Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications
Abstract
1. Introduction
2. Fundamentals of Plasma and Methods for Its Generation
3. Inactivation of Microorganisms by Plasma
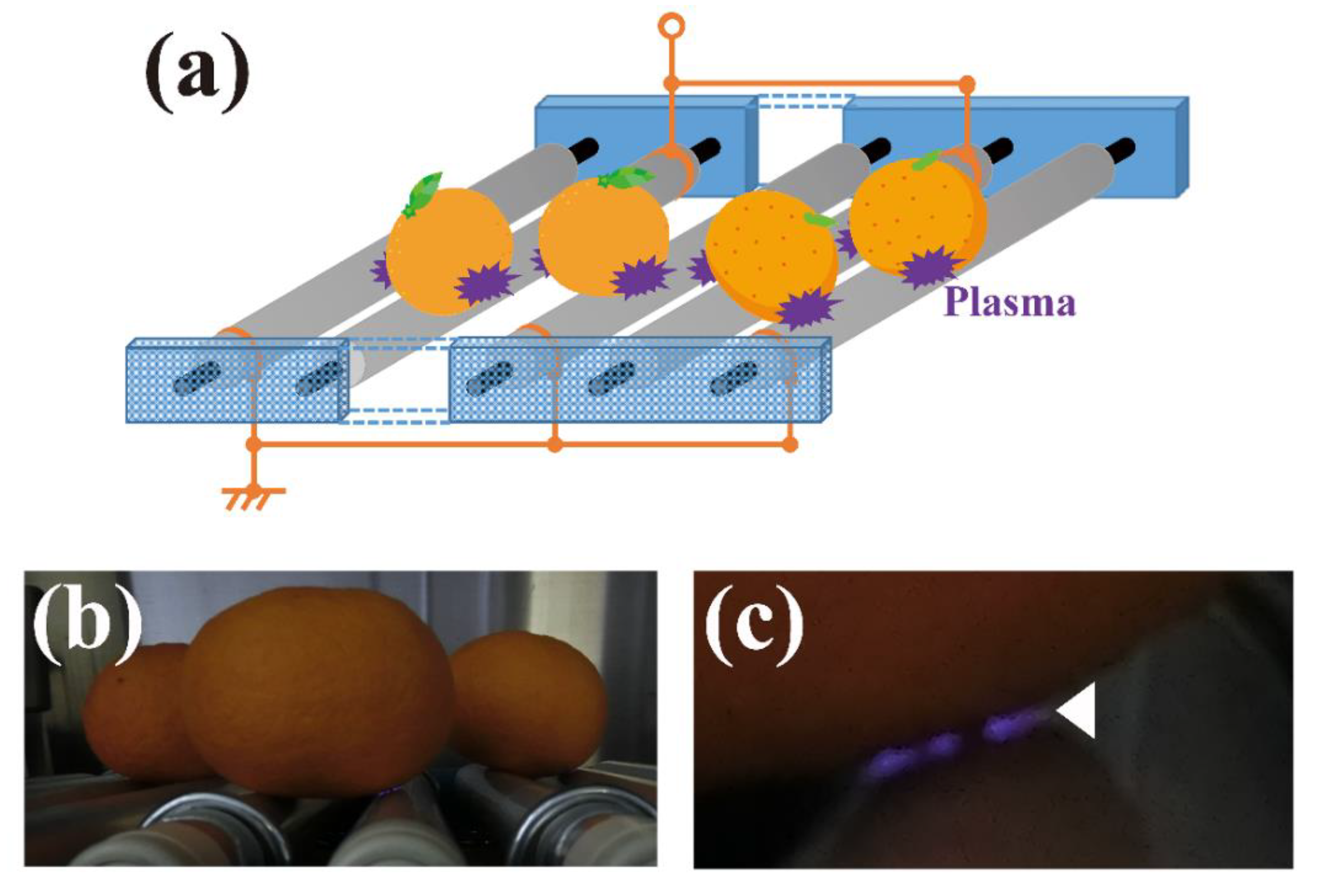
4. Future Perspectives in Agriculture and the Food Industry
5. Future Perspectives in Medicine and Dentistry
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Alternating current |
| APPJ | Atmospheric plasma jet |
| BLP-TES | Bi-polar and low- pressure plasma-triple effects sterilization |
| BSE | Bovine spongiform encephalopathy |
| CDC | Centers for Disease Control |
| CJD | Creutzfeldt-Jakob disease |
| CWD | Chronic wasting disease |
| DBD | Dielectric barrier discharge |
| DC | Direct current |
| ELISA | Enzyme-linked immunosorbent assay |
| FE-DBD | Floating electrode barrier discharge |
| HIV | Human immunodeficiency virus |
| LPS | Lipopolysaccharides |
| MAP | Mitogen-activated protein |
| MAPK | MAP kinase |
| MHCD | Micro hollow cathode discharge |
| ne | Electron density |
| NTP | Non-thermal plasma |
| PAM | Plasma-activated medium |
| PAW | Plasma-activated water |
| PHD | Pin-to-hole spark discharge |
| pPBS | Plasma-treated phosphate-buffered saline |
| PSM | Plasma-stimulated medium |
| PTW | Plasma-treated water |
| RF | Radio-frequency |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RSV | Respiratory syncytial virus |
| Te | Electron temperature |
| Tgas | Gas temperature |
| Tion | Ion temperature |
| Tn | Neutron temperature |
| USEPA | U.S. Environmental Protection Agency |
| UV | Ultraviolet |
| VOC | Volatile organic compound |
| WHO | World Health Organization |
References
- Langmuir, I. Oscillations in ionized gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, S. Nuclear fusion in dense plasmas. Rev. Mod. Phys. 1993, 65, 255–259. [Google Scholar] [CrossRef]
- Team, J. Fusion energy production from a deuterium-tritium plasma in the JET tokamak. Nuc. Fus. 1992, 32, 187–203. [Google Scholar] [CrossRef]
- Coburn, J.W.; Winters, H.F. Plasma etching-A discussion of mechanisms. J. Vac. Sci. Technol. 1979, 16, 391–403. [Google Scholar] [CrossRef]
- Sakudo, A.; Shintani, H. Sterilization and Disinfection by Plasma: Sterilization Mechanisms, Biological and Medical Applications (Medical Devices and Equipment); Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Shintani, H.; Sakudo, A. Gas Plasma Sterilization in Microbiology: Theory, Applications, Pitfalls and New Perspectives; Caister Academic Press: London, UK, 2016. [Google Scholar]
- Sakudo, A.; Shintani, H.; Yagyu, Y. Plasma sterilization. In Block’s Disinfection, Sterilization, and Preservation; McDonnell, G.E., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2019; in press. [Google Scholar]
- Nandkumar, N. Plasma-The fourth state of matter. Int. J. Sci. Technol. Res. 2014, 3, 49–52. [Google Scholar]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Nehra, V.; Kumar, A.; Dwivedi, H. Atmospheric non-thermal plasma sources. Int J. Eng. 2008, 2, 53–68. [Google Scholar]
- Boulos, M.I.; Fauchais, P.; Pfender, E. Thermal Plasma: Fundamental and Applications, Vol. 1; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Boulos, M.I. New frontiers in thermal plasma processing. Pure Appl. Chem. 1996, 68, 1007–1010. [Google Scholar] [CrossRef]
- Heberlein, J. New approaches in thermal plasma technology. Pure Appl. Chem. 2002, 74, 327–335. [Google Scholar] [CrossRef]
- Bonnizzoni, G.; Vassallo, E. Plasma physics and technology; industrial applications. Vacuum 2002, 64, 327–336. [Google Scholar] [CrossRef]
- Hippler, R.; Kersten, H.; Schmidt, M.; Schoenbach, K.H. Low Temperature Plasmas. Fundamentals, Technologies, and Techniques, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Meichsner, J.; Schmidt, M.; Schneider, R.; Wagner, H.E. Nonthermal Plasma Chemistry and Physics; CRC Press, Taylor & Francis Group: London, UK, 2013. [Google Scholar]
