ATP-Evoked Intracellular Ca2+ Responses in M-CSF Differentiated Human Monocyte-Derived Macrophage are Mediated by P2X4 and P2Y11 Receptor Activation
Abstract
:1. Introduction
2. Results
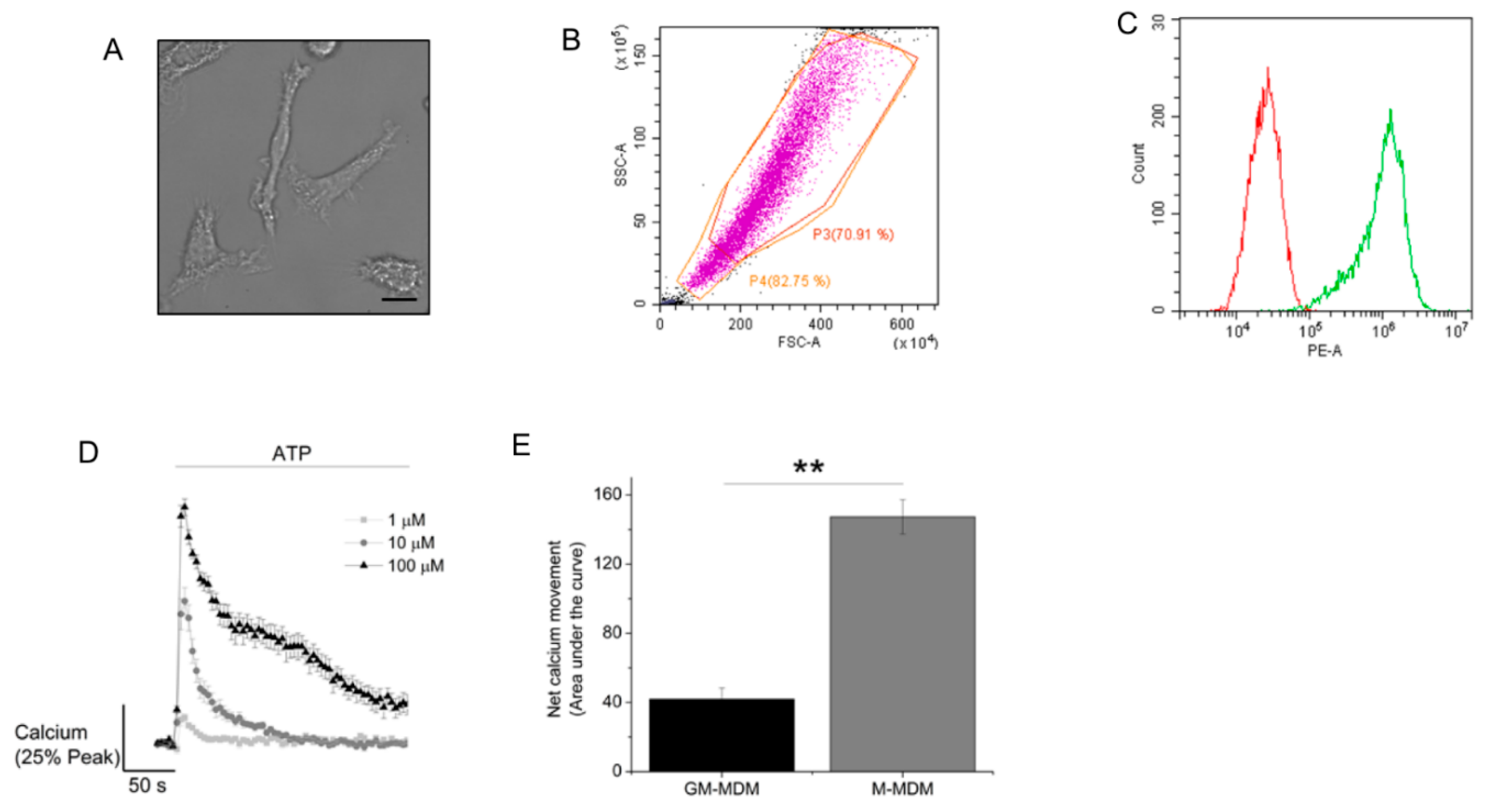
2.1. ATP Evokes Intracellular Ca2+ Responses in M-CSF Differentiated Macrophage
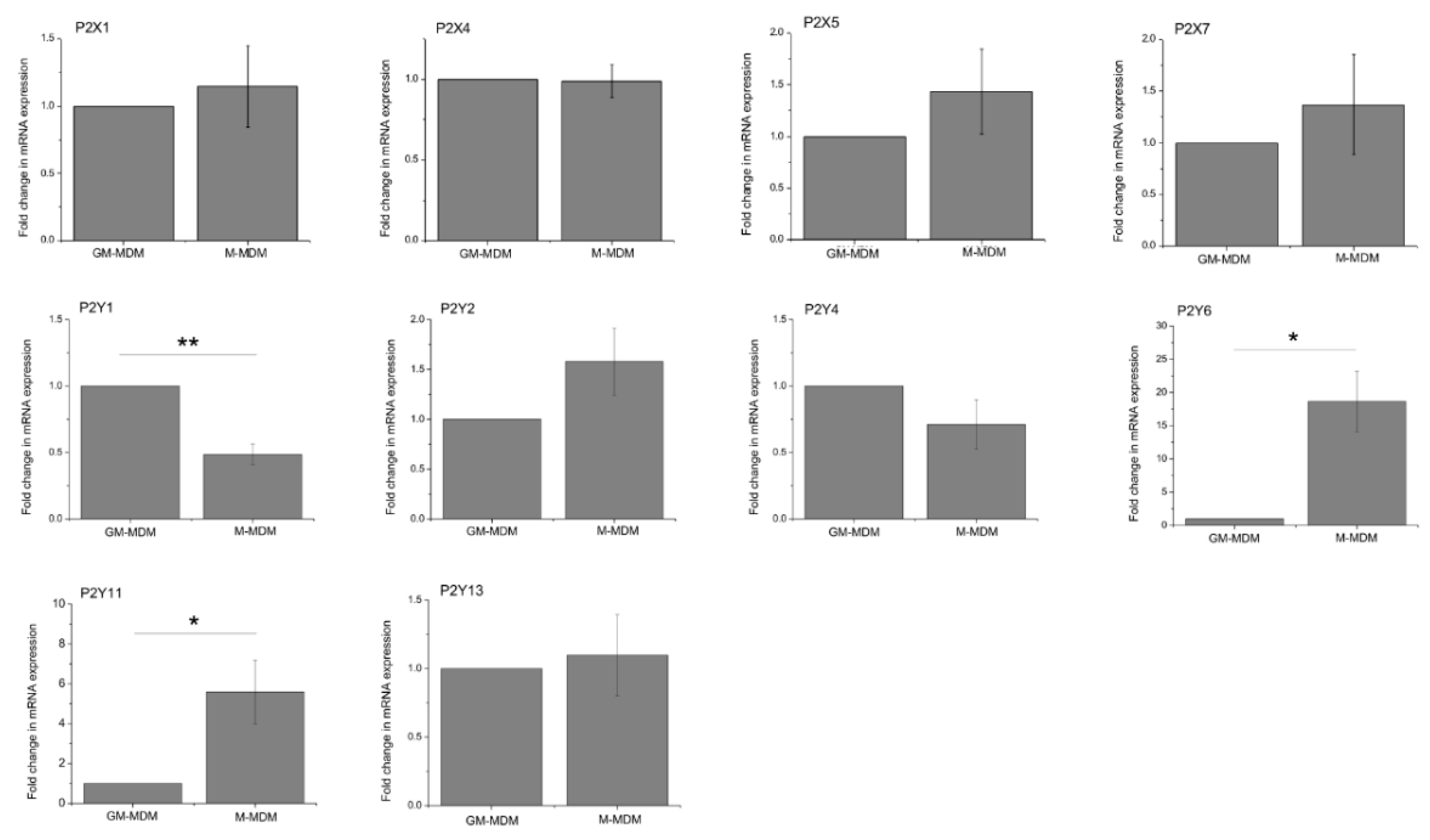
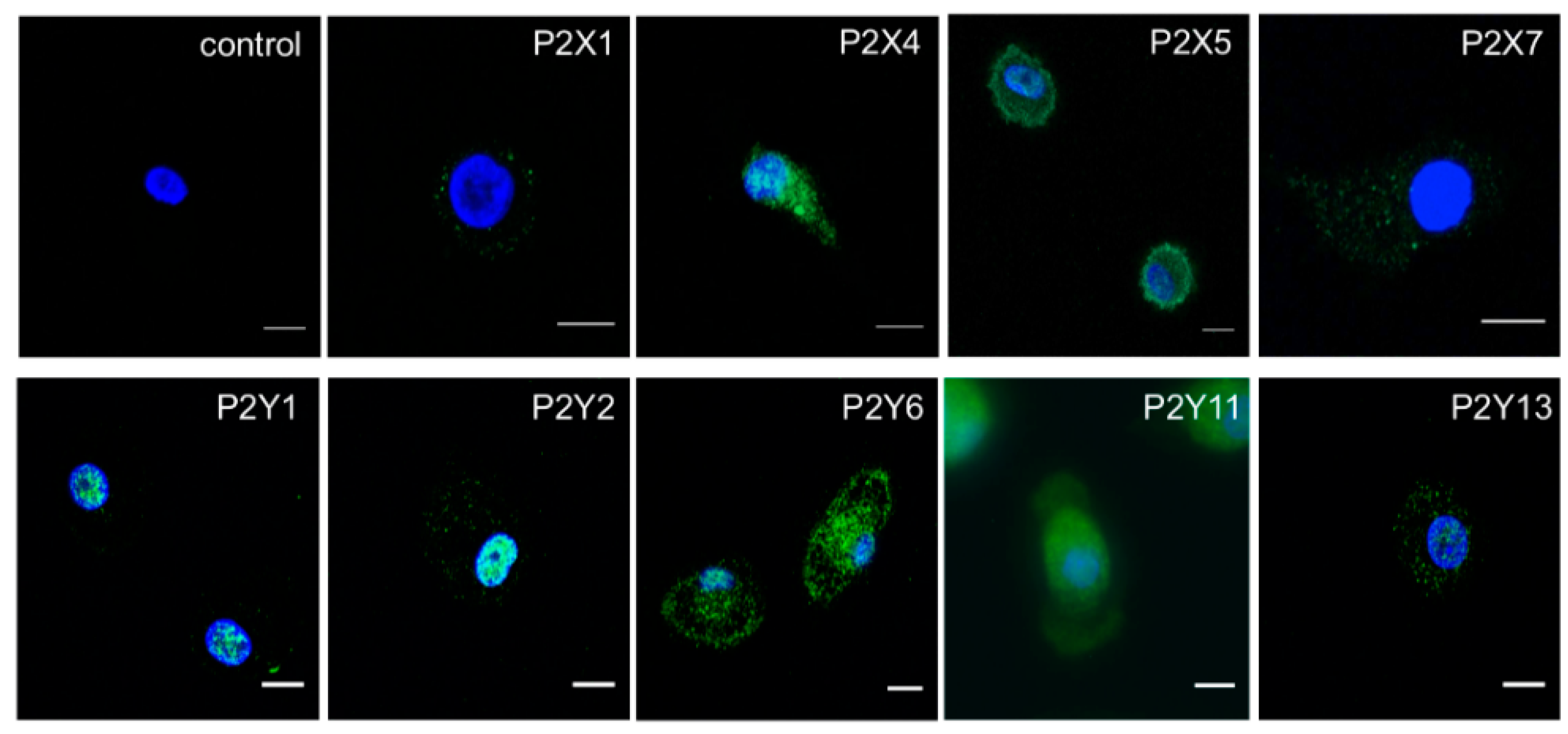
2.2. M-CSF Differentiated Macrophage Express Multiple P2Y and P2X Receptor Subtypes
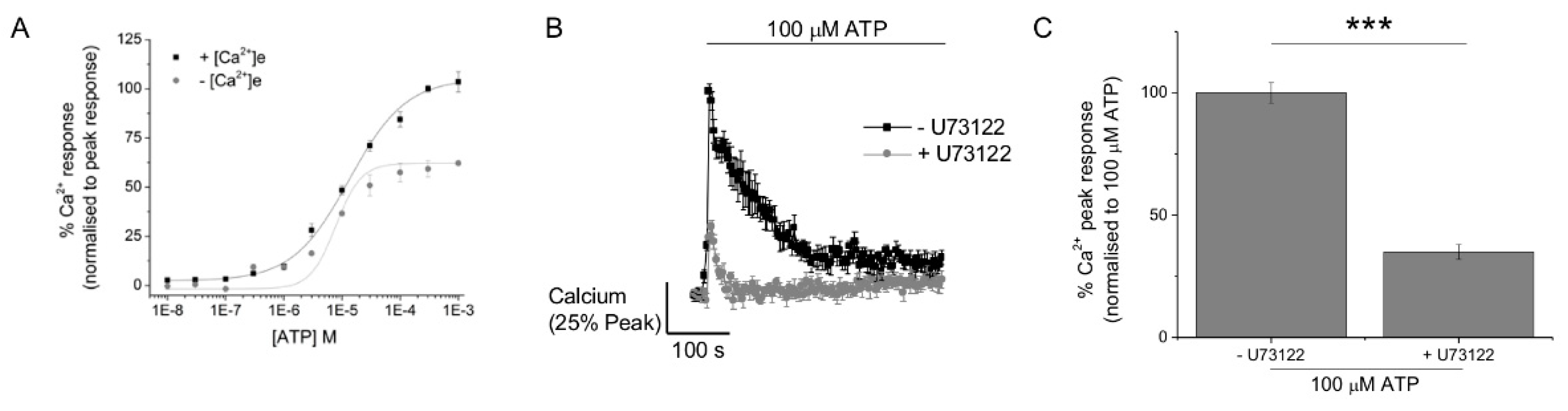
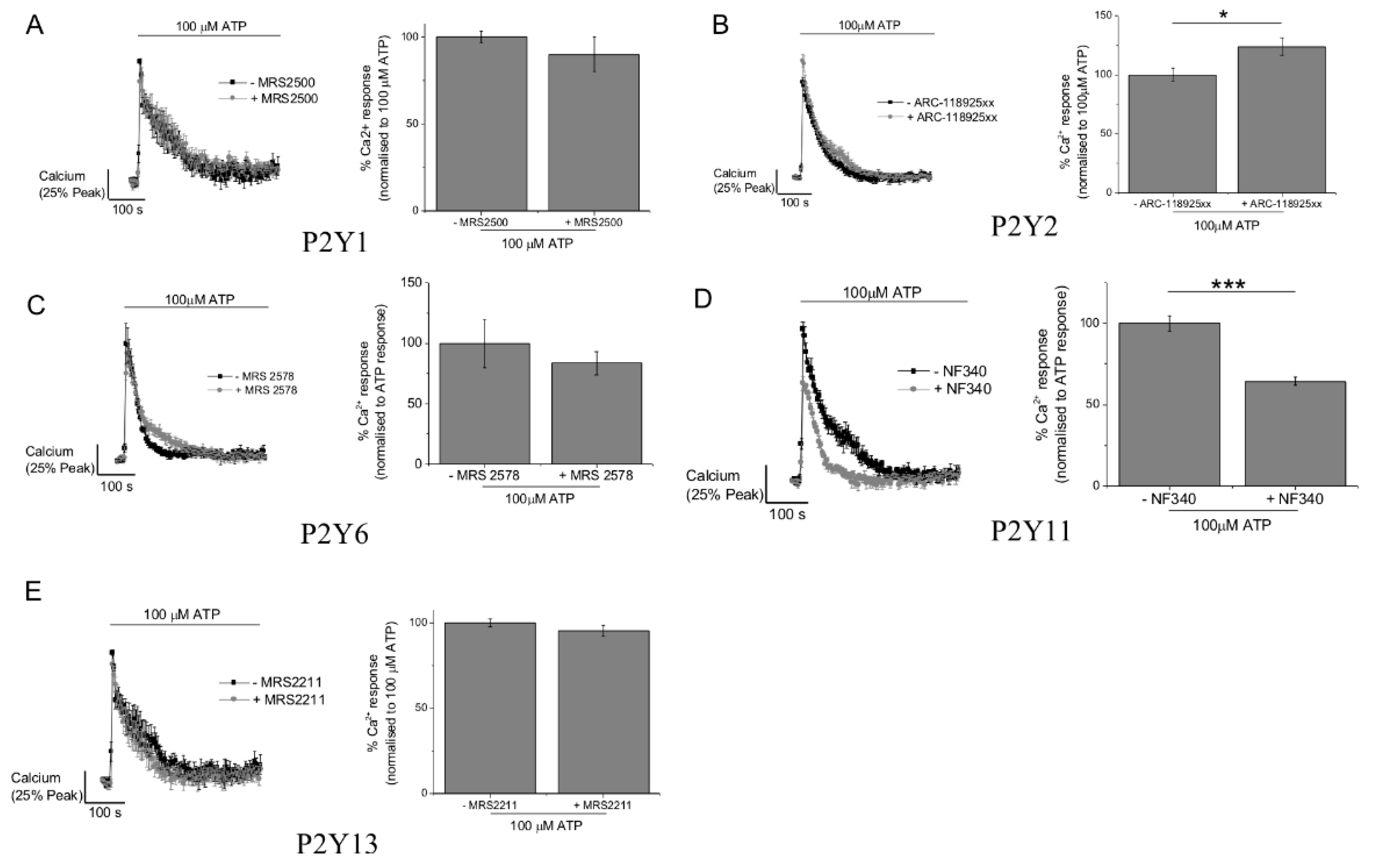
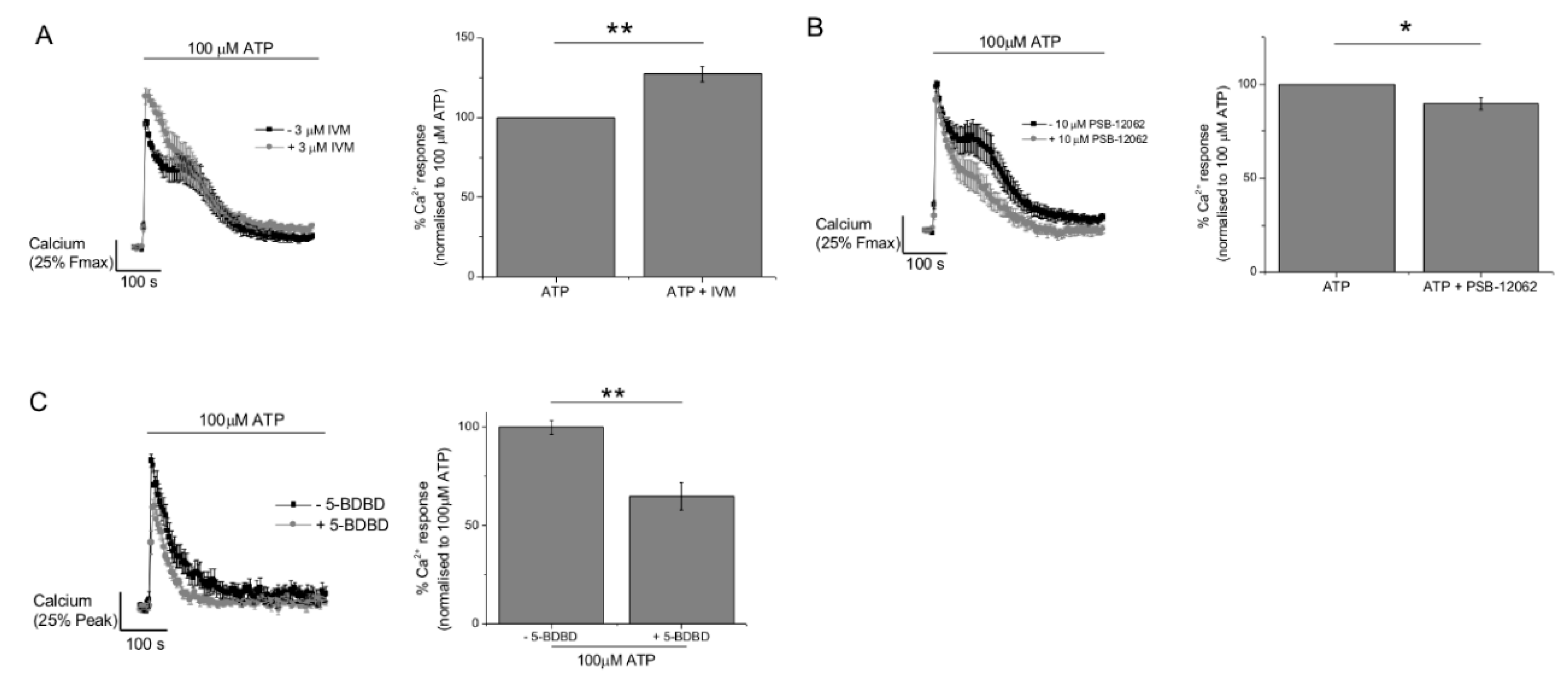
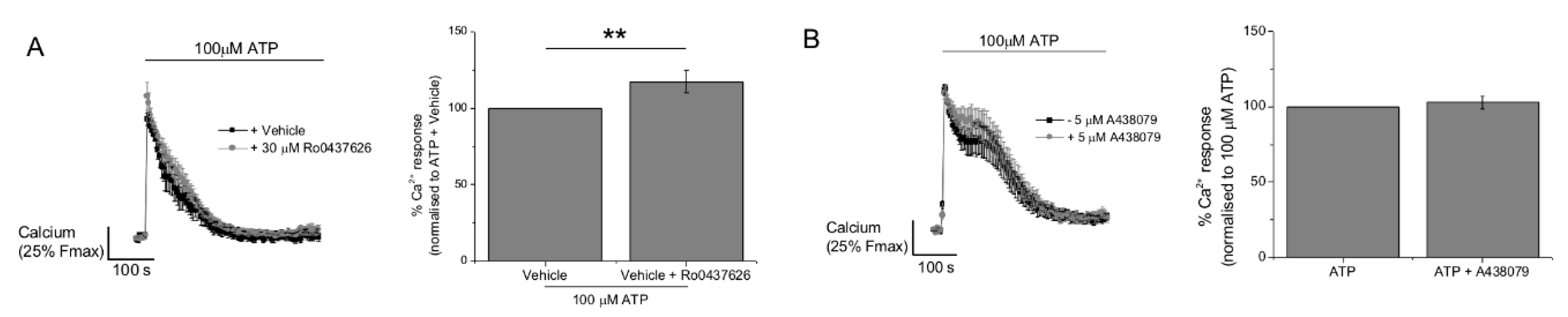
2.3. P2Y11 and P2X4 Mediate ATP-Evoked Ca2+ Responses
3. Discussion
4. Materials and Methods
4.1. Peripheral Blood Mononuclear Cells (PBMC) Isolation
4.2. Generation of Monocyte-Derived Macrophage
4.3. Quantitative RT-PCR
4.4. Immunocytochemistry
4.5. Flow Cytometry
4.6. Intracellular Ca2+ Measurement
4.7. Drugs and Application
