Chiral Cocrystal Solid Solutions, Molecular Complexes, and Salts of N-Triphenylacetyl-l-Tyrosine and Diamines
Abstract
:1. Introduction
2. Results and Discussion
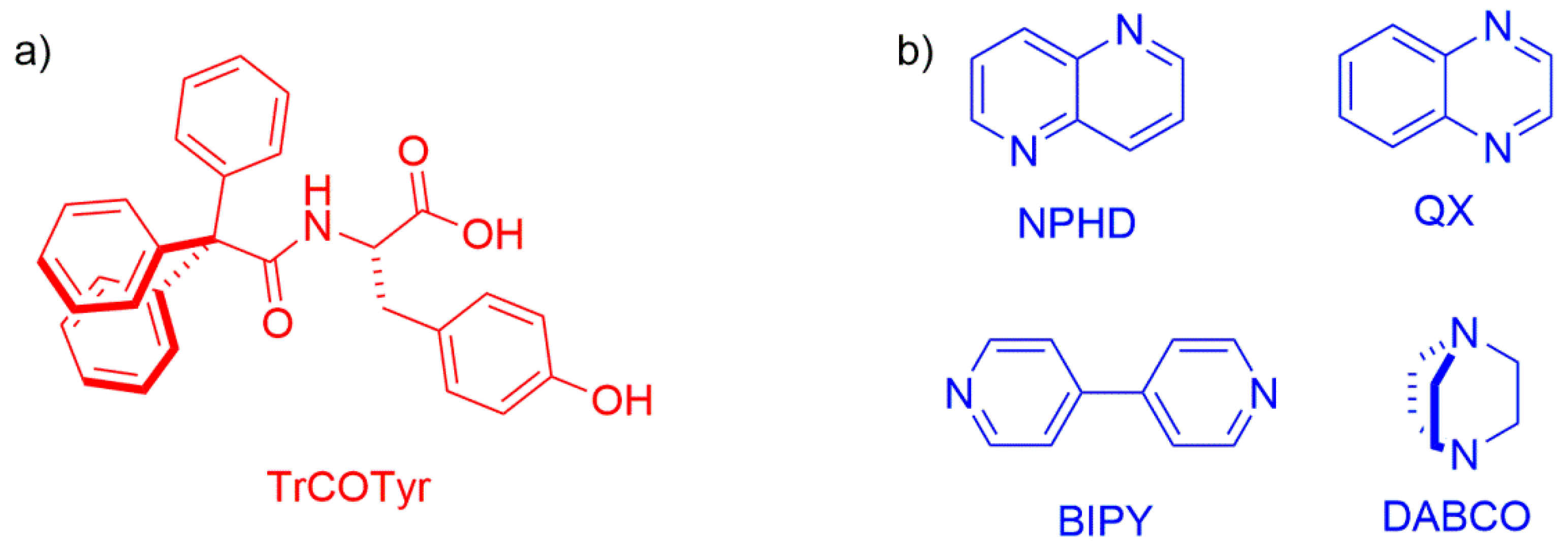
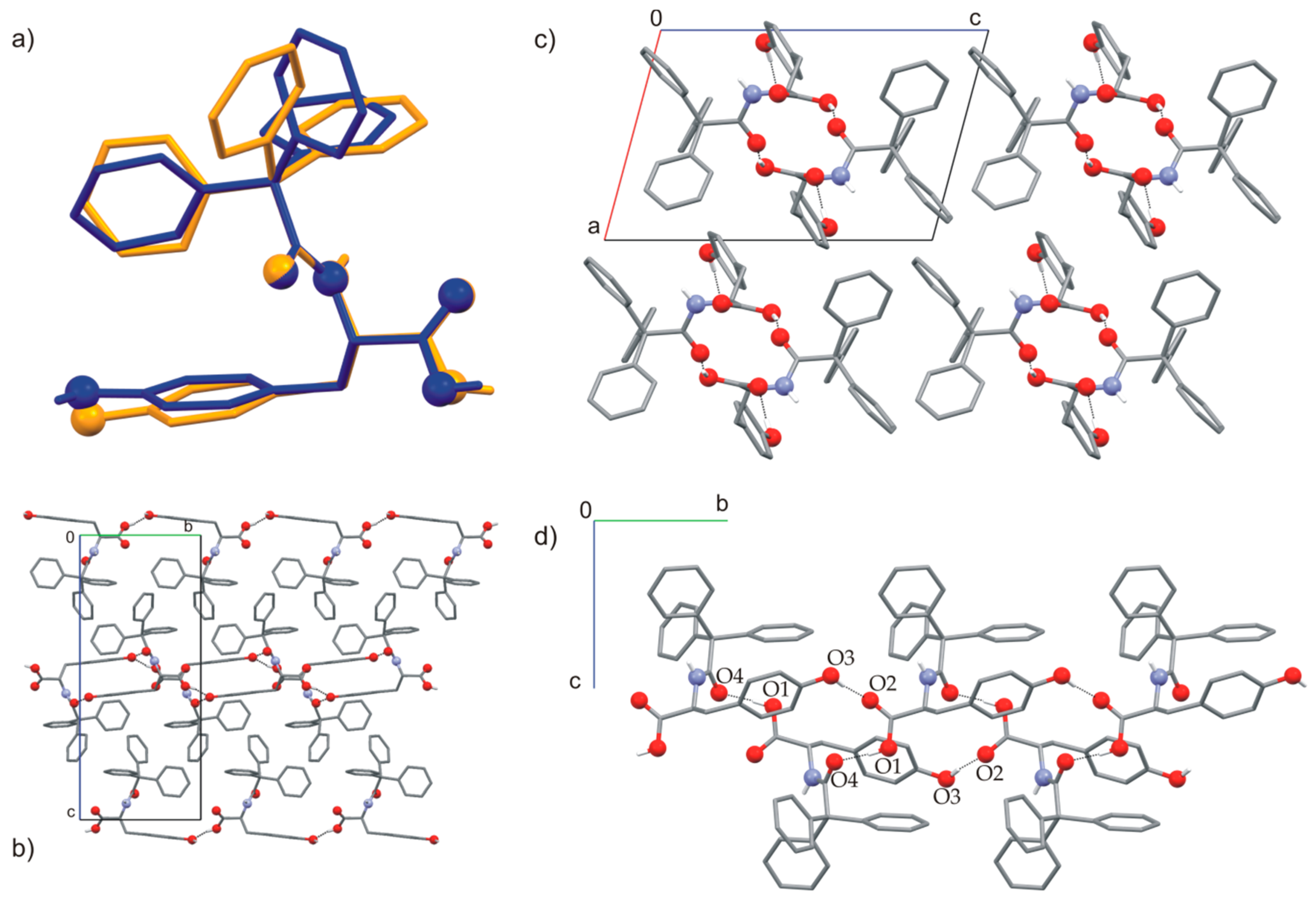
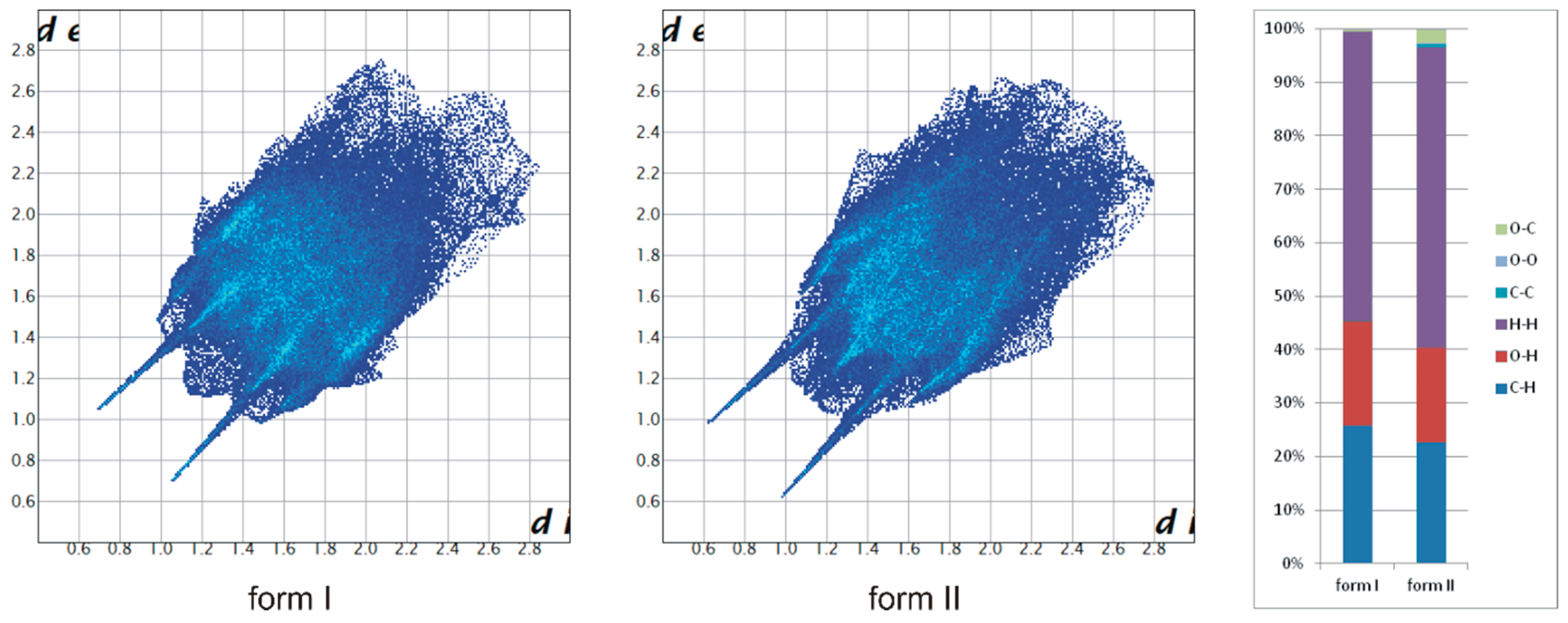
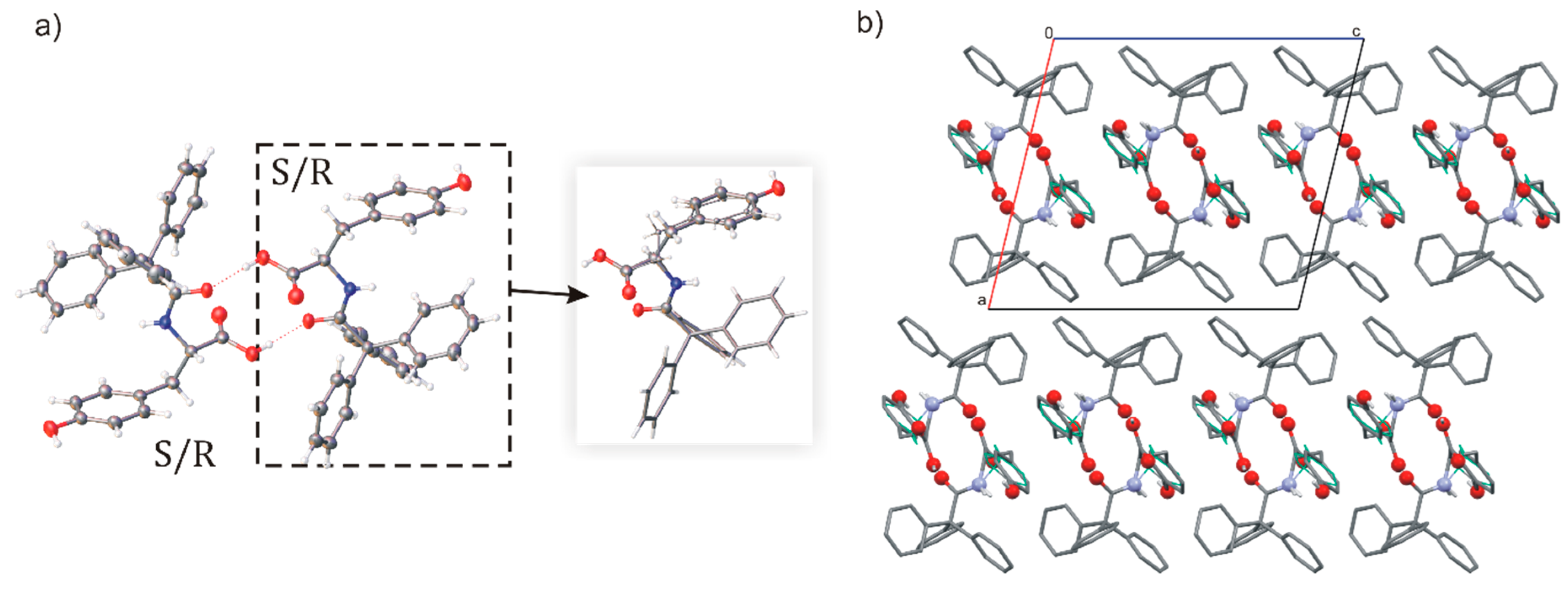
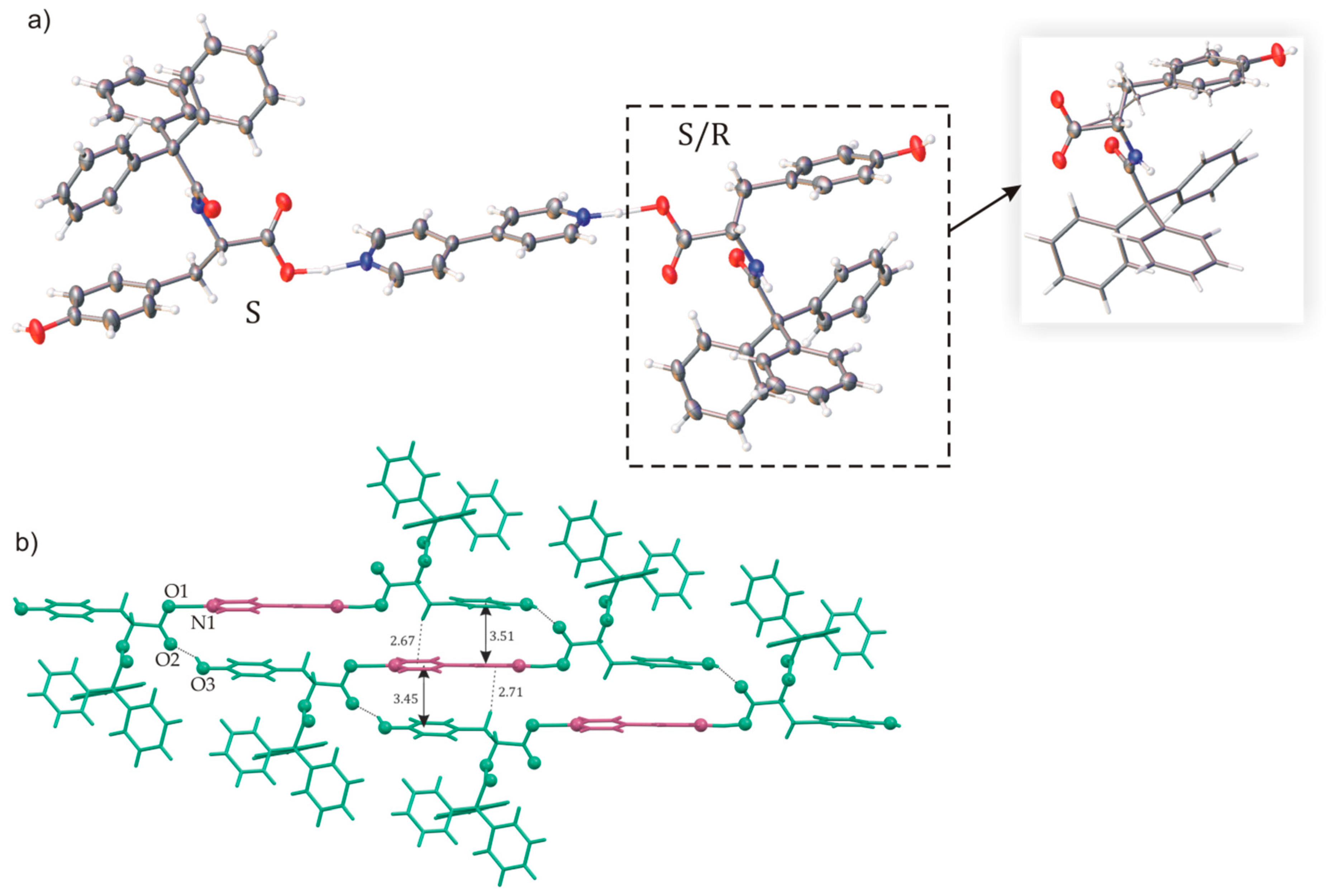
2.1. Polymorphs and Solvates of TrCOTyr·and rac-TrCOTyr
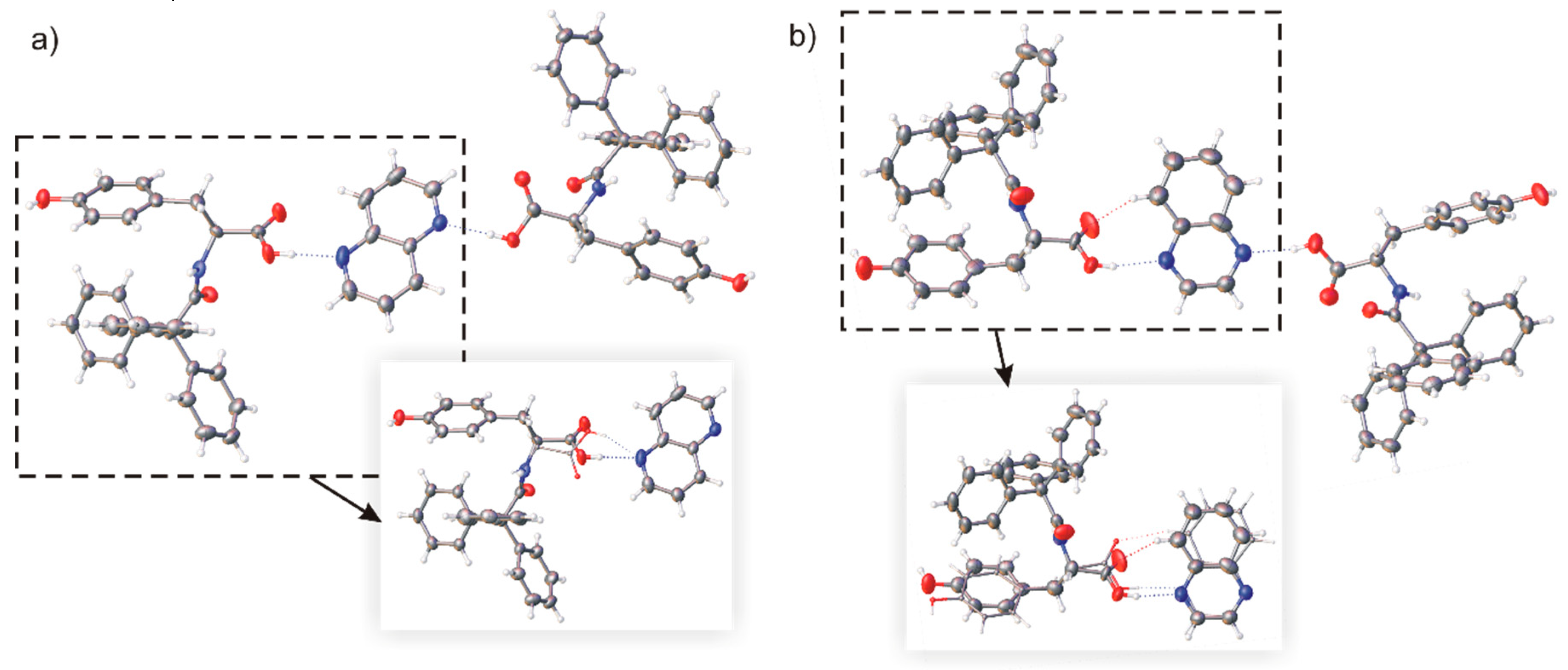
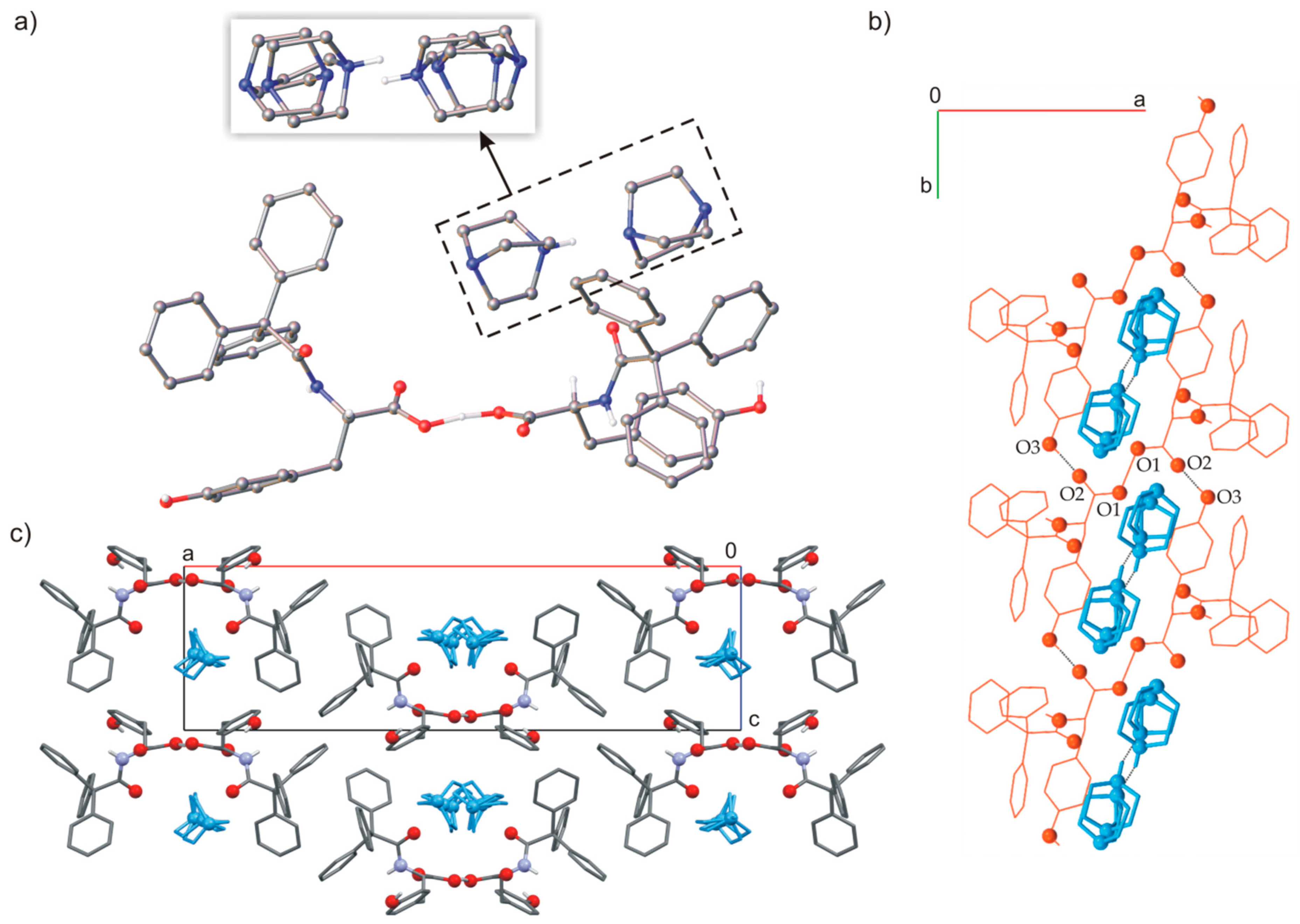
2.2. Host-Guest Complexes of TrCOTyr
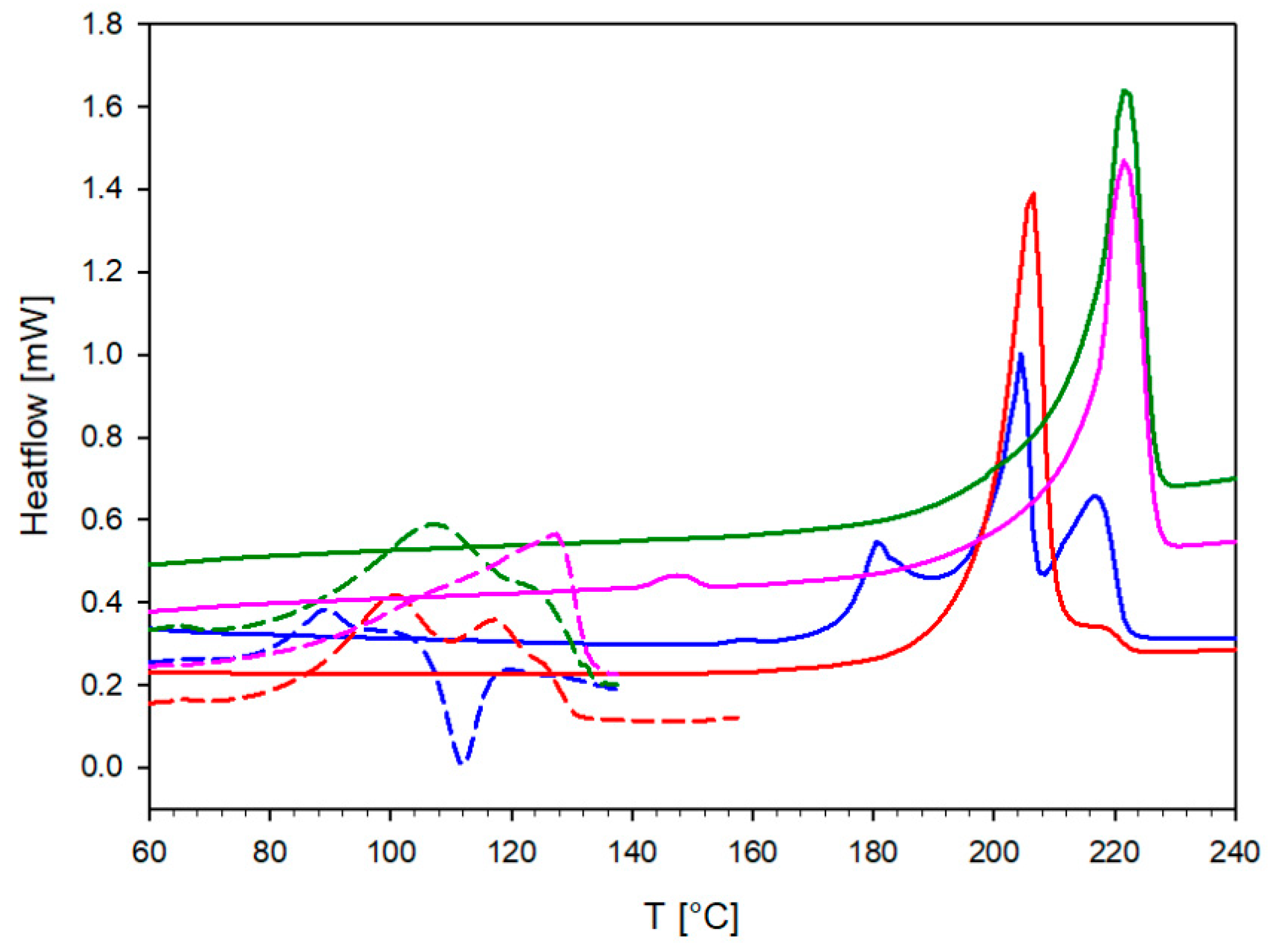
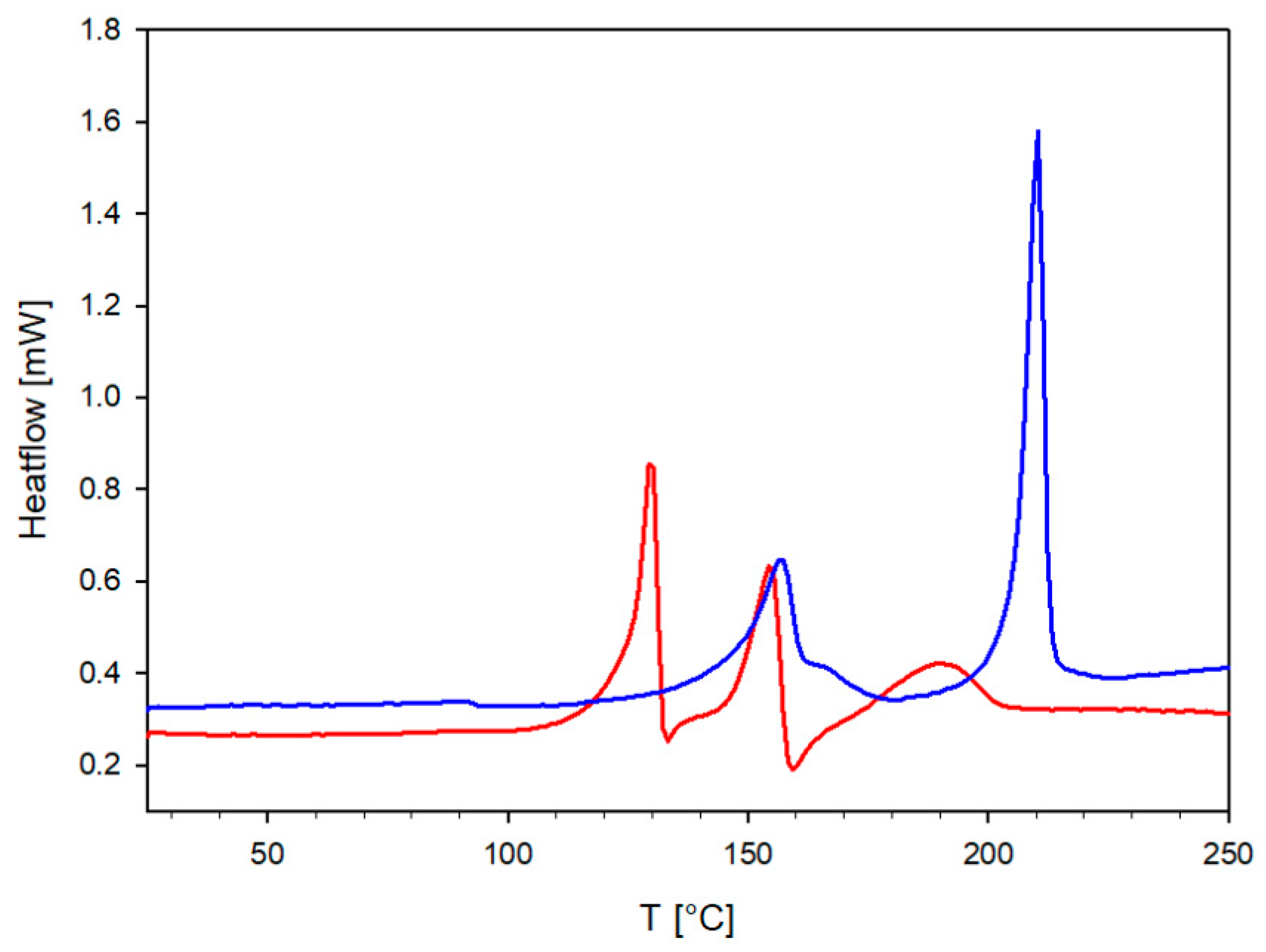
2.3. Differential Scanning Calorimetry
3. Materials and Methods
3.1. Synthesis and Crystallization
3.2. X-Ray Analysis
3.3. Differential Scanning Calorimetry (DSC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Tr | trityl group |
| TrCOTyr | N-triphenylacetyl-l-tyrosine |
| NPHD | 1,5- naphthyridine |
| QX | quinoxaline |
| BIPY | 4,4′- bipyridyl |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
References
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Fujita, M. Molecular Self-Assembly Organic Versus Inorganic Approaches; Springer: Heidelberg, Germany, 2000. [Google Scholar]
- Lawrence, D.S.; Jiang, T.; Levett, M. Self-Assembling Supramolecular Complexes. Chem. Rev. 1995, 95, 2229–2260. [Google Scholar] [CrossRef]
- Lindoy, L.F.; Atkinson, I.M. Self-Assembly in Supramolecular Systems; RSC: Cambridge, UK, 2000. [Google Scholar]
- MacGillivray, L.R. Comprehensive Supramolecular Chemistry II. In Supramolecular Engineering: Designing The Solid State; Atwood, J.L., Gokel, G.W., Barbour, L.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Campbell, N.A.; Reece, J.B.; Taylor, M.R.; Simon, E.J.; Dickey, J.L. Biology: Concepts and Connections, 6th ed.; Benjamin/Cummings Publishing Company: San Francisco, CA, USA, 2008. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L.; Stryer, L. Biochemistry; W.H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- Weber, E. (Ed.) Molecular Inclusion and Molecular Recognition-Clathrates I and I; Springer: Berlin, Germany, 1987/1988; Volumes 140 and 149.