- Taylor, P.R.; Pirzada, S.A. Thermal plasma processing of materials: A review. Adv. Perform. Mater. 1994, 1, 35–50. [Google Scholar] [CrossRef]
- Dobrynin, D.; Friedman, G.; Fridman, A.; Starikovskiy, A. Inactivation of bacteria using dc corona discharge: Role of ions and humidity. New J. Phys. 2011, 13, 103033. [Google Scholar] [CrossRef] [PubMed]
- Rupf, S.; Lehmann, A.; Hannig, M.; Schafer, B.; Schubert, A.; Feldmann, U.; Schindler, A. Killing of adherent oral microbes by a non-thermal atmospheric plasma jet. J. Med. Microbiol. 2010, 59, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Ono, Y.; Ogino, A.; Nagatsua, M. Low-temperature sterilization of wrapped materials using flexible sheet-type dielectric barrier discharge. Appl. Phys. Lett. 2008, 93, 221502. [Google Scholar] [CrossRef]
- Kolb, J.F.; Mohamed, A.A.H.; Price, R.O.; Swanson, R.J.; Bowman, A.; Chiavarini, R.L.; Stacey, M.; Schoenbach, K.H. Cold atmospheric pressure air plasma jet for medical applications. Appl. Phys. Lett. 2008, 92, 241501. [Google Scholar] [CrossRef]
- Dobrynin, D.; Arjunan, K.; Fridman, A.; Friedman, G.; Morss Clyne, A. Direct and controllable nitric oxide delivery into biological media and living cells by a pin-to hole spark discharge (PHD) plasma. J. Phys. D Appl. Phys. 2011, 44, 075201. [Google Scholar] [CrossRef]
- Rossi, F.; Kylian, O.; Rauscher, H.; Gilliland, D.; Sirghi, L. Use of a low-pressure plasma discharge for the decontamination and sterilization of medical devices. Pure Appl. Chem. 2008, 80, 1939–1951. [Google Scholar] [CrossRef]
- Stapelmann, K.; Fiebrandt, M.; Raguse, M.; Awakowicz, P.; Reitz, G.; Moeller, R. Utilization of low-pressure plasma to inactivate bacterial spores on stainless steel screws. Astrobiology 2013, 13, 597–606. [Google Scholar] [CrossRef]
- Teschke, M.; Kedzierski, J.; Finantu-Dinu, E.G.; Korzec, D.; Engemann, J. High-speed photographs of a dielectric barrier atmospheric pressure plasma jet. IEEE Trans. Plasma Sci. 2005, 33, 310–311. [Google Scholar] [CrossRef]
- Kalghatgi, S.U.; Fridman, G.; Cooper, M.; Nagaraj, G.; Peddinghaus, M.; Balasubramanian, M.; Vasilets, V.N.; Gutsol, A.F.; Fridman, A.; Friedman, G. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 2007, 35, 1559. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Balasubramanian, M.; Ayan, H.; Fridman, A.; Gutsol, A.; Brooks, A. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425. [Google Scholar] [CrossRef]
- Cooper, M.; Fridman, G.; Fridman, A.; Joshi, S. Biological responses of Bacillus stratosphericus to floating electrode-dielectric barrier discharge plasma treatment. J. Appl. Microbiol. 2010, 109, 2039. [Google Scholar] [CrossRef] [PubMed]
- Schoenbach, K.; Verhappen, R.; Tessnow, T.; Peterkin, F.; Byszewski, W. Microhollow cathode discharges. Appl. Phys. Lett. 1996, 68, 13. [Google Scholar] [CrossRef]
- Fridman, G.; Fridman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Shintani, H.; Sakudo, A.; Burke, P.; McDonnell, G. Gas plasma sterilization of microorganisms and mechanisms of action. Exp. Ther. Med. 2010, 1, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Wan, Z.; Keener, K.M. Effects of cold plasma on food quality: A review. Foods 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Kamgang-Youbi, G.; Herry, J.M.; Meylheuc, T.; Brisset, J.L.; Bellon-Fontaine, M.N.; Doubla, A.; Naitali, M. Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Lett. Appl. Microbiol. 2009, 48, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Lu, X.; He, G. The selective effect of plasma activated medium in an in vitro co-culture of liver cancer and normal cells. J. Appl. Phys. 2017, 121, 013302. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Cheng, X.; Canady, J.; Sherman, J.; Keidar, M. Principles of using cold atmospheric plasma stimulated media for cancer treatment. Sci. Rep. 2015, 5, 18339. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, S.; Choe, W.; Yong, H.I.; Jo, C.; Kim, K. Plasma-functionalized solution: A potent antimicrobial agent for biomedical applications from antibacterial therapeutics to biomaterial surface engineering. ACS Appl. Mater. Interfaces 2017, 9, 43470–43477. [Google Scholar] [CrossRef]
