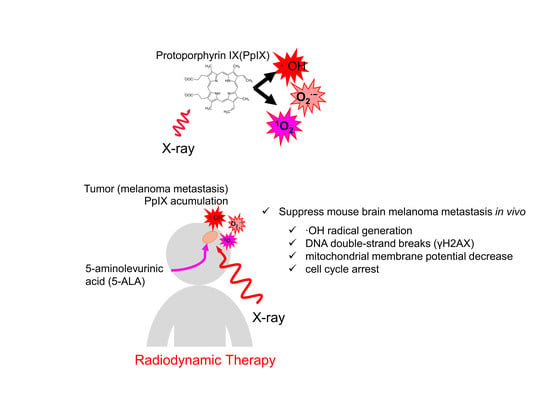
Verification of 5-Aminolevurinic Radiodynamic Therapy Using a Murine Melanoma Brain Metastasis Model
Abstract
:1. Introduction
2. Results
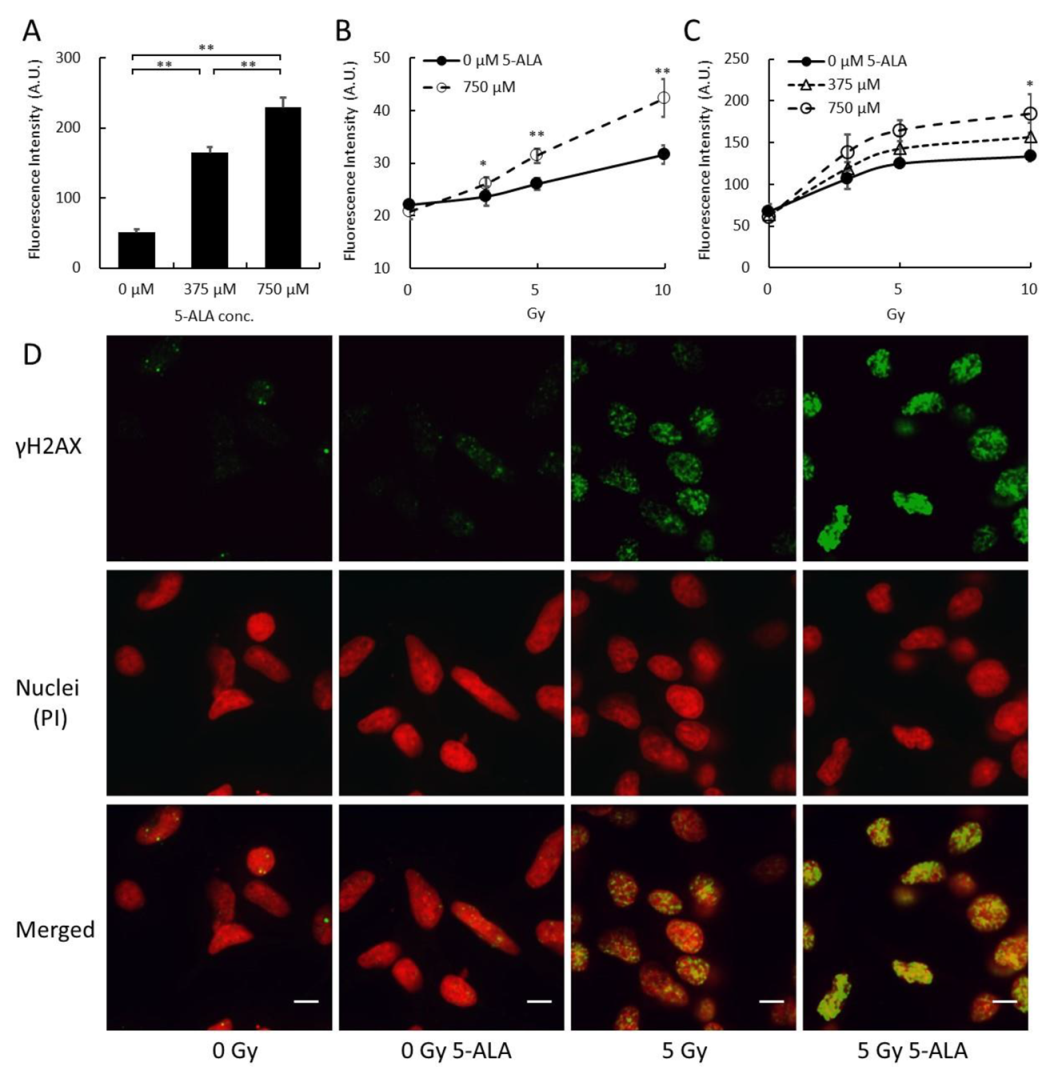
2.1. Cellular Response Immediately after Treatment by X-Ray Irradiation with 5-ALA
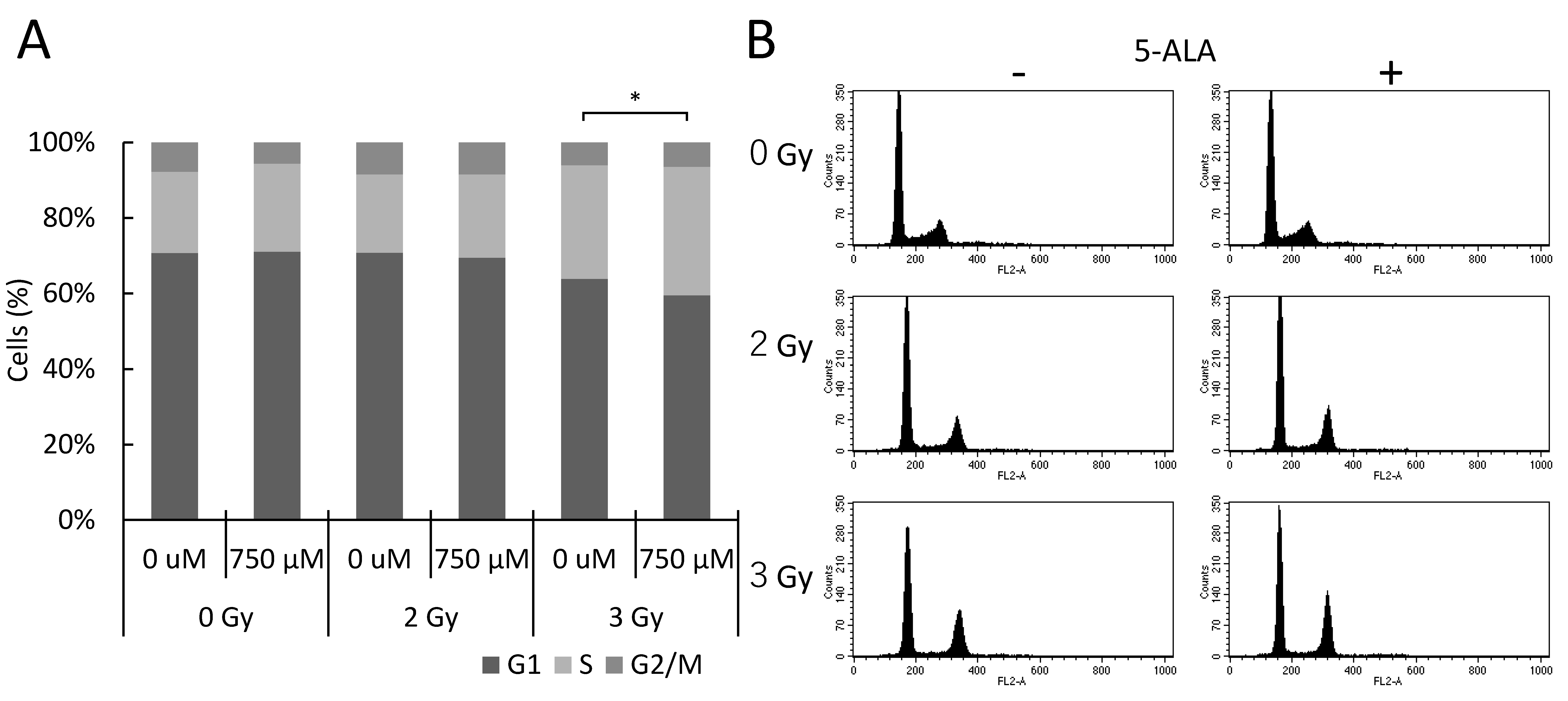
2.2. 5-ALA and X-Ray Irradiation Affect the Cell Cycle Progression In Vitro
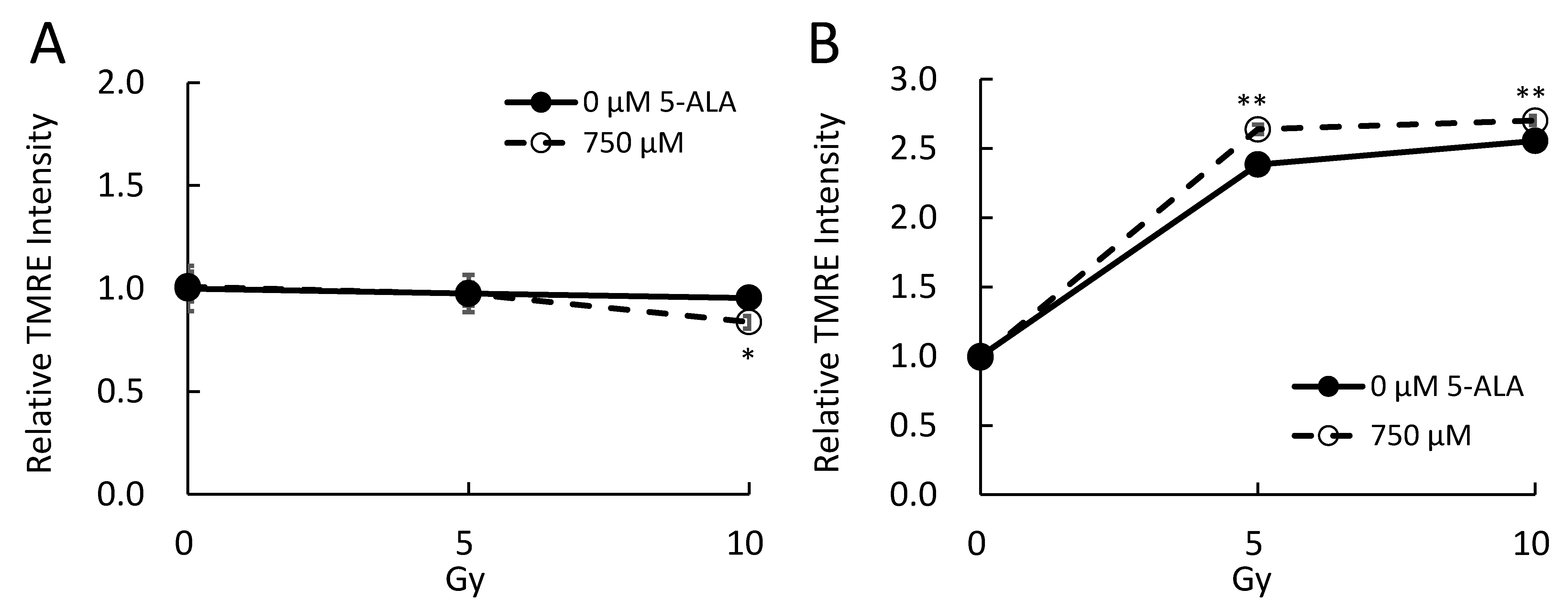
2.3. 5-ALA and X-Ray Irradiation Affect the Mitochondrial Membrane Potential In Vitro
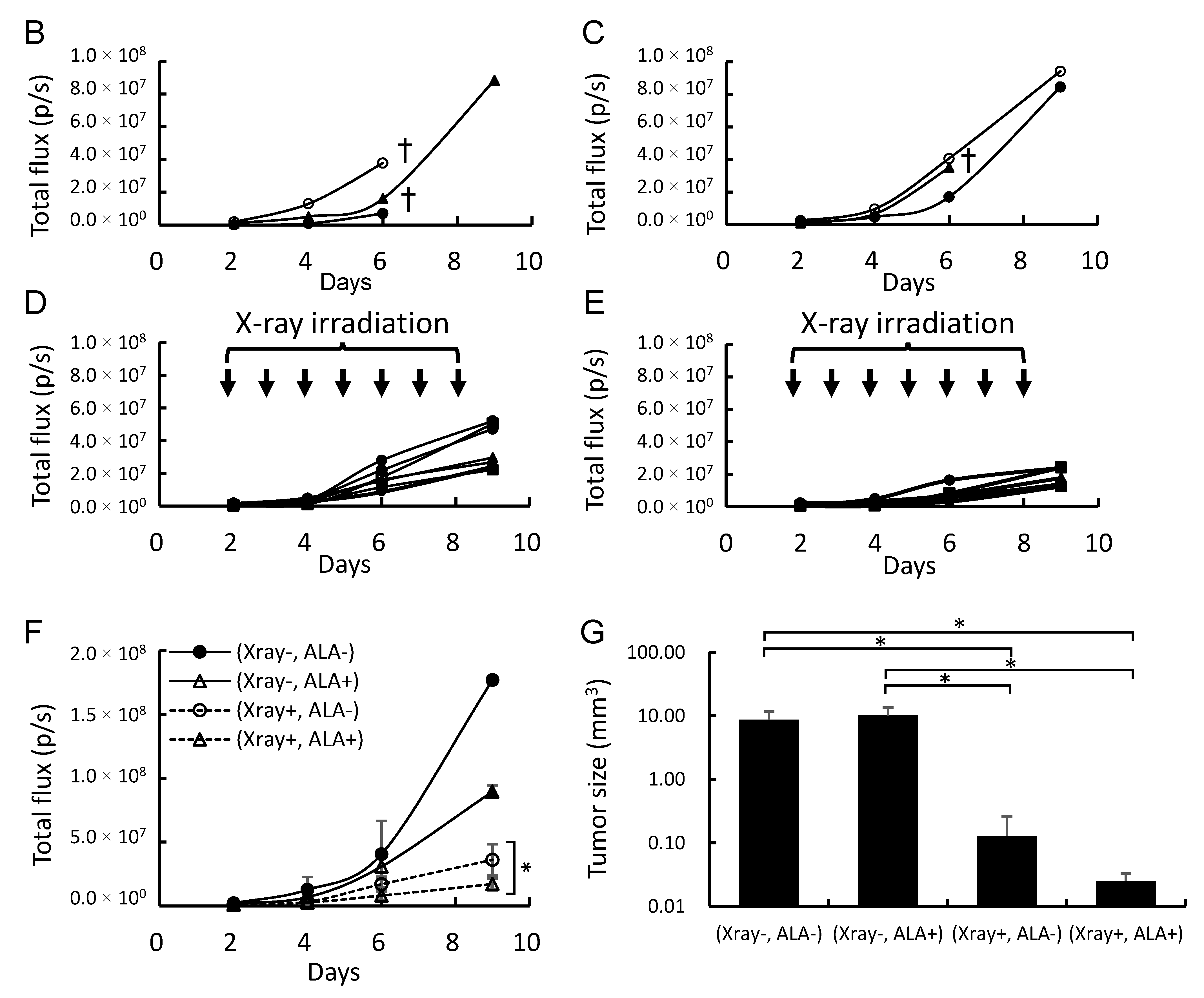
2.4. 5-ALA and X-Ray Irradiation Suppress Tumor Growth in a Brain Metastasis Melanoma Model Mouse
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. X-Ray Irradiation Conditions
4.4. Determination of PpIX Concentration in Cells and Tissue
4.5. Measurement of Intracellular ROS
4.6. γH2AX Detection
4.7. Cell Cycle Assay
4.8. Mitochondrial Membrane Potential
4.9. Intracranial Implantation of B16-Luc Melanoma Cells and X-Ray Irradiation in Mice
4.10. In Vivo Imaging of Intracranial Tumors
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-ALA | 5-aminolevurinic acid |
| DSB | double-strand break |
| MMP | mitochondrial membrane potential |
| PDD | photodynamic diagnosis |
| PDT | photodynamic therapy |
| PpIX | protoporphyrin IX |
| RDT | radiodynamic therapy |
| ROS | reactive oxygen species |
| RT | radiation therapy |
References