- Akazome, M.; Senda, K.; Ogura, K. Sheet Structure of an l,d-Dipeptide Aggregate: Inclusion Compounds of (S)-Phenylglycyl-(R)-phenylglycine with Amides. J. Org. Chem. 2002, 67, 8885–8889. [Google Scholar] [CrossRef] [PubMed]
- Akazome, M.; Takahashi, T.; Sonobe, R.; Ogura, K. An important role of water in construction and destruction of the sheet structure in dipeptide aggregate. Tetrahedron 2002, 58, 8857–8861. [Google Scholar] [CrossRef]
- Akazome, M.; Takahashi, T.; Sonobe, R.; Ogura, K. Inclusion of Poly(ethylene glycol)s by Crystalline (R)-(1-Naphthyl)glycyl-(R)-phenylglycine. Supramol. Chem. 2001, 13, 109–136. [Google Scholar] [CrossRef]
- Akazome, M.; Yanagita, Y.; Sonobe, R.; Ogura, K. Specific Inclusion of 1,2-Dimethoxybenzene Derivatives by Crystalline (R)-Arylglycyl-(R)-phenylglycines and Its Structure. Bull. Chem. Soc. Jp. 1997, 70, 2823–2827. [Google Scholar] [CrossRef]
- Akazome, M.; Sumikawa, A.; Sonobe, R.; Ogura, K. Optional formation of “parallel or antiparallel” β-sheet-like structures in (R)-(1-naphthyl)glycyl-(R)-phenylglycine crystals. Chem. Lett. 1996, 25, 995–996. [Google Scholar] [CrossRef]
- Akazome, M.; Toma, S.; Horiguchi, T.; Megumi, K.; Matsumoto, S. Inclusion of aliphatic alcohols in pockets of ( S)-threonyl-( S)-phenylglycine using grinding method. Tetrahedron 2011, 67, 2844–2848. [Google Scholar] [CrossRef]
- Akazome, M.; Doba, A.; Matsumoto, S.; Ogura, K. Predominant (S)-Enantioselective Inclusion of Aryl Methyl Sulfoxides by (S)-Isoleucyl-(S)-phenylglycines. J. Org. Chem. 2010, 75, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Akazome, M.; Hirabayashi, A.; Senda, K.; Ogura, K. Inclusion compounds of l,d-dipeptide with small sulfoxides: Flexible sheet structure of (S)-phenylglycyl-(R)-phenylglycine. Tetrahedron 2007, 63, 9933–9938. [Google Scholar] [CrossRef]