- Ikawa, S.; Tani, A.; Nakashima, Y.; Kitano, K. Physicochemical properties of bactericidal plasma-treated water. J. Phys. D Appl. Phys. 2016, 49, 425401. [Google Scholar] [CrossRef]
- Van Boxem, W.; Van der Paal, J.; Gorbanev, Y.; Vanuytsel, S.; Smits, E.; Dewilde, S.; Bogaerts, A. Anti-cancer capacity of plasma-treated PBS: Effect of chemical composition on cancer cell cytotoxicity. Sci Rep. 2017, 7, 16478. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.P.; Heidecke, C.D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Sci. Rep. 2017, 7, 8319. [Google Scholar] [CrossRef] [PubMed]
- Balan, G.G.; Rosca, I.; Ursu, E.L.; Doroftei, F.; Bostanaru, A.C.; Hnatiuc, E.; Nastasa, V.; Sandru, V.; Stefanescu, G.; Trifan, A.; et al. Plasma-activated water: A new and effective alternative for duodenoscope reprocessing. Infect. Drug Resist. 2018, 11, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Nakamura, K.; Utsumi, F.; Kajiyama, H.; Kano, H.; Maruyama, S.; Kikkawa, F.; Hori, M. Cell survival and proliferation signaling pathways are downregulated by plasma-activated medium in glioblastoma brain tumor cells. Plasma Med. 2012, 2, 207–220. [Google Scholar] [CrossRef]
- Sakudo, A.; Toyokawa, Y.; Imanishi, Y.; Murakami, T. Crucial roles of reactive chemical species in modification of respiratory syncytial virus by nitrogen gas plasma. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 131–136. [Google Scholar] [CrossRef]
- McDonnell, G.E. Antisepsis, Disinfection, and Sterilization; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Knipe, D.M.; Howley, P.M.; Griffin, D.E.; Lamb, R.A.; Martin, M.A. Fields Virology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Yokoyama, T.; Murai, K.; Murozuka, T.; Wakisaka, A.; Tanifuji, M.; Fujii, N.; Tomono, T. Removal of small non-enveloped viruses by nanofiltration. Vox Sang. 2004, 86, 225–229. [Google Scholar] [CrossRef]
- Niemira, B.A. Cold plasma reduction of Salmonella and Escherichia coli O157:H7 on almonds using ambient pressure gases. J. Food Sci. 2012, 77, M171–M175. [Google Scholar] [CrossRef]
- Klampfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef]
- Sung, S.J.; Huh, J.B.; Yun, M.J.; Chang, B.M.; Jeong, C.M.; Jeon, Y.C. Sterilization effect of atmospheric pressure non-thermal air plasma on dental instruments. J. Adv. Prosthodont 2013, 5, 2–8. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, P.; Wu, H.; Bai, N.; Wang, R.; Zhu, W.; Zhang, J.; Liu, F. Inactivation of Staphylococcus aureus and Enterococcus faecalis by a direct-current, cold atmospheric-pressure air plasma microjet. J. Biomed. Res. 2010, 24, 264–269. [Google Scholar] [CrossRef]
- Vandervoort, K.G.; Brelles-Marino, G. Plasma-mediated inactivation of Pseudomonas aeruginosa biofilms grown on borosilicate surfaces under continuous culture system. PLoS ONE 2014, 9, e108512. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A.; Boyd, G.; Sites, J. Cold plasma rapid decontamination of food contact surfaces contaminated with Salmonella biofilms. J. Food Sci. 2014, 79, M917–M922. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Antimicrobial Resistance. Available online: http://www.who.int/mediacentre/factsheets/fs194/en/ (accessed on 24 June 2019).
- CDC (The US Centers for Disease Control and Prevention). National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS). Available online: https://www.cdc.gov/narms/index.html (accessed on 24 June 2019).
