Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis
Abstract
1. Introduction
2. Thyroid Hormone Effects via Interactions with the Thyroid Hormone Receptor
2.1. Thyroid Hormone
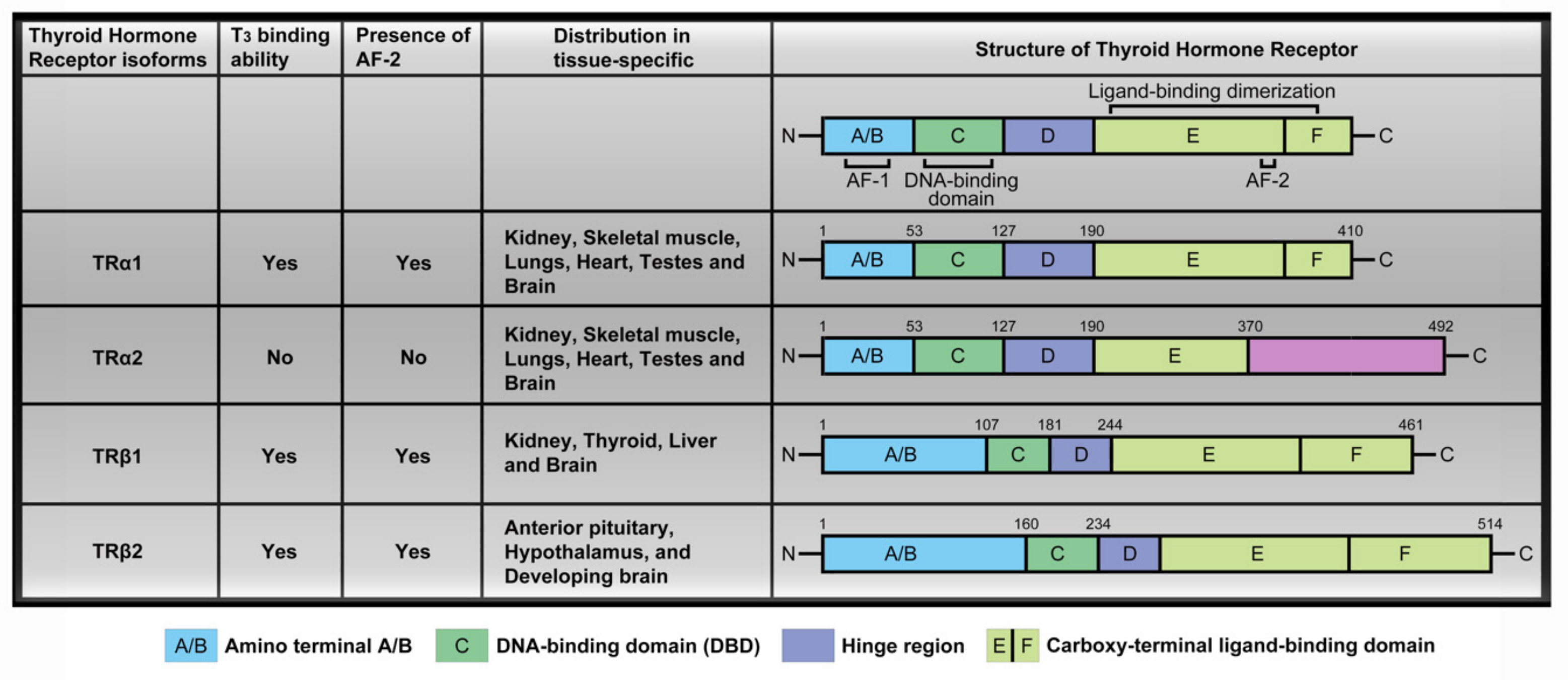
2.2. Thyroid Hormone Receptor
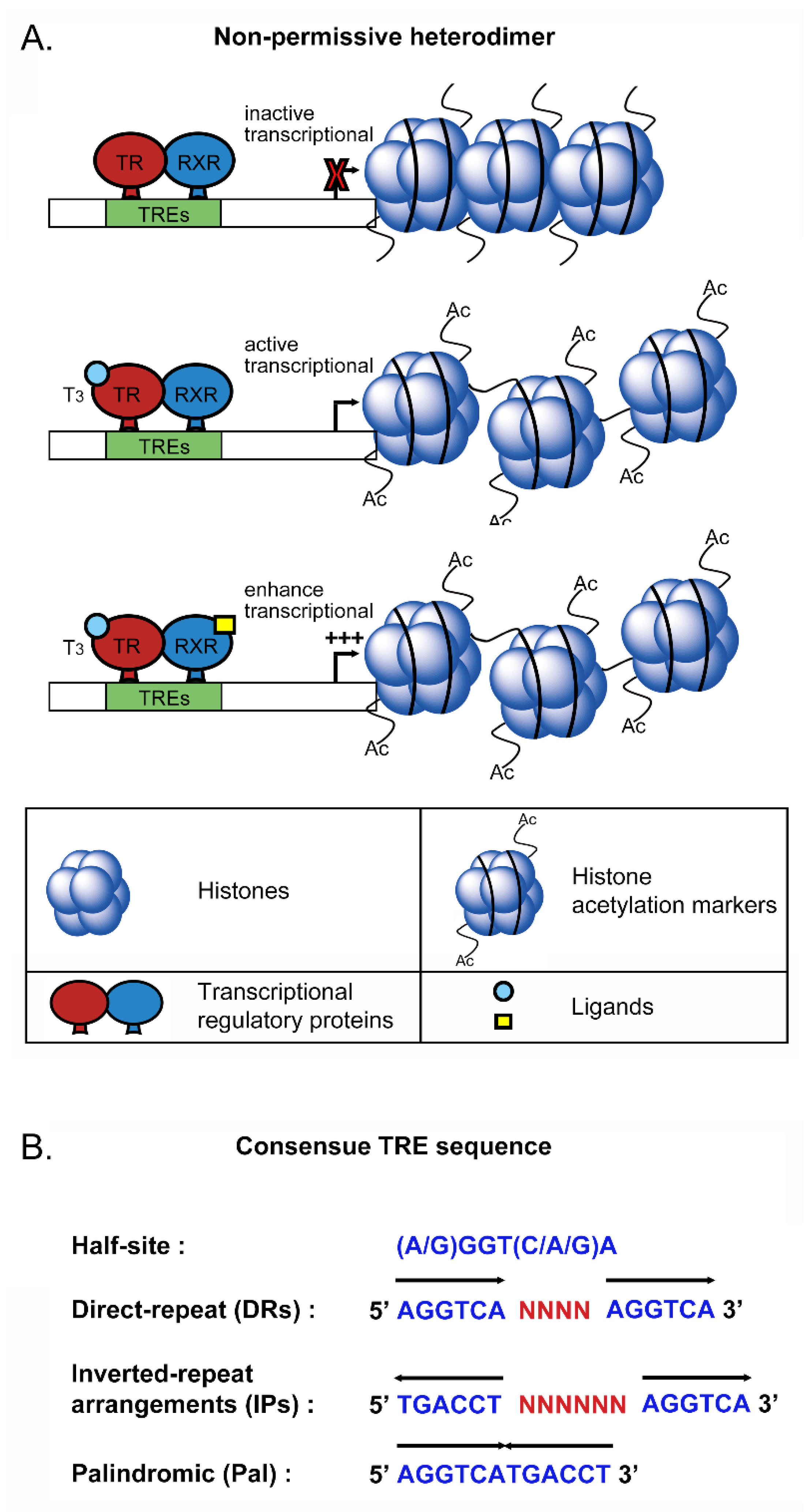
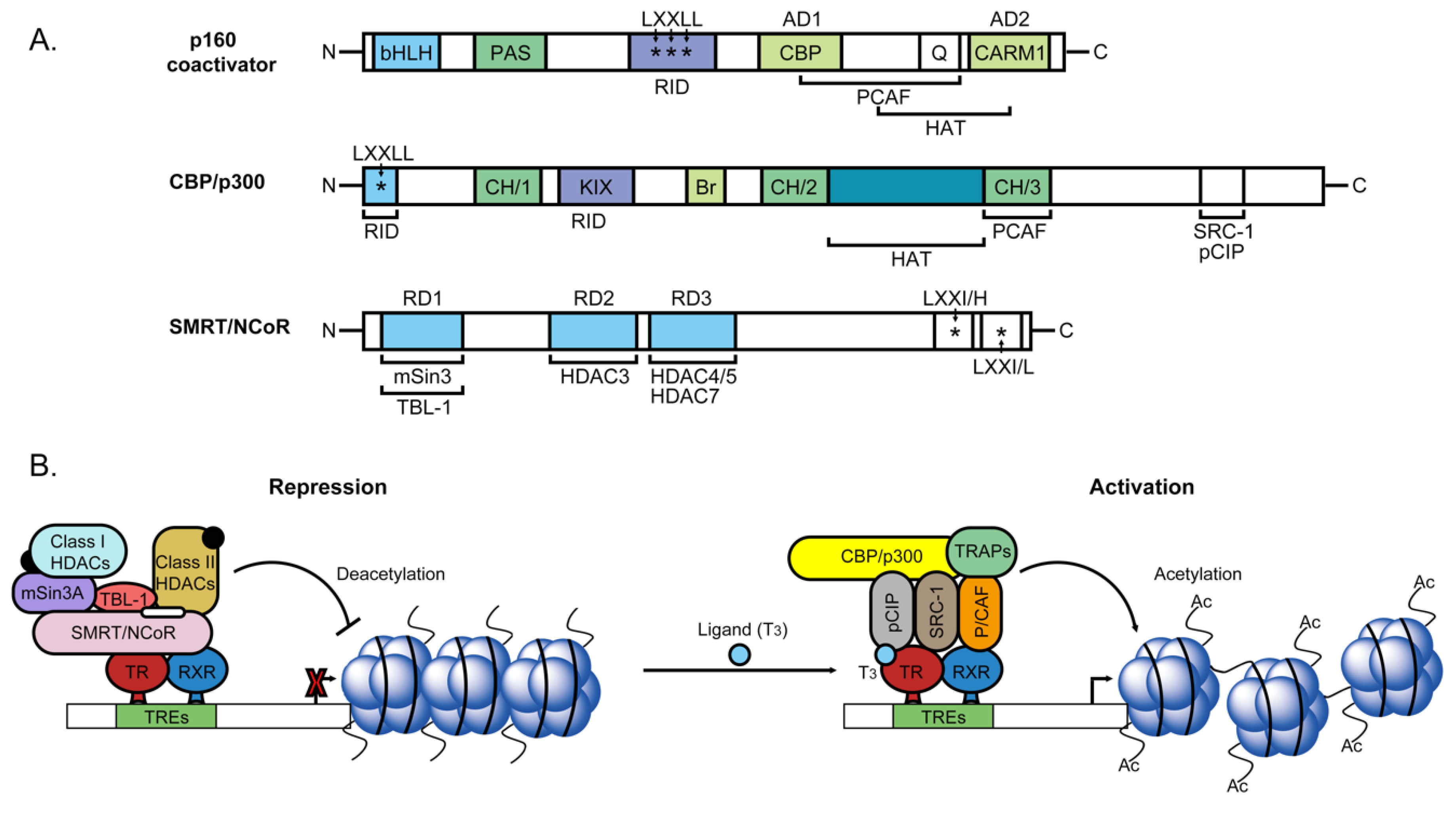
2.3. Nuclear Transcriptional Activity of Thyroid Hormone
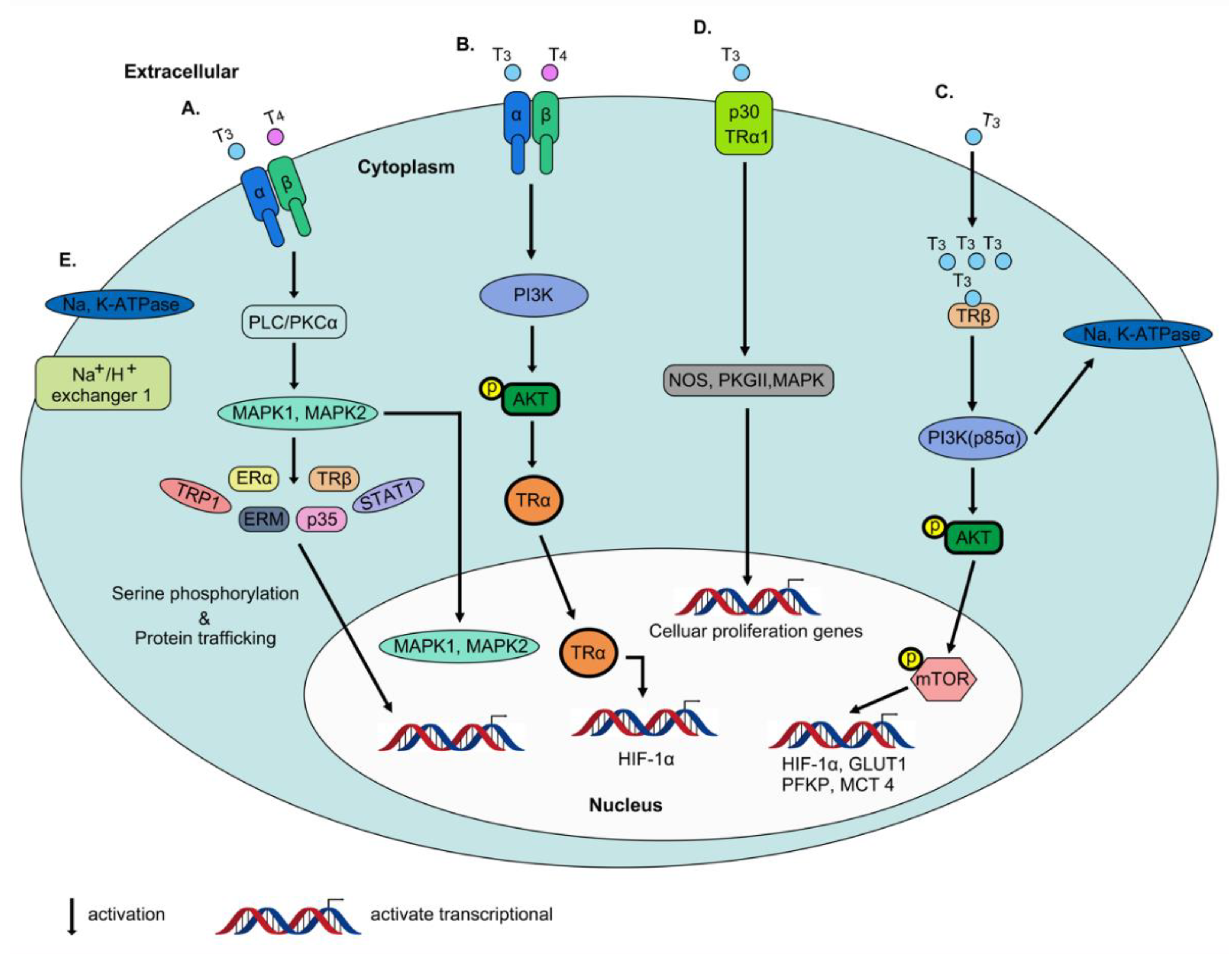
2.4. Non-Genomic Actions of Thyroid Hormone
3. Functional Significance of Thyroid Hormone and Receptors in Tumors
3.1. Breast Cancer
3.2. Thyroid Cancer
3.3. Lung Cancer
3.4. Brain Tumors
3.5. Liver Cancer
3.6. Colorectal Cancer
3.7. Thyroid Hormone Is Anti-Apoptosis in Cancer Cells
4. TH Analogs Exert Anti-Proliferative Effects on Cancer Cells
4.1. Tetraiodothyroacetic Acid (Tetrac)
4.2. Triiodothyroacetic Acid (Triac)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chi, H.C.; Chen, C.Y.; Tsai, M.M.; Tsai, C.Y.; Lin, K.H. Molecular functions of thyroid hormones and their clinical significance in liver-related diseases. Biomed. Res. Int. 2013, 2013, 601361. [Google Scholar] [CrossRef] [PubMed]
- Bassett, J.H.; Williams, G.R. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr. Rev. 2016, 37, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.C.; Tsai, C.Y.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J. Biomed. Sci. 2019, 26, 24. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.F.; Ando, T.; Lin, R.Y.; Tomer, Y.; Latif, R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J. Clin. Invest. 2005, 115, 1972–1983. [Google Scholar] [CrossRef] [PubMed]
- Visser, W.E.; Friesema, E.C.; Visser, T.J. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol. Endocrinol. 2011, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Fuhrer, D.; Biebermann, H. Molecules important for thyroid hormone synthesis and action - known facts and future perspectives. Thyroid Res. 2011, 4 (Suppl 1), S9. [Google Scholar] [CrossRef]
- Friesema, E.C.; Jansen, J.; Jachtenberg, J.W.; Visser, W.E.; Kester, M.H.; Visser, T.J. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol. Endocrinol. 2008, 22, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 249–259. [Google Scholar] [CrossRef]
- Bassett, J.H.; Williams, G.R. Critical role of the hypothalamic-pituitary-thyroid axis in bone. Bone 2008, 43, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y. Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev. Endocr. Metab. Disord. 2000, 1, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.R. Thyroid hormone analogs and metabolites: new applications for an old hormone? Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oppenheimer, J.H.; Schwartz, H.L.; Surks, M.I. Nuclear binding capacity appears to limit the hepatic response to L-triiodothyronine (T3). Endocr. Res. Commun. 1975, 2, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.L.; Lazar, M.A. Modulating nuclear receptor function: may the phos be with you. J. Clin. Investig. 1999, 103, 1617–1618. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, T.; Tennyson, G.E.; Nikodem, V.M. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc. Natl. Acad. Sci. USA 1988, 85, 5804–5808. [Google Scholar] [CrossRef]
- Sakurai, A.; Nakai, A.; DeGroot, L.J. Expression of three forms of thyroid hormone receptor in human tissues. Mol. Endocrinol. 1989, 3, 392–399. [Google Scholar] [CrossRef]
- Jones, I.; Ng, L.; Liu, H.; Forrest, D. An intron control region differentially regulates expression of thyroid hormone receptor beta2 in the cochlea, pituitary, and cone photoreceptors. Mol. Endocrinol. 2007, 21, 1108–1119. [Google Scholar] [CrossRef]
- Williams, G.R. Cloning and characterization of two novel thyroid hormone receptor beta isoforms. Mol. Cell Biol. 2000, 20, 8329–8342. [Google Scholar] [CrossRef]
- Ying, H.; Suzuki, H.; Zhao, L.; Willingham, M.C.; Meltzer, P.; Cheng, S.Y. Mutant thyroid hormone receptor beta represses the expression and transcriptional activity of peroxisome proliferator-activated receptor gamma during thyroid carcinogenesis. Cancer Res. 2003, 63, 5274–5280. [Google Scholar] [PubMed]
- Pascual, A.; Aranda, A. Thyroid hormone receptors, cell growth and differentiation. Biochim Biophys Acta 2013, 1830, 3908–3916. [Google Scholar] [CrossRef] [PubMed]
- Perlmann, T.; Rangarajan, P.N.; Umesono, K.; Evans, R.M. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993, 7, 1411–1422. [Google Scholar] [CrossRef]
- Yen, P.M.; Ikeda, M.; Wilcox, E.C.; Brubaker, J.H.; Spanjaard, R.A.; Sugawara, A.; Chin, W.W. Half-site arrangement of hybrid glucocorticoid and thyroid hormone response elements specifies thyroid hormone receptor complex binding to DNA and transcriptional activity. J. Biol. Chem. 1994, 269, 12704–12709. [Google Scholar]
- Mangelsdorf, D.J.; Evans, R.M. The RXR heterodimers and orphan receptors. Cell 1995, 83, 841–850. [Google Scholar] [CrossRef]
- Horlein, A.J.; Naar, A.M.; Heinzel, T.; Torchia, J.; Gloss, B.; Kurokawa, R.; Ryan, A.; Kamei, Y.; Soderstrom, M.; Glass, C.K. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 1995, 377, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Perissi, V.; Staszewski, L.M.; McInerney, E.M.; Kurokawa, R.; Krones, A.; Rose, D.W.; Lambert, M.H.; Milburn, M.V.; Glass, C.K.; Rosenfeld, M.G. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999, 13, 3198–3208. [Google Scholar] [CrossRef]
- Struhl, K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998, 12, 599–606. [Google Scholar] [CrossRef]
