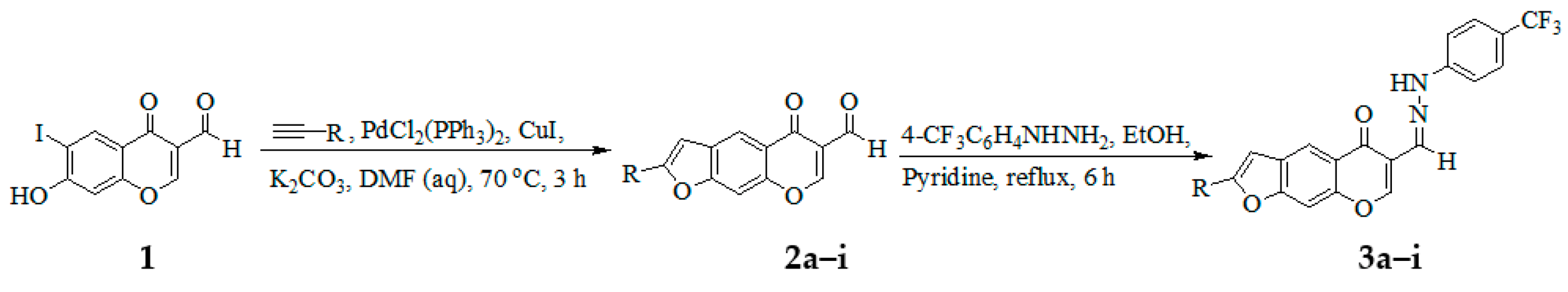
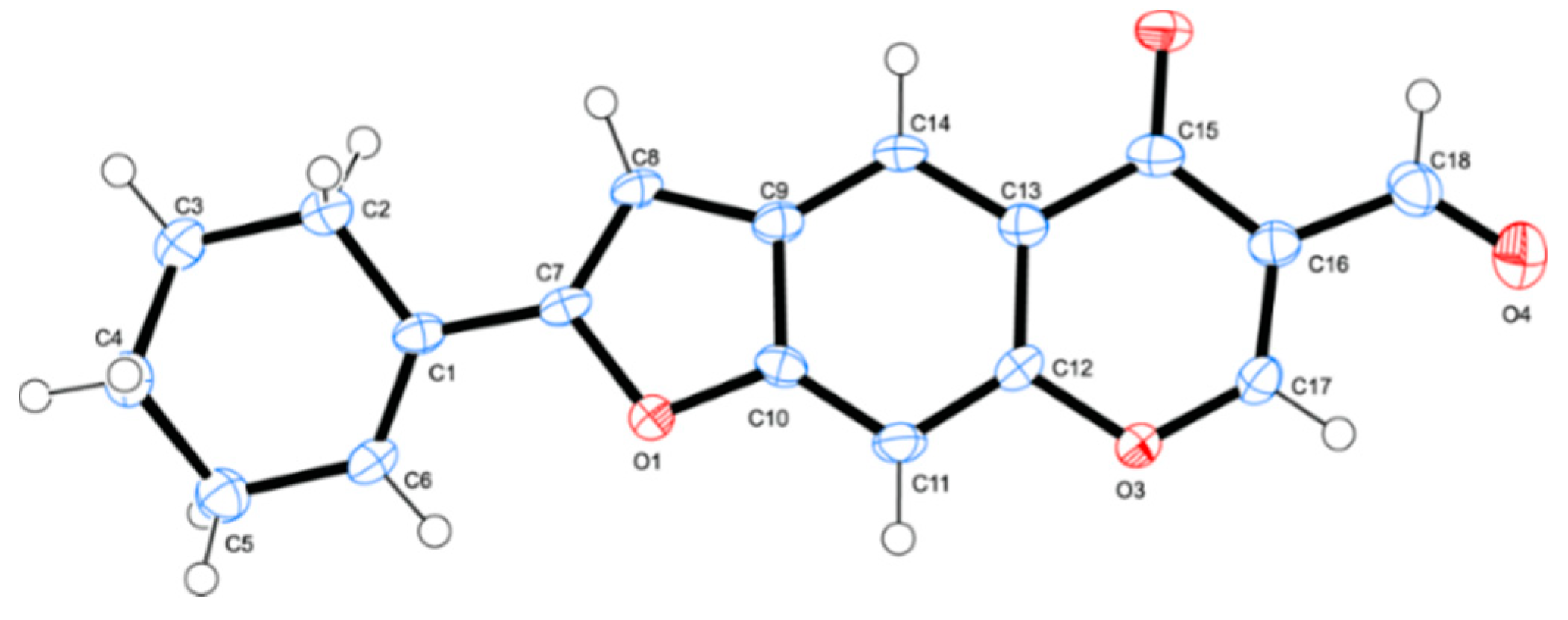
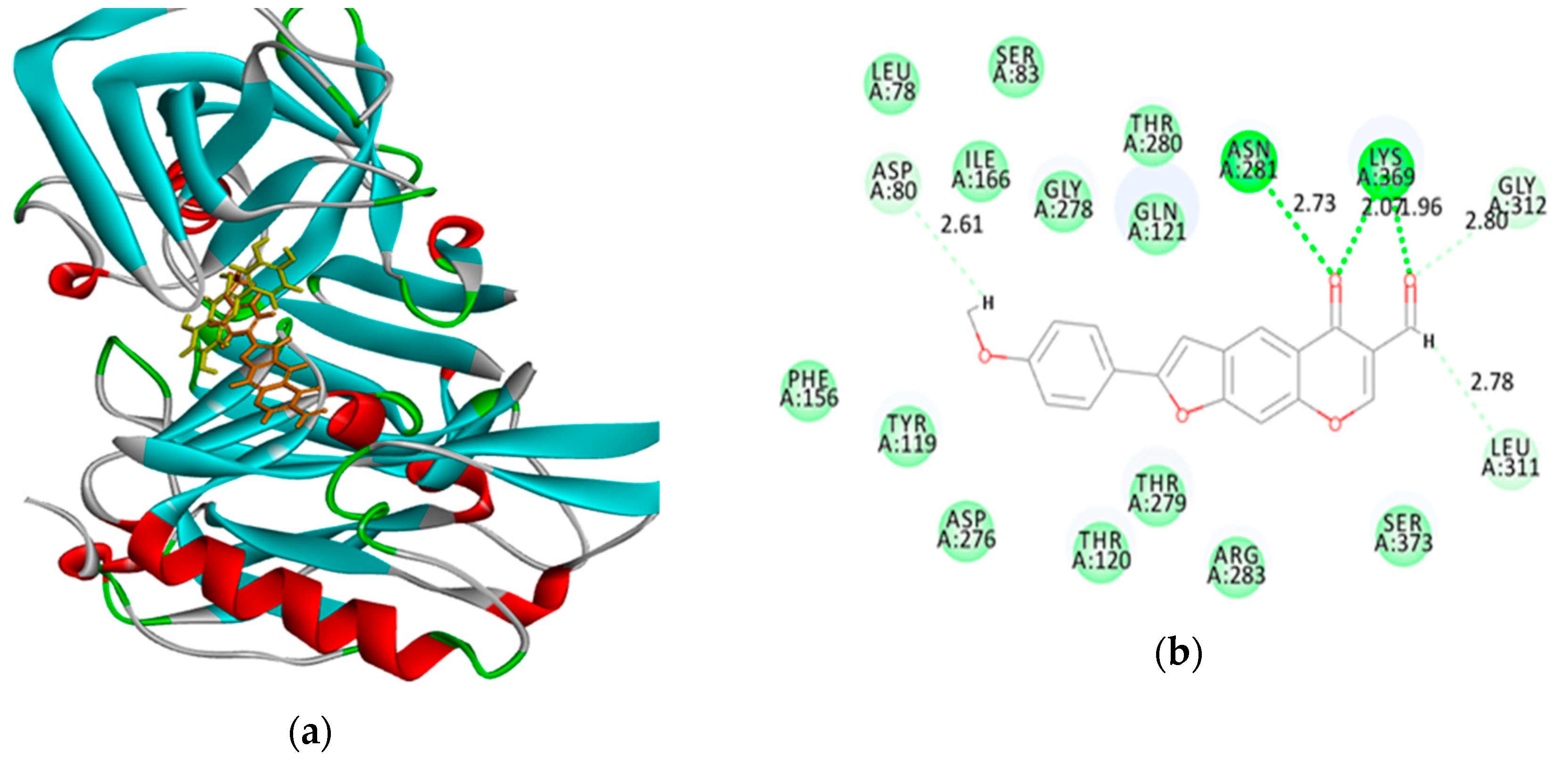
3.3. Typical Procedure for Tandem Sonogashira cross-coupling–heteroannulation of 1
A mixture of 1 (0.50 g, 1.58 mmol), PdCl2(PPh3)2 (0.06 g, 0.08 mmol), CuI (0.03 g, 0.16 mmol) and K2CO3 (0.33 g, 2.37 mmol) in 9:1 DMF-H2O (v/v, 20 mL) was placed in a two necked round-bottom flask equipped with a stirrer bar, condenser and rubber septum. The mixture was purged with argon for 30 min and a solution of phenylacetylene (0.19 g, 1.90 mmol) was introduced through the rubber septum with the aid of a syringe. The mixture was stirred at 70 °C for 3 h and then poured into an ice-cold water. The product was extracted into chloroform, and the combined organic layers were washed with brine and then dried over anhydrous MgSO4. The salt was filtered off, and the solvent was evaporated under reduced pressure on a rotary evaporator. The residue was purified by column chromatography on silica gel using 2:1 toluene-ethyl acetate (v/v) mixture as an eluent. Compounds 2a–i were prepared in this fashion.