4.8. Data and Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Stanley, E.R.; Berg, K.L.; Einstein, D.B.; Lee, P.S.; Pixley, F.J.; Wang, Y.; Yeung, Y.G. Biology and action of colony—Stimulating factor-1. Mol. Reprod. Dev. 1997, 46, 4–10. [Google Scholar] [CrossRef]
- Wiktor-Jedrzejczak, W.; Bartocci, A.; Ferrante, A.W., Jr.; Ahmed-Ansari, A.; Sell, K.W.; Pollard, J.W.; Stanley, E.R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA 1990, 87, 4828–4832. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.C.; Achuthan, A.; Fleetwood, A.J.; Dinh, H.; Roiniotis, J.; Scholz, G.M.; Chang, M.W.; Beckman, S.K.; Cook, A.D.; Hamilton, J.A. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J. Immunol. 2012, 188, 5752–5765. [Google Scholar] [CrossRef] [PubMed]
- Fleetwood, A.J.; Cook, A.D.; Hamilton, J.A. Functions of granulocyte-macrophage colony-stimulating factor. Crit. Rev. Immunol. 2005, 25, 405–428. [Google Scholar] [CrossRef]
- Chitu, V.; Stanley, E.R. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 2006, 18, 39–48. [Google Scholar] [CrossRef]
