Peptidic Antifreeze Materials: Prospects and Challenges
Abstract
:1. Introduction
2. Structure and Ice-Binding Sites of Antifreeze (Glyco) Proteins
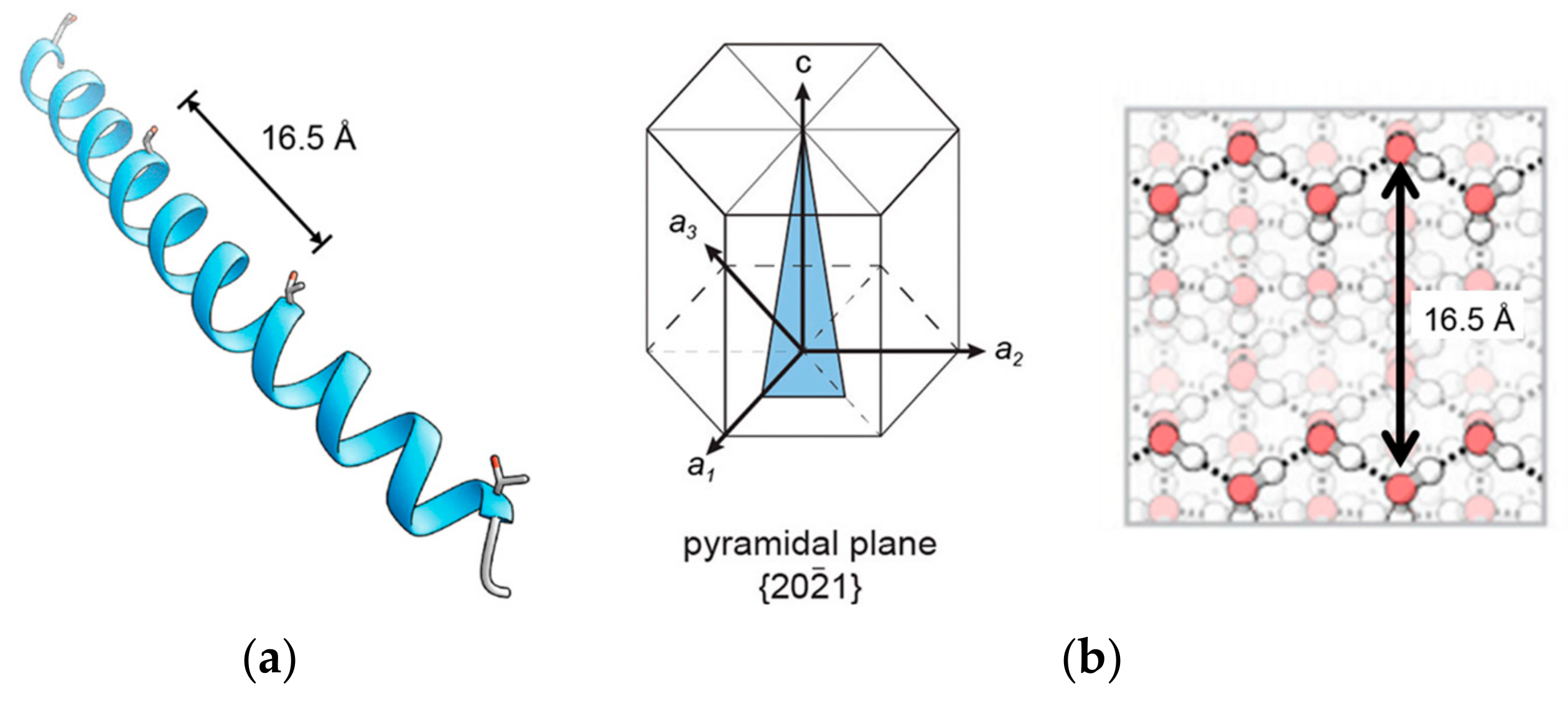
2.1. α-Helical AFPs
2.2. β-Strand AFPs
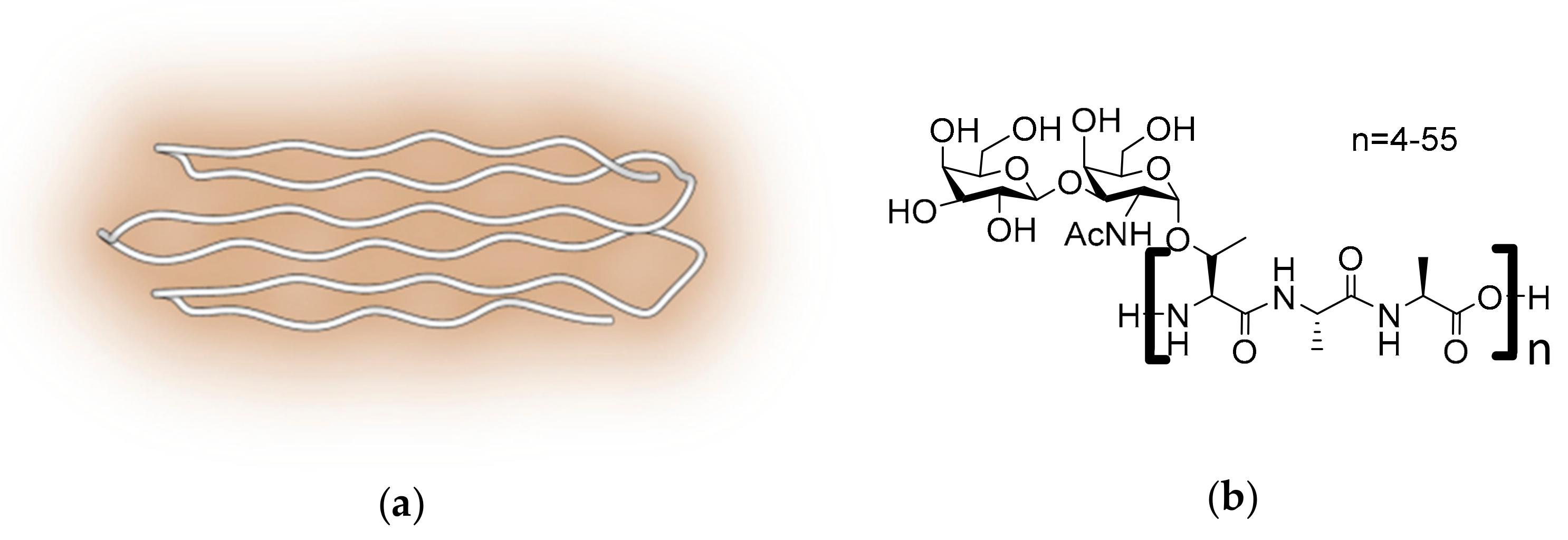
2.3. Antifreeze Glycoproteins
2.4. Polyproline II Containing AFPs
3. Ice-Binding and Activity
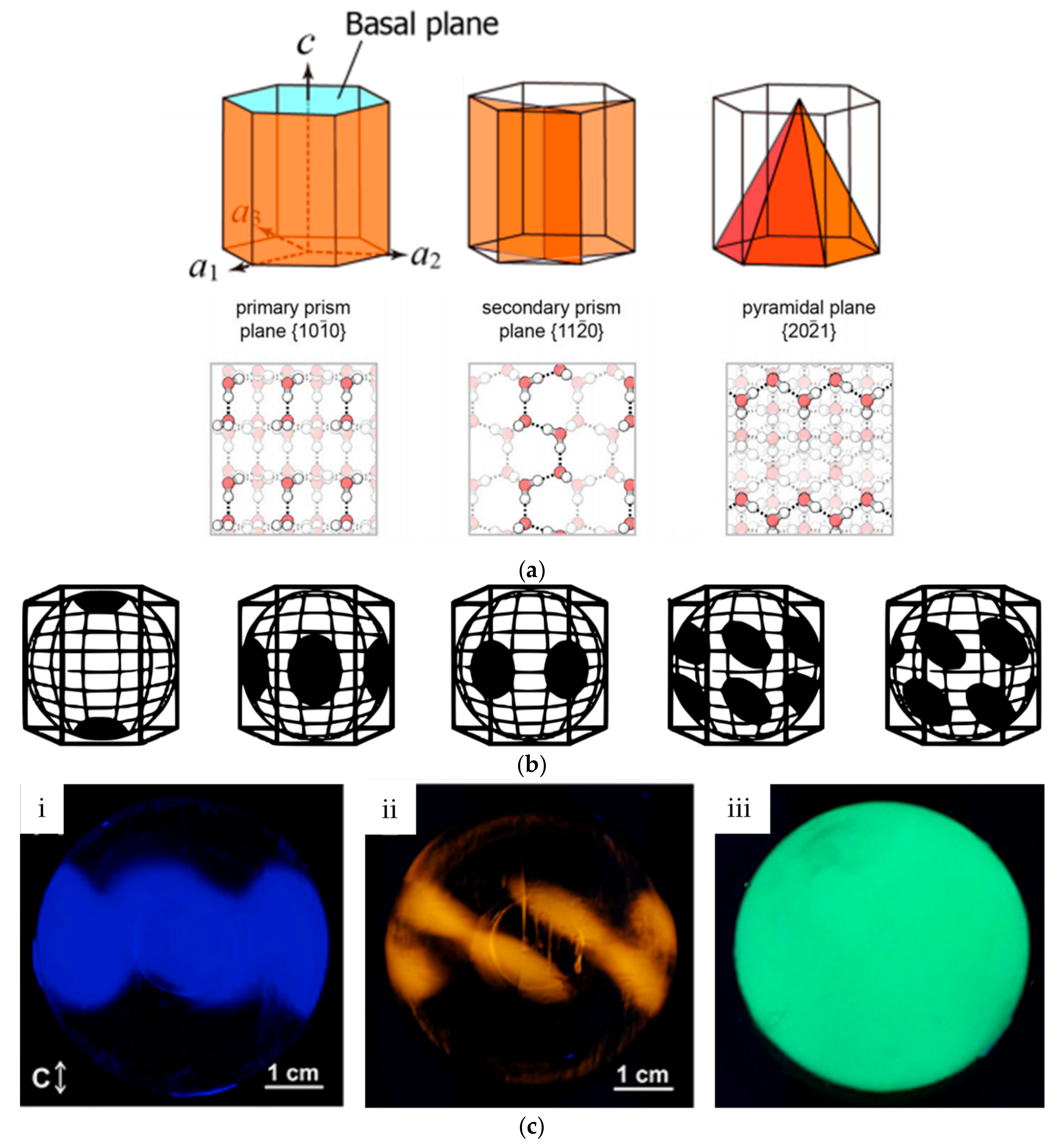
3.1. Ice Plane Recognition
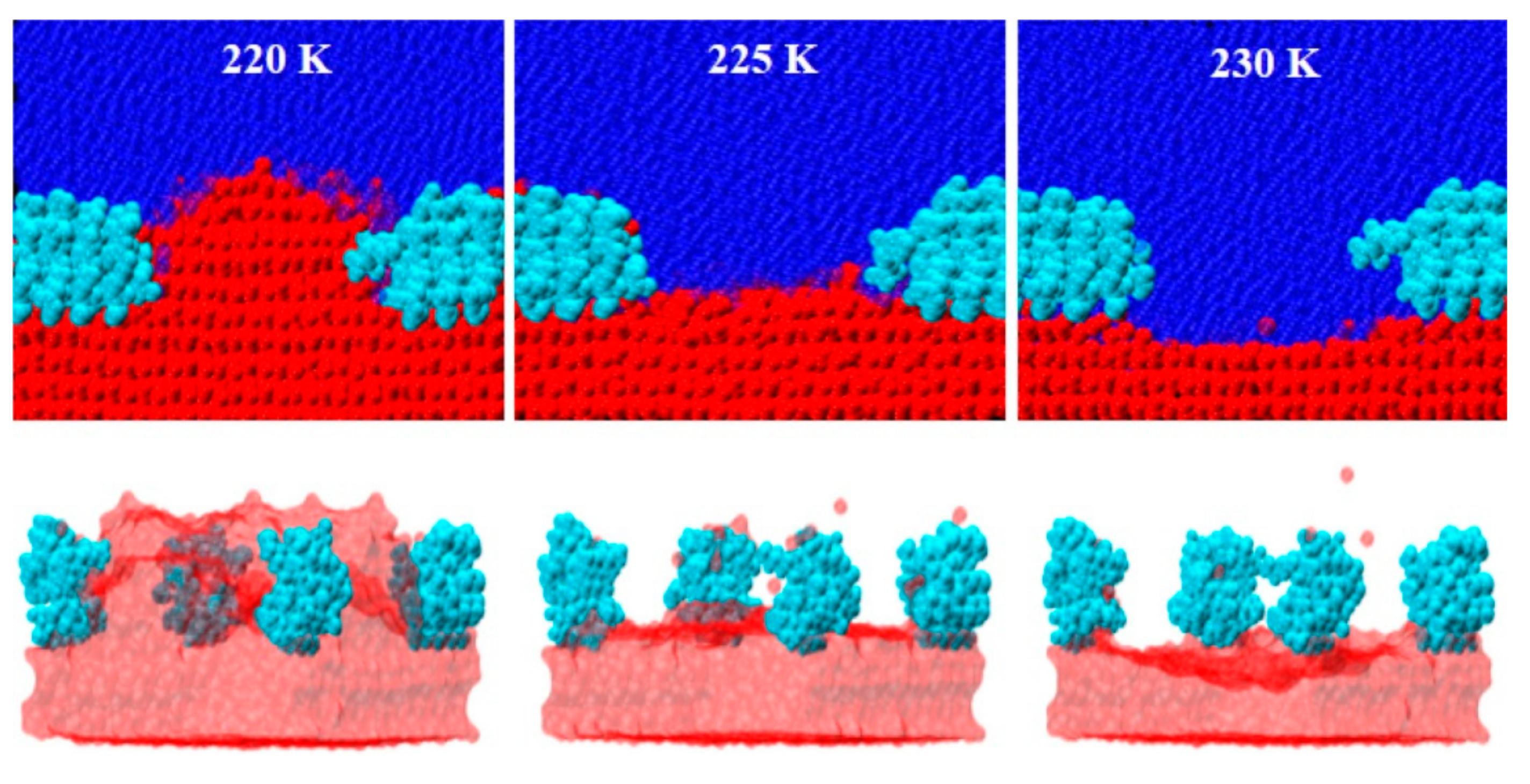
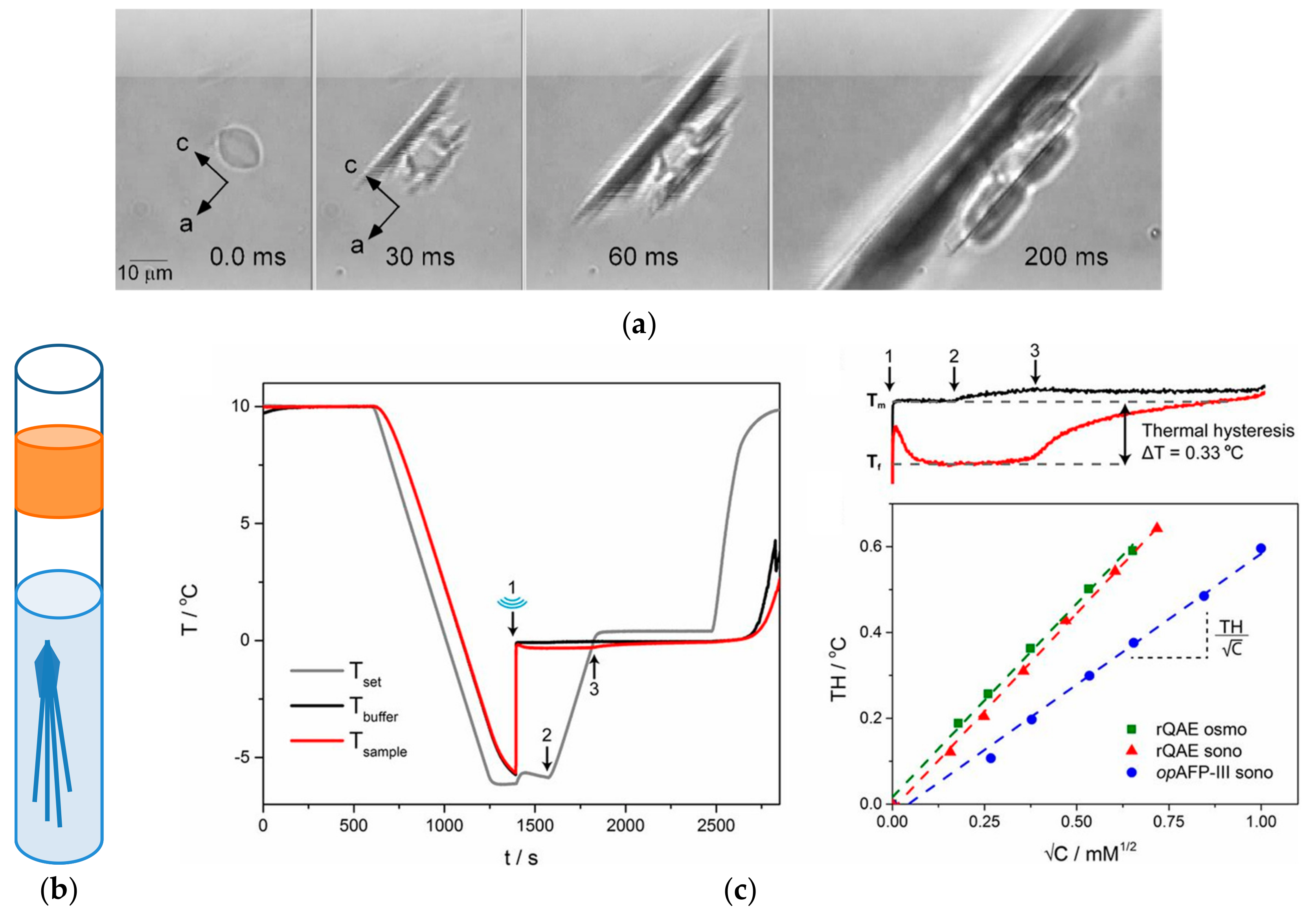
3.2. Thermal Hysteresis
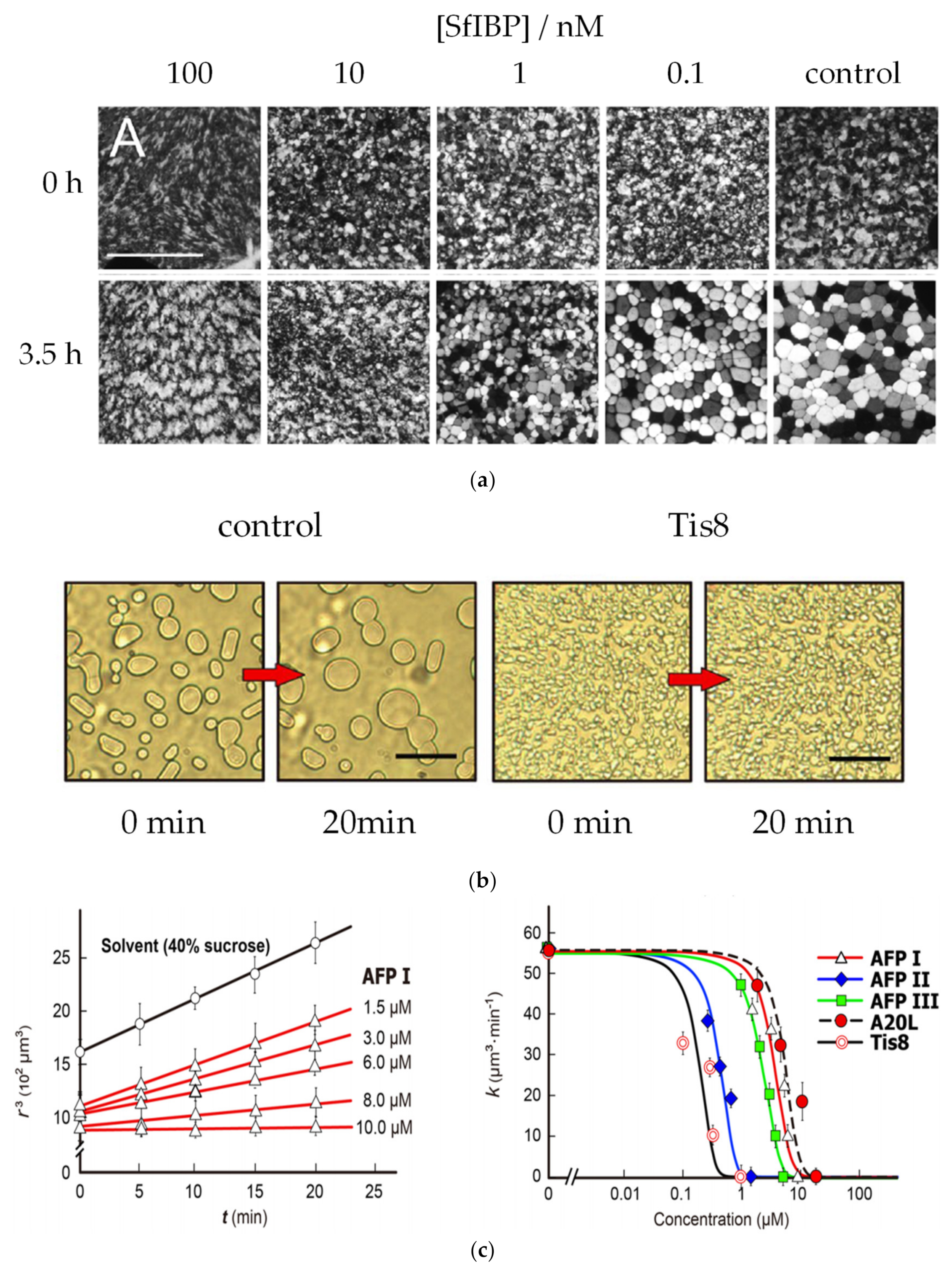
3.3. Ice Recrystallizaiton Inhibition
4. Production of AF(G)Ps and Synthetic Analogues: Design and Chemical Synthesis Approaches
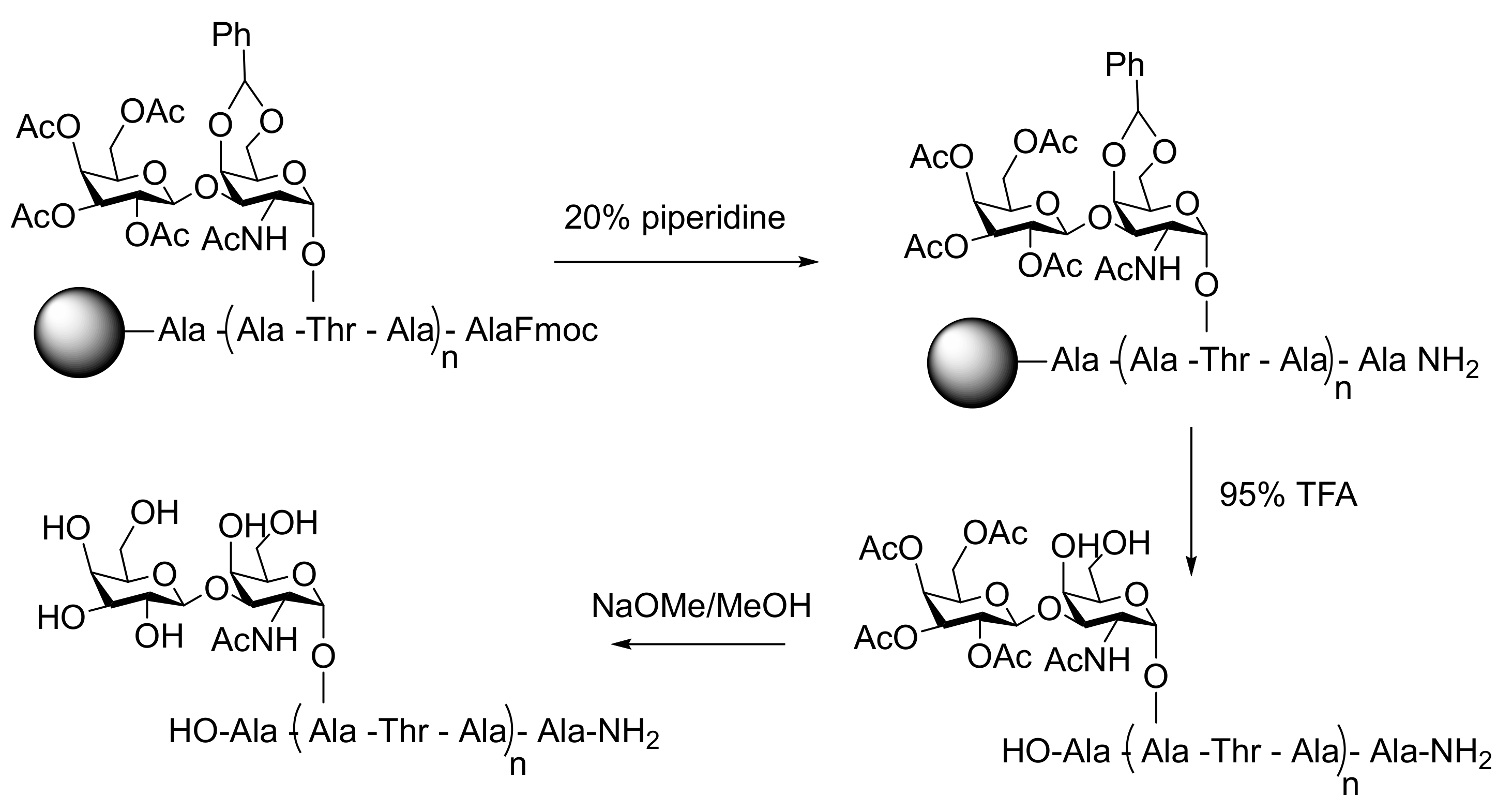
4.1. Molecular Analogues
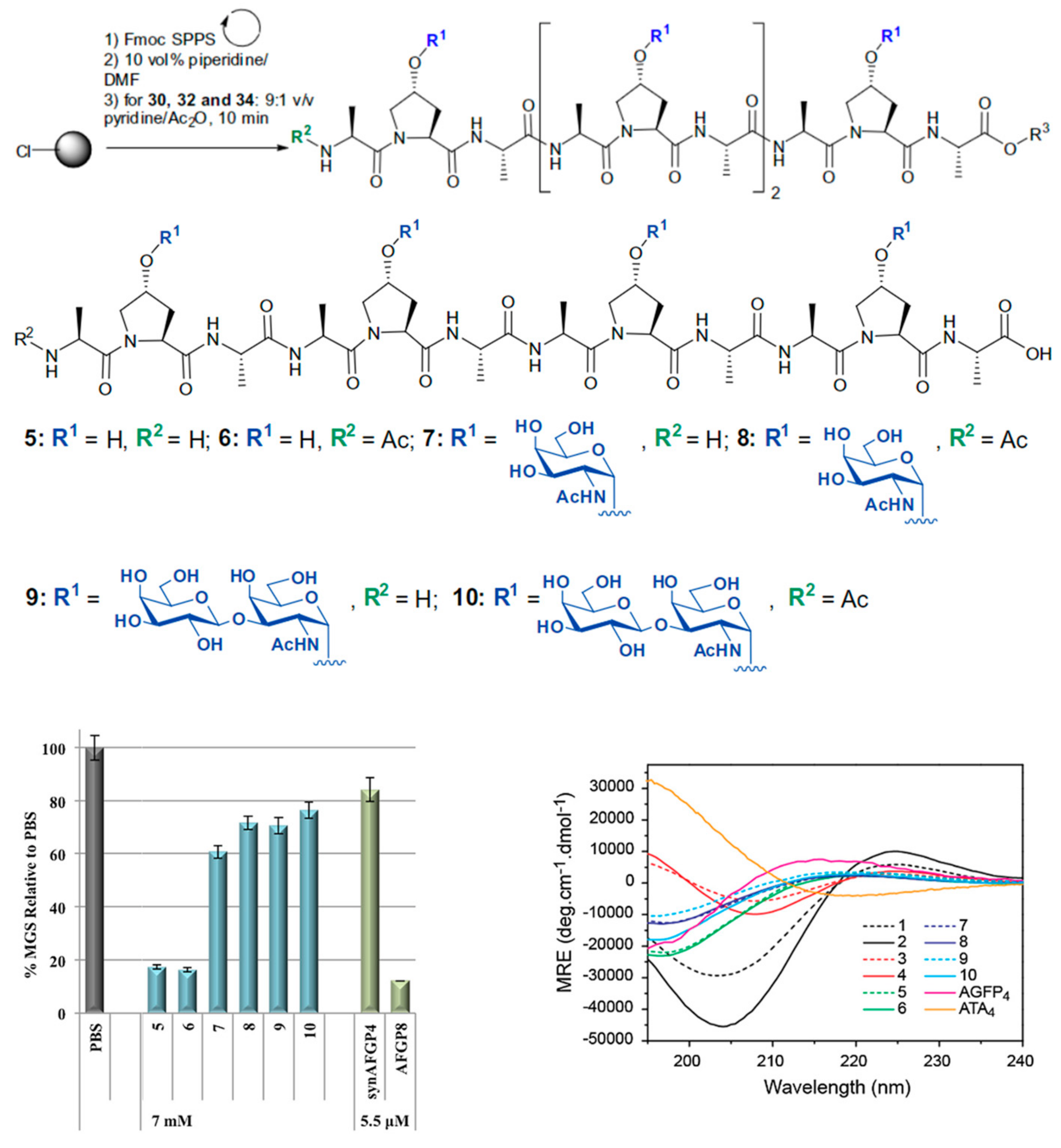
4.2. de Novo Design and Synthesis
4.3. Cyclic Peptide Analogues
4.4. Classifying Technologies to Aid de Novo Design
5. Applications of AF(G)Ps and Analogues
6. Prospects and Challenges
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AF(G)Ps | antifreeze (glyco)proteins |
| TH | thermal hysteresis |
| IRI | ice recrystallization inhibition |
| SPPS | solid-phase peptide synthesis |
| IBS | ice-binding site |
| PyBOP | benzotriazole-1-yloxytris(pyrrolidino)phosphonium |
| DMTMM | 4-(4-6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium |
| Fmoc | fluorenylmethyloxycarbonyl |
| FIPA | Fluorescence-based ice plane affinity |
| TRITC | tetramethylrhodamine |
| PDB | Protein Data Bank |
| GFP | green fluorescent protein |
References
- Bar Dolev, M.; Braslavsky, I.; Davies, P.L. Ice-Binding Proteins and Their Function. Annu. Rev. Biochem. 2016, 85, 515–542. [Google Scholar] [CrossRef] [PubMed]
- Mangiagalli, M.; Bar-Dolev, M.; Tedesco, P.; Natalello, A.; Kaleda, A.; Brocca, S.; Pascale, D.; Pucciarelli, S.; Miceli, C.; Braslavsky, I.; et al. Cryo-protective effect of an ice-binding protein derived from Antarctic bacteria. FEBS J. 2016, 284, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.; Bailey, T.L.; Healey, J.R.J.; Marcellini, M.; Deville, S.; Gibson, M.I. Polyproline as a Minimal Antifreeze Protein Mimic That Enhances the Cryopreservation of Cell Monolayers. Angew Chem. Int. Ed. Engl. 2017, 56, 15941–15944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcilius, L.; Santhakumar, G.; Stone, R.S.; Capicciotti, C.J.; Joseph, S.; Matthews, J.M.; Ben, R.N.; Payne, R.J. Synthesis of peptides and glycopeptides with polyproline II helical topology as potential antifreeze molecules. Bioorg. Med. Chem. 2013, 21, 3569–3581. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.K.; Lee, J.H.; Murugan, R.N.; Lee, S.G.; Do, H.; Koh, H.Y.; Shim, H.E.; Kim, H.C.; Kim, H.J. Antifreeze peptides and glycopeptides, and their derivatives: Potential uses in biotechnology. Mar. Drugs 2013, 11, 2013–2041. [Google Scholar] [CrossRef]
- Drori, R.; Li, C.; Hu, C.; Raiteri, P.; Rohl, A.L.; Ward, M.D.; Kahr, B. A Supramolecular Ice Growth Inhibitor. J. Am. Chem. Soc. 2016, 138, 13396–13401. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.E.; Clarkson, G.; Fox, D.J.; Vipond, R.A.; Scott, P.; Gibson, M.I. Antifreeze Protein Mimetic Metallohelices with Potent Ice Recrystallization Inhibition Activity. J. Am. Chem. Soc. 2017, 139, 9835–9838. [Google Scholar] [CrossRef] [Green Version]
- Sproncken, C.C.M.; Surís-Valls, R.; Cingil, H.E.; Detrembleur, C.; Voets, I.K. Complex Coacervate Core Micelles Containing Poly(vinyl alcohol) Inhibit Ice Recrystallization. Macromol. Rapid Commun. 2018, 39. [Google Scholar] [CrossRef]
- Wang, C.; Wiener, C.G.; Sepulveda-Medina, P.I.; Ye, C.; Simmons, D.S.; Li, R.; Fukuto, M.; Weiss, R.A.; Vogt, B.D. Antifreeze Hydrogels from Amphiphilic Statistical Copolymers. Chem. Mater. 2018, 31, 135–145. [Google Scholar] [CrossRef]
- Mitchell, D.E.; Fayter, A.E.R.; Deller, R.C.; Hasan, M.; Gutierrez-Marcos, J.; Gibson, M.I. Ice-recrystallization inhibiting polymers protect proteins against freeze-stress and enable glycerol-free cryostorage. Mater. Horiz. 2019, 6, 364–368. [Google Scholar] [CrossRef]