5-Oxo-2-phenyl-5H-furo[3,2-g]chromene-6-carbaldehyde (2a):
Brown solid (0.28 g, 61%), Rf (toluene-ethyl acetate) 0.69, mp. 145–146 °C; νmax (ATR) 495, 647, 682, 773, 950, 1083, 1230, 1301, 1379, 1446, 1469, 1567, 1616, 1644, 1698, 2853, 2922, 3068, 3097 cm-1; δH (300 MHz, DMSO-d6) 7.74–7.82 (3H, m, Ar), 7.90 (1H, s, H-3), 8.21 (2H, d, J = 6.3 Hz, Ar), 8.36 (1H, s, H-9), 8.66 (1H, s, H-7), 9.20 (1H, s, H-4), 10.41 (1H, s, –CHO); δC (75 MHz, DMSO-d6) 101.8, 112.3, 115.1, 119.4, 119.9, 121.2, 121.9, 127.2, 131.2, 150.3, 152.7, 157.4, 157.63, 160.8, 175.2, 188.9; HRMS (ES+): m/z [M + H]+ calc. for C18H11O4: 291.0650; found 291.0657.
2-(3-Fluorophenyl)-5-oxo-5H-furo[3,2-g]chromene-6-carbaldehyde (2b):
Yellow solid (0.31 g, 64%), Rf (toluene-ethyl acetate) 0.60, mp. 250–251 °C; νmax (ATR) 463, 524, 669, 781, 827, 868, 1229, 1318, 1447, 1476, 1569, 1619, 1644, 1700, 2853, 2923, 3096 cm−1; δH (300 MHz, DMSO-d6) 7.20 (1H, t, J = 7.0 Hz, H-5′), 7.47 (1H, d, J = 6.0 Hz, H-4′), 7.54 (1H, s, H-3), 7.64 (1H, s, H-2′), 7.65 (1H, d, J = 7.0 Hz, H-6′), 7.96 (1H, s, H-9), 8.26 (1H, s, H-7), 8.82 (1H, s, H-4), 10.0 (1H, s, –CHO); δC (75 MHz, DMSO-d6) 101.7, 103.8, 112.2 (d, 2JCF = 23.75 Hz), 117.0 (d, 2JCF = 20.8 Hz), 118.4, 119.6, 121.6, 121.8, 128.6 (d, 4JCF = 7.5 Hz), 129.4, 131.4 (d, 3JCF = 8.5 Hz), 131.8 (d, 3JCF = 8.5 Hz), 154.2, 157.4, 163.0 (d, 1JCF = 242.8 Hz), 163.8, 175.5, 188.8; HRMS (ES+): m/z [M + H]+ calc. for C18H10FO4: 309.0561; found 309.0563.
2-(4-Fluorophenyl)-5-oxo-5H-furo[3,2-g]chromene-6-carbaldehyde (2c):
Yellow solid (0.34 g, 71%), Rf (toluene-ethyl acetate) 0.61, mp. 260–261 °C; νmax (ATR) 511, 648, 767, 807, 1001, 1024, 1226, 1300, 1504, 1600, 1615, 1637, 1698, 3074, 3093, 3386 cm-1; δH (300 MHz, DMSO-d6) 7.34 (2H, t, J = 9.6 Hz, H-3′,5′), 7.55 (1H, s, H-3), 7.93 (2H, dd, JHH = 5.4 Hz and JHF = 8.1 Hz, H-2′,6′), 8.03 (1H, s, H-9), 8.31 (1H, s, H-7), 8.84 (1H, s, H-4), 10.10 (1H, s, –CHO); δC (75 MHz, DMSO-d6) 101.6, 102.3, 116.8 (d, 2JCF = 22.87 Hz), 118.0, 121.7 (d, 4JCF = 2.25 Hz), 125.8, 127.8, 127.9, 129.2, 129.3, 131.8, (d, 3JCF = 9.15 Hz), 154.0, 157.4, 163.2 (d, 1JCF = 246.2 Hz), 175.5, 188.9; HRMS (ES+): m/z [M + H]+ calc. for C18H9O4F: 309.0559; found 309.0552.