- Verreck, F.A.; de Boer, T.; Langenberg, D.M.; Hoeve, M.A.; Kramer, M.; Vaisberg, E.; Kastelein, R.; Kolk, A.; de Waal-Malefyt, R.; Ottenhoff, T.H. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 4560–4565. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, G.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Du, J. TGF-b induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef]
- Xu, S.; Shinohara, M.L. Tissue-resident macrophage in fungal infections. Front. Immunol. 2017, 8, 1798. [Google Scholar] [CrossRef]
- Akagawa, K.S.; Kamoshita, K.; Tokunaga, T. Effects of granulocyte-macrophage colony-stimulating factor and colony-stimulating factor-1 on the proliferation and differentiation of murine alveolar macrophages. J. Immunol. 1988, 141, 3383–3390. [Google Scholar]
- Murray, P.J. Macrophage polarization. Ann. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, X.; Daha, M.R.; van Kooten, C. Reversible differentiation of pro- and anti-inflammatory macrophages. Mol. Immunol. 2013, 53, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bodin, P.; Burnstock, G. Purinergic signalling: ATP release. Neurochem. Res. 2001, 26, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Sitkovsky, M.V.; Robson, S.C. Purinergic signaling during inflammation. N Engl. J. Med. 2012, 367, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Layhadi, J.A.; Turner, J.; Crossman, D.; Fountain, S.J. ATP Evokes Ca2+ Responses and CXCL5 Secretion via P2X4 Receptor Activation in Human Monocyte-Derived Macrophages. J. Immunol. 2018, 200, 1159–1168. [Google Scholar] [CrossRef]
- Cohen, H.B.; Briggs, K.T.; Marino, J.P.; Ravid, K.; Robson, S.C.; Mosser, D.M. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood 2013, 122, 1935–1945. [Google Scholar] [CrossRef] [Green Version]
- Eligini, S.; Crisci, M.; Bono, E.; Songia, P.; Tremoli, E.; Colombo, G.I.; Colli, S. Human monocyte-derived macrophages spontaneously differentiated in vitro show distinct phenotypes. J. Cell Physiol. 2013, 228, 1464–1472. [Google Scholar] [CrossRef]
- Kotnis, S.; Bingham, B.; Vasilyev, D.V.; Miller, S.W.; Bai, Y.; Yeola, S.; Chanda, P.K.; Bowlby, M.R.; Kaftan, E.J.; Samad, T.A.; et al. Genetic and functional analysis of human P2X5 reveals a distinct pattern of exon 10 polymorphism with predominant expression of the nonfunctional receptor isoform. Mol. Pharmacol. 2010, 77, 953–960. [Google Scholar] [CrossRef]