- Krauland, M.G.; Marsh, J.W.; Paterson, D.L.; Harrison, L.H. Integron-mediated multidrug resistance in a global collection of nontyphoidal Salmonella enterica isolates. Emerg Infect. Dis. 2009, 15, 388–396. [Google Scholar] [CrossRef] [PubMed]
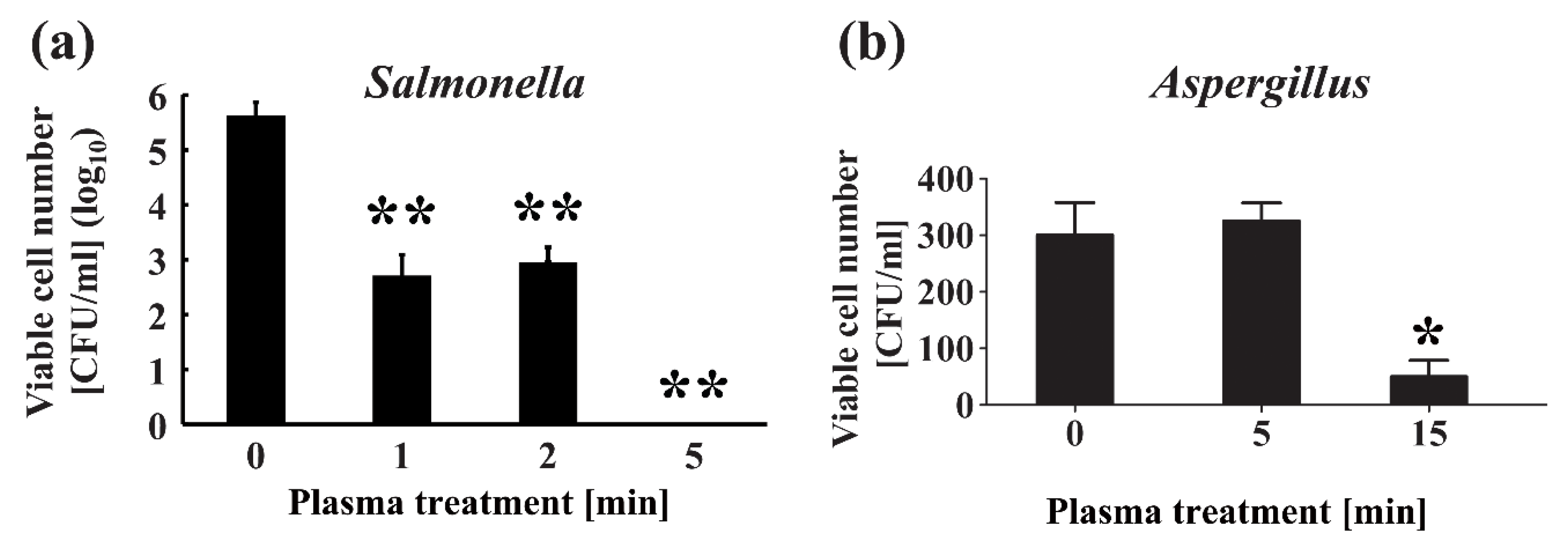
- Maeda, K.; Toyokawa, Y.; Shimizu, N.; Imanishi, Y.; Sakudo, A. Inactivation of Salmonella by nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply. Food Control. 2015, 52, 54–59. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Zhao, Y.; Liu, D.; Liu, Z.; Wang, X.; Chen, H.; Kong, M.G. Gas Plasma pre-treatment increases antibiotic sensitivity and persister eradication in methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2018, 9, 537. [Google Scholar] [CrossRef]
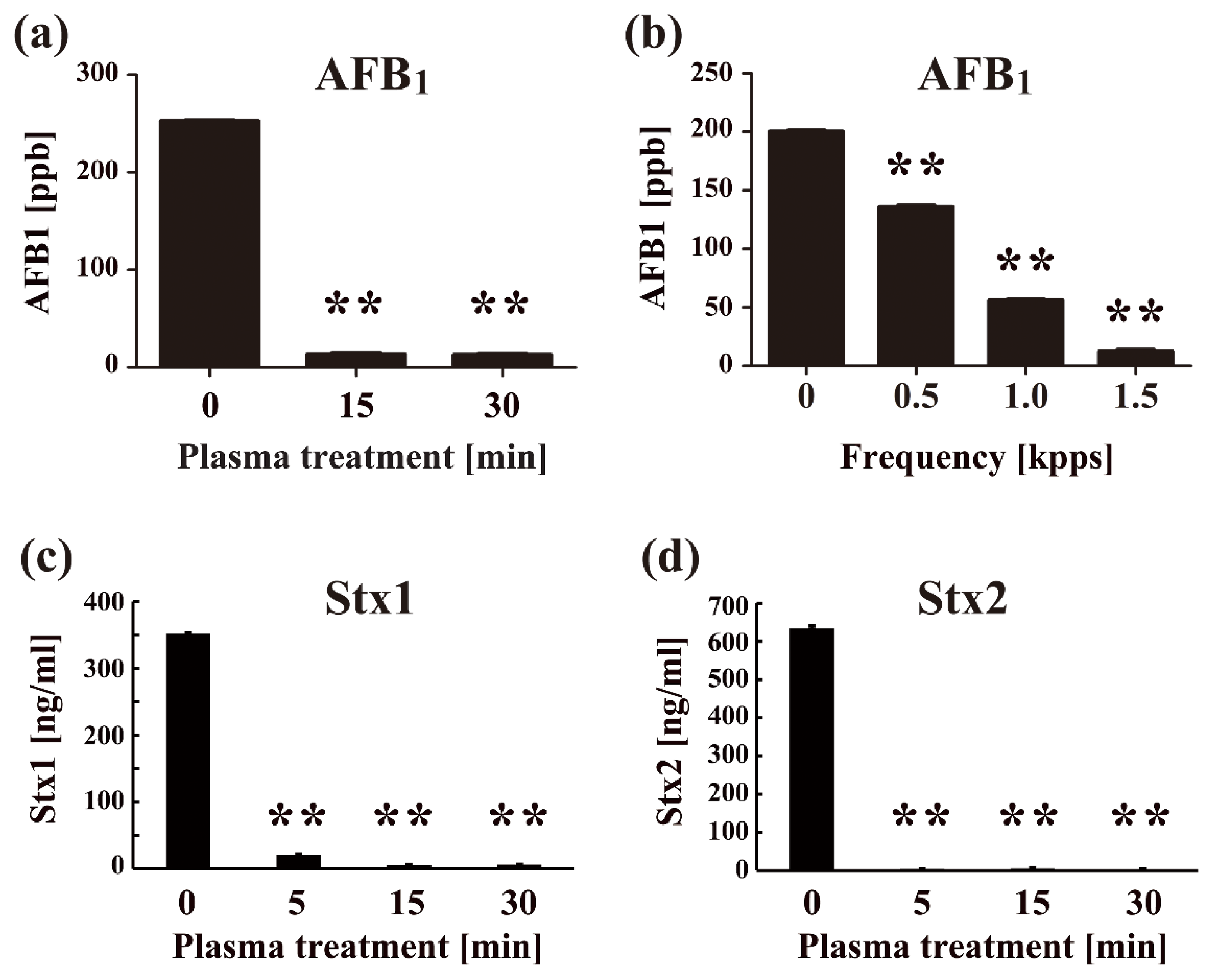
- Sakudo, A.; Toyokawa, Y.; Misawa, T.; Imanishi, Y. Degradation and detoxification of aflatoxin B1 using nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply. Food Control. 2017, 73B, 619–626. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma-A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Soušková, H.; Scholtz, V.; Julák, J.; Savická, D. The fungal spores survival under the low-temperature plasma. In Plasma for Bio-Decontamination, Medicine and Food Security (NATO Science for Peace and Security Series A: Chemistry and Biology); Machala, Z., Hensel, K., Akishev, Y., Eds.; Springer: New York, NY, USA, 2012; pp. 57–66. [Google Scholar]
- Souskova, H.; Scholtz, V.; Julak, J.; Kommova, L.; Savicka, D.; Pazlarova, J. The survival of micromycetes and yeasts under the low-temperature plasma generated in electrical discharge. Folia Microbiol. (Praha) 2011, 56, 77–79. [Google Scholar] [CrossRef]