- Perez, A.F.; Taing, K.R.; Quon, J.C.; Flores, A.; Yong, B. Effect of Type I Antifreeze Proteins on the Freezing and Melting Process of Cryoprotective Solutions Studied by Site-Directed Spin Labeling Technique. Crystals 2019, 9, 352. [Google Scholar] [CrossRef]
- Surís-Valls, R.; Voets, I.K. The Impact of Salts on the Ice Recrystallization Inhibition Activity of Antifreeze (Glyco)Proteins. Biomolecules 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.T.; Arai, T.; Yamauchi, A.; Miura, A.; Kondo, H.; Ohyama, Y.; Tsuda, S. Ice recrystallization is strongly inhibited when antifreeze proteins bind to multiple ice planes. Sci. Rep. 2019, 9, 2212. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, L.; Dreischmeier, K.; Zipori, A.; Sirotinskaya, V.; Adar, C.; Reicher, N.; Braslavsky, I.; Rudich, Y.; Koop, T. Contrasting Behavior of Antifreeze Proteins: Ice Growth Inhibitors and Ice Nucleation Promoters. J. Phys. Chem. Lett. 2019, 10, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A. Algal ice-binding proteins change the structure of sea ice. Proc. Natl. Acad. Sci. USA 2011, 108, E198. [Google Scholar] [CrossRef] [PubMed]
- Bar Dolev, M.; Bernheim, R.; Guo, S.; Davies, P.L.; Braslavsky, I. Putting life on ice: Bacteria that bind to frozen water. J. R. Soc. Interface 2016, 13. [Google Scholar] [CrossRef]
- Harding, M.M.; Ward, L.G.; Haymet, A.D.J. Type I ‘antifreeze’ proteins—Structure-activity studies and mechanisms of ice growth inhibition. Eur. J. Biochem. 1999, 264, 653–665. [Google Scholar] [CrossRef]
- Sicheri, F.; Yang, D.S. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature 1995, 375, 427–431. [Google Scholar] [CrossRef]
- Harding, M.M.; Anderberg, P.I.; Haymet, A.D.J. ‘Antifreeze’ glycoproteins from polar fish. Eur. J. Biochem. 2003, 270, 1381–1392. [Google Scholar] [CrossRef]
- Marqusee, S.; Robbins, V.H.; Baldwin, R.L. Unusually stable helix formation in short alanine-based peptides. Proc. Natl. Acad. Sci. USA 1989, 86, 5286–5290. [Google Scholar] [CrossRef]
- Mao, Y.; Ba, Y. Insight into the binding of antifreeze proteins to ice surfaces via 13C spin lattice relaxation solid-state NMR. Biophys. J. 2006, 91, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Olijve, L.L.; Meister, K.; DeVries, A.L.; Duman, J.G.; Guo, S.; Bakker, H.J.; Voets, I.K. Blocking rapid ice crystal growth through nonbasal plane adsorption of antifreeze proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 3740–3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, M.; Clark, A.H.; Lips, A.; Ruddock, J.N.; White, D.N.J. Inhibition of ice crystal growth by preferential peptide adsorption: A molecular modelling study. Faraday Discuss. 1993, 95. [Google Scholar] [CrossRef]
- Liou, Y.C.; Thibault, P.; Walker, V.K.; Davies, P.L.; Graham, L.A. A complex family of highly heterogeneous and internally repetitive hyperactive antifreeze proteins from the beetle Tenebrio molitor. Biochemistry 1999, 38, 11415–11424. [Google Scholar] [CrossRef]
- Graether, S.P.; Kuiper, M.J.; Gagne, S.M.; Walker, V.K.; Jia, Z.; Sykes, B.D.; Davies, P.L. Beta-helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature 2000, 406, 325–328. [Google Scholar] [CrossRef]
- Garnham, C.P.; Campbell, R.L.; Davies, P.L. Anchored clathrate waters bind antifreeze proteins to ice. Proc. Natl. Acad. Sci. USA 2011, 108, 7363–7367. [Google Scholar] [CrossRef] [Green Version]
- Middleton, A.J.; Marshall, C.B.; Faucher, F.; Bar-Dolev, M.; Braslavsky, I.; Campbell, R.L.; Walker, V.K.; Davies, P.L. Antifreeze protein from freeze-tolerant grass has a beta-roll fold with an irregularly structured ice-binding site. J. Mol. Biol 2012, 416, 713–724. [Google Scholar] [CrossRef]
- Bredow, M.; Walker, V.K. Ice-Binding Proteins in Plants. Front. Plant. Sci. 2017, 8, 2153. [Google Scholar] [CrossRef]