- Tyler, J.K.; Kadonaga, J.T. The "dark side" of chromatin remodeling: repressive effects on transcription. Cell 1999, 99, 443–446. [Google Scholar] [CrossRef]
- Knoepfler, P.S.; Eisenman, R.N. Sin meets NuRD and other tails of repression. Cell 1999, 99, 447–450. [Google Scholar] [CrossRef]
- Heinzel, T.; Lavinsky, R.M.; Mullen, T.M.; Soderstrom, M.; Laherty, C.D.; Torchia, J.; Yang, W.M.; Brard, G.; Ngo, S.D.; Davie, J.R.; et al. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 1997, 387, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Kao, H.Y.; Chakravarti, D.; Lin, R.J.; Hassig, C.A.; Ayer, D.E.; Schreiber, S.L.; Evans, R.M. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 1997, 89, 373–380. [Google Scholar] [CrossRef]
- Guenther, M.G.; Lane, W.S.; Fischle, W.; Verdin, E.; Lazar, M.A.; Shiekhattar, R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 2000, 14, 1048–1057. [Google Scholar] [PubMed]
- Fondell, J.D.; Ge, H.; Roeder, R.G. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc. Natl. Acad. Sci. USA 1996, 93, 8329–8333. [Google Scholar] [CrossRef] [PubMed]
- Fondell, J.D.; Guermah, M.; Malik, S.; Roeder, R.G. Thyroid hormone receptor-associated proteins and general positive cofactors mediate thyroid hormone receptor function in the absence of the TATA box-binding protein-associated factors of TFIID. Proc. Natl. Acad. Sci. USA 1999, 96, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ma, H.; Hong, H.; Koh, S.S.; Huang, S.M.; Schurter, B.T.; Aswad, D.W.; Stallcup, M.R. Regulation of transcription by a protein methyltransferase. Science 1999, 284, 2174–2177. [Google Scholar] [CrossRef] [PubMed]
- Onate, S.A.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 1995, 270, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.; Nguyen, P.; Shinsako, J.; Anderson, C.; Feng, W.; Nguyen, M.P.; Chen, D.; Huang, S.M.; Subramanian, S.; McKinerney, E.; et al. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol. Endocrinol. 1998, 12, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Leo, C.; Chen, J.D. The SRC family of nuclear receptor coactivators. Gene 2000, 245, 1–11. [Google Scholar] [CrossRef]
- Heery, D.M.; Kalkhoven, E.; Hoare, S.; Parker, M.G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 1997, 387, 733–736. [Google Scholar] [CrossRef]
- Leers, J.; Treuter, E.; Gustafsson, J.A. Mechanistic principles in NR box-dependent interaction between nuclear hormone receptors and the coactivator TIF2. Mol. Cell. Biol. 1998, 18, 6001–6013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McInerney, E.M.; Rose, D.W.; Flynn, S.E.; Westin, S.; Mullen, T.M.; Krones, A.; Inostroza, J.; Torchia, J.; Nolte, R.T.; Assa-Munt, N.; et al. Determinants of coactivator LXXLL motif specificity in nuclear receptor transcriptional activation. Gene Dev. 1998, 12, 3357–3368. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Ferrington, D.A.; Bigelow, D.J.; Michaelis, E.K. Protein half-lives of two subunits of an NMDA receptor-like complex, the 71-kDa glutamate-binding and the 80-kDa CPP-binding protein. Biochem. Bioph. Res. Co. 1997, 241, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Xu, L.; Heinzel, T.; Torchia, J.; Kurokawa, R.; Gloss, B.; Lin, S.C.; Heyman, R.A.; Rose, D.W.; Glass, C.K.; et al. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 1996, 85, 403–414. [Google Scholar] [CrossRef]
- McKenna, N.J.; Nawaz, Z.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 11697–11702. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Arita, T.; Asao, H.; Tanaka, N.; Higuchi, M.; Kuroda, H.; Kaneko, K.; Munakata, H.; Endo, Y.; Fujita, T.; et al. Cloning of a novel signal-transducing adaptor molecule containing an SH3 domain and ITAM. Biochem. Bioph. Res. Co. 1996, 225, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Rachez, C.; Gamble, M.; Chang, C.P.; Atkins, G.B.; Lazar, M.A.; Freedman, L.P. The DRIP complex and SRC-1/p160 coactivators share similar nuclear receptor binding determinants but constitute functionally distinct complexes. Mol. Cell Biol. 2000, 20, 2718–2726. [Google Scholar] [CrossRef]
- Llopis, J.; Westin, S.; Ricote, M.; Wang, Z.; Cho, C.Y.; Kurokawa, R.; Mullen, T.M.; Rose, D.W.; Rosenfeld, M.G.; Tsien, R.Y.; et al. Ligand-dependent interactions of coactivators steroid receptor coactivator-1 and peroxisome proliferator-activated receptor binding protein with nuclear hormone receptors can be imaged in live cells and are required for transcription. Proc. Natl. Acad. Sci. USA 2000, 97, 4363–4368. [Google Scholar] [CrossRef]
- Siegrist-Kaiser, C.A.; Juge-Aubry, C.; Tranter, M.P.; Ekenbarger, D.M.; Leonard, J.L. Thyroxine-dependent modulation of actin polymerization in cultured astrocytes. A novel, extranuclear action of thyroid hormone. J. Biol. Chem. 1990, 265, 5296–5302. [Google Scholar]
- Sterling, K.; Brenner, M.A.; Sakurada, T. Rapid effect of triiodothyronine on the mitochondrial pathway in rat liver in vivo. Science 1980, 210, 340–342. [Google Scholar] [CrossRef]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.F.; Haas, T.A.; Zhang, L.; Loftus, J.; Smith, J.W. Ligand binding to integrins. J. Biol. Chem. 2000, 275, 21785–21788. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Cody, V.; Davis, F.B.; Hercbergs, A.A.; Luidens, M.K.; Mousa, S.A.; Davis, P.J. Identification and functions of the plasma membrane receptor for thyroid hormone analogues. Discov Med. 2011, 11, 337–347. [Google Scholar] [PubMed]