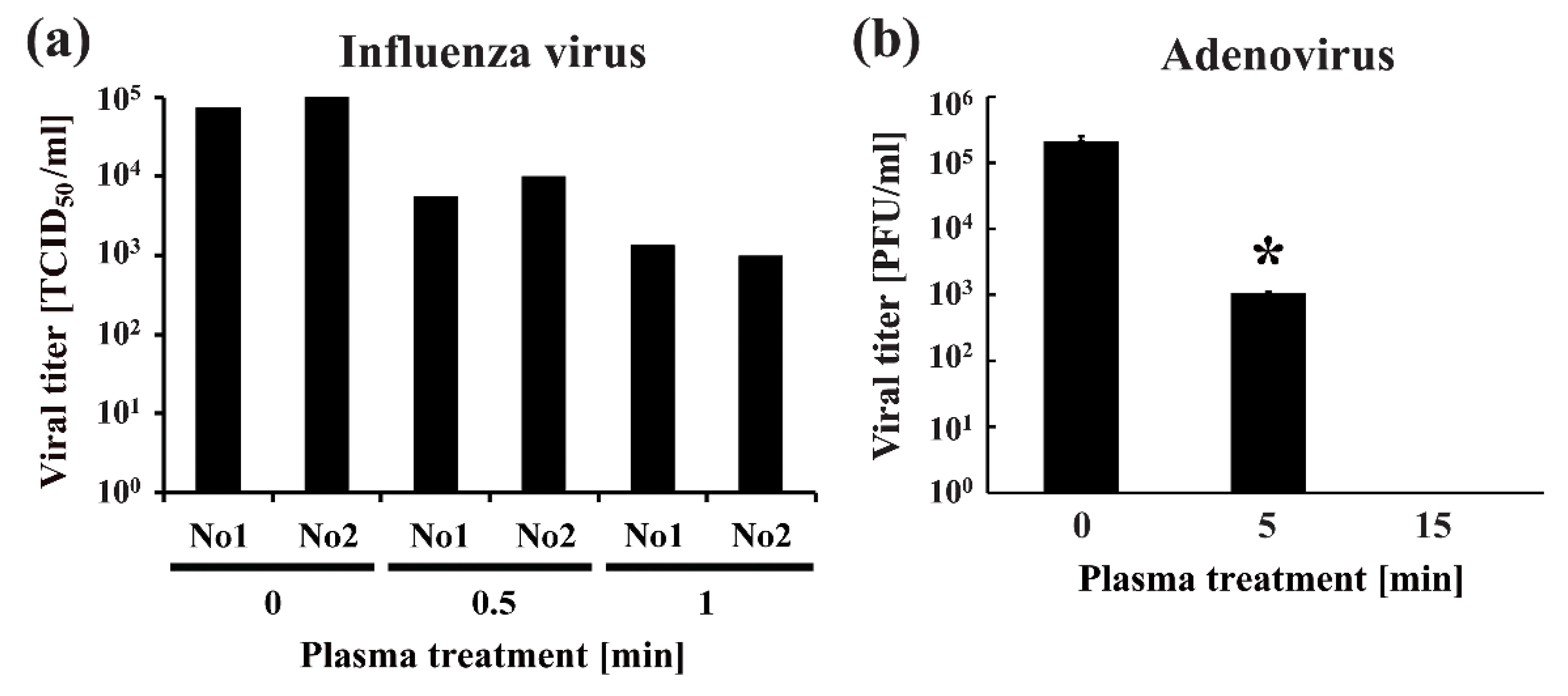
- Sakudo, A.; Shimizu, N.; Imanishi, Y.; Ikuta, K. N2 gas plasma inactivates influenza virus by inducing changes in viral surface morphology, protein, and genomic RNA. Biomed. Res. Int. 2013, 2013, 694269. [Google Scholar] [CrossRef]
- Sakudo, A.; Toyokawa, Y.; Imanishi, Y. Nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply inactivates adenovirus. PLoS ONE 2016, 11, e0157922. [Google Scholar] [CrossRef]
- Alshraiedeh, N.H.; Alkawareek, M.Y.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Atmospheric pressure, nonthermal plasma inactivation of MS2 bacteriophage: Effect of oxygen concentration on virucidal activity. J. Appl. Microbiol. 2013, 115, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H.; Kong, M.G. Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma-activated water. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, Y.; Wei, K.; Li, W.; Yao, M.; Zhang, J.; Grinshpun, S.A. MS2 virus inactivation by atmospheric-pressure cold plasma using different gas carriers and power levels. Appl. Environ. Microbiol. 2015, 81, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, R.; Misawa, T.; Sakudo, A. Key role of singlet oxygen and peroxynitrite in viral RNA damage during virucidal effect of plasma torch on feline calicivirus. Sci. Rep. 2018, 8, 17947. [Google Scholar] [CrossRef] [PubMed]
- USEPA (US Environmental Protection Agency). Registration Division Office of Pesticide Program, Criteria and Standards Division Office of Drinking Water, Guide Standard and Protocol for Testing Microbiological Water Purifiers. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/9102362E.PDF?Dockey=9102362E.pdf (accessed on 24 June 2019).