- Mochizuki, K.; Molinero, V. Antifreeze Glycoproteins Bind Reversibly to Ice via Hydrophobic Groups. J. Am. Chem. Soc. 2018, 140, 4803–4811. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Colvin, M.E.; Yeh, Y.; Feeney, R.E.; Fink, W.H. The Dynamics, Structure, and Conformational Free Energy of Proline-Containing Antifreeze Glycoprotein. Biophys. J. 2002, 82, 2892–2905. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.H.; Graham, L.A.; Campbell, R.L.; Davies, P.L. Structural modeling of snow flea antifreeze protein. Biophys. J. 2007, 92, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Pentelute, B.L.; Gates, Z.P.; Tereshko, V.; Dashnau, J.L.; Vanderkooi, J.M.; Kossiakoff, A.A.; Kent, S.B. X-ray structure of snow flea antifreeze protein determined by racemic crystallization of synthetic protein enantiomers. J. Am. Chem. Soc. 2008, 130, 9695–9701. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Jana, B. Ordered hydration layer mediated ice adsorption of a globular antifreeze protein: Mechanistic insight. Phys. Chem. Chem. Phys. 2019. [Google Scholar] [CrossRef] [PubMed]
- Naullage, P.M.; Qiu, Y.; Molinero, V. What Controls the Limit of Supercooling and Superheating of Pinned Ice Surfaces? J. Phys. Chem. Lett. 2018, 9, 1712–1720. [Google Scholar] [CrossRef]
- Hudait, A.; Odendahl, N.; Qiu, Y.; Paesani, F.; Molinero, V. Ice-Nucleating and Antifreeze Proteins Recognize Ice through a Diversity of Anchored Clathrate and Ice-like Motifs. J. Am. Chem. Soc. 2018, 140, 4905–4912. [Google Scholar] [CrossRef]
- Brotzakis, Z.F.; Voets, I.K.; Bakker, H.J.; Bolhuis, P.G. Water structure and dynamics in the hydration layer of a type III anti-freeze protein. Phys. Chem. Chem. Phys. 2018, 20, 6996–7006. [Google Scholar] [CrossRef] [Green Version]
- Naullage, P.M.; Lupi, L.; Molinero, V. Molecular Recognition of Ice by Fully Flexible Molecules. J. Phys. Chem. C 2017, 121, 26949–26957. [Google Scholar] [CrossRef]
- Bayer-Giraldi, M.; Sazaki, G.; Nagashima, K.; Kipfstuhl, S.; Vorontsov, D.A.; Furukawa, Y. Growth suppression of ice crystal basal face in the presence of a moderate ice-binding protein does not confer hyperactivity. Proc. Natl. Acad. Sci.USA 2018, 115, 7479–7484. [Google Scholar] [CrossRef] [Green Version]
- Kaleda, A.; Haleva, L.; Sarusi, G.; Pinsky, T.; Mangiagalli, M.; Bar Dolev, M.; Lotti, M.; Nardini, M.; Braslavsky, I. Saturn-Shaped Ice Burst Pattern and Fast Basal Binding of an Ice-Binding Protein from an Antarctic Bacterial Consortium. Langmuir 2018, 35, 7337–7346. [Google Scholar] [CrossRef]
- Meister, K.; DeVries, A.L.; Bakker, H.J.; Drori, R. Antifreeze Glycoproteins Bind Irreversibly to Ice. J. Am. Chem. Soc. 2018, 140, 9365–9368. [Google Scholar] [CrossRef]
- Midya, U.S.; Bandyopadhyay, S. Operation of Kelvin Effect in the Activities of an Antifreeze Protein: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2018, 122, 3079–3087. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.A.; Devries, A.L. Melting inhibition and superheating of ice by an antifreeze glycopeptide. Science 1989, 245, 505–507. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.L. The role of antifreeze glycopeptides and peptides in the freezing avoidance of antarctic fishes. Comp. Biochem. Physiol. Part. B Comp. Biochem. 1988, 90, 611–621. [Google Scholar] [CrossRef]
- Basu, K.; Garnham, C.P.; Nishimiya, Y.; Tsuda, S.; Braslavsky, I.; Davies, P. Determining the ice-binding planes of antifreeze proteins by fluorescence-based ice plane affinity. J. Vis. Exp. 2014, e51185. [Google Scholar] [CrossRef]
- Bredow, M.; Tomalty, H.E.; Walker, V.K. Identification of Plant Ice-binding Proteins Through Assessment of Ice-recrystallization Inhibition and Isolation Using Ice-affinity Purification. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Wilson, P.W.; Beaglehole, D.; DeVries, A.L. Antifreeze glycopeptide adsorption on single crystal ice surfaces using ellipsometry. Biophys. J. 1993, 64, 1878–1884. [Google Scholar] [CrossRef] [Green Version]