- Gugger, A.; Barnhill, R.L.; Seifert, B.; Dehler, S.; Moch, H.; Lugassy, C.; Marques-Maggio, E.; Rushing, E.J.; Mihic-Probst, D. Cutaneous Melanoma with Brain Metastasis: Report of 193 Patients with New Observations. PLoS ONE. 2016, 11, e0156115. [Google Scholar] [CrossRef] [PubMed]
- Madajewicz, S.; Karakousis, C.; West, C.R.; Caracandas, J.; Avellanosa, A.M. Malignant melanoma brain metastases. Review of Roswell Park Memorial Institute experience. Cancer 1984, 53, 2550–2552. [Google Scholar] [CrossRef]
- Chason, J.L.; Walker, F.B.; Landers, J.W. Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer 1963, 16, 781–787. [Google Scholar] [CrossRef]
- Krayem, M.; Sabbah, M.; Najem, A.; Wouters, A.; Lardon, F.; Simon, S.; Sales, F.; Journe, F.; Awada, A.; Ghanem, G.E.; et al. The Benefit of Reactivating p53 under MAPK Inhibition on the Efficacy of Radiotherapy in Melanoma. Cancers 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Chicas-Sett, R.; Morales-Orue, I.; Rodriguez-Abreu, D.; Lara-Jimenez, P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: A systematic review. Clin. Transl. Radiat. Oncol. 2017, 9, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Pottier, R.H.; Pross, D.C. Photodynamic therapy with endogenous protoporphyrin IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B. 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Morgan, J.; Oseroff, A.R. Mitochondria-based photodynamic anti-cancer therapy. Adv. Drug Deliv. Rev. 2001, 49, 71–86. [Google Scholar] [CrossRef]
- Lopez, R.F.; Lange, N.; Guy, R.; Bentley, M.V. Photodynamic therapy of skin cancer: Controlled drug delivery of 5-ALA and its esters. Adv. Drug Deliv. Rev. 2004, 56, 77–94. [Google Scholar] [CrossRef]