2-(3-Chlorophenyl)-5-oxo-5H-furo[3,2-g]chromene-6-carbaldehyde (2d):
Yellow solid (0.35 g, 69%), Rf (toluene-ethyl acetate) 0.67, mp. 198–199 °C; νmax (ATR) 436, 646, 680, 738, 778, 951, 1095, 1306, 1446, 1476, 1601, 1621, 1661, 1694, 2853, 2923, 3070, 3107 cm−1 δH (300 MHz, DMSO-d6) 7.45–7.60 (2H, m, H-4′ and H-5′), 7.71 (1H, s, H-3), 7.84 (1H, d, J = 7.0 Hz, H-6′), 7.94 (1H, s, H-2′), 8.06 (1H, s, H-9), 8.35 (1H, s, H-7), 8.89 (1H, s, H-4) 10.09 (1H, s, –CHO); δC (75 MHz, DMSO-d6) 101.0, 111.6, 119.3, 120.8, 121.0, 122.2, 124.1, 125.1, 129.8, 131.1, 131.7, 134.5, 150.3, 156.0, 158.1, 163.0, 175.0, 189.0; HRMS (ES+): m/z [M + H]+ calc. for C18H1035ClO4: 325.0268; found 325.0261.
2-(4-Chlorophenyl)-5-oxo-5H-furo[3,2-g]chromene-6-carbaldehyde (2e):
Yellow solid (0.34 g, 66%), Rf (toluene-ethyl acetate) 0.63, mp. 231–232 °C; max (ATR) 480, 644, 791, 811, 1011, 1092, 1296, 1444, 1471, 1489, 1567, 1609, 1651, 1696, 2852, 2921, 3078, 3102 cm−1; δH (300 MHz, DMSO-d6) 7.58 (2H, d, J = 8.1 Hz, H-2′,6′), 7.67 (1H, s, H-3), 7.93 (2H, d, J = 8.1 Hz, H-3′,5′), 8.10 (1H, s, H-9), 8.38 (1H, s, H-7), 8.94 (1H, s, H-4), 10.13 (1H, s, –CHO); δC (75 MHz, DMSO-d6) 100.2, 103.2, 118.3, 119.6, 127.1, 127.3, 128.1, 128.8, 129.2, 132.0, 134.8, 154.1, 157.4, 163.9, 175.5, 188.9; HRMS (ES+): m/z [M + H]+ calc. for C18H1035ClO4: 325.0268; found 325.0264.
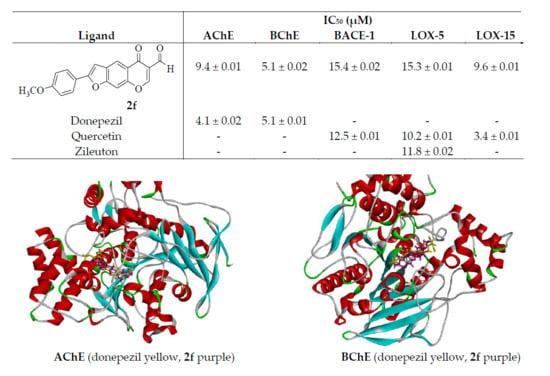
2-(4-Methoxyphenyl)-5-oxo-5H-furo[3,2-g]chromene-6-carbaldehyde (2f):
Yellow solid (0.30 g, 60%), Rf (toluene-ethyl acetate) 0.63, mp. 228–229 °C; max (ATR) 519, 651, 790, 833, 1021, 1175, 1251, 1302, 1505, 1568, 1615, 1645, 1697, 2847, 2925, 3069, 3110 cm−1; δH (300 MHz, DMSO-d6) 3.75 (3H, s, OCH3), 7.00 (2H, d, J = 8.1 Hz, H-3′,5′), 7.90 (1H, s, H-3), 7.77 (2H, d, J = 8.4 Hz, H-2′,6′), 7.96 (1H, s, H-9), 8.23 (1H, s, H-7), 8.84 (1H, s, H-4), 10.06 (1H, s, –CHO); δC (75 MHz, DMSO-d6) 56.4, 101.1, 102.0, 115.7, 117.9 (2×C), 120.1, 122.3, 127.8, 129.9, 154.3, 157.9, 159.9, 161.5, 164.3, 176.1, 189.5; HRMS (ES+): m/z [M + H]+ calc. for C19H12O5: 321.0763; found 321.0773.