- Davis, P.J.; Davis, F.B.; Mousa, S.A.; Luidens, M.K.; Lin, H.Y. Membrane receptor for thyroid hormone: physiologic and pharmacologic implications. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Shih, A.; Davis, F.B.; Davis, P.J. Thyroid hormone promotes the phosphorylation of STAT3 and potentiates the action of epidermal growth factor in cultured cells. Biochem J. 1999, 338 Pt 2, 427–432. [Google Scholar] [CrossRef]
- Cao, H.J.; Lin, H.Y.; Luidens, M.K.; Davis, F.B.; Davis, P.J. Cytoplasm-to-nucleus shuttling of thyroid hormone receptor-beta1 (Trbeta1) is directed from a plasma membrane integrin receptor by thyroid hormone. Endocr. Res. 2009, 34, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Sun, M.; Tang, H.Y.; Lin, C.; Luidens, M.K.; Mousa, S.A.; Incerpi, S.; Drusano, G.L.; Davis, F.B.; Davis, P.J. L-Thyroxine vs. 3,5,3’-triiodo-L-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am. J. Physiol. Cell Physiol. 2009, 296, C980–C991. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Lin, H.Y.; Mousa, S.A.; Luidens, M.K.; Hercbergs, A.A.; Wehling, M.; Davis, F.B. Overlapping nongenomic and genomic actions of thyroid hormone and steroids. Steroids 2011, 76, 829–833. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, X.; Kambe, F.; Moeller, L.C.; Refetoff, S.; Seo, H. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol. Endocrinol. 2005, 19, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Moeller, L.C.; Cao, X.; Dumitrescu, A.M.; Seo, H.; Refetoff, S. Thyroid hormone mediated changes in gene expression can be initiated by cytosolic action of the thyroid hormone receptor beta through the phosphatidylinositol 3-kinase pathway. Nucl. Recept. Signal. 2006, 4, e020. [Google Scholar] [CrossRef] [PubMed]
- Moeller, L.C.; Dumitrescu, A.M.; Refetoff, S. Cytosolic action of thyroid hormone leads to induction of hypoxia-inducible factor-1alpha and glycolytic genes. Mol. Endocrinol 2005, 19, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, M.; Lei, J.; Ingbar, D.H. Nongenomic actions of L-thyroxine and 3,5,3’-triiodo-L-thyronine. Focus on "L-Thyroxine vs. 3,5,3’-triiodo-L-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase". Am. J. Physiol. Cell Physiol. 2009, 296, C977–C979. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Glinsky, G.V.; Lin, H.Y.; Leith, J.T.; Hercbergs, A.; Tang, H.Y.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Cancer Cell Gene Expression Modulated from Plasma Membrane Integrin alphavbeta3 by Thyroid Hormone and Nanoparticulate Tetrac. Front. Endocrinol. (Lausanne) 2014, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Lin, K.H.; Hsu, Y.C. Alterations of thyroid hormone receptor alpha gene: frequency and association with Nm23 protein expression and metastasis in gastric cancer. Cancer Lett. 2002, 175, 121–127. [Google Scholar] [CrossRef]
- Lu, C.; Mishra, A.; Zhu, Y.J.; Meltzer, P.; Cheng, S.Y. Global expression profiling reveals gain-of-function oncogenic activity of a mutated thyroid hormone receptor in thyroid carcinogenesis. Am. J. Cancer Res. 2011, 1, 168–191. [Google Scholar] [PubMed]
- Lu, C.; Zhu, X.; Willingham, M.C.; Cheng, S.Y. Activation of tumor cell proliferation by thyroid hormone in a mouse model of follicular thyroid carcinoma. Oncogene 2012, 31, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Zhao, L.; Cheng, S.Y. Inhibition of estrogen-dependent tumorigenesis by the thyroid hormone receptor beta in xenograft models. Am. J. Cancer Res. 2013, 3, 302–311. [Google Scholar]
- Lin, K.H.; Zhu, X.G.; Shieh, H.Y.; Hsu, H.C.; Chen, S.T.; McPhie, P.; Cheng, S.Y. Identification of naturally occurring dominant negative mutants of thyroid hormone alpha 1 and beta 1 receptors in a human hepatocellular carcinoma cell line. Endocrinology 1996, 137, 4073–4081. [Google Scholar] [CrossRef]
- Kamiya, Y.; Puzianowska-Kuznicka, M.; McPhie, P.; Nauman, J.; Cheng, S.Y.; Nauman, A. Expression of mutant thyroid hormone nuclear receptors is associated with human renal clear cell carcinoma. Carcinogenesis 2002, 23, 25–33. [Google Scholar] [CrossRef]
- Heublein, S.; Mayr, D.; Meindl, A.; Angele, M.; Gallwas, J.; Jeschke, U.; Ditsch, N. Thyroid Hormone Receptors Predict Prognosis in BRCA1 Associated Breast Cancer in Opposing Ways. PloS ONE 2015, 10. [Google Scholar] [CrossRef]
- Plateroti, M.; Kress, E.; Mori, J.I.; Samarut, J. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol. Cell Biol. 2006, 26, 3204–3214. [Google Scholar] [CrossRef] [PubMed]
- Hellevik, A.I.; Asvold, B.O.; Bjoro, T.; Romundstad, P.R.; Nilsen, T.I.; Vatten, L.J. Thyroid function and cancer risk: a prospective population study. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Moeller, L.C.; Fuhrer, D. Thyroid hormone, thyroid hormone receptors, and cancer: a clinical perspective. Endocr. Relat. Cancer 2013, 20, R19–R29. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Yamamura, Y.; Kau, S.W.; Bevers, T.; Strom, S.; Patangan, M.; Hsu, L.; Krishnamurthy, S.; Theriault, R.L.; Hortobagyi, G.N. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer-Am. Cancer Soc. 2005, 103, 1122–1128. [Google Scholar] [CrossRef]
- Tang, H.Y.; Lin, H.Y.; Zhang, S.; Davis, F.B.; Davis, P.J. Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology 2004, 145, 3265–3272. [Google Scholar] [CrossRef] [PubMed]