- Takahashi, J.; Misawa, M. Characterization of reactive oxygen species generated by protoporphyrin IX under X-ray irradiation. Rad. Phys. Chem. 2009, 78, 889–898. [Google Scholar] [CrossRef]
- Takahashi, J.; Misawa, M.; Iwahashi, H. Combined treatment with X-ray irradiation and 5-aminolevulinic acid elicits better transcriptomic response of cell cycle-related factors than X-ray irradiation alone. Int. J. Radiat. Biol. 2016, 92, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Murakami, M.; Mori, T.; Iwahashi, H. Verification of radiodynamic therapy by medical linear accelerator using a mouse melanoma tumor model. Sci. Rep. 2018, 8, 2728. [Google Scholar] [CrossRef] [PubMed]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.; Schmidt, A. Rapid determination of urinary total porphyrins by ion exchange chromatography. Z. Klin. Chem. Klin. Biochem. 1971, 9, 415–418. [Google Scholar] [CrossRef]
- Sak, A.; Stuschke, M. Use of γH2AX and other biomarkers of double-strand breaks during radiotherapy. Semin. Radiat. Oncol. 2010, 20, 223–231. [Google Scholar] [CrossRef]
- Yamamori, T.; Yasui, H.; Yamazumi, M.; Wada, Y.; Nakamura, Y.; Nakamura, H.; Inanami, O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic. Biol. Med. 2012, 53, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Siu, T.L.; Huang, S. Cerebral metastases from malignant melanoma: Current treatment strategies, advances in novel therapeutics and future directions. Cancers 2010, 2, 364–375. [Google Scholar] [CrossRef]
