
| Inner nuclear | 49.4 ± 10.7 | 31.0 ± 10.1 | <0.0001 b |
| Inner plexiform | 53.6 ± 12.7 | 32.9 ± 16.7 | <0.0001 b |
| Ganglion cell | 12.9 ± 4.1 | 8.1 ± 4.1 | n.s. |
| Nuclei density per 100 µm2 | |||
| Outer nuclear 1 Inner nuclear 1 | 2.5 ± 0.4 1.6 ± 0.3 | 2.7 ± 0.3 1.96 ± 0.4 | 0.0033 <0.0001 |
| Solution of continuity in the H&E 3 | 13.0 ± 5.0 | 0.0 ± 3.0 | <0.0001 |
| Sg-Sspn Subunit | Median Difference (95% CI) Sgcd–/– vs. Sgcd+/+ | p-Value | H1 Based on IF |
|---|---|---|---|
| α-sarcoglycan | 0.1320 (0.0775, 0.2031) | 0.0286 | MKO > MWT |
| β-sarcoglycan | −0.1598 (−0.1986, −0.0572) | 0.0286 | MKO < MWT |
| γ-sarcoglycan | −0.7787 (−0.9732, −0.4996) | 0.0286 | MKO < MWT |
| δ-sarcoglycan | −1.0802 (−1.5383, −0.8444) | 0.0286 | MKO < MWT |
| ε-sarcoglycan | −0.0846 (−0.1108, −0.0260) | 0.0286 | MKO < MWT |
| Sspn | −0.18043 (−0.3132, 0.0668) | 0.1143 | MKO < MWT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Ortiz, A.C.; Peralta-Ildefonso, M.J.; Lira-Romero, E.; Moya-Albor, E.; Brieva, J.; Ramirez-Sanchez, I.; Clapp, C.; Luna-Angulo, A.; Rendon, A.; Adan-Castro, E.; et al. Lack of Delta-Sarcoglycan (Sgcd) Results in Retinal Degeneration. Int. J. Mol. Sci. 2019, 20, 5480. https://doi.org/10.3390/ijms20215480
Perez-Ortiz AC, Peralta-Ildefonso MJ, Lira-Romero E, Moya-Albor E, Brieva J, Ramirez-Sanchez I, Clapp C, Luna-Angulo A, Rendon A, Adan-Castro E, et al. Lack of Delta-Sarcoglycan (Sgcd) Results in Retinal Degeneration. International Journal of Molecular Sciences. 2019; 20(21):5480. https://doi.org/10.3390/ijms20215480
Chicago/Turabian StylePerez-Ortiz, Andric C., Martha J. Peralta-Ildefonso, Esmeralda Lira-Romero, Ernesto Moya-Albor, Jorge Brieva, Israel Ramirez-Sanchez, Carmen Clapp, Alexandra Luna-Angulo, Alvaro Rendon, Elva Adan-Castro, and et al. 2019. "Lack of Delta-Sarcoglycan (Sgcd) Results in Retinal Degeneration" International Journal of Molecular Sciences 20, no. 21: 5480. https://doi.org/10.3390/ijms20215480
APA StylePerez-Ortiz, A. C., Peralta-Ildefonso, M. J., Lira-Romero, E., Moya-Albor, E., Brieva, J., Ramirez-Sanchez, I., Clapp, C., Luna-Angulo, A., Rendon, A., Adan-Castro, E., Ramírez-Hernández, G., Díaz-Lezama, N., Coral-Vázquez, R. M., & Estrada-Mena, F. J. (2019). Lack of Delta-Sarcoglycan (Sgcd) Results in Retinal Degeneration. International Journal of Molecular Sciences, 20(21), 5480. https://doi.org/10.3390/ijms20215480