- Sar, P.; Peter, R.; Rath, B.; Das Mohapatra, A.; Mishra, S.K. 3, 3’5 Triiodo L thyronine induces apoptosis in human breast cancer MCF-7 cells, repressing SMP30 expression through negative thyroid response elements. PLoS ONE 2011, 6, e20861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzalez-Sancho, J.M.; Figueroa, A.; Lopez-Barahona, M.; Lopez, E.; Beug, H.; Munoz, A. Inhibition of proliferation and expression of T1 and cyclin D1 genes by thyroid hormone in mammary epithelial cells. Mol. Carcinog. 2002, 34, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Guigon, C.J.; Kim, D.W.; Willingham, M.C.; Cheng, S.Y. Mutation of thyroid hormone receptor-beta in mice predisposes to the development of mammary tumors. Oncogene 2011, 30, 3381–3390. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tang, H.Y.; Shih, A.; Keating, T.; Cao, G.; Davis, P.J.; Davis, F.B. Thyroid hormone is a MAPK-dependent growth factor for thyroid cancer cells and is anti-apoptotic. Steroids 2007, 72, 180–187. [Google Scholar] [CrossRef]
- Furuya, F.; Lu, C.; Willingham, M.C.; Cheng, S.Y. Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis 2007, 28, 2451–2458. [Google Scholar] [CrossRef]
- Meng, R.; Tang, H.Y.; Westfall, J.; London, D.; Cao, J.H.; Mousa, S.A.; Luidens, M.; Hercbergs, A.; Davis, F.B.; Davis, P.J.; et al. Crosstalk between integrin alphavbeta3 and estrogen receptor-alpha is involved in thyroid hormone-induced proliferation in human lung carcinoma cells. PLoS ONE 2011, 6, e27547. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.B.; Tang, H.Y.; Shih, A.; Keating, T.; Lansing, L.; Hercbergs, A.; Fenstermaker, R.A.; Mousa, A.; Mousa, S.A.; Davis, P.J.; et al. Acting via a cell surface receptor, thyroid hormone is a growth factor for glioma cells. Cancer Res. 2006, 66, 7270–7275. [Google Scholar] [CrossRef] [PubMed]
- Hercbergs, A.; Johnson, R.E.; Ashur-Fabian, O.; Garfield, D.H.; Davis, P.J. Medically induced euthyroid hypothyroxinemia may extend survival in compassionate need cancer patients: an observational study. Oncologist 2015, 20, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Tang, H.Y.; Keating, T.; Wu, Y.H.; Shih, A.; Hammond, D.; Sun, M.; Hercbergs, A.; Davis, F.B.; Davis, P.J. Resveratrol is pro-apoptotic and thyroid hormone is anti-apoptotic in glioma cells: both actions are integrin and ERK mediated. Carcinogenesis 2008, 29, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Lin, C.L.; Lieu, A.S.; Hwang, Y.F.; Howng, S.L.; Hong, Y.R.; Chang, D.S.; Lee, K.S. The expression of thyroid hormone receptor isoforms in human astrocytomas. Surg. Neurol. 2008, 70 (Suppl 1), S4–S8, discussion S1:8. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.C.; Huang, Y.H.; Liao, C.Y.; Liao, C.J.; Cheng, W.L.; Chen, W.J.; Lin, K.H. Mediation of the inhibitory effect of thyroid hormone on proliferation of hepatoma cells by transforming growth factor-beta. J. Mol. Endocrinol. 2006, 36, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Huang, Y.H.; Wu, M.H.; Wu, S.M.; Chi, H.C.; Liao, C.J.; Chen, C.Y.; Tseng, Y.H.; Tsai, C.Y.; Tsai, M.M.; et al. Thyroid hormone suppresses cell proliferation through endoglin-mediated promotion of p21 stability. Oncogene 2013, 32, 3904–3914. [Google Scholar] [CrossRef]
- Mishkin, S.Y.; Pollack, R.; Yalovsky, M.A.; Morris, H.P.; Mishkin, S. Inhibition of local and metastatic hepatoma growth and prolongation of survival after induction of hypothyroidism. Cancer Res. 1981, 41, 3040–3045. [Google Scholar]
- Chi, H.C.; Liao, C.H.; Huang, Y.H.; Wu, S.M.; Tsai, C.Y.; Liao, C.J.; Tseng, Y.H.; Lin, Y.H.; Chen, C.Y.; Chung, I.H.; et al. Thyroid hormone receptor inhibits hepatoma cell migration through transcriptional activation of Dickkopf 4. Biochem. Biophys. Res. Commun. 2013, 439, 60–65. [Google Scholar] [CrossRef]
- Ventura-Holman, T.; Mamoon, A.; Subauste, M.C.; Subauste, J.S. The effect of oncoprotein v-erbA on thyroid hormone-regulated genes in hepatocytes and their potential role in hepatocellular carcinoma. Mol. Biol. Rep. 2011, 38, 1137–1144. [Google Scholar] [CrossRef]
- Chung, I.H.; Chen, C.Y.; Lin, Y.H.; Chi, H.C.; Huang, Y.H.; Tai, P.J.; Liao, C.J.; Tsai, C.Y.; Lin, S.L.; Wu, M.H.; et al. Thyroid hormone-mediated regulation of lipocalin 2 through the Met/FAK pathway in liver cancer. Oncotarget 2015, 6, 15050–15064. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xia, L.; Ma, S.; Qi, X.; Li, Q.; Xia, Y.; Tang, X.; Cui, D.; Wang, Z.; Chi, J.; et al. Hepatocellular carcinoma: thyroid hormone promotes tumorigenicity through inducing cancer stem-like cell self-renewal. Sci. Rep. 2016, 6, 25183. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, G.V.; Rochira, A.; Leone, A.; Damiano, F.; Marsigliante, S.; Siculella, L. 3,5,3’triiodo-L-thyronine induces SREBP-1 expression by non-genomic actions in human HEP G2 cells. J. Cell Physiol. 2012, 227, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Yeh, C.T.; Huang, Y.H.; Wu, S.M.; Chi, H.C.; Tsai, M.M.; Tsai, C.Y.; Liao, C.J.; Tseng, Y.H.; Lin, Y.H.; et al. Dickkopf 4 positively regulated by the thyroid hormone receptor suppresses cell invasion in human hepatoma cells. Hepatology 2012, 55, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.T.; Hsieh, M.T.; Yang, S.H.; Tsai, P.W.; Wang, S.H.; Wang, C.C.; Lee, Y.S.; Cheng, G.Y.; HuangFu, W.C.; London, D.; et al. Anti-proliferative and gene expression actions of resveratrol in breast cancer cells in vitro. Oncotarget 2014, 5, 12891–12907. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Chin, Y.T.; Yang, Y.S.H.; Wei, P.L.; Wu, H.C.; Shih, A.; Lu, Y.T.; Pedersen, J.Z.; Incerpi, S.; Liu, L.F.; et al. The combination of tetraiodothyroacetic acid and cetuximab inhibits cell proliferation in colorectal cancers with different K-ras status. Steroids 2016, 111, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Dentice, M.; Luongo, C.; Ambrosio, R.; Sibilio, A.; Casillo, A.; Iaccarino, A.; Troncone, G.; Fenzi, G.; Larsen, P.R.; Salvatore, D. beta-Catenin regulates deiodinase levels and thyroid hormone signaling in colon cancer cells. Gastroenterology 2012, 143, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Kress, E.; Skah, S.; Sirakov, M.; Nadjar, J.; Gadot, N.; Scoazec, J.Y.; Samarut, J.; Plateroti, M. Cooperation between the thyroid hormone receptor TRalpha1 and the WNT pathway in the induction of intestinal tumorigenesis. Gastroenterology 2010, 138, 1863–1874. [Google Scholar] [CrossRef]
- Glushakov, R.I.; Proshin, S.N.; Tapil’skaya, N.I. The incidence of breast tumor during experimental hyperthyroidism. Bull. Exp. Biol Med. 2013, 156, 245–247. [Google Scholar] [CrossRef]
- Conde, S.J.; Luvizotto Rde, A.; de Sibio, M.T.; Nogueira, C.R. Thyroid hormone status interferes with estrogen target gene expression in breast cancer samples in menopausal women. ISRN Endocrinol. 2014, 2014, 317398. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, P.P.; Figueiredo, N.B.; Padovani, C.R.; Brentani, M.M.; Nogueira, C.R. Profile of thyroid hormones in breast cancer patients. Braz. J. Med. Biol. Res. 2005, 38, 761–765. [Google Scholar] [CrossRef]
- Lopez-Fontana, C.M.; Sasso, C.V.; Maselli, M.E.; Santiano, F.E.; Semino, S.N.; Cuello Carrion, F.D.; Jahn, G.A.; Caron, R.W. Experimental hypothyroidism increases apoptosis in dimethylbenzanthracene-induced mammary tumors. Oncol. Rep. 2013, 30, 1651–1660. [Google Scholar] [CrossRef]
- Huang, J.; Jin, L.; Ji, G.; Xing, L.; Xu, C.; Xiong, X.; Li, H.; Wu, K.; Ren, G.; Kong, L. Implication from thyroid function decreasing during chemotherapy in breast cancer patients: chemosensitization role of triiodothyronine. BMC Cancer 2013, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Dinda, S.; Sanchez, A.; Moudgil, V. Estrogen-like effects of thyroid hormone on the regulation of tumor suppressor proteins, p53 and retinoblastoma, in breast cancer cells. Oncogene 2002, 21, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.C.; Salazar, E.P.; Kane, S.R.; Liu, N. Effects of thyroid hormones on human breast cancer cell proliferation. J. Steroid Biochem. Mol. Biol. 2008, 109, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.M.; Sheikh, M.S.; Rishi, A.K.; Dawson, M.I.; Li, X.S.; Wilber, J.F.; Feng, P.; Fontana, J.A. Thyroid hormone enhancement of estradiol stimulation of breast carcinoma proliferation. Exp. Cell Res. 1995, 218, 1–8. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- Davis, F.B.; Mousa, S.A.; O’Connor, L.; Mohamed, S.; Lin, H.Y.; Cao, H.J.; Davis, P.J. Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ. Res. 2004, 94, 1500–1506. [Google Scholar] [CrossRef]
- Lombardi, A.; Moreno, M.; de Lange, P.; Iossa, S.; Busiello, R.A.; Goglia, F. Regulation of skeletal muscle mitochondrial activity by thyroid hormones: focus on the "old" triiodothyronine and the "emerging" 3,5-diiodothyronine. Front. Physiol. 2015, 6, 237. [Google Scholar] [CrossRef]
- Ditsch, N.; Toth, B.; Himsl, I.; Lenhard, M.; Ochsenkuhn, R.; Friese, K.; Mayr, D.; Jeschke, U. Thyroid hormone receptor (TR)alpha and TRbeta expression in breast cancer. Histol Histopathol 2013, 28, 227–237. [Google Scholar] [CrossRef]
- Jerzak, K.J.; Cockburn, J.; Pond, G.R.; Pritchard, K.I.; Narod, S.A.; Dhesy-Thind, S.K.; Bane, A. Thyroid hormone receptor alpha in breast cancer: prognostic and therapeutic implications. Breast Cancer Res. Treat. 2015, 149, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Zarebczan, B.; Chen, H. Multi-targeted approach in the treatment of thyroid cancer. Minerva Chir. 2010, 65, 59–69. [Google Scholar] [PubMed]
- Ocak, S.; Akten, A.O.; Tez, M. Thyroid cancer in hyperthyroid patients: is it different clinical entity? Endocr. Regul. 2014, 48, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Hercbergs, A.; Luidens, M.K.; Lin, H.Y. Recurrence of differentiated thyroid carcinoma during full TSH suppression: is the tumor now thyroid hormone dependent? Horm. Cancer 2015, 6, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Romitti, M.; Wajner, S.M.; Zennig, N.; Goemann, I.M.; Bueno, A.L.; Meyer, E.L.; Maia, A.L. Increased type 3 deiodinase expression in papillary thyroid carcinoma. Thyroid 2012, 22, 897–904. [Google Scholar] [CrossRef]
- Faber, J.; Poulsen, S.; Iversen, P.; Kirkegaard, C. Thyroid hormone turnover in patients with small cell carcinoma of the lung. Acta Endocrinol. (Copenh) 1988, 118, 460–464. [Google Scholar] [CrossRef]
- Ratcliffe, J.G.; Stack, B.H.; Burt, R.W.; Radcliffe, W.A.; Spilg, W.G.; Cuthbert, J.; Kennedy, R.S. Thyroid function in lung cancer. Br. Med. J. 1978, 1, 210–212. [Google Scholar] [CrossRef]
- Yasar, Z.A.; Kirakli, C.; Yilmaz, U.; Ucar, Z.Z.; Talay, F. Can non-thyroid illness syndrome predict mortality in lung cancer patients? A prospective cohort study. Horm. Cancer 2014, 5, 240–246. [Google Scholar] [CrossRef]
- Kinoshita, S.; Sone, S.; Yamashita, T.; Tsubura, E.; Ogura, T. Effects of experimental hyper- and hypothyroidism on natural defense activities against Lewis lung carcinoma and its spontaneous pulmonary metastases in C57BL/6 mice. Tokushima J. Exp. Med. 1991, 38, 25–35. [Google Scholar]