- Śniegocka, M.; Podgórska, E.; Płonka, P.M.; Elas, M.; Romanowska-Dixon, B.; Szczygieł, M.; Żmijewski, M.A.; Cichorek, M.; Markiewicz, A.; Brożyna, A.A.; et al. Transplantable Melanomas in Hamsters and Gerbils as Models for Human Melanoma. Sensitization in Melanoma Radiotherapy-From Animal Models to Clinical Trials. Int. J. Mol. Sci. 2018, 19, 1048. [Google Scholar]
- Sparsa, A.; Bellaton, S.; Naves, T.; Jauberteau, M.O.; Bonnetblanc, J.M.; Sol, V.; Verdier, M.; Ratinaud, M.H. Photodynamic treatment induces cell death by apoptosis or autophagy depending on the melanin content in two B16 melanoma cell lines. Oncol. Rep. 2013, 29, 1196–1200. [Google Scholar] [CrossRef]
- Box, N.F.; Vukmer, T.O.; Terzian, T. Targeting p53 in melanoma. Pigment. Cell Melanoma Res. 2014, 27, 8–10. [Google Scholar] [CrossRef]
- Hocker, T.; Tsao, H. Ultraviolet radiation and melanoma: A systematic review and analysis of reported sequence variants. Hum. Mutat. 2007, 28, 578–588. [Google Scholar] [CrossRef]
- Takahashi, J.; Misawa, M.; Iwahashi, H. Transcriptome Analysis of Porphyrin-Accumulated and X-Ray-Irradiated Cell Cultures under Limited Proliferation and Non-Lethal Conditions. Microarrays 2015, 4, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Cavalieri, S.; Di Guardo, L.; Cimminiello, C.; Nichetti, F.; Corti, F.; Garcia, M.A.; Pappalardi, B.; Fallai, C.; de Braud, F.; et al. Combination of Immunotherapy and Brain Radiotherapy in Metastatic Melanoma: A Retrospective Analysis. Oncol. Res. Treat. 