- Raymond, J.A.; Wilson, P.; DeVries, A.L. Inhibition of growth of nonbasal planes in ice by fish antifreezes. Proc. Natl. Acad. Sci. USA 1989, 86, 881–885. [Google Scholar] [CrossRef]
- Antson, A.A.; Smith, D.J.; Roper, D.I.; Lewis, S.; Caves, L.S.; Verma, C.S.; Buckley, S.L.; Lillford, P.J.; Hubbard, R.E. Understanding the mechanism of ice binding by type III antifreeze proteins. J. Mol. Biol. 2001, 305, 875–889. [Google Scholar] [CrossRef]
- Knight, C.A.; Cheng, C.C.; DeVries, A.L. Adsorption of alpha-helical antifreeze peptides on specific ice crystal surface planes. Biophys. J. 1991, 59, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Liou, Y.C.; Tocilj, A.; Davies, P.L.; Jia, Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature 2000, 406, 322–324. [Google Scholar] [CrossRef]
- Celik, Y.; Drori, R.; Pertaya-Braun, N.; Altan, A.; Barton, T.; Bar-Dolev, M.; Groisman, A.; Davies, P.L.; Braslavsky, I. Microfluidic experiments reveal that antifreeze proteins bound to ice crystals suffice to prevent their growth. Proc. Natl. Acad. Sci. USA 2013, 110, 1309–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drori, R.; Davies, P.L.; Braslavsky, I. When are antifreeze proteins in solution essential for ice growth inhibition? Langmuir 2015, 31, 5805–5811. [Google Scholar] [CrossRef] [PubMed]
- Haleva, L.; Celik, Y.; Bar-Dolev, M.; Pertaya-Braun, N.; Kaner, A.; Davies, P.L.; Braslavsky, I. Microfluidic Cold-Finger Device for the Investigation of Ice-Binding Proteins. Biophys. J. 2016, 111, 1143–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertaya, N.; Marshall, C.B.; DiPrinzio, C.L.; Wilen, L.; Thomson, E.S.; Wettlaufer, J.S.; Davies, P.L.; Braslavsky, I. Fluorescence microscopy evidence for quasi-permanent attachment of antifreeze proteins to ice surfaces. Biophys. J. 2007, 92, 3663–3673. [Google Scholar] [CrossRef] [PubMed]
- Braslavsky, I.; Celik, Y.; Drori, R.; Bar, M.; Davies, P.L. The Case for Irreversible Binding of Ice-Binding Proteins to Ice. Biophys. J. 2012, 102. [Google Scholar] [CrossRef]
- DeVries, A. Antifreeze glycopeptides and peptides: Interactions with ice and water. Methods Enzymol. 1986, 127, 293–303. [Google Scholar] [CrossRef]
- Gaede-Koehler, A.; Kreider, A.; Canfield, P.; Kleemeier, M.; Grunwald, I. Direct measurement of the thermal hysteresis of antifreeze proteins (AFPs) using sonocrystallization. Anal. Chem. 2012, 84, 10229–10235. [Google Scholar] [CrossRef]
- Bar, M.; Celik, Y.; Fass, D.; Braslavsky, I. Interactions of β-Helical Antifreeze Protein Mutants with Ice. Cryst. Growth Design 2008, 8, 2954–2963. [Google Scholar] [CrossRef]
- Graham, L.A.; Agrawal, P.; Oleschuk, R.D.; Davies, P.L. High-capacity ice-recrystallization endpoint assay employing superhydrophobic coatings that is equivalent to the ‘splat’ assay. Cryobiology 2018, 81, 138–144. [Google Scholar] [CrossRef]
- Biggs, C.I.; Stubbs, C.; Graham, B.; Fayter, A.E.R.; Hasan, M.; Gibson, M.I. Mimicking the Ice Recrystallization Activity of Biological Antifreezes. When is a New Polymer “Active”? Macromol. Biosci. 2019, 19, e1900082. [Google Scholar] [CrossRef]
- Budke, C.; Heggemann, C.; Koch, M.; Sewald, N.; Koop, T. Ice recrystallization kinetics in the presence of synthetic antifreeze glycoprotein analogues using the framework of LSW theory. J. Phys. Chem. B 2009, 113, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Recrystallization Kinetics Using the Circle Hough Transform Algorithm. Cryst. Growth Des. 2016, 16, 4190–4195. [CrossRef]
- DeVries, A.L.; Wohlschlag, D.E. Freezing resistance in some Antarctic fishes. Science 1969, 163, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Voets, I.K. From ice-binding proteins to bio-inspired antifreeze materials. Soft Matter 2017, 13, 4808–4823. [Google Scholar] [CrossRef] [Green Version]
- Oude Vrielink, A.S.; Aloi, A.; Olijve, L.L.; Voets, I.K. Interaction of ice binding proteins with ice, water and ions. Biointerphases 2016, 11, 018906. [Google Scholar] [CrossRef]