- Iwasaki, Y.; Sunaga, N.; Tomizawa, Y.; Imai, H.; Iijima, H.; Yanagitani, N.; Horiguchi, K.; Yamada, M.; Mori, M. Epigenetic inactivation of the thyroid hormone receptor beta1 gene at 3p24.2 in lung cancer. Ann. Surg. Oncol. 2010, 17, 2222–2228. [Google Scholar] [CrossRef]
- Bunevicius, A.; Deltuva, V.; Tamasauskas, S.; Tamasauskas, A.; Laws, E.R., Jr.; Bunevicius, R. Low triiodothyronine syndrome as a predictor of poor outcomes in patients undergoing brain tumor surgery: a pilot study: clinical article. J. Neurosurg. 2013, 118, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; Deltuva, V.P.; Tamasauskas, S.; Smith, T.; Laws, E.R.; Bunevicius, R.; Iervasi, G.; Tamasauskas, A. Preoperative low tri-iodothyronine concentration is associated with worse health status and shorter five year survival of primary brain tumor patients. Oncotarget 2017, 8, 8648–8656. [Google Scholar] [CrossRef] [PubMed]
- Monden, T.; Nakajima, Y.; Hashida, T.; Ishii, S.; Tomaru, T.; Shibusawa, N.; Hashimoto, K.; Satoh, T.; Yamada, M.; Mori, M.; et al. Expression of thyroid hormone receptor isoforms down-regulated by thyroid hormone in human medulloblastoma cells. Endocr. J. 2006, 53, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Kaseb, A.; Li, D.; Patt, Y.Z.; Vauthey, J.N.; Thomas, M.B.; Curley, S.A.; Spitz, M.R.; Sherman, S.I.; Abdalla, E.K.; et al. Association between hypothyroidism and hepatocellular carcinoma: a case-control study in the United States. Hepatology 2009, 49, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Huang, Y.H.; Lin, K.H.; Chu, Y.D.; Yeh, C.T. Identification of Functional Thyroid Stimulating Hormone Receptor and TSHR Gene Mutations in Hepatocellular Carcinoma. Anticancer Res. 2018, 38, 2793–2802. [Google Scholar] [CrossRef]
- Chan, I.H.; Privalsky, M.L. Thyroid hormone receptors mutated in liver cancer function as distorted antimorphs. Oncogene 2006, 25, 3576–3588. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Shieh, H.Y.; Chen, S.L.; Hsu, H.C. Expression of mutant thyroid hormone nuclear receptors in human hepatocellular carcinoma cells. Mol. Carcinog. 1999, 26, 53–61. [Google Scholar] [CrossRef]
- Lin, K.H.; Zhu, X.G.; Hsu, H.C.; Chen, S.L.; Shieh, H.Y.; Chen, S.T.; McPhie, P.; Cheng, S.Y. Dominant negative activity of mutant thyroid hormone alpha1 receptors from patients with hepatocellular carcinoma. Endocrinology 1997, 138, 5308–5315. [Google Scholar] [CrossRef] [PubMed]
- Barlow, C.; Meister, B.; Lardelli, M.; Lendahl, U.; Vennstrom, B. Thyroid abnormalities and hepatocellular carcinoma in mice transgenic for v-erbA. EMBO J. 1994, 13, 4241–4250. [Google Scholar] [CrossRef]
- Rennert, G.; Rennert, H.S.; Pinchev, M.; Gruber, S.B. A case-control study of levothyroxine and the risk of colorectal cancer. J. Natl. Cancer Inst. 2010, 102, 568–572. [Google Scholar] [CrossRef]
- Rose, D.P.; Davis, T.E. Plasma thyronine levels in carcinoma of the breast and colon. Arch. Intern. Med. 1981, 141, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, A.; Wiedle, G.; Borisch, B.; Birrer, S.; Luder, P.; Imhof, B.A. Integrin alpha(v)beta(3) expression in colon carcinoma correlates with survival. Mod. Pathol. 2001, 14, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Iishi, H.; Tatsuta, M.; Baba, M.; Okuda, S.; Taniguchi, H. Enhancement by thyroxine of experimental carcinogenesis induced in rat colon by azoxymethane. Int. J. Cancer 1992, 50, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Iishi, H.; Tatsuta, M.; Baba, M.; Taniguchi, H. Monoamine oxidase B inhibitor enhances experimental carcinogenesis in rat colon induced by azoxymethane. Cancer Lett. 1994, 76, 177–183. [Google Scholar] [CrossRef]
- Kress, E.; Rezza, A.; Nadjar, J.; Samarut, J.; Plateroti, M. The thyroid hormone receptor-alpha (TRalpha) gene encoding TRalpha1 controls deoxyribonucleic acid damage-induced tissue repair. Mol. Endocrinol. 2008, 22, 47–55. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Markowitz, S.; Haut, M.; Stellato, T.; Gerbic, C.; Molkentin, K. Expression of the ErbA-beta class of thyroid hormone receptors is selectively lost in human colon carcinoma. J. Clin. Invest. 1989, 84, 1683–1687. [Google Scholar] [CrossRef]
- Pietrzak, M.; Puzianowska-Kuznicka, M. Triiodothyronine utilizes phosphatidylinositol 3-kinase pathway to activate anti-apoptotic myeloid cell leukemia-1. J. Mol. Endocrinol. 2008, 41, 177–186. [Google Scholar] [CrossRef]
- Ho, Y.; Lin, Y.S.; Liu, H.L.; Shih, Y.J.; Lin, S.Y.; Shih, A.; Chin, Y.T.; Chen, Y.R.; Lin, H.Y.; Davis, P.J. Biological Mechanisms by Which Antiproliferative Actions of Resveratrol Are Minimized. Nutrients 2017, 9, 1046. [Google Scholar] [CrossRef]
- Mousa, S.A.; Lin, H.Y.; Tang, H.Y.; Hercbergs, A.; Luidens, M.K.; Davis, P.J. Modulation of angiogenesis by thyroid hormone and hormone analogues: implications for cancer management. Angiogenesis 2014, 17, 463–469. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chin, Y.T.; Yang, Y.C.; Lai, H.Y.; Wang-Peng, J.; Liu, L.F.; Tang, H.Y.; Davis, P.J. Thyroid Hormone, Cancer, and Apoptosis. Compr. Physiol. 2016, 6, 1221–1237. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Sudha, T.; Lin, H.Y.; Mousa, S.A. Thyroid Hormone, Hormone Analogs, and Angiogenesis. Compr. Physiol. 2015, 6, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Peeters, R.P.; Visser, T.J. Metabolism of Thyroid Hormone, Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; South Dartmouth, MA, USA, 2000.
