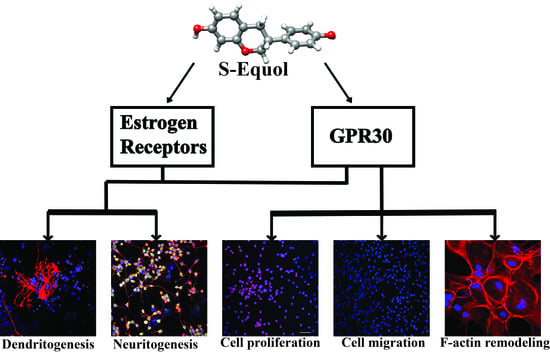
A Novel Mechanism of S-equol Action in Neurons and Astrocytes: The Possible Involvement of GPR30/GPER1
Abstract
:1. Introduction
2. Results
2.1. S-equol Augmented the Dendrite Arborization of Purkinje Cells in the Primary Cerebellar Culture
2.2. S-equol Augmented Neurite Outgrowth in Neuro-2A Cells
2.3. S-equol Increased the Proliferation of Astrocytes
2.4. S-equol Increased the Invasion, Lamellipodia Formation, and Rearrangement of Cortical F-actin Activity in Astrocytes
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Primary Cerebellar Culture
4.3. Mouse Neuro-2A Culture and Induction of Differentiation
4.4. Primary Culture of Cerebellar Astrocyte
4.5. BrdU Incorporation Assay
4.6. Cell Proliferation Assay
4.7. Matrigel Invasion Assay
4.8. Lamellipodial Formation and CSF Index
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Setchell, K.D.R.; Clerici, C. Equol: History, Chemistry, and Formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.R.; Clerici, C. Equol: Pharmacokinetics and Biological Actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setchell, K.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a potent ligand for estrogen receptor B, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Shinkaruk, S.; Carreau, C.; Flouriot, G.; Bennetau-pelissero, C.; Potier, M. Comparative Effects of R- and S-equol and Implication of Transactivation Functions (AF-1 and AF-2) in Estrogen Receptor-Induced Transcriptional Activity. Nutrients 2010, 2, 340–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, T.D.; Munson, D.J.; Haldy, M.E.; Setchell, K.D.R.; Lephart, E.D.; Handa, R.J. Equol Is a Novel Anti-Androgen that Inhibits Prostate Growth and Hormone Feedback. Biol. Reprod. 2004, 70, 1188–1195. [Google Scholar] [CrossRef]
- Aso, T. Equol Improves Menopausal Symptoms in Japanese Women. J. Nutr. 2010, 140, 1386S–1389S. [Google Scholar] [CrossRef] [Green Version]
- Onoda, A.; Ueno, T.; Uchiyama, S.; Hayashi, S.; Kato, K.; Wake, N. Effects of S -equol and natural S -equol supplement (SE5-OH) on the growth of MCF-7 in vitro and as tumors implanted into ovariectomized athymic mice. Food Chem. Toxicol. 2011, 49, 2279–2284. [Google Scholar] [CrossRef]
- Koibuchi, N.; Chin, W.W. Thyroid hormone action and brain development. Trends Endocrinol Metab. 2000, 11, 123–128. [Google Scholar] [CrossRef]
- Koibuchi, N. The role of thyroid hormone on cerebellar development. Cerebellum 2008, 7, 530–533. [Google Scholar] [CrossRef]
- Hedges, V.L.; Ebner, T.J.; Meisel, R.L.; Mermelstein, P.G. The cerebellum as a target for estrogen action. Front. Neuroendocrinol. 2012, 33, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Zsarnovszky, A.; Kiss, D.; Jocsak, G.; Nemeth, G.; Toth, I. Thyroid hormone- and estrogen receptor interactions with natural ligands and endocrine disruptors in the cerebellum. Front. Neuroendocrinol. 2018, 48, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, Y.; Zhou, D.-X.; Zhao, L.-M.; Li, G.-R.; Deng, X.-L. Equol is neuroprotective during focal cerebral ischemia and reperfusion that involves p-Src and gp91phox. Curr. Neurovasc. Res. 2014, 11, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sullivan, J.C.; Schreihofer, D.A. Dietary genistein and equol (4′, 7 isoflavandiol) reduce oxidative stress and protect rats against focal cerebral ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R871–R877. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, S.J.; Hu, C.; Aksenova, M.V.; Mactutus, C.F.; Booze, R.M. HIV-1 Tat and cocaine mediated synaptopathy in cortical and midbrain neurons is prevented by the isoflavone Equol. Front. Microbiol. 2015, 6, 894. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Filardo, E.J.; Lolait, S.J.; Thomas, P.; Maggiolini, M.; Prossnitz, E.R. Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives. J. Steroid Biochem. Mol. Biol. 2018, 176, 4–15. [Google Scholar] [CrossRef]
- Maggiolini, M.; Picard, D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J. Endocrinol. 2010, 204, 105–114. [Google Scholar] [CrossRef]