- Filipic, A.; Primc, G.; Zaplotnik, R.; Mehle, N.; Gutierrez-Aguirre, I.; Ravnikar, M.; Mozetic, M.; Zel, J.; Dobnik, D. Cold atmospheric plasma as a novel method for inactivation of potato virus Y in water samples. Food Environ. Virol. 2019, 11, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Tyczkowska-Sieron, E.; Markiewicz, J.; Grzesiak, B.; Krukowski, H.; Glowacka, A.; Tyczkowski, J. Short communication: Cold atmospheric plasma inactivation of Prototheca zopfii isolated from bovine milk. J. Dairy Sci. 2018, 101, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Wang, F.P.; Chen, W.; Huang, J.; Bazaka, K.; Ostrikov, K.K. Non-equilibrium plasma prevention of Schistosoma japonicum transmission. Sci. Rep. 2016, 6, 35353. [Google Scholar] [CrossRef]
- Hayes, J.; Kirf, D.; Garvey, M.; Rowan, N. Disinfection and toxicological assessments of pulsed UV and pulsed-plasma gas-discharge treated-water containing the waterborne protozoan enteroparasite Cryptosporidium parvum. J. Microbiol. Methods 2013, 94, 325–337. [Google Scholar] [CrossRef]
- Baxter, H.C.; Campbell, G.A.; Whittaker, A.G.; Jones, A.C.; Aitken, A.; Simpson, A.H.; Casey, M.; Bountiff, L.; Gibbard, L.; Baxter, R.L. Elimination of transmissible spongiform encephalopathy infectivity and decontamination of surgical instruments by using radio-frequency gas-plasma treatment. J. Gen. Virol. 2005, 86, 2393–2399. [Google Scholar] [CrossRef]
- von Keudell, A.; Awakowicz, P.; Benedikt, J.; Raballand, V.; Yanguas-Gil, A.; Opretzka, J.; Flötgen, C.; Reuter, R.; Byelykh, L.; Halfmann, H.; et al. Inactivation of bacteria and biomolecules by low pressure plasma discharges. Plasma Process. Polym. 2010, 7, 327–352. [Google Scholar] [CrossRef]
- Sakudo, A.; Imanishi, Y. Degradation and inactivation of Shiga toxins by nitrogen gas plasma. AMB Express 2017, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- McCombs, G.B.; Darby, M.L. New discoveries and directions for medical, dental and dental hygiene research: Low temperature atmospheric pressure plasma. Int. J. Dent. Hyg. 2010, 8, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Shintani, H.; Shimizu, N.; Imanishi, Y.; Sekiya, T.; Tamazawa, K.; Taniguchi, A.; Kido, N. Inactivation of microorganisms and endotoxins by low temperature nitrogen gas plasma exposure. Biocontrol. Sci. 2007, 12, 131–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control. 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Traba, C.; Chen, L.; Liang, J.F. Low power gas discharge plasma mediated inactivation and removal of biofilms formed on biomaterials. Curr. Appl. Phys. 2013, 13, S12–S18. [Google Scholar] [CrossRef][Green Version]
- Kylian, O.; Sasaki, T.; Rossi, F. Plasma sterilization of Geobacillus stearothermophilus by O2:N2 RF inductively coupled plasma. Eur Phys. J. Appl. Phys. 2006, 34, 139–142. [Google Scholar] [CrossRef]
- Rossi, F.; Kylian, O.; Hasiwa, M. Decontamination of surfaces by low pressure plasma discharges. Plasma Process. Polym. 2006, 3, 431–442. [Google Scholar] [CrossRef]
- Rossi, F.; Kylian, O.; Hasiwa, M. Mechanisms of sterilization and decontamination of surfaces by low-pressure plasma. In Advanced Plasma Technology; D’Agostino, R., Favia, P., Kawai, Y., Ikegami, H., Sato, N., Arefi-Khonsari, F., Eds.; Wiley-VCH Verlag GmbH &Co.: Weinheim, Germany, 2008; pp. 319–340. [Google Scholar]
- Pignata, C.; D’Angelo, D.; Fea, E.; Gilli, G. A review on microbiological decontamination of fresh produce with nonthermal plasma. J. Appl. Microbiol. 2017, 122, 1438–1455. [Google Scholar] [CrossRef]
- Toyokawa, Y.; Yagyu, Y.; Misawa, T.; Sakudo, A. A new roller conveyer system of non-thermal gas plasma as a potential control measure of plant pathogenic bacteria in primary food production. Food Control. 2017, 72A, 62–72. [Google Scholar] [CrossRef]
- European Commission. Plasma gas technique as electronic preservation practice of organic food and feed, EGTOP/2014, Directorate-General for Agriculture and Rural Development, Directorate, B. Multilateral relations, quality policy, B.4. Organics, Expert Group for Technical Advice on Organic Production EGTOP, Final Report on Food (III). Available online: https://ec.europa.eu/agriculture/organic/sites/orgfarming/files/docs/body/egtop-final-report-food-iii_en.pdf (accessed on 26 April 2018).