- Senese, R.; Cioffi, F.; de Lange, P.; Goglia, F.; Lanni, A. Thyroid: biological actions of ’nonclassical’ thyroid hormones. J. Endocrinol. 2014, 221, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; de Lange, P.; Lombardi, A.; Silvestri, E.; Lanni, A.; Goglia, F. Metabolic effects of thyroid hormone derivatives. Thyroid 2008, 18, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Brenta, G.; Danzi, S.; Klein, I. Potential therapeutic applications of thyroid hormone analogs. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 632–640. [Google Scholar] [CrossRef]
- Webb, P. Selective activators of thyroid hormone receptors. Expert. Opin. Investig. Drugs 2004, 13, 489–500. [Google Scholar] [CrossRef]
- Baxter, J.D.; Webb, P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat. Rev. Drug Discov. 2009, 8, 308–320. [Google Scholar] [CrossRef]
- Glinskii, A.B.; Glinsky, G.V.; Lin, H.Y.; Tang, H.Y.; Sun, M.; Davis, F.B.; Luidens, M.K.; Mousa, S.A.; Hercbergs, A.H.; Davis, P.J. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle 2009, 8, 3562–3570. [Google Scholar] [CrossRef]
- Yalcin, M.; Dyskin, E.; Lansing, L.; Bharali, D.J.; Mousa, S.S.; Bridoux, A.; Hercbergs, A.H.; Lin, H.Y.; Davis, F.B.; Glinsky, G.V.; et al. Tetraiodothyroacetic acid (tetrac) and nanoparticulate tetrac arrest growth of medullary carcinoma of the thyroid. J. Clin. Endocrinol. Metab. 2010, 95, 1972–1980. [Google Scholar] [CrossRef]
- Davidson, B.; Berner, A.; Nesland, J.M.; Risberg, B.; Berner, H.S.; Trope, C.G.; Kristensen, G.B.; Bryne, M.; Ann Florenes, V. E-cadherin and alpha-, beta-, and gamma-catenin protein expression is up-regulated in ovarian carcinoma cells in serous effusions. J. Pathol. 2000, 192, 460–469. [Google Scholar] [CrossRef]
- Imai, T.; Horiuchi, A.; Shiozawa, T.; Osada, R.; Kikuchi, N.; Ohira, S.; Oka, K.; Konishi, I. Elevated expression of E-cadherin and alpha-, beta-, and gamma-catenins in metastatic lesions compared with primary epithelial ovarian carcinomas. Hum. Pathol. 2004, 35, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- King, T.D.; Suto, M.J.; Li, Y. The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J. Cell Biochem. 2012, 113, 13–18. [Google Scholar] [CrossRef] [PubMed]
- White, B.D.; Chien, A.J.; Dawson, D.W. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology 2012, 142, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, P.; Vermeulen, S.; Mareel, M. The role of the E-cadherin/catenin complex in gastrointestinal cancer. Acta Gastroenterol Belg 1999, 62, 393–402. [Google Scholar] [PubMed]
- Fanjul-Fernandez, M.; Quesada, V.; Cabanillas, R.; Cadinanos, J.; Fontanil, T.; Obaya, A.; Ramsay, A.J.; Llorente, J.L.; Astudillo, A.; Cal, S.; et al. Cell-cell adhesion genes CTNNA2 and CTNNA3 are tumour suppressors frequently mutated in laryngeal carcinomas. Nat. Commun. 2013, 4, 2531. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.N.; Huang, Y.H.; Lin, Y.C.; Yeh, C.T.; Liang, Y.; Chen, S.L.; Lin, K.H. Thyroid hormone promotes cell invasion through activation of furin expression in human hepatoma cell lines. Endocrinology 2008, 149, 3817–3831. [Google Scholar] [CrossRef] [PubMed]
- de Franciscis, S.; Serra, R. Matrix metalloproteinases and endothelial dysfunction: The search for new prognostic markers and for new therapeutic targets for vascular wall imbalance. Thromb. Res. 2015, 136, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.X.; Wei, D.; Liu, M.; Gao, A.C.; Ali-Osman, F.; Sawaya, R.; Huang, S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 2004, 23, 3550–3560. [Google Scholar] [CrossRef]
- Lu, C.; Cheng, S.Y. Extranuclear signaling of mutated thyroid hormone receptors in promoting metastatic spread in thyroid carcinogenesis. Steroids 2011, 76, 885–891. [Google Scholar] [CrossRef]
- Takeda, T.; Suzuki, S.; Liu, R.T.; DeGroot, L.J. Triiodothyroacetic acid has unique potential for therapy of resistance to thyroid hormone. J. Clin. Endocrinol. Metab. 1995, 80, 2033–2040. [Google Scholar] [CrossRef]
- Shinderman-Maman, E.; Cohen, K.; Moskovich, D.; Hercbergs, A.; Werner, H.; Davis, P.J.; Ellis, M.; Ashur-Fabian, O. Thyroid hormones derivatives reduce proliferation and induce cell death and DNA damage in ovarian cancer. Sci. Rep. 2017, 7, 16475. [Google Scholar] [CrossRef] [PubMed]
- Stavreva, D.A.; Varticovski, L.; Levkova, L.; George, A.A.; Davis, L.; Pegoraro, G.; Blazer, V.; Iwanowicz, L.; Hager, G.L. Novel cell-based assay for detection of thyroid receptor beta-interacting environmental contaminants. Toxicology 2016, 368–369, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, S.; Peeters, R.P.; Visser, T.J.; Visser, W.E. Triiodothyroacetic acid in health and disease. J. Endocrinol. 2017, 234, R99–R121. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, J.M.; Hartman, N.; Henson, L.C.; Lieberman, J.; Williams, D.E.; Wong, M.; Hershman, J.M. 3,5,3’-triiodothyroacetic acid therapy for thyroid hormone resistance. J. Clin. Endocrinol. Metab. 1989, 69, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Dulgeroff, A.J.; Geffner, M.E.; Koyal, S.N.; Wong, M.; Hershman, J.M. Bromocriptine and Triac therapy for hyperthyroidism due to pituitary resistance to thyroid hormone. J. Clin. Endocrinol. Metab. 1992, 75, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Radetti, G.; Persani, L.; Molinaro, G.; Mannavola, D.; Cortelazzi, D.; Chatterjee, V.K.; Beck-Peccoz, P. Clinical and hormonal outcome after two years of triiodothyroacetic acid treatment in a child with thyroid hormone resistance. Thyroid 1997, 7, 775–778. [Google Scholar] [CrossRef]
- Salmela, P.I.; Wide, L.; Juustila, H.; Ruokonen, A. Effects of thyroid hormones (T4,T3), bromocriptine and Triac on inappropriate TSH hypersecretion. Clin. Endocrinol. (Oxf.) 1988, 28, 497–507. [Google Scholar] [CrossRef]