- Briard, J.G.; Fernandez, M.; De Luna, P.; Woo, T.K.; Ben, R.N. QSAR Accelerated Discovery of Potent Ice Recrystallization Inhibitors. Sci. Rep. 2016, 6, 26403. [Google Scholar] [CrossRef] [Green Version]
- Chao, H.; Sonnichsen, F.D.; DeLuca, C.I.; Sykes, B.D.; Davies, P.L. Structure-function relationship in the globular type III antifreeze protein: Identification of a cluster of surface residues required for binding to ice. Protein Sci. 1994, 3, 1760–1769. [Google Scholar] [CrossRef] [Green Version]
- Chao, H.; Davies, P.L.; Sykes, B.D.; Sonnichsen, F.D. Use of proline mutants to help solve the NMR solution structure of type III antifreeze protein. Protein Sci. 1993, 2, 1411–1428. [Google Scholar] [CrossRef] [Green Version]
- Baardsnes, J.; Kondejewski, L.H.; Hodges, R.S.; Chao, H.; Kay, C.; Davies, P.L. New ice-binding face for type I antifreeze protein. FEBS Lett. 1999, 463, 87–91. [Google Scholar] [CrossRef]
- Meister, K.; Strazdaite, S.; DeVries, A.L.; Lotze, S.; Olijve, L.L.; Voets, I.K.; Bakker, H.J. Observation of ice-like water layers at an aqueous protein surface. Proc. Natl. Acad. Sci. USA 2014, 111, 17732–17736. [Google Scholar] [CrossRef] [Green Version]
- Olijve, L.L.C.; Hendrix, M.M.R.M.; Voets, I.K. Influence of Polymer Chain Architecture of Poly(vinyl alcohol) on the Inhibition of Ice Recrystallization. Macromol. Chem. Phys. 2016, 217, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Stevens, C.A.; Drori, R.; Zalis, S.; Braslavsky, I.; Davies, P.L. Dendrimer-Linked Antifreeze Proteins Have Superior Activity and Thermal Recovery. Bioconjug. Chem. 2015, 26, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Baardsnes, J.; Kuiper, M.J.; Davies, P.L. Antifreeze protein dimer - When two ice-binding faces are better than one. J. Biol. Chem. 2003, 278, 38942–38947. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.K.; Jarrett-Wilkins, C.; Beards, M.; Staykov, E.; MacFarlane, L.R.; Bell, T.D.M.; Matthews, J.M.; Manners, I.; Faul, C.F.J.; Moens, P.D.J.; et al. 1D Self-Assembly and Ice Recrystallization Inhibition Activity of Antifreeze Glycopeptide-Functionalized Perylene Bisimides. Chemistry 2018, 24, 7834–7839. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Stubbs, C.; Murray, K.; Tomas, R.M.F.; Otten, L.; Gibson, M.I. Synthetically Scalable Poly(ampholyte) Which Dramatically Enhances Cellular Cryopreservation. Biomacromolecules 2019, 20, 3104–3114. [Google Scholar] [CrossRef]
- Ampaw, A.; Charlton, T.A.; Briard, J.G.; Ben, R.N. Designing the next generation of cryoprotectants - From proteins to small molecules. Pept. Sci. 2019, 111. [Google Scholar] [CrossRef]
- Nagao, M.; Sengupta, J.; Diaz-Dussan, D.; Adam, M.; Wu, M.; Acker, J.; Ben, R.; Ishihara, K.; Zeng, H.; Miura, Y.; et al. Synthesis of Highly Biocompatible and Temperature-Responsive Physical Gels for Cryopreservation and 3D Cell Culture. Acs Appl. Bio Mater. 2018, 1, 356–366. [Google Scholar] [CrossRef]
- Jain, N.; Friedman, S.H. A Tetra-Orthogonal Strategy for the Efficient Synthesis of Scaffolds Based on Cyclic Peptides. Int. J. Pept. Res. 2018, 24, 535–542. [Google Scholar] [CrossRef]
- Mezzato, S.; Schaffrath, M.; Unverzagt, C. An orthogonal double-linker resin facilitates the efficient solid-phase synthesis of complex-type N-glycopeptide thioesters suitable for native chemical ligation. Angew. Chem. Int. Ed. Engl. 2005, 44, 1650–1654. [Google Scholar] [CrossRef]
- LeValley, P.J.; Ovadia, E.M.; Bresette, C.A.; Sawicki, L.A.; Maverakis, E.; Bai, S.; Kloxin, A.M. Design of functionalized cyclic peptides through orthogonal click reactions for cell culture and targeting applications. Chem. Commun. 2018, 54, 6923–6926. [Google Scholar] [CrossRef]
- Zhang, W.; Laursen, R.A. Structure-function relationships in a type I antifreeze polypeptide. The role of threonine methyl and hydroxyl groups in antifreeze activity. J. Biol. Chem. 1998, 273, 34806–34812. [Google Scholar] [CrossRef] [PubMed]