- Akazome, M.; Hirabayashi, A.; Takaoka, K.; Nomura, S.; Ogura, K. Molecular recognition of l-leucyl-l-alanine: Enantioselective inclusion of alkyl methyl sulfoxides. Tetrahedron 2005, 61, 1107–1113. [Google Scholar] [CrossRef]
- Akazome, M.; Hirabayashi, A.; Ogura, K. Enantioselective inclusion of (R)-phenylglycyl-(R)-phenylglycine with benzyl methyl sulfoxides. Tetrahedron 2004, 60, 12085–12093. [Google Scholar] [CrossRef]
- Akazome, M.; Ueno, Y.; Ooiso, H.; Ogura, K. Enantioselective inclusion of methyl phenyl sulfoxides and benzyl methyl sulfoxides by (R)-phenylglycyl-(R)-phenylglycine and the crystal structures of the inclusion cavities. J. Org. Chem. 2000, 65, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Akazome, M.; Takahashi, T.; Ogura, K. Enantiomeric Inclusion of α-Hydroxy Esters by (R)-(1-Naphthyl)glycyl-(R)-phenylglycine and the Crystal Structures of the Inclusion Cavities. J. Org. Chem. 1999, 64, 2293–2300. [Google Scholar] [CrossRef]
- Akazome, M.; Noguchi, M.; Tanaka, O.; Sumikawa, A.; Uchida, T.; Ogura, K. Enantiomeric recognition of alkyl phenyl sulfoxides by crystalline (R)-phenylglycyl-(R)-phenylglycine. Tetrahedron 1997, 53, 8315–8322. [Google Scholar] [CrossRef]
- Görbitz, C.H. Microporous Organic Materials from Hydrophobic Dipeptides. Chem. Eur. J. 2007, 13, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Görbitz, C.H. L-Alanyl-L-phenylalanine–2-propanol (1/2) (α-form), L-valyl-L-phenylalanine–2-propanol (1/1) and L-leucyl-L-phenylalanine–2-propanol (1/1) (β-form). Acta Cryst. 1999, 55, 2171–2177. [Google Scholar] [CrossRef]
- Görbitz, C.H. L-Leucyl-L-leucine 2-methyl-1-propanol solvate. Acta Cryst. 1999, 55, 670–672. [Google Scholar] [CrossRef]
- Görbitz, C.H.; Torgersen, E. Symmetry, pseudosymmetry and packing disorder in the alcohol solvates of L-leucyl-L-valine. Acta Cryst. 1999, 55, 104–113. [Google Scholar] [CrossRef]