2-(3,5-Dimethoxyphenyl)-5-oxo-5H-furo[3,2-g]chromene-6-carbaldehyde (2g):
Yellow solid (0.39 g, 71%), Rf (toluene-ethyl acetate) 0.52, mp. 236–237 °C; νmax (ATR) 575, 602, 669, 791, 863, 1053, 1363, 1581, 1631, 1673, 2896, 2938 cm−1; δH(300 MHz, DMSO-d6) 3.81 (6H, s, -OCH3), 6.55 (1H, t, J = 1.8 Hz, H-4′), 7.04 (2H, d, J = 1.8 Hz, H-2′,6′), 7.66 (1H, s, H-3), 8.07 (1H, s, H-9), 8.34 (1H, s, H-7), 8.91 (1H, s, H-4), 10.12 (1H, s, –CHO); δC (75 MHz, DMSO-d6) 56.0 (2×C), 101.7, 103.2, 118.3, 119.6, 121.9, 128.1, 128.8, 129.2, 129.3, 129.8, 131.9, 134.8, 154.1, 157.4, 157.8, 163.9, 175.5, 188.9; HRMS (ES+): m/z [M + H]+ calc. for C20H13O6 350.0788; found 350.0790.
5-Oxo-2-(p-tolyl)-5H-furo[3,2-g]chromene-6-carbaldehyde (2h):
Yellow solid (0.36 g, 76%), Rf (toluene-ethyl acetate) 0.52, mp. 214–215 °C; νmax (ATR) 499, 582, 777, 831, 1025, 1175, 1251, 1305, 1505, 1572, 1606, 1650, 2858, 3059 cm−1; δH(300 MHz, DMSO-d6) ; 2.36 (3H, s, –CH3), 7.33 (2H, d, J = 8.1 Hz, H-3′,5′), 7.57 (1H, s, H-3), 7.83 (2H, d, J = 7.5 Hz, H-2′,6′), 8.10 (1H, s, H-9), 8.38 (1H, s, H-7), 8.95 (1H, s, H-4), 10.15 (1H, s, –CHO); δC (75 MHz, DMSO-d6) 21.5, 101.5, 117.7, 119.6, 121.7, 125.5, 126.5, 129.1, 129.4, 130.3, 140.1, 153.9, 157.4, 159.3, 163.9, 175.6, 189.9; HRMS (ES+): m/z [M + H]+ calc. for C19H11O4 305.0814; found 305.0810.
2-(Cyclohex-1-en-1-yl)-5-oxo-5H-furo[3,2-g]chromene-6-carbaldehyde (2i):
Yellow solid (0.34 g, 74%), Rf (toluene-ethyl acetate) 0.52, mp. 183–184 °C; νmax (ATR) 467, 560, 764, 1223, 1312, 1418, 1573, 1621, 1651, 1702, 2924, 3080 cm−1; δH (300 MHz, DMSO-d6) 1.63 (2H, t, J = 1.5 Hz, –CH2), 1.73 (2H, t, J = 3.9 Hz, –CH2), 2.25 (2H, t, J = 3.6 Hz, –CH2), 2.39 (2H, t, J = 1.6 Hz, –CH2), 6.64 (1H, t, J = 3.9 Hz, –CH), 7.12 (1H, s, H-3), 7.72 (1H, s, H-9), 8.00 (1H, s, H-8), 8.96 (1H, s, H-5), 10.15 (1H, s, –CHO); δC (75 MHz, DMSO-d6) 21.9, 22.1, 24.6, 25.4, 101.0, 117.5, 119.5, 121.4, 126.8, 128.7, 128.9, 134.3 153.9, 157.2, 160.3, 163.7, 175.5, 189.0; HRMS (ES+): m/z [M + H]+ calc. for C18H14O4: 294.0889; found 294.0892.
3.4. Typical Procedure for the Synthesis of Hydrazones 3a–i from 2a–i
A stirred mixture of 3a (0.20 g, 0.63 mmol) and pyridine (0.05 g, 0.63 mmol) in EtOH (15 mL) was treated with 4-trifluoromethylphenyhydrazine (0.13 g, 0.76 mmol). The mixture was heated under reflux for 6 h and then poured onto crush ice. The precipitate was filtered off and purified by silica gel column chromatography to afford compound 3a as a brown solid. Compounds 3b–i were prepared in this fashion.
2-Phenyl-6-[(4-trifluoromethylphenyl)hydrazonomethyl]furo[3,2-g]chromen-5-one (3a):
Brown solid (0.18 g, 61%), Rf (ethyl acetate-hexane) 0.65, mp. 254–255 °C; max (ATR) 418, 469, 596, 795, 830, 1062, 1102, 1209, 1327, 1573, 1606, 1641, 2361, 3041, 3266 cm−1; δH (300 MHz, DMSO-d6) 7.18 (2H, d, J = 7.5 Hz, H-3″,5″), 7.40 7.60 (5H, m, Ar), 7.63 (1H, s, H-3), 7.85–7.93 (2H, m, Ar), 8.03 (1H, s, H-7), 8.07 (1H, s, H-8), 8.36 (1H, s, H-4), 8.84 (1H, s, –CH=N), 10.87 (1H, s, NH); δC (75 MHz, DMSO-d6) 99.7, 110.9, 112.2, 118.7, 119.2 (q, 2J = 25.2 Hz), 120.0, 121.7, 123.7, 125.4 (q, 1J = 269.1 Hz), 126.9, 127.2, 129.3, 129.6, 130.0, 131.1, 148.4 150.4, 152.7, 157.2, 157.6, 175.1; δF (282 MHz, DMSO-d6) -59.3; HRMS (ES+): m/z [M + H]+ calc. for C25H16 N2O3F3: 449.1113; found. 449.1117.