- Howard, E.I.; Blakeley, M.P.; Haertlein, M.; Haertlein, I.P.; Mitschler, A.; Fisher, S.J.; Siah, A.C.; Salvay, A.G.; Popov, A.; Dieckmann, C.M.; et al. Neutron structure of type-III antifreeze protein allows the reconstruction of AFP-ice interface. J. Mol. Recognit. 2011, 24, 724–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudait, A.; Qiu, Y.; Odendahl, N.; Molinero, V. Hydrogen-Bonding and Hydrophobic Groups Contribute Equally to the Binding of Hyperactive Antifreeze and Ice-Nucleating Proteins to Ice. J. Am. Chem. Soc. 2019, 141, 7887–7898. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Nishimura, S. Synthesis of an antifreeze glycoprotein analogue: Efficient preparation of sequential glycopeptide polymers. Chem. Commun. 1996, 2779–2780. [Google Scholar] [CrossRef]
- Hachisu, M.; Hinou, H.; Takamichi, M.; Tsuda, S.; Koshida, S.; Nishimura, S. One-pot synthesis of cyclic antifreeze glycopeptides. Chem. Commun. 2019, 1641–1643. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Monde, K.; Nishimura, S.-I. Sequential Glycoproteins: Practical Method for the Synthesis of Antifreeze Glycoprotein Models Containing Base Labile Groups. Macromolecules 2004, 37, 6771–6779. [Google Scholar] [CrossRef]
- Wilkinson, B.L.; Stone, R.S.; Capicciotti, C.J.; Thaysen-Andersen, M.; Matthews, J.M.; Packer, N.H.; Ben, R.N.; Payne, R.J. Total synthesis of homogeneous antifreeze glycopeptides and glycoproteins. Angew. Chem. Int. Ed. Engl. 2012, 51, 3606–3610. [Google Scholar] [CrossRef]
- Tseng, P.-H.; Jiaang, W.-T.; Chang, M.-Y.; Chen, S.-T. Facile Solid-Phase Synthesis of an Antifreeze Glycoprotein. Chemistry 2001, 7, 585–590. [Google Scholar] [CrossRef]
- Kao, M.H.; Fletcher, G.L.; Wang, N.C.; Hew, C.L. The relationship between molecular weight and antifreeze polypeptide activity in marine fish. Can. J. Zool. 1986, 64, 578–582. [Google Scholar] [CrossRef]
- Burcham, T.S.; Knauf, M.J.; Osuga, D.T.; Feeney, R.E.; Yeh, Y. Antifreeze glycoproteins: Influence of polymer length and ice crystal habit on activity. Biopolymers 1984, 23, 1379–1395. [Google Scholar] [CrossRef]
- Kong, C.H.Z.; Leung, I.K.H.; Sarojini, V. Synthetic insect antifreeze peptides modify ice crystal growth habit. CrystEng.Comm. 2017, 19, 2163–2167. [Google Scholar] [CrossRef] [Green Version]
- Wierzbicki, A.; Knight, C.A.; Rutland, T.J.; Muccio, D.D.; Pybus, B.S.; Sikes, C.S. Structure−Function Relationship in the Antifreeze Activity of Synthetic Alanine−Lysine Antifreeze Polypeptides. Biomacromolecules 2000, 1, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Laursen, R.A. Artificial antifreeze polypeptides: α-helical peptides with KAAK motifs have antifreeze and ice crystal morphology modifying properties. FEBS Lett. 1999, 455, 372–376. [Google Scholar] [CrossRef]
- Nagel, L.; Plattner, C.; Budke, C.; Majer, Z.; DeVries, A.L.; Berkemeier, T.; Koop, T.; Sewald, N. Synthesis and characterization of natural and modified antifreeze glycopeptides: Glycosylated foldamers. Amino. Acids. 2011, 41, 719–732. [Google Scholar] [CrossRef]
- Leclere, M.; Kwok, B.K.; Wu, L.K.; Allan, D.S.; Ben, R.N. C-linked antifreeze glycoprotein (C-AFGP) analogues as novel cryoprotectants. Bioconjug. Chem. 2011, 22, 1804–1810. [Google Scholar] [CrossRef]
- Czechura, P.; Tam, R.Y.; Dimitrijevic, E.; Murphy, A.V.; Ben, R.N. The importance of hydration for inhibiting ice recrystallization with C-linked antifreeze glycoproteins. J. Am. Chem. Soc. 2008, 130, 2928–2929. [Google Scholar] [CrossRef]
- Liu, S.; Ben, R.N. C-linked galactosyl serine AFGP analogues as potent recrystallization inhibitors. Org. Lett. 2005, 7, 2385–2388. [Google Scholar] [CrossRef]
- Brotzakis, Z.F.; Gehre, M.; Voets, I.K.; Bolhuis, P.G. Stability and growth mechanism of self-assembling putative antifreeze cyclic peptides. Phys. Chem. Chem. Phys. 2017, 19, 19032–19042. [Google Scholar] [CrossRef] [Green Version]