- Görbitz, C.H. Solvent Site Preferences in the Crystal Structures of L-Leucyl-L-Leucine Alcohol (1: 1) Complexes. Acta Chem. Scand. 1998, 52, 1343–1349. [Google Scholar] [CrossRef]
- Muller, S.; Ariaans, G.J.A.; Kaptein, B.; Broxterman, Q.B.; Formaggio, F.; Battan, E.; Crisma, M.; Toniolo, C.; Bruggink, A. Exploring new dipeptides based on phenylglycine and C-alpha-methyl phentylglycine as hosts in inclusion resolutions. Tetrahedron Asymmetry 2004, 15, 1919–1927. [Google Scholar] [CrossRef]
- Bučar, D.-K. Engineering Molecular Crystals: Backbreaking, yet Gratifying. Cryst. Growth Des. 2017, 17, 2913–2918. [Google Scholar] [CrossRef] [Green Version]
- Tumanova, N.; Tumanov, N.; Robeyns, K.; Fischer, F.; Fusaro, L.; Morelle, F.; Ban, V.; Hautier, G.; Filinchuk, Y.; Wouters, J.; et al. Opening Pandora’s Box: Chirality, Polymorphism, and Stoichiometric Diversity in Flurbiprofen/Proline Cocrystals. Cryst. Growth Des. 2018, 18, 954–961. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.O.; Voronin, A.P.; Vener, M.V.; Churakov, A.V.; Perlovich, G.L. Specific features of supramolecular organisation and hydrogen bonding in proline cocrystals: A case study of fenamates and diclofenac. CrystEngComm 2018, 20, 6970–6981. [Google Scholar] [CrossRef]
- Bracco, S.; Asnaghi, D.; Negroni, M.; Sozzani, P.; Comotti, A. Porous dipeptide crystals as volatile-drug vessels. Chem. Commun. 2018, 54, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Diez, S.J.; Eddleston, M.D.; Arhangelskis, M.; Milbled, M.; Müller, M.J.; Bond, A.D.; Bučar, D.-K.; Jones, W. Crystallization at Solvent Interfaces Enables Access to a Variety of Cocrystal Polymorphs and Hydrates. Cryst. Growth Des. 2018, 18, 3263–3268. [Google Scholar] [CrossRef]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous Organic Materials: Strategic Design and Structure–Function Correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef]