2-(3-Fluorophenyl)-6-[(4-trifluoromethylphenyl)hydrazonomethyl]furo[3,2-g]chromen-5-one (3b):
Yellow solid (0.17 g, 59%), Rf (ethyl acetate-hexane) 0.61, mp. 250–251 °C; νmax (ATR) 469, 595, 627, 781, 830, 1058, 1100, 1288, 1321, 1603, 1640, 2361, 3040, 3263 cm−1; δH (300 MHz, DMSO-d6) 7.13 (2H, d, J = 7.2 Hz, H-3″,5″), 7.28 (1H, t, J = 7.2 Hz, H-5′), 7.51 (2H, d, J = 7.5 Hz, H-2″,6″), 7.53 (1H, s, H-7), 7.18 (1H, s, H-9), 7.74 (2H, d, J = 13.5 Hz, H-4′), 8.01 (2H, d, J = 12.3 Hz, H-4′), 8.37 (1H, s, H-4), 8.84 (1H, s, –CH = N), 10.87 (1H, s, NH); δC (75 MHz, DMSO-d6) 101.1, 103.9, 112.1, 112.2 (d, 2JCF = 23.75 Hz), 116.8 (d, 2JCF = 20.8 Hz), 118.2, 118.7 (q, 2J = 46.5 Hz), 121.6, 125.9 (q, 1J = 262.6 Hz), 127.0 (d, 4JCF = 3.87 Hz), 127.3 (d, 3JCF = 3.75 Hz), 128.1, 131.2, 131.9 (d, 3JCF = 8.5 Hz), 133.0, 143.6, 148.5, 153.6, 154.4, 157.2 (d, 3JCF = 8.6 Hz), 163.0 (d, 1JCF = 242.8 Hz), 175.5; δF (282 MHz, DMSO-d6) -112.1 (1F, d, J = 5.9 Hz,), -59.4; HRMS (ES+): m/z [M + H]+ calc. for C25H15N2O3F4: 467.1019; found 467.1009..
2-(4-Fluorophenyl)-6-[(4-trifluoromethylphenyl)hydrazonomethyl]furo[3,2-g]chromen-5-one (3c):
Yellow solid (0.19 g, 68%), Rf (ethyl acetate-hexane) 0.65, mp. 252–253 °C; νmax (ATR) 516, 600, 657, 796, 837, 1064, 1111, 1160, 1236, 1305, 1327, 1504, 1620, 1643, 2360, 3077, 3259 cm−1; δH (300 MHz, DMSO-d6) 7.17 (2H, d, J = 8.7 Hz, H-3″,5″), 7.34 (2H, t, J = 8.7 Hz, H-3′,5′), 7.51 (2H, d, J = 8.7 Hz, H-2″,6″), 7.60 (1H, s, H-7), 8.00 (2H, dd, JHH = 3.3 Hz and JHF = 6.9 Hz, H-2′,6′), 8.06 (1H, s, H-9), 8.35 (1H, s, H-4), 8.85 (1H, s, –CH = N), 10.86 (1H, s, -NH); δC (75 MHz, DMSO-d6) 99.4, 102.7, 116.9 (d, 2JCF = 22.87 Hz), 119.2 (q, 2J = 35.3 Hz), 120.8, 121.9, 122.0 (d, 4JCF = 2.25 Hz), 124.3, 124.7 (d, 3JCF = 8.6 Hz), 125.3 (q, 1J = 270.2 Hz), 129.2, 129.3, 129.6, 129.9, 131.9, 132.5, 135.9, 136.0, 143.0, 155.8, 158.2, 158.4, 161.4 (d, 1JCF = 242.8 Hz), 175.4; δF (282 MHz, DMSO-d6) –111.0 (1F, ddd, J = 2.8, 9.0, 13.8), –59.3; HRMS (ES+): m/z [M + H]+ calc. for C25H15N2O3F4: 467.1019; found 467.1014.