- Torre, P.; Bertoli, M.; Di Giovanni, S.; Scommegna, S.; Conte, C.; Novelli, G.; Cianfarani, S. Endocrine and neuropsychological assessment in a child with a novel mutation of thyroid hormone receptor: response to 12-month triiodothyroacetic acid (TRIAC) therapy. J. Endocrinol. Invest. 2005, 28, 657–662. [Google Scholar] [CrossRef]
- Rivolta, C.M.; Mallea Gil, M.S.; Ballarino, C.; Ridruejo, M.C.; Miguel, C.M.; Gimenez, S.B.; Bernacchi, S.S.; Targovnik, H.M. A novel 1297-1304delGCCTGCCA mutation in the exon 10 of the thyroid hormone receptor beta gene causes resistance to thyroid hormone. Mol. Diagn. 2004, 8, 163–169. [Google Scholar]
- Darendeliler, F.; Bas, F. Successful therapy with 3,5,3’-triiodothyroacetic acid (TRIAC) in pituitary resistance to thyroid hormone. J. Pediatr. Endocrinol. Metab. 1997, 10, 535–538. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Piscitelli, G.; Cattaneo, M.G.; Faglia, G. Successful treatment of hyperthyroidism due to nonneoplastic pituitary TSH hypersecretion with 3,5,3’-triiodothyroacetic acid (TRIAC). J. Endocrinol. Invest. 1983, 6, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Beck-Peccoz, P.; Mariotti, S.; Guillausseau, P.J.; Medri, G.; Piscitelli, G.; Bertoli, A.; Barbarino, A.; Rondena, M.; Chanson, P.; Pinchera, A. Treatment of hyperthyroidism due to inappropriate secretion of thyrotropin with the somatostatin analog SMS 201-995. J. Clin. Endocrinol. Metab. 1989, 68, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.; Eber, O. Treatment of inappropriate TSH secretion with Triac. Acta Med. Austriaca 1986, 13, 13–16. [Google Scholar] [PubMed]
- Medeiros-Neto, G.; Kallas, W.G.; Knobel, M.; Cavaliere, H.; Mattar, E. Triac (3,5,3’-triiodothyroacetic acid) partially inhibits the thyrotropin response to synthetic thyrotropin-releasing hormone in normal and thyroidectomized hypothyroid patients. J. Clin. Endocrinol. Metab. 1980, 50, 223–225. [Google Scholar] [CrossRef]
- Falcone, M.; Miyamoto, T.; Fierro-Renoy, F.; Macchia, E.; DeGroot, L.J. Antipeptide polyclonal antibodies specifically recognize each human thyroid hormone receptor isoform. Endocrinology 1992, 131, 2419–2429. [Google Scholar] [CrossRef]
- Hodin, R.A.; Lazar, M.A.; Chin, W.W. Differential and tissue-specific regulation of the multiple rat c-erbA messenger RNA species by thyroid hormone. J. Clin. Invest. 1990, 85, 101–105. [Google Scholar] [CrossRef]
- Strait, K.A.; Schwartz, H.L.; Perez-Castillo, A.; Oppenheimer, J.H. Relationship of c-erbA mRNA content to tissue triiodothyronine nuclear binding capacity and function in developing and adult rats. J. Biol. Chem. 1990, 265, 10514–10521. [Google Scholar]
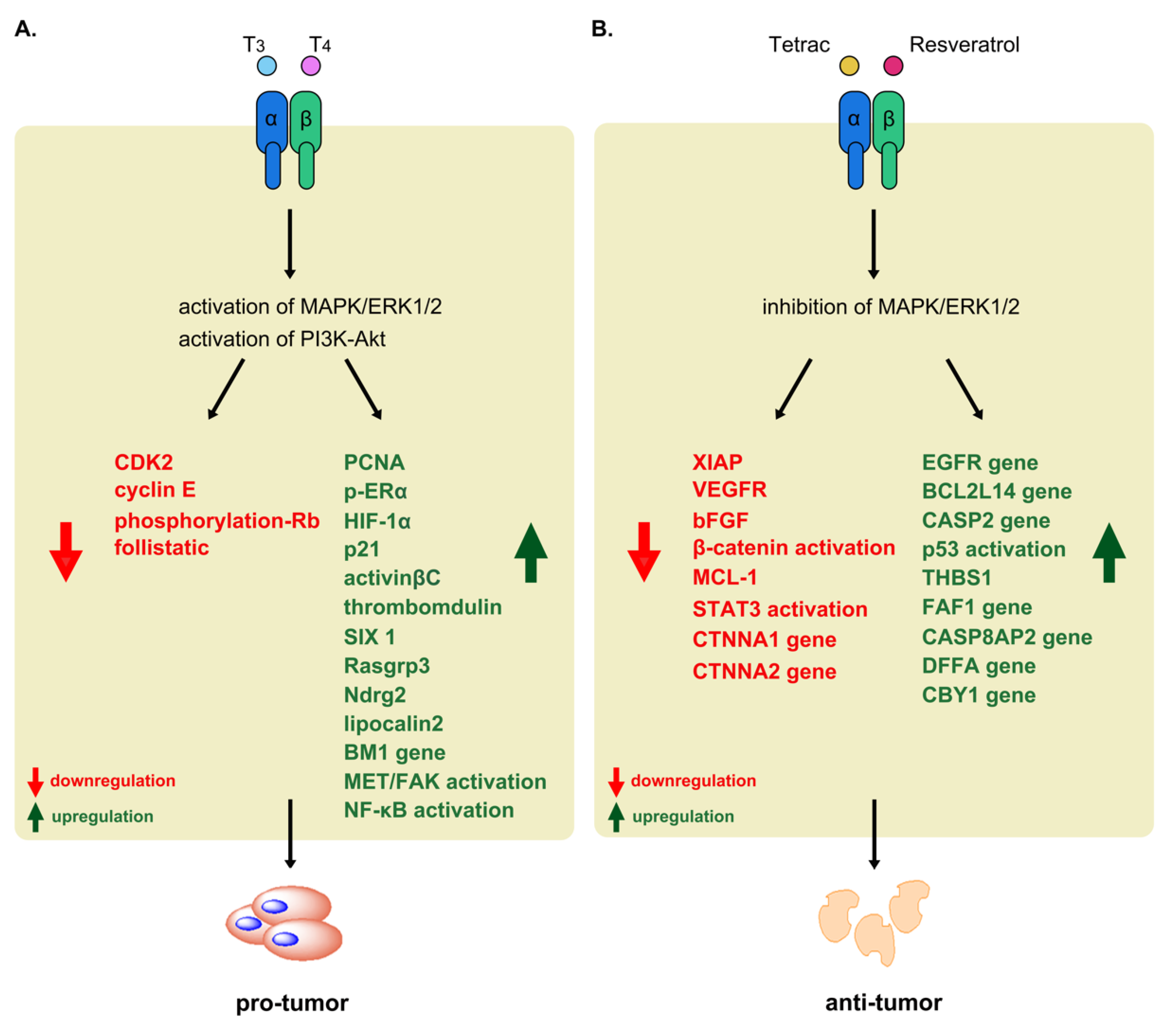
| Cancer Types | Thyroxine | Binding Receptor | Molecular Mechanisms | Physiological Processes | Ref. |
|---|---|---|---|---|---|
| Breast cancer | T3, T4 | αvβ3 | activation of MAPK/ERK1/2 | proliferation↑ | [58,75] |
| T3 | αvβ3 | downregulation of SMP30 gene | anti-cancer↑, apoptosis↑ | [76] | |
| T3 | TRβ | downregulation of T1 gene | proliferation↓ | [77] | |
| T3 | TRβ | inhibition of STAT5 signaling | development↓ | [78] | |
| T3 | TRβ | downregulation of β-catenin | prognosis↑ | [70] | |
| Thyroid cancer | T3, T4 | αvβ3 | activation of MAPK/ERK1/2 | proliferation↑, anti-apoptosis↑ | [79] |
| T3 | TRβΔ | activation of PI3K-Akt | metastatic↓, development↑ | [80] | |
| T3 | TRβ | inhibition of PI3K-Akt | tumor growth↑ | [80] | |
| increase p27 | proliferation↓ | ||||
| decrease cyclin D | |||||
| Lung cancer | T3, T4 | αvβ3 | increase proliferating cell nuclear antigen (PCNA) | proliferation↑ | [81] |
| induce ERα phosphorylation | |||||
| activation of MAPK/ERK1/2 | |||||
| Brain tumor | T3, T4 | αvβ3 | increase proliferating cell nuclear antigen (PCNA) | tumor growth↑ | [57,82,83,84] |
| activation of MAPK/ERK1/2 | |||||
| T3 | TRβ | activation of PI3K-Akt | proliferation↑ | [57] | |
| upregulation of HIF-1α gene | |||||
| T3 | TRα | expression of TRα1 and TRα2 | tumor grade↓, tumor malignancy↓ | [85] | |
| Liver cancer | T3 | TRα, TRβ | downregulation of CDK2, cyclin E, phosphorylation-Rb | proliferation↑ | [86,87] |
| upregulation of p21 | |||||
| T3, T4 | αvβ3 | induction of DKK4 | cell invasion↓ | [88,89] | |
| reduction of MMP2 | metastatic↓ | ||||
| downregulation of ELF2 | |||||
| T3 | TRαΔ | dysregulation of follistatin, activinβC, thrombomodulin, SIX1, Rasgrp3, Ndrg2 | development↑, carcinogenesis↑ | [90] | |
| T3 | TRα | upregulation of lipocalin 2 | invasion↑, metastasis↑ | [91] | |
| T4 | TRα, TRβ | activation of NF-κB | cancer stem like cell↑ | [92] | |
| activation of BM1 gene | drug resistance↑ | ||||
| T3 | αvβ3 | activation of ERK1/2/Akt | tumor growth↑ | [93] | |
| T3, T4 | TRα | activation of MET/FAK | invasion↑, metastasis↑ | [94] | |
| Colorectal cancer | T4 | αvβ3 | increase proliferating cell nuclear antigen (PCNA), cyclin D1, c-myc | proliferation↑ | [95,96] |
| T4 | TRα | activation of NF-κB | Tumor progression↑, metastasis↑ | [97] | |
| T3 | TRα1 | activation of Frizzled-related protein, sFRP2 | proliferation↑ | [98] | |
| modulation of β-catenin |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-C.; Yeh, C.-T.; Lin, K.-H. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int. J. Mol. Sci. 2019, 20, 4986. https://doi.org/10.3390/ijms20204986
Liu Y-C, Yeh C-T, Lin K-H. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. International Journal of Molecular Sciences. 2019; 20(20):4986. https://doi.org/10.3390/ijms20204986
Chicago/Turabian StyleLiu, Yu-Chin, Chau-Ting Yeh, and Kwang-Huei Lin. 2019. "Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis" International Journal of Molecular Sciences 20, no. 20: 4986. https://doi.org/10.3390/ijms20204986
APA StyleLiu, Y.-C., Yeh, C.-T., & Lin, K.-H. (2019). Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. International Journal of Molecular Sciences, 20(20), 4986. https://doi.org/10.3390/ijms20204986