- Tiwari, P.; Biswas, S.; Verma, R.; Sharma, A.; Konar, A.D. Porous Biomaterials via Side Chain-Side Chain Interactions of Tyrosine Analogue of Pyridine Carboxamides. ChemistrySelect 2018, 3, 262–272. [Google Scholar] [CrossRef]
- Georgilis, E.; Gessmann, R.; Mitraki, A.; Petratos, K. Diphenylalanine in tetrahydrofuran: A highly potent candidate for the development of novel nanomaterials. Acta Cryst. 2017, 73, 447–450. [Google Scholar] [CrossRef]
- Kodama, K.; Kanai, H.; Shimomura, Y.; Hirose, T. Enantioseparation of Sulfoxides and Nitriles by Inclusion Crystallization with Chiral Organic Salts Based on l -Phenylalanine. Eur. J. Org. Chem. 2018, 14, 1726–1729. [Google Scholar] [CrossRef]
- Puglisi, R.; Ballistreri, F.P.; Gangemi, C.M.A.; Toscano, R.M.; Tomaselli, G.A.; Pappalardo, A.; Sfrazzetto, G.T. Chiral Zn–salen complexes: A new class of fluorescent receptors for enantiodiscrimination of chiral amines. New J. Chem. 2017, 41, 911–915. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, C.; Jin, Y.; Li, B. Visual chiral recognition of D/L-leucine using cube-shaped gold nanoparticles as colorimetric probes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 17263. [Google Scholar] [CrossRef] [PubMed]
- Chatziefthimiou, S.D.; Inclán, M.; Giastas, P.; Papakyriakou, A.; Yannakopoulou, K.; Mavridis, I.M. Molecular recognition of N-acetyltryptophan enantiomers by β-cyclodextrin. Beilstein J. Org. Chem. 2017, 13, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Fraschetti, C.; Montagna, M.; Crestoni, M.E.; Calcaterra, A.; Aiello, F.; Santi, L.; Filippi, A. Kinetic enantioselectivity of a protonated bis(diamido)-bridged basket resorcin[4]arene towards alanine peptides. Org Biomol Chem. 2017, 15, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Carotti, A.; Ianni, F.; Camaioni, E.; Pucciarini, L.; Marinozzi, M.; Sardella, R.; Natalini, B. N-Decyl-S-trityl-(R)-cysteine, a new chiral selector for “green” ligand- exchange chromatography applications. J. Pharm. Biomed. Anal. 2017, 144, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis; Wiley: New York, NY, USA, 2006; pp. 152–156. [Google Scholar]