- Ghadiri, M.R.; Granja, J.R.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 1993, 366, 324–327. [Google Scholar] [CrossRef]
- Hourani, R.; Zhang, C.; van der Weegen, R.; Ruiz, L.; Li, C.; Keten, S.; Helms, B.A.; Xu, T. Processable cyclic peptide nanotubes with tunable interiors. J. Am. Chem. Soc. 2011, 133, 15296–15299. [Google Scholar] [CrossRef]
- Vijayaraj, R.; Sundar Raman, S.; Mahesh Kumar, R.; Subramanian, V. Studies on the structure and stability of cyclic peptide based nanotubes using oligomeric approach: A computational chemistry investigation. J. Phys. Chem. B 2010, 114, 16574–16583. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.J.; Braga, R.C.; Melo-Filho, C.C.; Moreira-Filho, J.T.; Muratov, E.N.; Andrade, C.H. QSAR-Based Virtual Screening: Advances and Applications in Drug Discovery. Front. Pharm. 2018, 9, 1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, U.; Uzairu, A.; Ebuka Arthur, D. Review on: Quantitative structure activity relationship (QSAR) modeling. J. Anal. Pharm. Res. 2018, 7. [Google Scholar] [CrossRef]
- Pratiwi, R.; Malik, A.A.; Schaduangrat, N.; Prachayasittikul, V.; Wikberg, J.E.S.; Nantasenamat, C.; Shoombuatong, W. CryoProtect: A Web Server for Classifying Antifreeze Proteins from Nonantifreeze Proteins. J. Chem. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, C.; Gao, R.; Zhang, L. An Effective Antifreeze Protein Predictor with Ensemble Classifiers and Comprehensive Sequence Descriptors. Int. J. Mol. Sci. 2015, 16, 21191–21214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozuch, D.J.; Stillinger, F.H.; Debenedetti, P.G. Combined molecular dynamics and neural network method for predicting protein antifreeze activity. Proc. Natl. Acad. Sci. USA 2018, 115, 13252–13257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huelsz-Prince, G.; DeVries, A.L.; Bakker, H.J.; van Zon, J.S.; Meister, K. Effect of Antifreeze Glycoproteins on Organoid Survival during and after Hypothermic Storage. Biomolecules 2019, 9. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Wang, L.; Gao, H.; Guo, X.N.; Yao, H.Y. Improvement of texture properties and flavor of frozen dough by carrot (Daucus carota) antifreeze protein supplementation. J. Agric. Food Chem. 2007, 55, 9620–9626. [Google Scholar] [CrossRef]
- Yeh, C.M.; Kao, B.Y.; Peng, H.J. Production of a recombinant type 1 antifreeze protein analogue by L. lactis and its applications on frozen meat and frozen dough. J. Agric. Food Chem. 2009, 57, 6216–6223. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, H.; Liu, W.; Wang, L.; Qian, H.; Qi, X. Extraction of Carrot (Daucus carota) Antifreeze Proteins and Evaluation of Their Effects on Frozen White Salted Noodles. Food Bioprocess. Technol. 2013, 7, 842–852. [Google Scholar] [CrossRef]
- Delesky, E.A.; Frazier, S.D.; Wallat, J.D.; Bannister, K.L.; Heveran, C.M.; Srubar, W.V., 3rd. Ice-Binding Protein from Shewanella frigidimarinas Inhibits Ice Crystal Growth in Highly Alkaline Solutions. Polymer 2019, 11. [Google Scholar] [CrossRef]
- Takeshita, Y.; Waku, T.; Wilson, P.W.; Hagiwara, Y. Effects of Winter Flounder Antifreeze Protein on the Growth of Ice Particles in an Ice Slurry Flow in Mini-Channels. Biomolecules 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Biggs, C.I.; Bailey, T.L.; Ben, G.; Stubbs, C.; Fayter, A.; Gibson, M.I. Polymer mimics of biomacromolecular antifreezes. Nat. Commun. 2017, 8, 1546. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.H.; Koo, B.W. Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, J.-h.; Li, L.; Wang, S.-y. The cryoprotective effects of antifreeze peptides from pigskin collagen on texture properties and water mobility of frozen dough subjected to freeze–thaw cycles. Eur. Food Res. Technol. 2016, 243, 1149–1156. [Google Scholar] [CrossRef]
- Kong, C.H.; Hamid, N.; Liu, T.; Sarojini, V. Effect of Antifreeze Peptide Pretreatment on Ice Crystal Size, Drip Loss, Texture, and Volatile Compounds of Frozen Carrots. J. Agric. Food Chem. 2016, 64, 4327–4335. [Google Scholar] [CrossRef]