2-(3-Chlorophenyl)-6-[(4-trifluoromethylphenyl)hydrazonomethyl]furo[3,2-g]chromen-5-one (3d):
Yellow solid (0.18 g, 65%), Rf (ethyl acetate-hexane) 0.62, mp. 239–240 °C; νmax (ATR) 443, 508, 796, 826, 1052, 1085, 1222, 1320, 1602, 1640, 2353, 3035, 3267 cm−1; δH (300 MHz, DMSO-d6) 7.14 (2H, d, J = 8.1 Hz, H-3″,5″), 7.26 (1H, t, J = 9.0 Hz, H-3′,5′) 7.50 (2H, d, J = 8.1 Hz, H-3′,5′), 7.53 (1H, s, H-3), 7.69–7.77 (3H, m, H-2″,6″), 7.80 (1H, s, H-7), 8.04 (1H, s, H-9), 8.34 (1H, s, H-4), 8.81 (1H, s, –CH = N), 10.84 (1H, s, -NH); δC (75 MHz, DMSO-d6) 102.5, 112.2, 117.0, 118.3, 120.5 (q, 2J = 64.30 Hz), 125.5, 125.8 (q, 1J = 260.0 Hz), 126.8, 127.4, 128.4, 129.3, 129.7, 130.1, 131.3, 131.6, 143.6, 148.5, 153.4, 154.2, 157.3, 159.6, 175.6; δF (282 MHz, DMSO-d6) –59.4; HRMS (ES+): m/z [M + H]+ calc. for C25H15N2O3F335Cl: 483.0723; found 483.0720.
2-(4-Chlorophenyl)-6-[(4-trifluoromethylphenyl)hydrazonomethyl]furo[3,2-g]chromen-5-one (3e):
Yellow solid (0.17 g, 60%), Rf (ethyl acetate-hexane) 0.63, mp. 230–231 °C; νmax (ATR) 437, 503, 594, 796, 827, 1062, 1095, 1224, 1325, 1606, 1642, 2359, 3045, 3262 cm−1; δH (300 MHz, DMSO-d6) 7.15 (2H, d, J = 8.0 Hz, H-3″,5″), 7.50 (2H, d, J = 8.0 Hz, H-3′,5′), 7.56 (2H, d, J = 8.0 Hz, H-2″,6″), 7.62 (1H, s, H-3), 7.91 (2H, d, J = 8.0 Hz, H-2′,6′), 7.96 (1H, s, H-7), 8.03 (1H, s, H-9), 8.33 (1H, s, H-4), 8.80 (1H, s, –CH=N), 10.83 (1H, s, –NH); δC (75 MHz, DMSO-d6) 101.0, 103.1, 112.1, 118.0, 118.5 119.0 (q, 2J = 31.4 Hz), 120.6, 126.9, 127.1, 127.0 (q, 2J = 276.1 Hz), 129.5, 129.6, 129.7 131.2, 134.6, 148.4, 153.4, 154.2, 157.2, 157.4, 175.4; δF (282 MHz, DMSO-d6) –59.3; HRMS (ES+): m/z [M + H]+ calc. for C25H15N2O3F335Cl: 483.0723; found 483.0731.
2-(4-Methoxyphenyl)-6-[(4-trifluoromethylphenyl)hydrazonomethyl]furo[3,2-g]chromen-5-one (3f):
Brown solid (0.24 g, 78%), Rf (ethyl acetate-hexane) 0.58, mp. 233–234 °C; max (ATR) 510, 632, 792, 831, 1064, 1109, 1172, 1222, 1258, 1299, 1473, 1506, 1568, 1615, 1640, 1698, 2840, 2934, 3075, 3258 cm−1 1H (300 MHz, DMSO-d6) 3.81 (3H, s, -OCH3), 7.05 (2H, d, J = 8.4 Hz, H-3″,5″), 7.77 (2H, d, J = 8.1 Hz, H-3′,5′), 7.45 (1H, s, H-7), 7.51 (2H, d, J = 8.1 Hz, H-2″,6″), 7.86 (2H, d, J = 8.1 Hz, H-2′,6′), 7.97 (1H, s, 8-H), 8.07 (1H, s, 5-H), 8.30 (1H, s, 2-H), 8.84 (1H, s,), 10.87 (1H, s,); 13C-NMR (75 MHz, DMSO-d6) 55.8, 100.5, 100.8, 112.1, 115.1, 117.1, 118.4 (q, 2J = 36.4 Hz), 120.5, 121.9, 125.3 (q, 1J = 272.3 Hz), 127.0, 127.1, 127.9, 128.7, 148.5, 153.5, 153.9, 157.2, 158.9, 160.8, 175.5; δF (282 MHz, DMSO-d6) –59.3; HRMS (ES+): m/z [M + H]+ calc. for C26H18N2O4F3 479.1219; found 479.1211.