- Pandey, D.P.; Lappano, R.; Albanito, L.; Madeo, A.; Maggiolini, M.; Picard, D. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. EMBO J. 2009, 28, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Madeo, A.; Maggiolini, M. Nuclear Alternate Estrogen Receptor GPR30 Mediates 17β-Estradiol–Induced Gene Expression and Migration in Breast Cancer–Associated Fibroblasts.pdf. Tumor Stem Cell Biol. Nucl. 2010, 70, 6036–6046. [Google Scholar]
- Hazell, G.G.J.; Yao, S.T.; Roper, J.A.; Prossnitz, E.R.; Carroll, A.O.; Lolait, S.J. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol. 2009, 202, 223–236. [Google Scholar] [CrossRef]
- Alexander, A.; Irving, A.J.; Harvey, J. Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology 2017, 113, 652–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Zhang, Q.; Yang, L.; Dong, Y.; Khan, M.; Yang, F.; Brann, D.W.; Wang, R. GPR30 mediates estrogen rapid signaling and neuroprotection. Mol. Cell Endocrinol. 2014, 387, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, M.; Hashimoto, A.; Satoh, H.; Kawabe, K.; Ogawa, M.; Takano, K.; Nakamura, Y. S-Equol, a Major Isoflavone from Soybean, Inhibits Nitric Oxide Production in Lipopolysaccharide-Stimulated Rat Astrocytes Partially via the GPR30-Mediated Pathway. Int. J. Inflam. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, M.A.; Azcoitia, I.; Garcia-Segura, L.M. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 2015, 16, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.C.; Winuthayanon, W.; Korach, K.S. What’s new in estrogen receptor action in the female reproductive tract. J. Mol. Endocrinol. 2016, 56, R55–R71. [Google Scholar] [CrossRef] [PubMed]
- Dauvois, S.; White, R.; Parker, M.G. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J. Cell Sci. 1993, 106, 1377–1388. [Google Scholar]
- Akama, K.T.; Thompson, L.I.; Milner, T.A.; McEwen, B.S. Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J. Biol. Chem. 2013, 288, 6438–6450. [Google Scholar] [CrossRef]
- Brailoiu, E.; Dun, S.L.; Brailoiu, G.C.; Mizuo, K.; Sklar, L.A.; Oprea, T.I.; Prossnitz, E.R.; Dun, N.J. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 2007, 193, 311–321. [Google Scholar] [CrossRef]
- Funakoshi, T.; Yanai, A.; Shinoda, K.; Kawano, M.M.; Mizukami, Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem. Biophys. Res. Commun. 2006, 346, 904–910. [Google Scholar] [CrossRef]
- Hammond, R.; Nelson, D.; Gibbs, R.B. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology 2011, 36, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.; Dong, J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Hadjimarkou, M.M.; Vasudevan, N. GPER1/GPR30 in the brain: Crosstalk with classical estrogen receptors and implications for behavior. J. Steroid Biochem. Mol. Biol. 2018, 176, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science 2005, 307, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Albanito, L.; Madeo, A.; Lappano, R.; Vivacqua, A.; Rago, V.; Carpino, A.; Oprea, T.I.; Prossnitz, E.R.; Musti, A.M.; Ando, S.; et al. G Protein–Coupled Receptor 30 (GPR30) Mediates Gene Expression Changes and Growth Response to 17B-Estradiol and Selective GPR30 Ligand G-1 in Ovarian Cancer Cells. Cancer Res. 2007, 67, 1859–1867. [Google Scholar] [CrossRef]
- Pelekanou, V.; Kampa, M.; Kiagiadaki, F.; Deli, A.; Theodoropoulos, P.; Agrogiannis, G.; Patsouris, E.; Tsapis, A.; Castanas, E.; Notas, G. Estrogen anti-inflammatory activity on human monocytes is mediated through cross-talk between estrogen receptor ERa36 and GPR30/GPER1. J. Leukoc Biol. 2016, 99, 333–347. [Google Scholar] [CrossRef]
- Hoffman, X.J.F.; Wright, X.C.L.; Mccarthy, X.M.M. A Critical Period in Purkinje Cell Development Is Mediated by Local Estradiol Synthesis, Disrupted by Inflammation, and Has Enduring Consequences Only for Males. J. Neurosci. 2016, 36, 10039–10049. [Google Scholar] [CrossRef]
- Tsutsui, K.; Ukena, K.; Sakamoto, H.; Okuyama, S.I.; Haraguchi, S. Biosynthesis, mode of action, and functional significance of neurosteroids in the purkinje cell. Front. Endocrinol. (Lausanne) 2011, 2, 1–9. [Google Scholar] [CrossRef]
- Sasahara, K.; Shikimi, H.; Haraguchi, S.; Sakamoto, H.; Honda S -i Harada, N.; Tsutsui, K. Mode of Action and Functional Significance of Estrogen-Inducing Dendritic Growth, Spinogenesis, and Synaptogenesis in the Developing Purkinje Cell. J. Neurosci. 2007, 27, 7408–7417. [Google Scholar] [CrossRef]