- MacMillan, D.S.; Murray, J.; Sneddon, H.F.; Jamieson, C.; Watson, A.J.B. Evaluation of alternative solvents in common amide coupling reactions: Replacement of dichloromethane and N,N-dimethylformamide. Green Chem. 2013, 15. [Google Scholar] [CrossRef]
- Jad, Y.E.; Acosta, G.A.; Khattab, S.N.; de la Torre, B.G.; Govender, T.; Kruger, H.G.; El-Faham, A.; Albericio, F. Peptide synthesis beyond DMF: THF and ACN as excellent and friendlier alternatives. Org. Biomol. Chem. 2015, 13, 2393–2398. [Google Scholar] [CrossRef]
- Subiros-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion. Chemistry 2009, 15, 9394–9403. [Google Scholar] [CrossRef]
- El-Faham, A.; Subiros Funosas, R.; Prohens, R.; Albericio, F. COMU: A safer and more effective replacement for benzotriazole-based uronium coupling reagents. Chemistry 2009, 15, 9404–9416. [Google Scholar] [CrossRef]

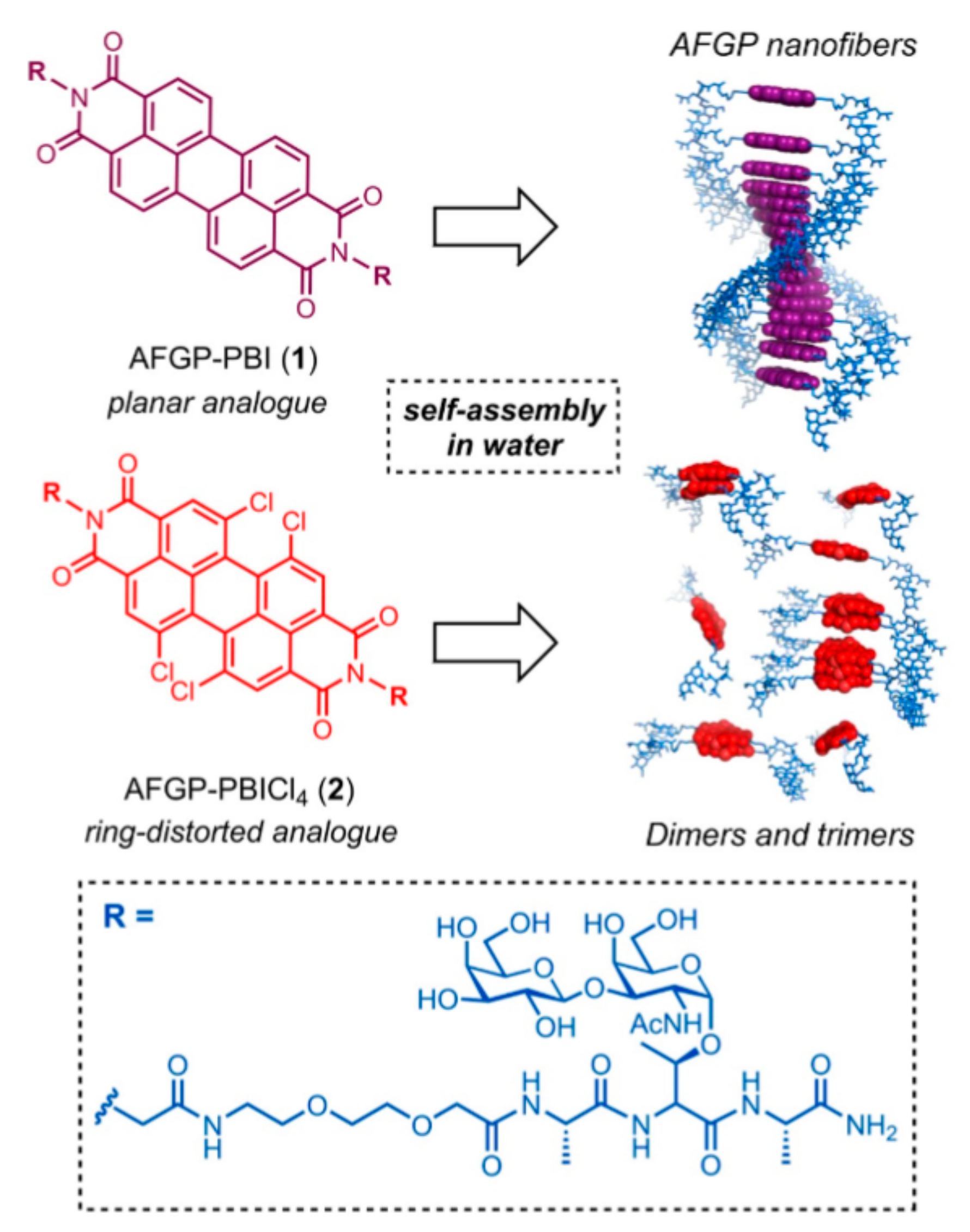
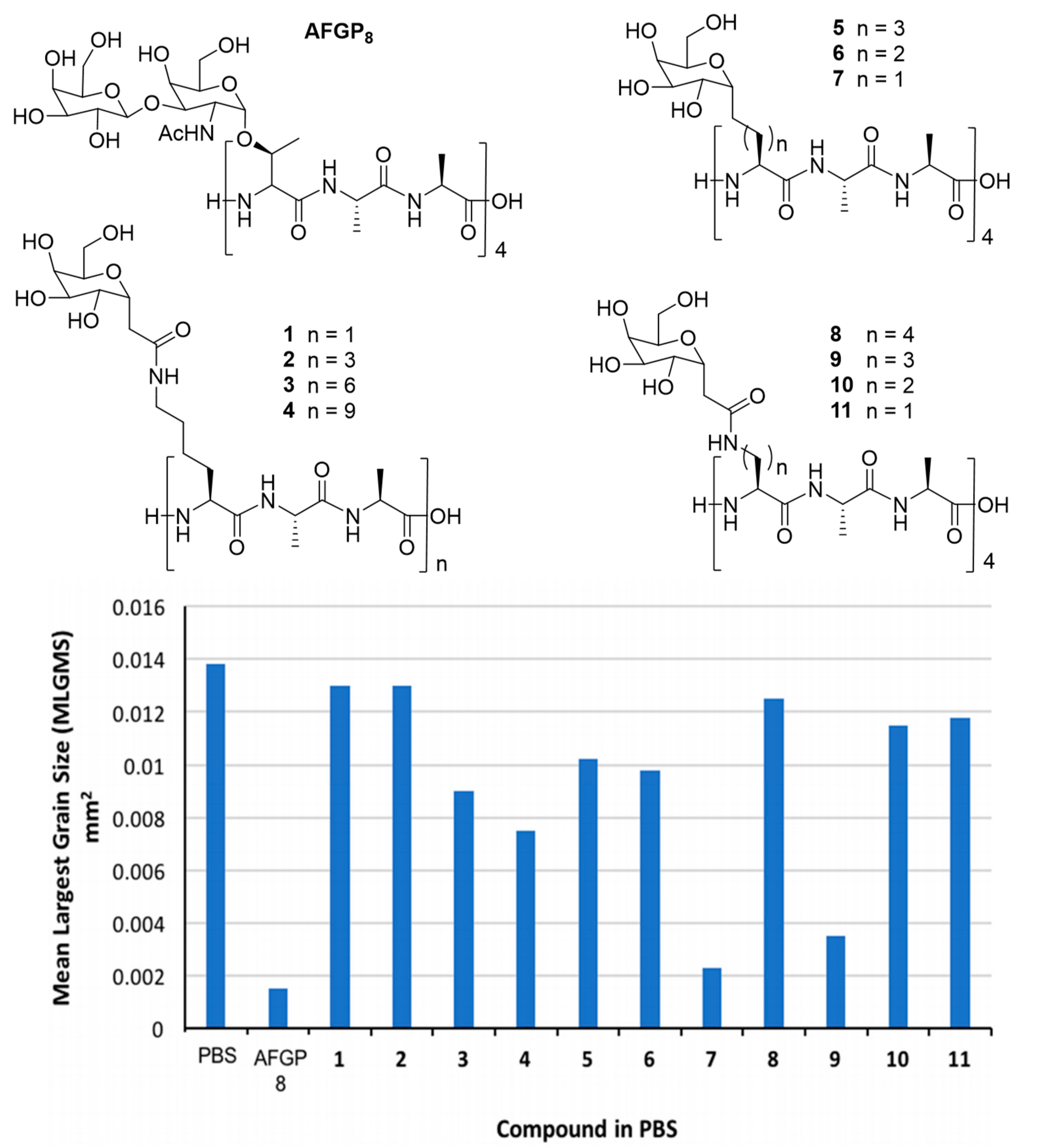
| AF(G)P Type | Synthesis Method | Activity Assay | [A] (mg mL−1 and/or μM) | Ref. | |
|---|---|---|---|---|---|
| Molecular analogues | I | SPPS of mutant wfAFPs (A17L and A20L) substituting the alanine by leucine | TH | 1, 2, 4 and 8 mg mL−1 | [69] |
| I | SPPS of analogues substituting threonine (T) residues by serine (S) or valine (V) | TH | 0.5, 1, 1.5, 2, 4, 5.5 and 7 mM | [81] | |
| AFGP8 | In-solution coupling of ATA building blocks with DPPA | TH | 10 mg mL−1 | [85] | |
| AFGP8 | In-solution coupling of ATA building blocks using DPPA, DMTMM and IIDQ | TH | 10 mg mL−1 | [86] | |
| AFGP2 and AFGP8 | Fmoc SPPS of AFGP building blocks, desulfurization of Thz and Cys residues | TH | 5 and 10 mg mL−1 | [87] | |
| AFGP8 | Fmoc SPPS coupling with DCC/4-DMAP and PyBOP | Not tested | [88] | ||
| AFGP8 | SPPS of AAT building blocks with DPPA resulting in native AFGP molecular analogue | Not tested | [84] | ||
| AFGP8 | Automated Fmoc SPPS at 40 °C using TBTU as coupling reagent | TH IRI | 30 and 40 mg mL−1 0.5 μg mL−1 | [94] | |
| DcAFP | SPPS of peptide fragments of DcAFP chemically ligated afterwards | TH | 10 mM | [91] | |
| de novo Analogues | polyAAK | SPPS of type I analogues based on lysine instead of threonine | TH | from 25 to 250 mg mL−1 | [92] |
| polyAAK | TH | 23 and 31 mM | [93] | ||
| PPII | SPPS | Cryopres IRI | 200 mM 20 mg mL−1 | [3] | |
| PPII | SPPS of Hydroxyproline peptides functionalized with disaccharides | TH IRI | 10 mg mL−1 7 mM | [4] | |
| AFGP | SPPS synthesis of AFGP analogues attached to supramolecular organic dyes | TH IRI | 22 mM | [74] | |
| AFGP8 | Cyclized AFGP analogues synthetized by SPPS using DPPA | TH | 10 mg mL−1 | [85] | |
| AFGP8 | SPPS of C-linked AFGPs to avoid O-linked degradation of disaccharides | Cyropres | From 1 to 5 mg mL−1 | [95] | |
| polyGGK | SPPS of poly GGK followed by the attachment of diverse sugar moieties through C-linking | TH | From 5 μM to 0.05 nM | [96] | |
| polyGGK | IRI | From 5 μM to 0.05 nM | [97] | ||
| DcAFP | SPPS of mutant DcAFP substituting the disulfide bonds for lactam bonds | TH | 10 mM | [91] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surís-Valls, R.; Voets, I.K. Peptidic Antifreeze Materials: Prospects and Challenges. Int. J. Mol. Sci. 2019, 20, 5149. https://doi.org/10.3390/ijms20205149
Surís-Valls R, Voets IK. Peptidic Antifreeze Materials: Prospects and Challenges. International Journal of Molecular Sciences. 2019; 20(20):5149. https://doi.org/10.3390/ijms20205149
Chicago/Turabian StyleSurís-Valls, Romà, and Ilja K. Voets. 2019. "Peptidic Antifreeze Materials: Prospects and Challenges" International Journal of Molecular Sciences 20, no. 20: 5149. https://doi.org/10.3390/ijms20205149
APA StyleSurís-Valls, R., & Voets, I. K. (2019). Peptidic Antifreeze Materials: Prospects and Challenges. International Journal of Molecular Sciences, 20(20), 5149. https://doi.org/10.3390/ijms20205149