- Khazaei, A.; Zolfigol, M.A.; Moosavi-Zare, A.R.; Zare, A.; Parhami, A.; Khalafi-Nezhad, A. Trityl chloride as an efficient organic catalyst for the synthesis of 1-amidoalkyl-2-naphtols in neutral media at room temperature. Appl. Catal. A 2010, 386, 179–187. [Google Scholar] [CrossRef]
- Khazaei, A.; Zolfigol, M.A.; Moosavi-Zare, A.R.; Abi, F.; Zare, A.; Kaveh, H.; Khakyzadeh, V.; Kazem-Rostami, M.; Parhami, A.; Torabi-Monfared, H. Discovery of an in situ carbocationic system using trityl chloride as a homogeneous organocatalyst for the solvent-free condensation of β-naphthol with aldehydes and amides/thioamides/alkyl carbamates in neutral media. Tetrahedron 2013, 69, 212–218. [Google Scholar] [CrossRef]
- Khazaei, A.; Zolfigol, M.A.; Moosavi-Zare, A.R.; Zare, A.; Khojasteh, M.; Asgari, Z.; Khakyzadeh, V.; Khalafi-Nezhad, A. Organocatalyst trityl chloride efficiently promoted the solvent-free synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-ones by in situ formation of carbocationic system in neutral media. Catal. Commun. 2012, 20, 54–57. [Google Scholar] [CrossRef]
- Mislow, K. Stereochemical consequences of correlated rotation in molecular propellers. Accounts Chem. Res. 1976, 9, 26–33. [Google Scholar] [CrossRef]
- Iwamura, H.; Mislow, K. Stereochemical consequences of dynamic gearing. Accounts Chem. Res. 1988, 21, 175–182. [Google Scholar] [CrossRef]
- Ściebura, J.; Skowronek, P.; Gawroński, J. Trityl ethers: Molecular bevel gears reporting chirality through circular dichroism spectra. Angew. Chem. Int. Ed. 2009, 48, 7069–7072. [Google Scholar] [CrossRef] [PubMed]
- Ściebura, J.; Gawroński, J. Double Chirality Transmission in Trityl Amines: Sensing Molecular Dynamic Stereochemistry by Circular Dichroism and DFT Calculations. Chem. Eur. J. 2011, 17, 13138–13141. [Google Scholar] [CrossRef] [PubMed]