2-(3,5-Dimethoxyphenyl)-6-[(4-trifluoromethylphenyl)hydrazonomethyl]furo[3,2-g]chromen-5-one (3g):
Yellow solid (1.77 g, 63%), Rf (ethyl acetate-hexane) 0.52, mp.166–167 °C; νmax (ATR) 680, 793, 831, 1063, 1110,1155, 1205,1322, 1456, 1600, 1637, 2934, 3255 cm−1; δH (300 MHz, DMSO-d6) 3.81 (6H, s, –OCH3), 6.53 (1H, t, J = 1.8 Hz, H-4′), 7.01 (2H, d, J = 1.8 Hz, H-2′,6′), 7.21 (1H, s, H-3), 7.46 (1H, s, H-9), 7.91 (2H, d, J = 8.7 Hz, H-2″,6″), 8.06 (1H, s, H-7), 8.21 (2H, d, J = 8.7 Hz, H-3″,5″) 8.31 (1H, s, H-4), 9.30 (1H, s, –CH=N), 11.36 (1H, s, -NH); δC (75 MHz, DMSO-d6) 56.0 (2×C), 100.5, 102.2, 103.4, 110.9, 112.3, 119.2 (q, 2J = 36.0 Hz), 120.1, 121.9, 125.8, 127.0, 127.6, 127.9 (q, 1J = 265.5 Hz), 129.4, 131.1, 131.2, 148.4, 150.5, 152.6, 157.1, 157.6, 161.4, 175.1; δF (282 MHz, DMSO-d6) –59.3; HRMS (ES+): m/z [M + H]+ calc. for C27H20N2O5F3 509.1324; found 509.1317.
2-(4-p-Tolyl)-6-[(4-trifluoromethylphenyl)hydrazonomethyl]furo[3,2-g]chromen-5-one (3h):
Yellow solid (0.16 g, 74%), Rf (ethyl acetate-hexane) 0.68, mp. 258–259 °C; max (ATR) 490, 774, 835, 1064, 1119, 1156, 1210, 1326, 1418, 1597, 1616, 1632, 2926, 3259 cm−1 1H (300 MHz, DMSO-d6) 2.31 (3H, s, –CH3), 7.16 (2H, d, J = 8.7 Hz, H-3″,5″), 7.33 (2H, d, J = 8.7 Hz, H-3′,5′), 7.50 (1H, s), 7.52 (2H, d, J = 8.1 Hz, H-2″,6″), 7.83 (2H, d, J = 8.1 Hz, H-2′,6′), 8.00 (1H, s, 8-H), 8.07 (1H, s, 5-H), 8.33 (1H, s, 2-H), 8.86 (1H, s,), 10.90 (1H, s,); δC (75 MHz, DMSO-d6) 31.2, 104.2, 110.8, 112.2, 119.4 (q, 2J = 32.1 Hz), 120.0, 120.2, 121.5, 124.2, 125.1, 125.4, 126.9, 127.6 (q, 1J = 273.8 Hz), 130.1, 131.2, 139.2, 143.6, 152.7, 155.9, 157.5, 158.9, 175.1; δF (282 MHz, DMSO-d6) –59.4; HRMS (ES+): m/z [M + H]+ calc. for C26H18N2O3F3; 460.1267; found. 463.1270.
2-(Cyclohex-1-en-1-yl)-6-((2-(4-(trifluoromethyl)phenyl)hydrazono)methyl)-5H-furo[3,2-g]chromen-5-one (3i):
Brown solid (1.90 g, 62%), Rf (ethyl acetate-hexane) 0.67, mp. 232–233 °C; νmax (ATR) 501, 597, 827, 1004, 1025, 1060, 1105, 1155, 1323, 1472, 1584, 1605, 1637, 1719, 2924, 3259 cm−1; δH (300 MHz, DMSO-d6) 1.65 (4H, t, J = 34.5 Hz –CH2), 2.27 (4H, t, J = 34.5 Hz, –CH2), 6.60 (1H, t, J = 3.9 Hz, –CH), 6.93 (1H, s, H-3), 7.16 (2H, d, J = 8.4 Hz, H-2″,6″), 7.52 (2H, d, J = 8.7 Hz, H-3″,5″) 7.88 (1H, s, H-9), 8.02 (1H, s, H-8), 8.26 (1H, s, H-5), 8.83 (1H, s, –CH=N), 10.87 (1H, s, –NH); δC (75 MHz, DMSO-d6) 21.9, 22.1, 24.7, 25.4, 29.4, 99.1, 100.5, 101.1, 112.3, 117.3, 118.6 (q, 2J = 38.2 Hz), 120.1, 122.7, 127.9 (q, 1J = 264.5 Hz), 126.1, 126.9, 128.4, 131.5, 132.9, 148.5, 153.5, 154.2, 160.0, 175.5; δF (282 MHz, DMSO-d6) –59.3; HRMS (ES+): m/z [M + H]+ calc. for C25H20N2O3F3: 453.1426; found 453.1424..