- Kim, H.J.; Casadesus, G. Estrogen-mediated effects on cognition and synaptic plasticity: What do estrogen receptor knockout models tell us? Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 1090–1093. [Google Scholar] [CrossRef] [Green Version]
- Chamniansawat, S.; Chongthammakun, S. Genomic and non-genomic actions of estrogen on synaptic plasticity in SH-SY5Y cells. Neurosci. Lett. 2010, 470, 49–54. [Google Scholar] [CrossRef]
- Mccarthy, M.M. Estradiol and the developing brain. Physiol. Rev. 2009, 88, 91–124. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Kim, H. Cautions and Research Needs Identified at the Equol, Soy, and Menopause Research Leadership Conference. J. Nutr. 2010, 140, 1390S–1394S. [Google Scholar] [CrossRef] [Green Version]
- Lampe, J.W. Emerging Research on Equol and Cancer. J. Nutr. 2010, 140, 1369S–1372S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiwata, N.; Melby, M.K.; Mizuno, S.; Watanabe, S. New equol supplement for relieving menopausal symptoms: Randomized, placebo-controlled trial of Japanese women. Menopause 2009, 16, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. (Oxf.) 2013, 78, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Kimura-Kuroda, J.; Nagata, I.; Kuroda, Y. Hydroxylated metabolites of polychlorinated biphenyls inhibit thyroid-hormone-dependent extension of cerebellar Purkinje cell dendrites. Dev. Brain Res. 2005, 154, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Ariyani, W.; Iwasaki, T.; Miyazaki, W.; Khongorzul, E.; Nakajima, T.; Kameo, S.; Koyama, H.; Tsushima, Y.; Koibuchi, N. Effects of gadolinium-based contrast agents on thyroid hormone receptor action and thyroid hormone-induced cerebellar purkinje cell morphogenesis. Front. Endocrinol. (Lausanne) 2016, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Ariyani, W.; Iwasaki, T.; Miyazaki, W.; Yu, L.; Takeda, S.; Koibuchi, N. A possible novel mechanism of action of genistein and daidzein for activating thyroid hormone receptor-mediated transcription. Toxicol. Sci. 2018, 164, 2. [Google Scholar] [CrossRef]
- Evangelopoulos, M.E.; Weis, J.; Kru, A. Signalling pathways leading to neuroblastoma differentiation after serum withdrawal: HDL blocks neuroblastoma differentiation by inhibition of EGFR. Oncogene 2005, 24, 3309–3318. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, R.G.; Sikorska, M.; Sandhu, J.K.; Lanthier, P.; Ribecco-lutkiewicz, M.; Bani-yaghoub, M. Differentiation of mouse Neuro 2A cells into dopamine neurons. J. Neurosci Methods 2010, 186, 60–67. [Google Scholar] [CrossRef]
- Schildge, S.; Bohrer, C.; Beck, K.; Schachtrup, C. Isolation and Culture of Mouse Cortical Astrocytes. J. Vis. Exp. 2013, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stogsdill, J.A.; Ramirez, J.; Liu, D.; Kim, Y.; Baldwin, K.T. Astrocytic Neuroligins Control Astrocyte Morphogenesis and Synaptogenesis. Nature 2017, 551, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Hoffmann, S.; Endlich, N.; Velic, A.; Schwab, A.; Weide, T.; Schlatter, E.; Pavenstädt, H. Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J. Mol. Med. 2008, 86, 1379–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariyani, W.; Miyazaki, W.; Koibuchi, N. A Novel Mechanism of S-equol Action in Neurons and Astrocytes: The Possible Involvement of GPR30/GPER1. Int. J. Mol. Sci. 2019, 20, 5178. https://doi.org/10.3390/ijms20205178
Ariyani W, Miyazaki W, Koibuchi N. A Novel Mechanism of S-equol Action in Neurons and Astrocytes: The Possible Involvement of GPR30/GPER1. International Journal of Molecular Sciences. 2019; 20(20):5178. https://doi.org/10.3390/ijms20205178
Chicago/Turabian StyleAriyani, Winda, Wataru Miyazaki, and Noriyuki Koibuchi. 2019. "A Novel Mechanism of S-equol Action in Neurons and Astrocytes: The Possible Involvement of GPR30/GPER1" International Journal of Molecular Sciences 20, no. 20: 5178. https://doi.org/10.3390/ijms20205178
APA StyleAriyani, W., Miyazaki, W., & Koibuchi, N. (2019). A Novel Mechanism of S-equol Action in Neurons and Astrocytes: The Possible Involvement of GPR30/GPER1. International Journal of Molecular Sciences, 20(20), 5178. https://doi.org/10.3390/ijms20205178