- Ściebura, J.; Janiak, A.; Stasiowska, A.; Grajewski, J.; Gawrońska, K.; Rychlewska, U.; Gawroński, J. Intramolecular interactions of trityl groups. ChemPhysChem 2014, 15, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, P.; Ścianowski, J.; Pacuła, A.J.; Gawroński, J. Chirality transfer through sulfur or selenium to chiral propellers. RSC Adv. 2015, 5, 69441–69444. [Google Scholar] [CrossRef]
- Skowronek, P.; Czapik, A.; Rajska, Z.; Kwit, M. Molecular and supramolecular helicity induction in trityl group-containing compounds: The case of chiral 3,3,3-triphenylpropionic acid derivatives. Tetrahedron 2019, 75, 4497–4505. [Google Scholar] [CrossRef]
- Akazome, M. Chiral Recognition by Inclusion Crystals of Amino-Acid Derivatives Having Trityl Groups. In Advances in Organic Crystal Chemistry; Tamura, R., Miyata, M., Eds.; Springer: Berlin, Germany, 2015; pp. 463–482, references therein, ch. 23. [Google Scholar]
- Bendzińska-Berus, W.; Warżajtis, B.; Gajewy, J.; Kwit, M.; Rychlewska, U. Trityl Group as an Crystal Engineering Tool for Construction of Inclusion Compounds and for Suppression of Amide NH···O=C Hydrogen Bonds. Cryst. Growth Des. 2017, 17, 2560–2568. [Google Scholar] [CrossRef]
- Bendzińska-Berus, W.; Jelecki, M.; Kwit, M.; Rychlewska, U. Transfer of chirality in N-triphenylacetylamino acids and chiral derivatives of N-triphenylacetyl Gly–Gly dipeptide and control of their assembly with steric constraints. CrystEngComm 2019, 21, 3420–3430. [Google Scholar] [CrossRef]
- Prusinowska, N.; Bendzińska-Berus, W.; Jelecki, M.; Rychlewska, U.; Kwit, M. Triphenylacetic acid amides: Molecular propellers with induced chirality. Eur. J. Org. Chem. 2015, 4, 738–749. [Google Scholar] [CrossRef]
- Prusinowska, N.; Bendzińska-Berus, W.; Szymkowiak, J.; Warżajtis, B.; Gajewy, J.; Jelecki, M.; Rychlewska, U.; Kwit, M. Double helicity induction in chiral bis (triphenylacetamides). RSC Adv. 2015, 5, 83448–83458. [Google Scholar] [CrossRef]