- Micklewright, J.J.; Layhadi, J.A.; Fountain, S.J. P2Y12 receptor modulation of ADP-evoked intracellular Ca2+ signalling in THP-1 human monocytic cells. Br. J. Pharmacol. 2018, 175, 2483–2491. [Google Scholar] [CrossRef]
- Ali, S.B.; Turner, J.J.O.; Fountain, S.J. Constitutive P2Y2 receptor activity regulates basal lipolysis in human adipocytes. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Layhadi, J.A.; Fountain, S.J. P2X4 Receptor-Dependent Ca2+ Influx in Model Human Monocytes and Macrophages. Int J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Li, J.; Fountain, S.J. Fluvastatin suppresses native and recombinant human P2X4 receptor function. Purinergic Signal. 2012, 8, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wilgenburg, B.; Browne, C.; Vowles, J.; Cowley, S.A. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS ONE 2013, 8, e71098. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Schlichter, L.C. Selective activation of KCa3.1 and CRAC channels by P2Y2 receptors promotes Ca2+ signalling, store refilling and migration of rat microglial cells. PLoS ONE 2013, 8, e62345. [Google Scholar]
- Vaeth, M.; Zee, I.; Concepcion, A.R.; Maus, M.; Shaw, P.; Portal-Celhay, C.; Zahra, A.; Kozhaya, L.; Weidinger, C.; Phillips, J.; et al. Ca2+ signalling but not store-operated Ca2+ entry is required for the function of macrophages and dendritic cells. J. Immunol. 2015, 1403013. [Google Scholar]
- Sakaki, H.; Tsukimoto, M.; Harada, H.; Moriyama, Y.; Kojima, S. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PLoS ONE 2013, 8, e59778. [Google Scholar] [CrossRef]
- Perez-Sen, R.; Gomez-Villafuertes, R.; Ortega, F.; Gualix, J.; Delicado, E.G.; Miras-Portugal, M.T. An Update on P2Y13 Receptor Signalling and Function. Adv. Exp. Med. Biol. 2017, 1051, 139–168. [Google Scholar]
- Marteau, F.; Le Poul, E.; Communi, D.; Communi, D.; Labouret, C.; Savi, P.; Boeynaems, J.M.; Gonzalez, N.S. Pharmacological characterization of the human P2Y13 receptor. Mol. Pharmacol. 2003, 64, 104–112. [Google Scholar] [CrossRef]