- Naitali, M.; Kamgang-Youbi, G.; Herry, J.M.; Bellon-Fontaine, M.N.; Brisset, J.L. Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef]
- Ito, M.; Oh, J.S.; Ohta, T.; Shiratani, M.; Hori, M. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Process. Polym. 2017, 15, e1700073. [Google Scholar] [CrossRef]
- Nishimura, J.; Takahashi, K.; Takaki, K.; Koide, S.; Suga, M.; Orikasa, T.; Teramoto, Y.; Uchino, T. Removal of ethylene and by-products using dielectric barrier discharge with Ag nanoparticle-loaded Zeolite for keeping freshness of fruits and vegetables. Trans. Mater. Res. Soc. Jpn. 2016, 41, 41–45. [Google Scholar] [CrossRef]
- Itarashiki, T.; Ohshiro, S.; Sakudo, A.; Hayashi, N. Current trend of medical sterilization and disinfection methods using plasmas. J. Plasma Fus. Res. 2015, 91, 505–513. [Google Scholar]
- Shiely, F.; Fallon, D.; Casey, C.; Kerins, D.M.; Eustace, J.A. Trial of a novel plasma gas disinfection system (Radica) to reduce mattress residual bacterial contamination in the acute hospital setting: A preliminary study. Ir J. Med. Sci. 2017, 186, 17–21. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef]
- Fridman, A.; Friedman, G. Plasma Medicine; Wiley-Blackwell: Oxford, UK, 2013. [Google Scholar]
- Laroussi, M.; Kong, M.G.; Morfill, G.; Stolz, W. Plasma Medicine: Applications of Low-Temperature Gas. Plasmas in Medicine and Biology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Wu, A.S.; Kalghatgi, S.; Dobrynin, D.; Sensenig, R.; Cerchar, E.; Podolsky, E.; Dulaimi, E.; Paff, M.; Wasko, K.; Arjunan, K.P.; et al. Porcine intact and wounded skin responses to atmospheric nonthermal plasma. J. Surg. Res. 2013, 179, e1–e12. [Google Scholar] [CrossRef]
- Shahbazi Rad, Z.; Abbasi Davani, F. Experimental investigation on electrical characteristics and dose measurement of dielectric barrier discharge plasma device used for therapeutic application. Rev. Sci. Instrum. 2017, 88, 043504. [Google Scholar] [CrossRef]
- Ballout, H.; Hertel, M.; Doehring, J.; Kostka, E.; Hartwig, S.; Paris, S.; Preissner, S. Effects of plasma jet, dielectric barrier discharge, photodynamic therapy and sodium hypochlorite on infected curved root canals. J. Biophotonics 2018, 11, e201700186. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.; Poorun, D.; Liu, D.; Lu, X.; He, M.; Duan, X.; Chen, H. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J. Dtsch. Dermatol. Ges. 2018, 16, 7–13. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.; Hong, Y.J.; Bae, W.Y.; Choi, E.H.; Jeong, J.W.; Park, H.K. Evaluation of non-thermal plasma-induced anticancer effects on human colon cancer cells. Biomed. Opt. Express 2017, 8, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemiere, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-thermal plasma as a unique delivery system of short-lived reactive oxygen and nitrogen species for immunogenic cell death in melanoma cells. Adv. Sci. (Weinh) 2019, 6, 1802062. [Google Scholar] [CrossRef] [PubMed]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G.; et al. Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Mizuno, M.; Toyokuni, S.; Maruyama, S. Cancer therapy using non-thermal atmospheric pressure plasma with ultra-high electron density. Phys. Plasmas 2015, 22, 122004. [Google Scholar] [CrossRef]
- Hattori, N.; Yamada, S.; Torii, K.; Takeda, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Fujii, T.; Nakayama, G.; et al. Effectiveness of plasma treatment on pancreatic cancer cells. Int. J. Oncol. 2015, 47, 1655–1662. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef]
- Nakamura, K.; Peng, Y.; Utsumi, F.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F.; Kajiyama, H. Novel Intraperitoneal Treatment With Non-thermal plasma-activated medium inhibits metastatic potential of ovarian cancer cells. Sci. Rep. 2017, 7, 6085. [Google Scholar] [CrossRef]