- Prusinowska, N.; Czapik, A.; Wojciechowska, M.; Kwit, M. Dynamic optical activity induction in the N-alkyl-N’-trityl ureas and thioureas. Org. Biomol. Chem. 2019, 17, 7782–7793. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.; Collet, A.; Wilen, S. Enantiomers, Racemates and Resolutions; Wiley: New York, NY, USA, 1981. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Etter, M. Hydrogen Bonds as Design Elements in Organic Chemistry. J. Phys. Chem. 1991, 95, 4601–4610. [Google Scholar] [CrossRef]
- Chen, S.; Xi, H.; Henry, R.F.; Marsden, I.; Zhang, G.G.Z. Chiral co-crystal solid solution: Structures, melting point phase diagram, and chiral enrichment of (ibuprofen)2(4,4-dipyridyl). CrystEngComm 2010, 12, 1485–1493. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Cryst. 2013, 69, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czapik, A.; Jelecki, M.; Kwit, M. Chiral Cocrystal Solid Solutions, Molecular Complexes, and Salts of N-Triphenylacetyl-l-Tyrosine and Diamines. Int. J. Mol. Sci. 2019, 20, 5004. https://doi.org/10.3390/ijms20205004
Czapik A, Jelecki M, Kwit M. Chiral Cocrystal Solid Solutions, Molecular Complexes, and Salts of N-Triphenylacetyl-l-Tyrosine and Diamines. International Journal of Molecular Sciences. 2019; 20(20):5004. https://doi.org/10.3390/ijms20205004
Chicago/Turabian StyleCzapik, Agnieszka, Maciej Jelecki, and Marcin Kwit. 2019. "Chiral Cocrystal Solid Solutions, Molecular Complexes, and Salts of N-Triphenylacetyl-l-Tyrosine and Diamines" International Journal of Molecular Sciences 20, no. 20: 5004. https://doi.org/10.3390/ijms20205004
APA StyleCzapik, A., Jelecki, M., & Kwit, M. (2019). Chiral Cocrystal Solid Solutions, Molecular Complexes, and Salts of N-Triphenylacetyl-l-Tyrosine and Diamines. International Journal of Molecular Sciences, 20(20), 5004. https://doi.org/10.3390/ijms20205004