- Myrtek, D.; Muller, T.; Geyer, V.; Derr, N.; Ferrari, D.; Zissel, G.; Durk, T.; Sorichter, S.; Luttmann, W.; Kuepper, M.; et al. Activation of human alveolar macrophages via P2 receptors: Coupling to intracellular Ca2+ increases and cytokine secretion. J. Immunol 2008, 181, 2181–2188. [Google Scholar] [CrossRef]
- Norenberg, W.; Hempel, C.; Urban, N.; Sobottka, H.; Illes, P.; Schaefer, M. Clemastine potentiates the human P2X7 receptor by sensitizing it to lower ATP concentrations. J. Biol. Chem. 2011, 286, 11067–11081. [Google Scholar] [CrossRef]
- Rassendren, F.; Buell, G.N.; Virginio, C.; Collo, G.; North, R.A.; Surprenant, A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem. 1997, 272, 5482–5486. [Google Scholar] [CrossRef]
- Stokes, L.; Layhadi, J.A.; Bibic, L.; Dhuna, K.; Fountain, S.J. P2X4 Receptor Function in the Nervous System and Current Breakthroughs in Pharmacology. Front. Pharmacol. 2017, 8, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Layhadi, J.A.; Fountain, S.J. ATP-Evoked Intracellular Ca2+ Responses in M-CSF Differentiated Human Monocyte-Derived Macrophage are Mediated by P2X4 and P2Y11 Receptor Activation. Int. J. Mol. Sci. 2019, 20, 5113. https://doi.org/10.3390/ijms20205113
Layhadi JA, Fountain SJ. ATP-Evoked Intracellular Ca2+ Responses in M-CSF Differentiated Human Monocyte-Derived Macrophage are Mediated by P2X4 and P2Y11 Receptor Activation. International Journal of Molecular Sciences. 2019; 20(20):5113. https://doi.org/10.3390/ijms20205113
Chicago/Turabian StyleLayhadi, Janice A., and Samuel J. Fountain. 2019. "ATP-Evoked Intracellular Ca2+ Responses in M-CSF Differentiated Human Monocyte-Derived Macrophage are Mediated by P2X4 and P2Y11 Receptor Activation" International Journal of Molecular Sciences 20, no. 20: 5113. https://doi.org/10.3390/ijms20205113
APA StyleLayhadi, J. A., & Fountain, S. J. (2019). ATP-Evoked Intracellular Ca2+ Responses in M-CSF Differentiated Human Monocyte-Derived Macrophage are Mediated by P2X4 and P2Y11 Receptor Activation. International Journal of Molecular Sciences, 20(20), 5113. https://doi.org/10.3390/ijms20205113