2019, 42, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Ogura, S.; Shimajiri, S.; Nakano, Y.; Akiba, D.; Kitagawa, T.; Ueta, K.; Tanaka, T.; Nishizawa, S. 5-aminolevulinic acid-induced protoporphyrin IX with multi-dose ionizing irradiation enhances host antitumor response and strongly inhibits tumor growth in experimental glioma in vivo. Mol. Med. Rep. 2015, 11, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Nemoto, K.; Ogawa, Y.; Hareyama, M.; Yoshida, H.; Takamura, A.; Ohmori, K.; Hamamoto, Y.; Sugita, T.; Saitoh, M.; et al. A multi-institutional retrospective analysis of external radiotherapy for mucosal melanoma of the head and neck in Northern Japan. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 495–500. [Google Scholar] [CrossRef]
- Chandra, R.A.; Wilhite, T.J.; Balboni, T.A.; Alexander, B.M.; Spektor, A.; Ott, P.A.; Ng, A.K.; Hodi, F.S.; Schoenfeld, J.D. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. 2015, 4, e1046028. [Google Scholar] [CrossRef] [Green Version]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Mascaraque, M.; Delgado-Wicke, P.; Damian, A.; Lucena, S.R.; Carrasco, E.; Juarranz, Á. Mitotic Catastrophe Induced in HeLa Tumor Cells by Photodynamic Therapy with Methyl-aminolevulinate. Int. J. Mol. Sci. 2019, 20, 1229. [Google Scholar] [CrossRef]
- Li, P.T.; Tsai, Y.J.; Lee, M.J.; Chen, C.T. Increased Histone Deacetylase Activity Involved in the Suppressed Invasion of Cancer Cells Survived from ALA-Mediated Photodynamic Treatment. Int. J. Mol. Sci. 2015, 16, 23994–24010. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.E.; Hopper, C.; MacRobert, A.J.; Speight, P.M.; Bown, S.G. Photodynamic therapy of oral cancer: Photosensitisation with systemic aminolaevulinic acid. Lancet 1993, 342, 147–148. [Google Scholar] [CrossRef]