- Sato, T.; Yokoyama, M.; Johkura, K. A key inactivation factor of HeLa cell viability by a plasma flow. J. Phys. D Appl. Phys. 2011, 44, 372001. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabova, B.; Hensel, K.; Spetlikova, E.; Sikurova, L.; Lukes, P. Formation of ROS and RNS in water electro-sprayed through transient spark discharge in air and their bactericidal effects. Plasma Process. Polym. 2013, 2013, 649–659. [Google Scholar] [CrossRef]
- Kurake, N.; Tanaka, H.; Ishikawa, K.; Kondo, T.; Sekine, M.; Nakamura, K.; Kajiyama, H.; Kikkawa, F.; Mizuno, M.; Hori, M. Cell survival of glioblastoma grown in medium containing hydrogen peroxide and/or nitrite, or in plasma-activated medium. Arch. Biochem. Biophys. 2016, 605, 102–108. [Google Scholar] [CrossRef]
- Hashim, S.A.; Samsudin, F.N.; Wong, C.S.; Abu Bakar, K.; Yap, S.L.; Mohd Zin, M.F. Non-thermal plasma for air and water remediation. Arch. Biochem. Biophys. 2016, 605, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Magureanu, M.; Piroi, D.; Mandache, N.B.; David, V.; Medvedovici, A.; Bradu, C.; Parvulescu, V.I. Degradation of antibiotics in water by non-thermal plasma treatment. Water Res. 2011, 45, 3407–3416. [Google Scholar] [CrossRef] [PubMed]
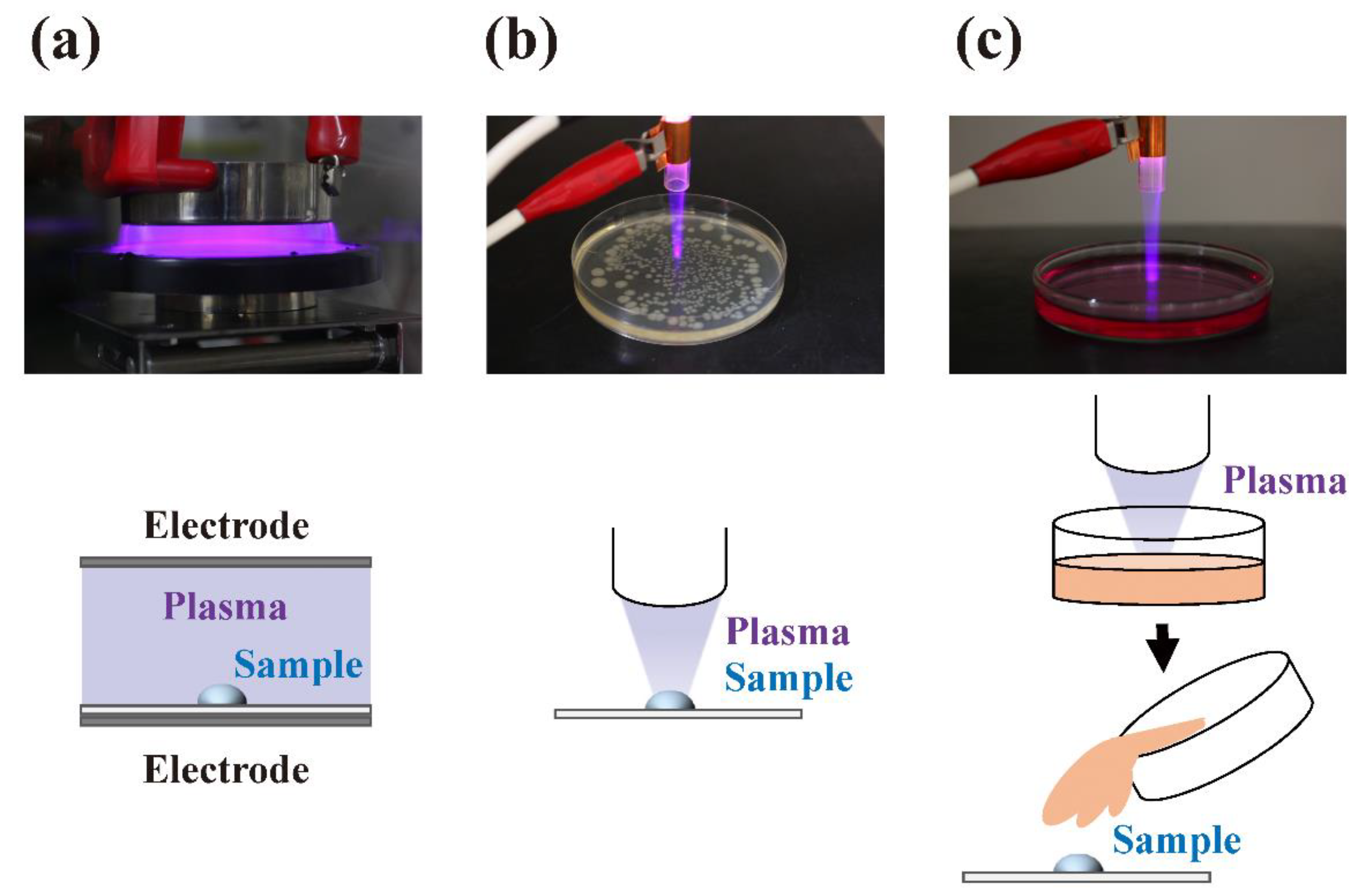

| Classification | Temperature [K] | Electron Density [m−3] | Discharge Type | Examples |
|---|---|---|---|---|
| High-temperature plasma (Equilibrium plasma) | Te ≈ Tion ≈ Tgas = 106–108 | ne ≥ 1020 | Laser fusion Tokamak | Fusion plasma for energy |
| Thermal plasma (Quasi-equilibrium plasma) | Te ≈ Tion ≈ Tn ≈ Tgas ≤ 2 × 104 | ne ≥ 1020 | Arc plasma, Plasma torch, Radio-frequency (RF) Plasma, Microwave plasma etc. | Radiation, welding and cutting, Waste treatment, Material processing, etc. |
| Non-thermal plasma (Non-equilibrium plasma) | Te ≥ Tion ≥ Tn ≈ Tgas = 300–1000 | ne ≈ 1010 | Glow discharge, Corona discharge, atmospheric pressure plasma jet (APPJ), dielectric barrier discharge (DBD), micro-hollow cathode discharge (MHCD), Plasma needle, Low-pressure plasma etc. | Ozonizer, Plasma medicine, Volatile organic compound (VOC) treatment, Plasma agriculture, Surface modifications (coating, etching, activation, cleaning, nitration, etc.), Illumination (plasma screen, fluorescent lamps, etc.) |
| Discharge Type * | Representative Conditions (V, A, Freq, Gas) | Pressure | Gas Temperature | Application | References |
|---|---|---|---|---|---|
| Direct current (DC) corona discharge | 5–30 kV direct current (DC) (positive and negative); 10–250 µA; dry or wet; O2, N2, Ar, He at 10 L/min | 1 atm | Room temperature | Biomedical applications | [18] |
| Atmospheric pressure plasma jet (APPJ) microwave | P = 2.5 W; 2.45 GHz; He/O2/N2 at 2.0/1.2/1.5 L/min | 1 atm | Max. 50.8 °C on a dentin surface; 20 °C on an agar surface | Biomedical applications | [19] |
| Dielectric barrier discharge (DBD) (Flexible sheet-type) | ±2.5 kV; 5 kHz; air, humidity 64.4% | 1 atm | Approximately 50 °C | Biomedical applications | [20] |
| Micro-hollow cathode discharge (MHCD) jet | 1.5–2.5 kV DC; 20 mA; air (0.1–8 L/min) | >1 atm | Room temperature (220 mL/min); >55 °C (5 mm from nozzle, 220 mL/min) | Medical applications | [21] |
| Pin-to-hole spark discharge (PHD) plasma | 4 kV DC; average ~1.8 J/pulse | 1 atm | 9030 ± 320 K (by Boltzmann calculation) | Medical applications (wound healing) | [22] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. https://doi.org/10.3390/ijms20205216
Sakudo A, Yagyu Y, Onodera T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. International Journal of Molecular Sciences. 2019; 20(20):5216. https://doi.org/10.3390/ijms20205216
Chicago/Turabian StyleSakudo, Akikazu, Yoshihito Yagyu, and Takashi Onodera. 2019. "Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications" International Journal of Molecular Sciences 20, no. 20: 5216. https://doi.org/10.3390/ijms20205216
APA StyleSakudo, A., Yagyu, Y., & Onodera, T. (2019). Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. International Journal of Molecular Sciences, 20(20), 5216. https://doi.org/10.3390/ijms20205216