- Setsukinai, K.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2002, 278, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Kramp, T.R.; Camphausen, K. Combination radiotherapy in an orthotopic mouse brain tumor model. J. Vis. Exp. 2012, 6, e3397. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, J.; Nagasawa, S.; Ikemoto, M.J.; Sato, C.; Sato, M.; Iwahashi, H. Verification of 5-Aminolevurinic Radiodynamic Therapy Using a Murine Melanoma Brain Metastasis Model. Int. J. Mol. Sci. 2019, 20, 5155. https://doi.org/10.3390/ijms20205155
Takahashi J, Nagasawa S, Ikemoto MJ, Sato C, Sato M, Iwahashi H. Verification of 5-Aminolevurinic Radiodynamic Therapy Using a Murine Melanoma Brain Metastasis Model. International Journal of Molecular Sciences. 2019; 20(20):5155. https://doi.org/10.3390/ijms20205155
Chicago/Turabian StyleTakahashi, Junko, Shinsuke Nagasawa, Mitsushi J. Ikemoto, Chikara Sato, Mari Sato, and Hitoshi Iwahashi. 2019. "Verification of 5-Aminolevurinic Radiodynamic Therapy Using a Murine Melanoma Brain Metastasis Model" International Journal of Molecular Sciences 20, no. 20: 5155. https://doi.org/10.3390/ijms20205155
APA StyleTakahashi, J., Nagasawa, S., Ikemoto, M. J., Sato, C., Sato, M., & Iwahashi, H. (2019). Verification of 5-Aminolevurinic Radiodynamic Therapy Using a Murine Melanoma Brain Metastasis Model. International Journal of Molecular Sciences, 20(20), 5155. https://doi.org/10.3390/ijms20205155